Plant Nitrogen and Phosphorus Resorption in Response to Varied Legume Proportions in a Restored Grassland
Abstract
:1. Introduction
2. Results
2.1. Precipitation during Growing Season
2.2. Soil Moisture and [N, P] Availability
2.3. Plant Density and Biomass
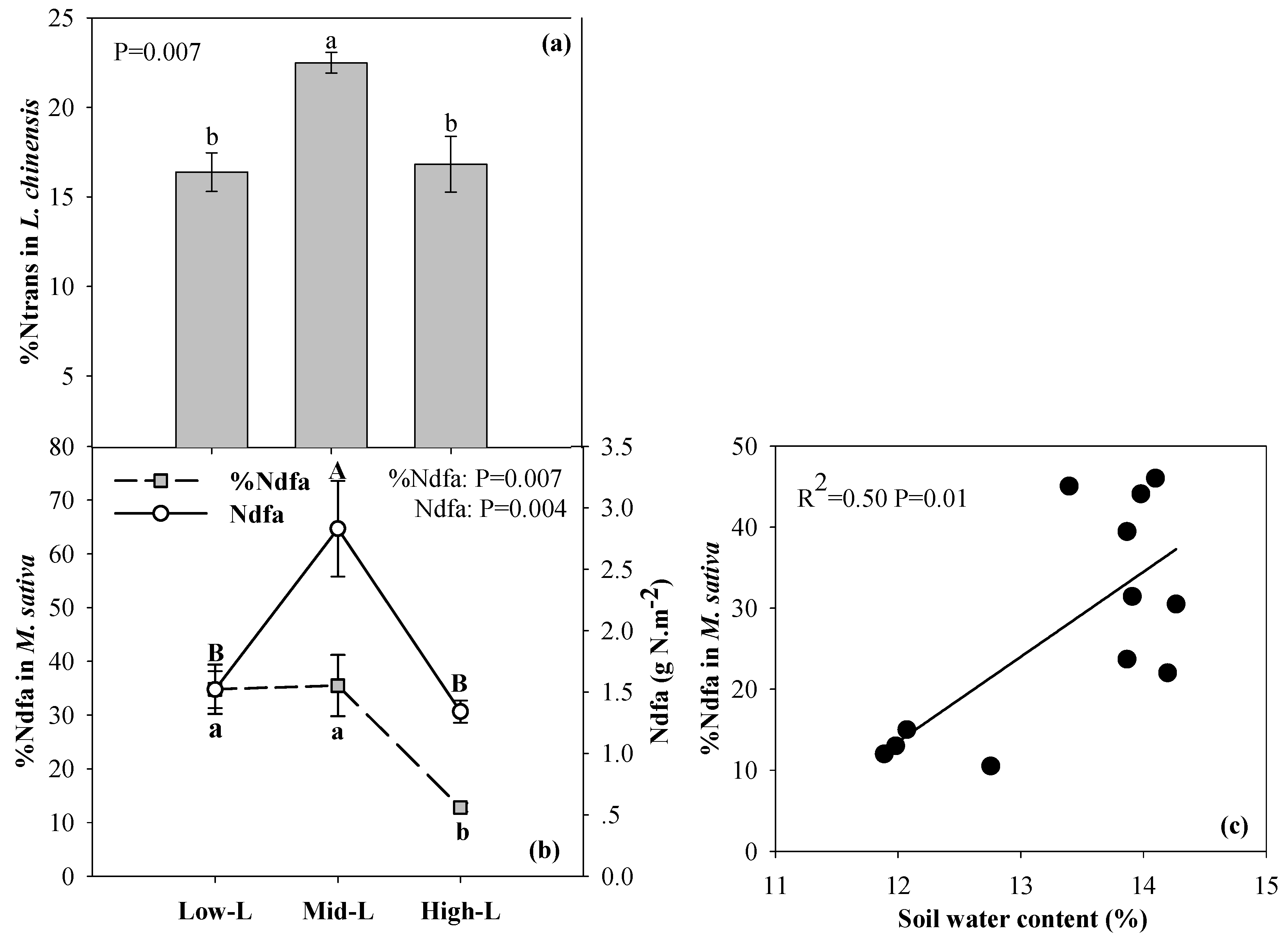
2.4. N Transfer in L. Chinensis and Biological N Fixation of M. Sativa
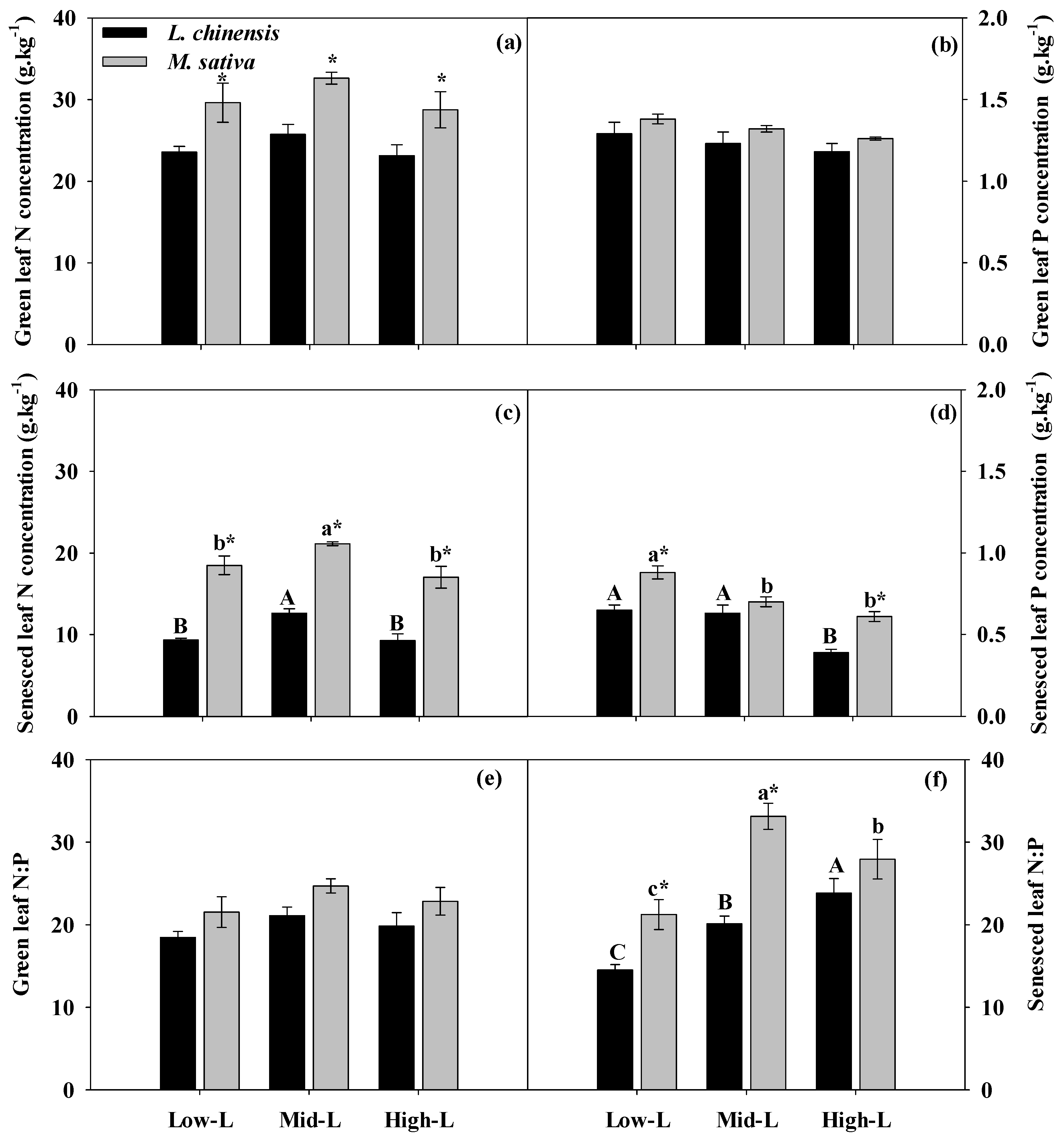
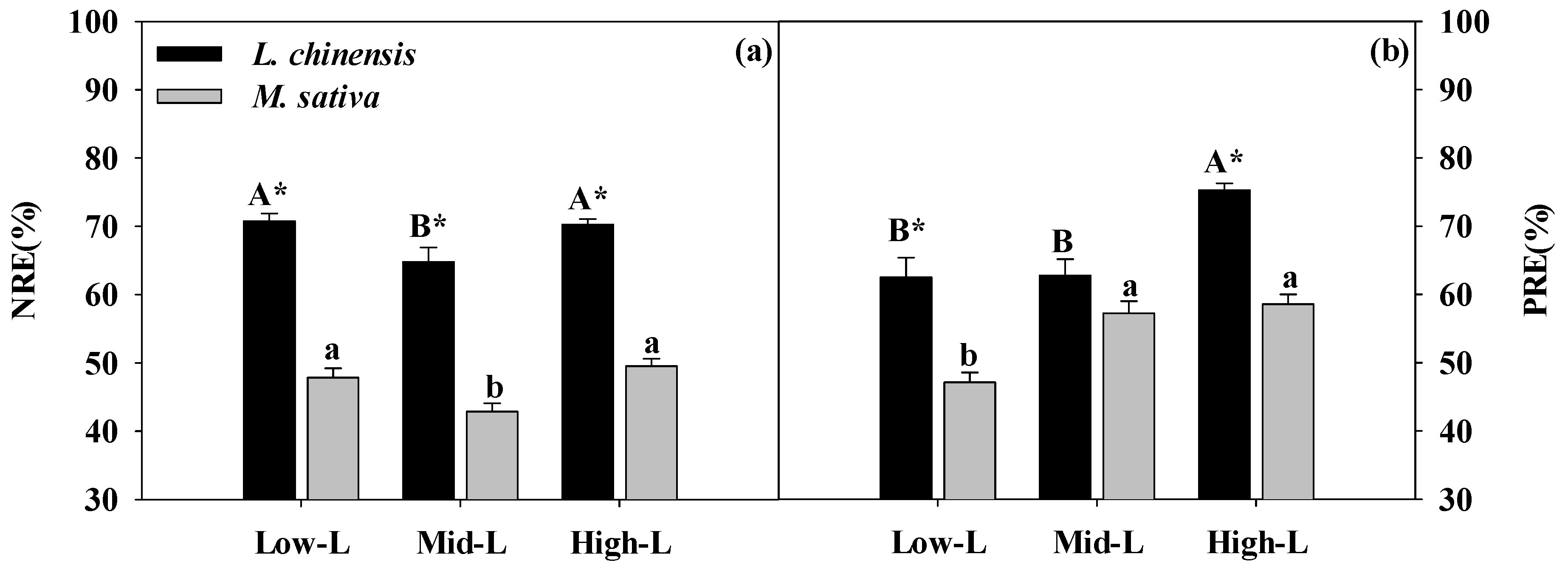
2.5. Leaf [N, P] concentrations and [N, P] resorption
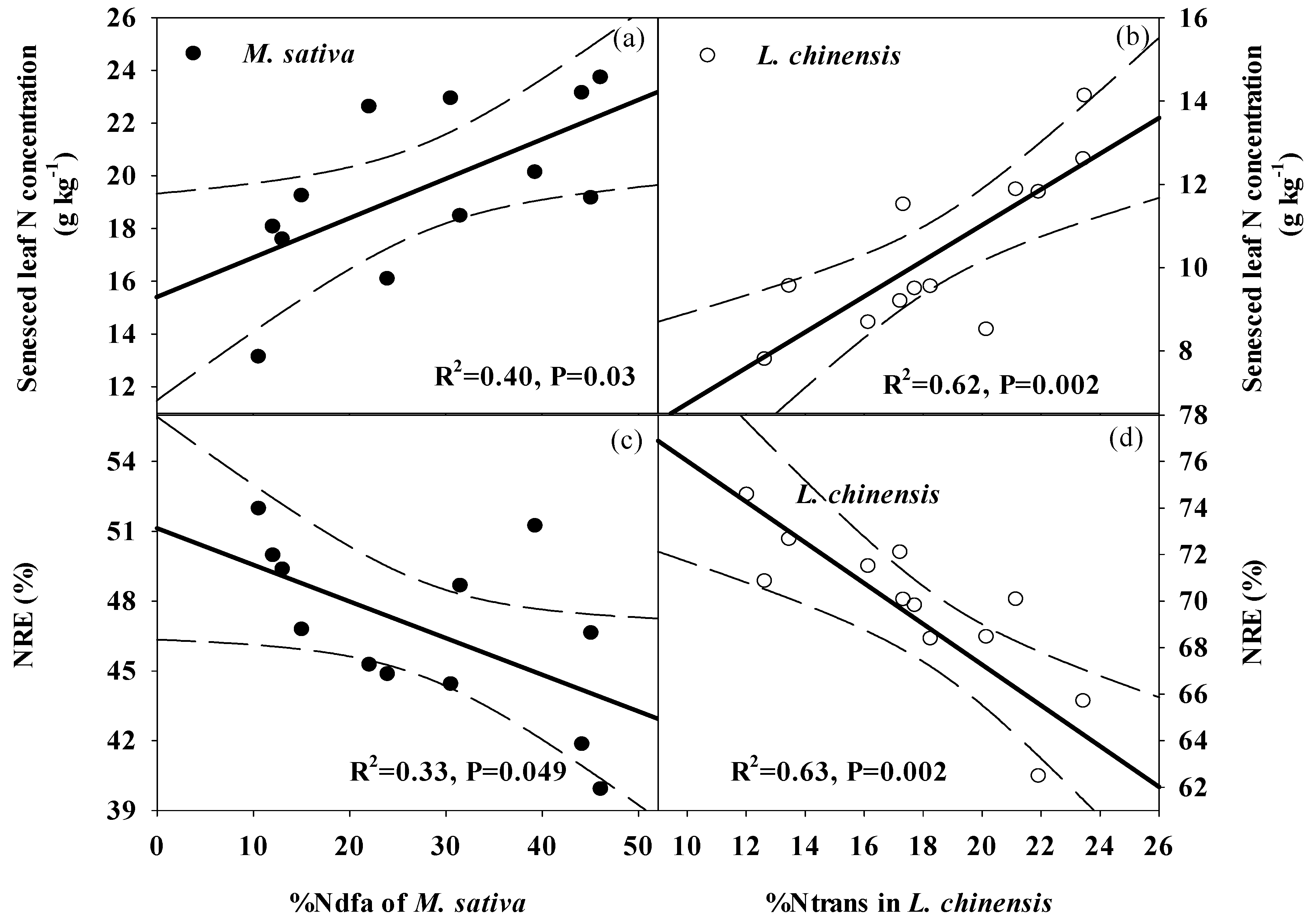
2.6. The Correlations between Nutrients Resorption and Legume-Driven N, and Environmental Factors
3. Discussion
3.1. Legume Proportions Influence Soil Water, N Fixation of M. Sativa, and N and P Availability in Soil
3.2. Effects of Legume Proportions on Growth and Nutrient Uptake
3.3. Effects of Legume Proportions on N and P Resorption
4. Materials and Methods
4.1. Study Site
4.2. Experiment design
4.3. Experimental Set up
4.4. Samples Collection and Measurement
4.5. Calculations
4.6. Statistic Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Killingbeck, K.T. The terminological jungle revisited-making a case for use of the term resorption. Oikos 1986, 46, 263–264. [Google Scholar] [CrossRef]
- Yuan, Z.Y.; Li, L.H.; Han, X.G.; Chen, S.P.; Wang, Z.W.; Chen, Q.S.; Bai, W.M. Nitrogen response efficiency increased monotonically with decreasing soil resource availability: A case study from a semiarid grassland in northern China. Oecologia 2006, 148, 564–572. [Google Scholar] [CrossRef]
- Aerts, R. Nutrient resorption from senescing leaves of perennials: Are there general patterns? J. Ecol. 1996, 84, 597–608. [Google Scholar] [CrossRef]
- Yuan, Z.Y.; Chen, H.Y. Global-scale patterns of nutrient resorption associated with latitude, temperature and precipitation. Glob. Ecol. Bio. 2009, 18, 11–18. [Google Scholar] [CrossRef]
- Aerts, R.; Chapin, F.S. The mineral nutrition of wild plants revisited: A re-evaluation of processes and patterns. Adv. Ecol. Res. 2000, 30, 1–67. [Google Scholar]
- Denton, M.D.; Veneklaas, E.J.; Freimoser, F.M.; Lambers, H. Banksia species (Proteaceae) from severely phosphorus-impoverished soils exhibit extreme efficiency in the use and re-mobilisation of phosphorus. Plant Cell Environ. 2007, 30, 1557–1565. [Google Scholar] [CrossRef]
- Venterink, H.O.; Güsewell, S. Competitive interactions between two meadow grasses under nitrogen and phosphorus limitation. Funct. Ecol. 2010, 24, 877–886. [Google Scholar] [CrossRef]
- Shi, M.; Fisher, J.B.; Brzostek, E.R.; Phillips, R.P. Carbon cost of plant nitrogen acquisition: Global carbon cycle impact from an improved plant nitrogen cycle in the Community Land Model. Glob. Chang. Biol. 2016, 22, 1299–1314. [Google Scholar] [CrossRef]
- Manzoni, S.; Trofymow, J.A.; Jackson, R.B.; Porporato, A. Stoichiometric controls on carbon, nitrogen, and phosphorus dynamics in decomposing litter. Ecol. Monogr. 2010, 80, 89–106. [Google Scholar] [CrossRef]
- Vergutz, L.; Manzoni, S.; Porporato, A.; Novais, R.F.; Jackson, R.B. Global resorption efficiencies and concentrations of carbon and nutrients in leaves of terrestrial plants. Ecol. Monogr. 2012, 82, 205–220. [Google Scholar] [CrossRef] [Green Version]
- Killingbeck, K.T. Nutrients in senesced leaves: Keys to the search for potential resorption and resorption proficiency. Ecology 1996, 77, 1716–1727. [Google Scholar] [CrossRef]
- Freschet, G.T.; Cornelissen, J.H.; Van Logtestijn, R.S.; Aerts, R. Substantial nutrient resorption from leaves, stems and roots in a subarctic flora: What is the link with other resource economics traits? New Phytol. 2010, 186, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Lü, X.T.; Reed, S.; Yu, Q.; He, N.P.; Wang, Z.W.; Han, X.G. Convergent responses of nitrogen and phosphorus resorption to nitrogen inputs in a semiaridgrassland. Glob. Chang. Biol. 2013, 19, 2775–2784. [Google Scholar]
- Lü, X.T.; Han, X.G. Nutrient resorption responses to water and nitrogen amendment in semi-arid grassland of Inner Mongolia, China. Plant Soil 2010, 327, 481–491. [Google Scholar] [CrossRef]
- Li, L.; Gao, X.P.; Li, X.Y.; Lin, L.S.; Zeng, F.J.; Gui, D.W.; Lu, Y. Nitrogen (N) and phosphorus (P) resorption of two dominant alpine perennial grass species in response to contrasting N and P availability. Environ. Exp. Bot. 2016, 127, 37–44. [Google Scholar] [CrossRef]
- Ågren, G.I.; Wetterstedt, J.M.; Billberger, M.F. Nutrient limitation on terrestrial plant growth–modeling the interaction between nitrogen and phosphorus. New Phytol. 2012, 194, 953–960. [Google Scholar] [CrossRef]
- Li, H.; Li, M.C.; Luo, J.; Cao, X.; Qu, L.; Gai, Y.; Jiang, X.N.; Liu, T.X.; Bai, H.; Janz, D.; et al. N-fertilization has different effects on the growth, carbon and nitrogen physiology, and wood properties of slow-and fast-growing Populus species. J. Exp. Bot. 2012, 63, 6173–6185. [Google Scholar] [CrossRef]
- Brant, A.N.; Chen, H.Y. Patterns and mechanisms of nutrient resorption in plants. Crit. Rev. Plant Sci. 2015, 34, 471–486. [Google Scholar] [CrossRef]
- Chapin, F.S.; Moilanen, L. Nutritional controls over nitrogen and phosphorus resorption from Alaskan birch leaves. Ecology 1991, 72, 709–715. [Google Scholar] [CrossRef]
- Li, J.; Guo, Q.; Zhang, J.; Korpelainen, H.; Li, C. Effects of nitrogen and phosphorus supply on growth and physiological traits of two Larix species. Environ. Exp. Bot. 2016, 130, 206–215. [Google Scholar] [CrossRef]
- Herridge, D.F.; Peoples, M.B.; Boddey, R.M. Global inputs of biological nitrogen fixation in agricultural systems. Plant Soil 2008, 311, 1–18. [Google Scholar] [CrossRef]
- Li, Q.; Song, Y.T.; Li, G.D.; Yu, P.J.; Wang, P.; Zhou, D.W. Grass-legume mixtures impact soil N, species recruitment, and productivity in temperate steppe grassland. Plant Soil 2015, 394, 271–285. [Google Scholar] [CrossRef]
- Trannin, W.S.; Urquiaga, S.; Guerra, G.; Ibijbijen, J.; Cadisch, G. Interspecies competition and N transfer in a tropical grass-legume mixture. Biol. Fertil. Soils 2000, 32, 441–448. [Google Scholar] [CrossRef]
- Peoples, M.B.; Chalk, P.M.; Unkovich, M.J.; Boddey, R.M. Can differences in 15N natural abundance be used to quantify the transfer of nitrogen from legumes to neighbouring non-legume plant species? Soil Biol. Biochem. 2015, 87, 97–109. [Google Scholar] [CrossRef]
- Peoples, M.B.; Swan, A.D.; Goward, L.; Kirkegaaard, J.A.; Hunt, J.R.; Li, G.D.; Schwenke, G.D.; Herridge, D.F.; Moodie, M.; Wilhelm, N.; et al. Soil mineral nitrogen benefits derived from legumes and comparisons of the apparent recovery of legume or fertiliser nitrogen by wheat. Soil Res. 2017, 55, 600–615. [Google Scholar] [CrossRef] [Green Version]
- Mortenson, M.C.; Schuman, G.E.; Ingram, L.J. Carbon sequestration in rangelands interseeded with yellow-flowering alfalfa (Medicago sativa ssp. falcata). Environ. Manag. 2004, 33, 475–481. [Google Scholar] [CrossRef]
- Fornara, D.; Tilman, D. Plant functional composition influences rates of soil carbon and nitrogen accumulation. J. Ecol. 2008, 96, 314–322. [Google Scholar] [CrossRef]
- Li, Q.; Yu, P.J.; Li, G.D.; Zhou, D.W. Grass-legume ratio can change soil carbon and nitrogen storage in a temperate steppe grassland. Soil Tillage Res. 2016, 157, 23–31. [Google Scholar] [CrossRef]
- Peoples, M.B.; Baldock, J.A. Nitrogen dynamics of pastures: Nitrogen fixation inputs, the impact of legumes on soil nitrogen fertility, and the contributions of fixed nitrogen to Australian farming systems. Aust. J. Exp. Agric. 2001, 41, 327–346. [Google Scholar] [CrossRef]
- Lü, X.T.; Hu, Y.Y.; Wolf, A.A.; Han, X.G. Species richness mediates within-species nutrient resorption: Implications for the biodiversity-productivity relationship. J. Ecol. (In press).
- Nyfeler, D.; Huguenin-Elie, O.; Suter, M.; Frossard, E.; Lüscher, A. Grass-legume mixtures can yield more nitrogen than legume pure stands due to mutual stimulation of nitrogen uptake from symbiotic and non-symbiotic sources. Agric. Ecosyst. Environ. 2011, 140, 155–163. [Google Scholar] [CrossRef]
- Yuan, Z.Q.; Yu, K.L.; Epstein, H.; Fang, C.; Li, J.T.; Liu, Q.Q.; Liu, X.W.; Gao, W.J.; Li, F.M. Effects of legume species introduction on vegetation and soil nutrient development on abandoned croplands in a semi-arid environment on the Loess Plateau, China. Sci. Total Environ. 2016, 541, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Siddique, I.; Engel, V.L.; Parrotta, J.A.; Lamb, D.; Nardoto, G.B.; Ometto, J.P.; Martinelli, L.A.; Schmidt, S. Dominance of legume trees alters nutrient relations in mixed species forest restoration plantings within seven years. Biogeochemisty 2008, 88, 89–101. [Google Scholar] [CrossRef]
- Augusto, L.; Delerue, F.; Gallet-Budynek, A.; Achat, D.L. Global assessment of limitation to symbiotic nitrogen fixation by phosphorus availability in terrestrial ecosystems using a meta-analysis approach. Glob. Biogeochem. Cycles 2011, 27, 804–815. [Google Scholar] [CrossRef]
- Crème, A.; Rumpel, C.; Gastal, F.; Gil, M.D.; Chabbi, A. Effects of grasses and a legume grown in monoculture or mixture on soil organic matter and phosphorus forms. Plant Soil 2016, 402, 117–128. [Google Scholar] [CrossRef] [Green Version]
- Nuruzzaman, M.; Lambers, H.; Bolland, M.D.A.; Veneklaas, E.J. Phosphorus benefits of different legume crops to subsequent wheat grown in different soils of Western Australia. Plant Soil 2005, 271, 175–187. [Google Scholar] [CrossRef]
- Li, L.; Li, S.M.; Sun, J.H.; Zhou, L.L.; Bao, X.G.; Zhang, H.G.; Zhang, F.S. Diversity enhances agricultural productivity via rhizosphere phosphorus facilitation on phosphorus-deficient soils. Proc. Natl. Acad. Sci. USA 2007, 104, 11192–11196. [Google Scholar] [CrossRef] [Green Version]
- Mayor, J.R.; Wright, S.J.; Turner, B.L. Species-specific responses of foliar nutrients to long--term nitrogen and phosphorus additions in a lowland tropical forest. J. Ecol. 2014, 102, 36–44. [Google Scholar] [CrossRef]
- Li, Q.; Chen, X.Y.; Zhou, D.W. Shoot nutrient content and nutrient resorption of Leymus chinensis in various legume mixtures. Front. Plant Sci. 2018, 9, 1483. [Google Scholar] [CrossRef]
- Ledgard, S.F.; Steele, K.W. Biological nitrogen fixation in mixed legume/grass pastures. Plant Soil 1992, 141, 137–153. [Google Scholar] [CrossRef]
- Yu, Q.; Chen, Q.S.; Elser, J.J.; He, N.P.; Wu, H.H.; Zhang, G.M.; Wu, J.G.; Bai, Y.F.; Han, X.G. Linking stoichiometric homeostasis with ecosystem structure, functioning, and stability. Ecol. Lett. 2010, 13, 1390–1399. [Google Scholar] [CrossRef] [PubMed]
- Kobe, R.K.; Lepczyk, C.A.; Iyer, M. Resorption efficiency decreases with increasing green leaf nutrients in a global data set. Ecology 2005, 86, 2780–2792. [Google Scholar] [CrossRef]
- Liu, P.; Sun, O.J.; Huang, J.; Li, L.; Han, X. Nonadditive effects of litter mixtures on decomposition and correlation with initial litter N and P concentrations in grassland plant species of northern China. Biol. Fertil. Soil 2007, 44, 211–216. [Google Scholar] [CrossRef]
- Sparks, D.L.; Page, A.L.; Helmke, P.A.; Loeppert, R.H.; Soltanpour, P.N.; Tabatabai, M.A.; Johnston, C.T.; Sumner, M.E. Methods of Soil Analysis. Part 3-Chemical Methods. Am. Soc. Agron. 1996, 5, 1085–1121. [Google Scholar]
- Schade, J.D.; Kyle, M.; Hobbie, S.E.; Fagan, W.F.; Elser, J.J. Stoichiometric tracking of soil nutrients by a desert insect herbivore. Ecol. Lett. 2003, 6, 96–101. [Google Scholar] [CrossRef] [Green Version]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S.; Dean, L.A. Estimation of available phosphorus in soils by extraction with sodium bicarbonate. In USDA Circular 939; US Department of Agriculture: Washington, DC, USA, 1954. [Google Scholar]
- Unkovich, M.; Herridge, D.; Peoples, M.; Cadish, G.; Boddey, B.; Giller, K.; Alves, B.; Chalk, P. Measuring Plant Associated Nitrogen Fixation in Agricultural Systems; Australian Centre for International Agricultural Research: Canberra, Australia, 2008.
- Snoeck, D.; Zapata, F.; Domenach, A.M. Isotopic evidence of the transfer of nitrogen fixed by legumes to coffee. Biotechnol. Agron. Soc. Environ. 2000, 4, 95–100. [Google Scholar]



| S | LP | S × LP | ||||
|---|---|---|---|---|---|---|
| F Value | p Value | F Value | p Value | F Value | p Value | |
| Green leaf N | 22.993 | <0.001 | 2.371 | 0.122 | 0.082 | 0.921 |
| Green leaf P | 5.204 | 0.035 | 2.833 | 0.085 | 0.009 | 0.991 |
| Green leaf N:P | 8.243 | 0.010 | 2.223 | 0.137 | 0.028 | 0.972 |
| Senesced leaf N | 181.779 | <0.001 | 18.680 | <0.001 | 1.372 | 0.279 |
| Senesced leaf P | 41.867 | <0.001 | 31.603 | <0.001 | 3.779 | 0.043 |
| Senesced leaf N:P | 35.466 | <0.001 | 17.658 | <0.001 | 3.897 | 0.039 |
| NRE | 388.294 | <0.001 | 12.219 | <0.001 | 0.323 | 0.728 |
| PRE | 62.602 | <0.001 | 19.651 | <0.001 | 4.981 | 0.019 |
| Response Variable | Regression Parameters for Each Dependent Variable | |||||||
|---|---|---|---|---|---|---|---|---|
| Intercept | Soil Water Content (%) | Soil Inorganic N (mg.kg−1) | Soil Available p (mg.kg−1) | Plant Available N:P in Soil | Overall R2 | Overall F Value | ||
| L. chinensis | Senesced leaf N (g.kg−1) | −5.763 | −0.180 | 0.567 ** | 0.112 | −0.138 | 0.657 | 19.187 ** |
| NRE(%) | 93.430 *** | 0.156 | −0.871 * | 0.097 | −0.117 | 0.383 | 6.198 * | |
| Senesced leaf P (g.kg−1) | −0.914 * | 0.441 | 0.184 | 0.461 ** | 0.184 | 0.681 | 21.323 ** | |
| PRE (%) | 152.126 *** | −6.386 ** | 0.185 | −0.190 | 0.151 | 0.630 | 17.039 ** | |
| M. sativa | Senesced leaf N (g.kg−1) | −5.871 | 0.278 | 0.892 ** | 0.184 | −0.207 | 0.562 | 12.813 ** |
| NRE (%) | 74.783 *** | −0.235 | −0.983 ** | −0.192 | 0.213 | 0.541 | 11.799 ** | |
| Senesced leaf P (g.kg−1) | −0.537 | −0.219 | −0.416 | 0.398 ** | −0.387 | 0.585 | 14.113 ** | |
| PRE (%) | 38.148 | −2.697 * | −0.204 | −0.191 | 5.824** | 0.742 | 12.937 ** | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Zhou, D.; Denton, M.D. Plant Nitrogen and Phosphorus Resorption in Response to Varied Legume Proportions in a Restored Grassland. Plants 2020, 9, 292. https://doi.org/10.3390/plants9030292
Li Q, Zhou D, Denton MD. Plant Nitrogen and Phosphorus Resorption in Response to Varied Legume Proportions in a Restored Grassland. Plants. 2020; 9(3):292. https://doi.org/10.3390/plants9030292
Chicago/Turabian StyleLi, Qiang, Daowei Zhou, and Matthew D. Denton. 2020. "Plant Nitrogen and Phosphorus Resorption in Response to Varied Legume Proportions in a Restored Grassland" Plants 9, no. 3: 292. https://doi.org/10.3390/plants9030292





