Growth under Fluctuating Light Reveals Large Trait Variation in a Panel of Arabidopsis Accessions
Abstract
:1. Introduction
2. Results
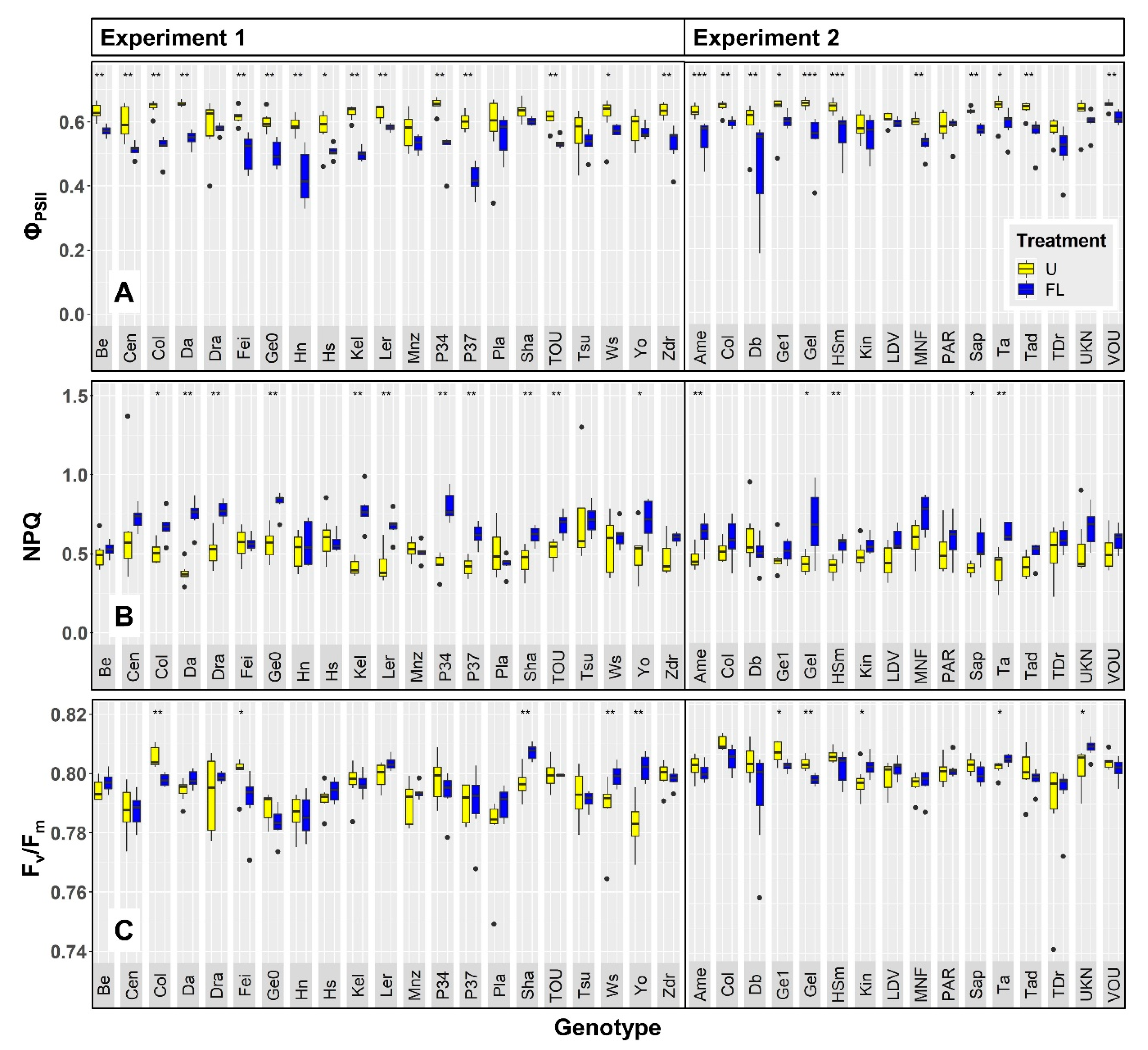
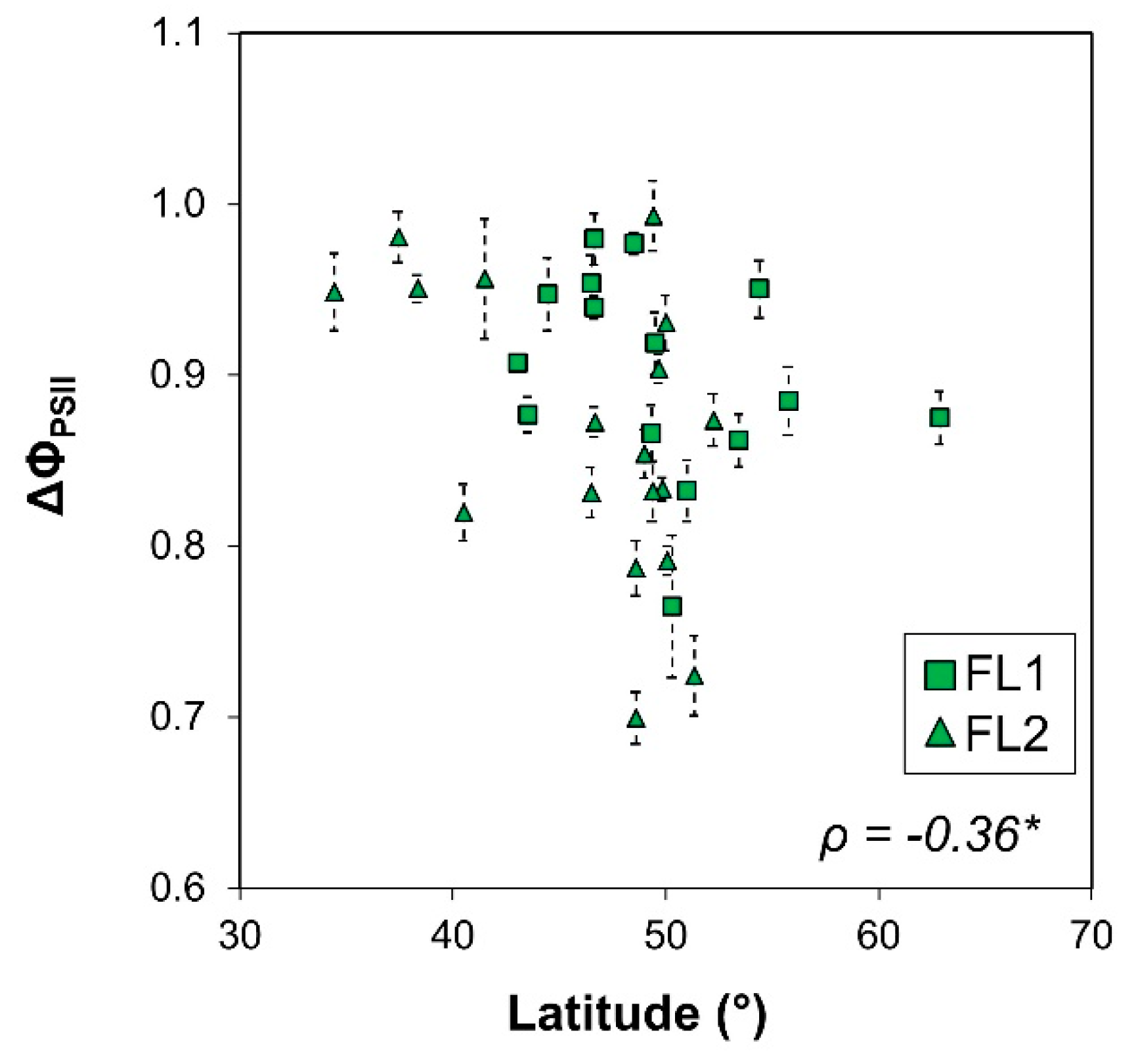
2.1. Chlorophyll a Fluorescence
2.2. Growth and Development
2.3. Principle Component Analysis
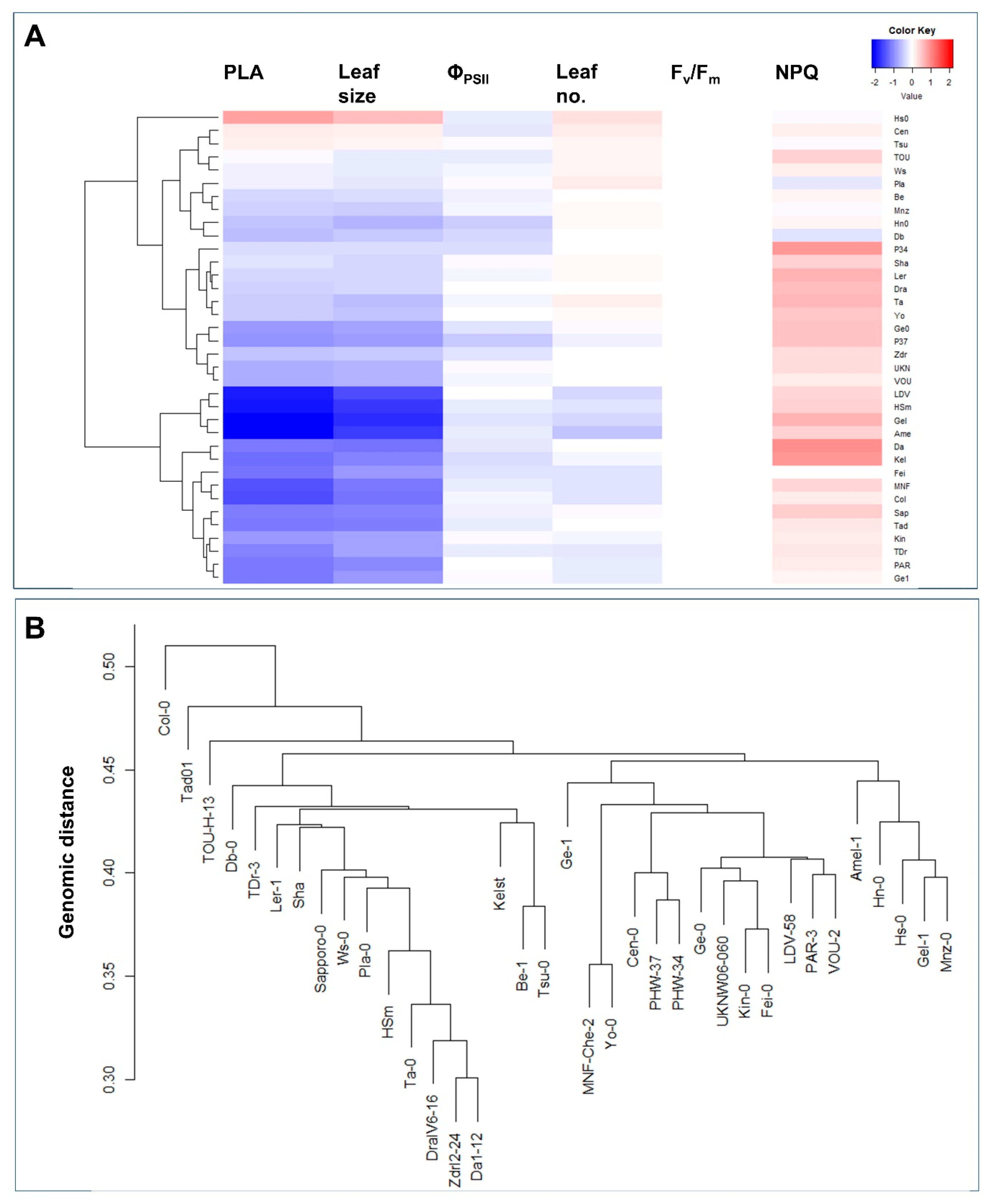
2.4. Phenotypic and Genomic Cluster Analysis
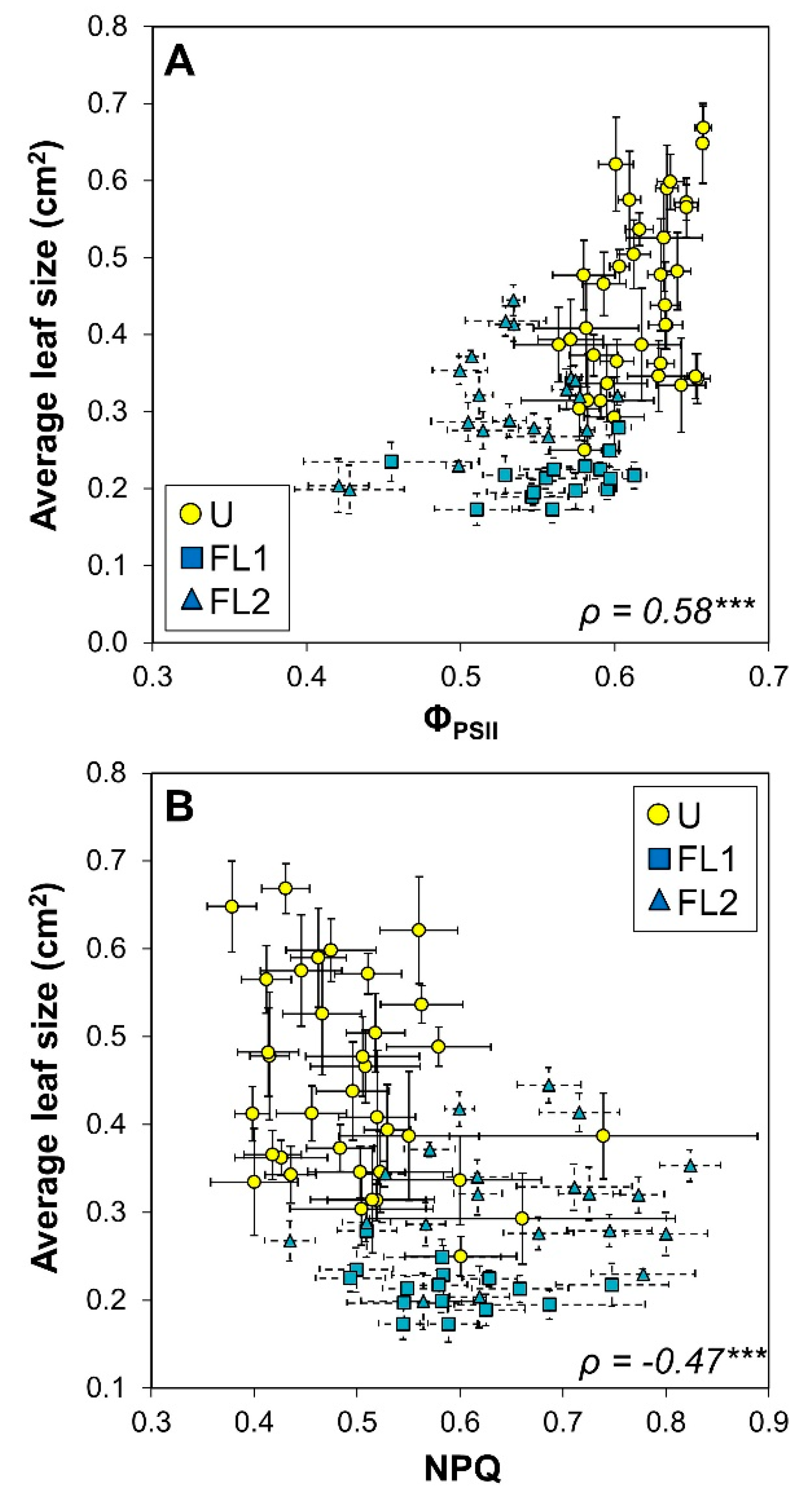
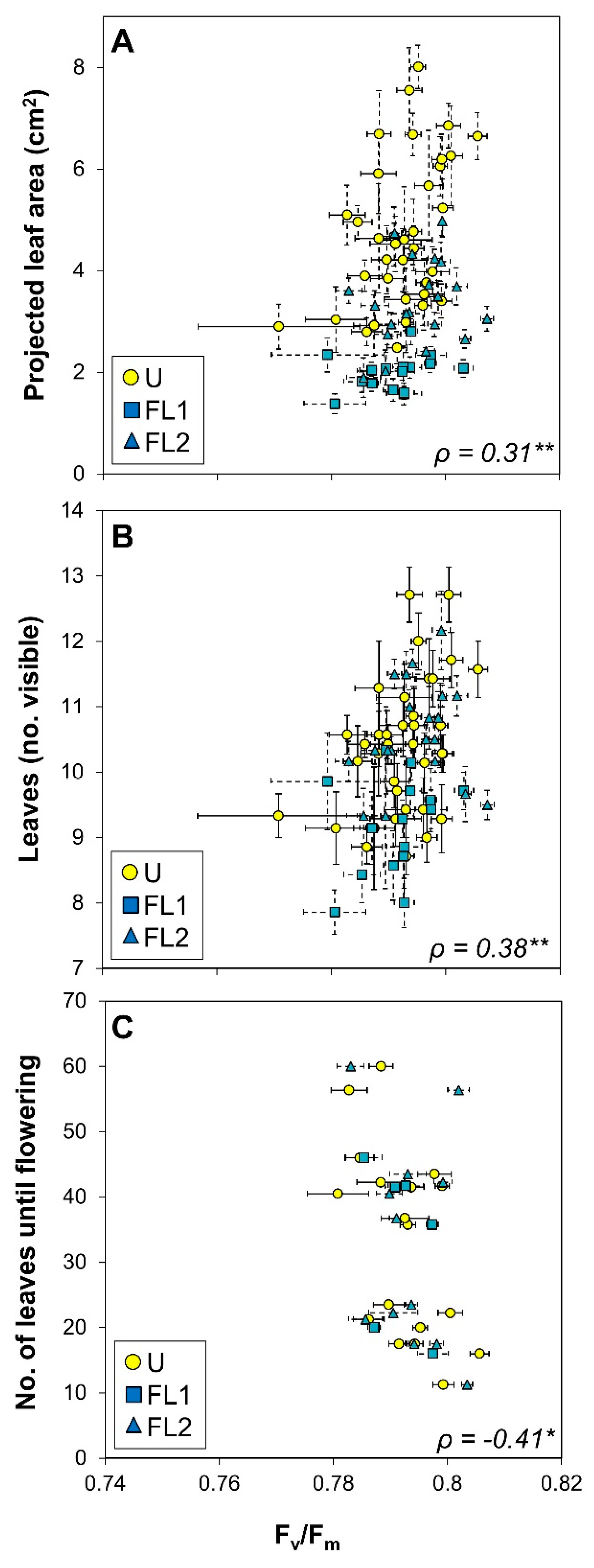
2.5. Correlation Analysis
3. Discussion
3.1. Fluctuating Light Reduces Visible Leaf Area Most Strongly in Plants with High PLA Under Uniform Light
3.2. Large Natural Genetic Variation for PLA and Chlorophyll a Fluorescence Traits
3.3. Correlations between Growth and Fluorescence: A Case for Rapid Phenotyping
3.4. Can Evolutionary Adaptation Explain the Phenotype of an Accession?
4. Materials and Methods
4.1. Plant Material, Growth Conditions and Treatments
4.2. Chlorophyll a Fluorescence
4.3. Growth and Development
4.4. Clustering of Accessions by Phenotypic Differences
4.5. Principal Component Analysis
4.6. Genomic Distances
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alter, P.; Dreissen, A.; Luo, F.L.; Matsubara, S. Acclimatory responses of Arabidopsis to fluctuating light environment: Comparison of different sunfleck regimes and accessions. Photosynth. Res. 2012, 113, 221–237. [Google Scholar] [CrossRef] [Green Version]
- Matthews, J.S.A.; Vialet-Chabrand, S.; Lawson, T. Acclimation to fluctuating light impacts the rapidity of response and diurnal rhythm of stomatal conductance. Plant Physiol. 2018, 176, 939–1951. [Google Scholar] [CrossRef] [Green Version]
- Schneider, T.; Bolger, A.; Zeier, J.; Preiskowski, S.; Benes, V.; Trenkamp, S.; Usadel, B.; Farré, E.M.; Matsubara, S. Fluctuating light interacts with time of day and leaf development stage to reprogram gene expression. Plant Physiol. 2019, 179, 1632–1657. [Google Scholar] [CrossRef] [Green Version]
- Rooijen, R.V.; Aarts, M.G.M.; Harbinson, J. Natural genetic variation for acclimation of photosynthetic light use efficiency to growth irradiance in Arabidopsis. Plant Physiol. 2015, 167, 1412–1429. [Google Scholar] [CrossRef] [Green Version]
- Van Rooijen, R.; Kruijer, W.; Boesten, R.; Van Eeuwijk, F.A.; Harbinson, J.; Aarts, M.G.M. Natural variation of YELLOW SEEDLING1 affects photosynthetic acclimation of Arabidopsis thaliana. Nat. Commun. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Cruz, J.A.; Savage, L.J.; Zegarac, R.; Hall, C.C.; Satoh-Cruz, M.; Davis, G.A.; Kovac, W.K.; Chen, J.; Kramer, D.M. Dynamic Environmental Photosynthetic Imaging Reveals Emergent Phenotypes. Cell Syst. 2016, 2, 365–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vialet-Chabrand, S.; Matthews, J.S.A.; Simkin, A.J.; Raines, C.A.; Lawson, T. Importance of fluctuations in light on plant photosynthetic acclimation. Plant Physiol. 2017, 173, 2163–2179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubásek, J.; Urban, O.; Šantrůček, J. C4 plants use fluctuating light less efficiently than do C3 plants: A study of growth, photosynthesis and carbon isotope discrimination. Physiol. Plant. 2013, 149, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Leakey, A.D.B.; Press, M.C.; Scholes, J.D.; Watling, J.R. Relative enhancement of photosynthesis and growth at elevated CO2 is greater under sunflecks than uniform irradiance in a tropical rain forest tree seedling. Plant, Cell Environ. 2002, 25, 1701–1714. [Google Scholar] [CrossRef]
- Vaseghi, M.J.; Chibani, K.; Telman, W.; Liebthal, M.F.; Gerken, M.; Schnitzer, H.; Mueller, S.M.; Dietz, K.J. The chloroplast 2-cysteine peroxiredoxin functions as thioredoxin oxidase in redox regulation of chloroplast metabolism. Elife 2018, 7, 1–28. [Google Scholar] [CrossRef]
- Kaiser, E.; Matsubara, S.; Harbinson, J.; Heuvelink, E.; Marcelis, L.F.M. Acclimation of photosynthesis to lightflecks in tomato leaves: Interaction with progressive shading in a growing canopy. Physiol. Plant. 2018, 162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaiser, E.; Galvis, V.C.; Armbruster, U. Efficient photosynthesis in dynamic light environments: A chloroplast’ s perspective. Biochem. J. 2019, 476, 2725–2741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearcy, R.W.; Krall, J.P.; Sassenrath-Cole, G.F. Photosynthesis in fluctuating light environments. In Photosynthesis and the Environment; Baker, N.R., Ed.; Kluwer Academic: Dordrecht, The Netherlands, 1996; pp. 321–346. [Google Scholar]
- Zhu, X.G.; Ort, D.R.; Whitmarsh, J.; Long, S.P. The slow reversibility of photosystem II thermal energy dissipation on transfer from high to low light may cause large losses in carbon gain by crop canopies: A theoretical analysis. J. Exp. Bot. 2004, 55, 1167–1175. [Google Scholar] [CrossRef] [PubMed]
- Suorsa, M.; Järvi, S.; Grieco, M.; Nurmi, M.; Pietrzykowska, M.; Rantala, M.; Kangasjärvi, S.; Paakkarinen, V.; Tikkanen, M.; Jansson, S.; et al. PROTON GRADIENT REGULATION5 is essential for proper acclimation of Arabidopsis photosystem I to naturally and artificially fluctuating light conditions. Plant Cell 2012, 24, 2934–2948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armbruster, U.; Carrillo, L.R.; Venema, K.; Pavlovic, L.; Schmidtmann, E.; Kornfeld, A.; Jahns, P.; Berry, J.A.; Kramer, D.M.; Jonikas, M.C. Ion antiport accelerates photosynthetic acclimation in fluctuating light environments. Nat. Commun. 2014, 5, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, S.; Badger, M.R. Photoprotection in plants: A new light on photosystem II damage. Trends Plant Sci. 2011, 16, 53–60. [Google Scholar] [CrossRef]
- Kanazawa, A.; Kramer, D.M. In vivo modulation of nonphotochemical exciton quenching (NPQ) by regulation of the chloroplast ATP synthase. PNAS 2002, 99, 12789–12794. [Google Scholar] [CrossRef] [Green Version]
- Armbruster, U.; Leonelli, L.; Galvis, V.C.; Strand, D.; Quinn, E.H.; Jonikas, M.C.; Niyogi, K.K. Regulation and levels of the thylakoid K+/H+ antiporter KEA3 shape the dynamic response of photosynthesis in fluctuating light. Plant Cell Physiol. 2016, 57, 1557–1567. [Google Scholar] [CrossRef] [Green Version]
- Caliandro, R.; Nagel, K.A.; Kastenholz, B.; Bassi, R.; Li, Z.; Niyogi, K.K.; Pogson, B.J.; Schurr, U.; Matsubara, S. Effects of altered α- and β-branch carotenoid biosynthesis on photoprotection and whole-plant acclimation of Arabidopsis to photo-oxidative stress. Plant, Cell Environ. 2013, 36, 438–453. [Google Scholar] [CrossRef]
- Kaiser, E.; Morales, A.; Harbinson, J.; Kromdijk, J.; Heuvelink, E.; Marcelis, L.F.M. Dynamic photosynthesis in different environmental conditions. J. Exp. Bot. 2015, 66. [Google Scholar] [CrossRef] [Green Version]
- Kaiser, E.; Morales, A.; Harbinson, J. Fluctuating light takes crop photosynthesis on a rollercoaster ride. Plant Physiol. 2018, 176. [Google Scholar] [CrossRef] [Green Version]
- Armbruster, U.; Correa Galvis, V.; Kunz, H.H.; Strand, D.D. The regulation of the chloroplast proton motive force plays a key role for photosynthesis in fluctuating light. Curr. Opin. Plant Biol. 2017, 37, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Körner, C. Paradigm shift in plant growth control. Curr. Opin. Plant Biol. 2015, 25, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Driever, S.M.; Lawson, T.; Andralojc, P.J.; Raines, C.A.; Parry, M.A.J. Natural variation in photosynthetic capacity, growth, and yield in 64 field-grown wheat genotypes. J. Exp. Bot. 2014, 65, 4959–4973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carmo-Silva, E.; Andralojc, P.J.; Scales, J.C.; Driever, S.M.; Mead, A.; Lawson, T.; Raines, C.A.; Parry, M.A.J. Phenotyping of field-grown wheat in the UK highlights contribution of light response of photosynthesis and flag leaf longevity to grain yield. J. Exp. Bot. 2017, 68, 3473–3486. [Google Scholar] [CrossRef]
- Qu, M.; Zheng, G.; Hamdani, S.; Essemine, J.; Song, Q.; Wang, H.; Chu, C.; Sirault, X.; Zhu, X.G. Leaf photosynthetic parameters related to biomass accumulation in a global rice diversity survey. Plant Physiol. 2017, 175, 248–258. [Google Scholar] [CrossRef] [Green Version]
- Murchie, E.H.; Kefauver, S.; Araus, J.L.; Muller, O.; Rascher, U.; Flood, P.J.; Lawson, T. Measuring the dynamic photosynthome. Ann. Bot. 2018, 122, 207–220. [Google Scholar] [CrossRef] [Green Version]
- van Bezouw, R.F.H.M.; Keurentjes, J.J.B.; Harbinson, J.; Aarts, M.G.M. Converging phenomics and genomics to study natural variation in plant photosynthetic efficiency. Plant J. 2019, 97, 112–133. [Google Scholar]
- Fiorani, F.; Schurr, U. Future Scenarios for Plant Phenotyping. Annu. Rev. Plant Biol. 2013, 64, 267–291. [Google Scholar] [CrossRef] [Green Version]
- Dutta, S.; Cruz, J.A.; Jiao, Y.; Chen, J.; Kramer, D.M.; Osteryoung, K.W. Non-invasive, whole-plant imaging of chloroplast movement and chlorophyll fluorescence reveals photosynthetic phenotypes independent of chloroplast photorelocation defects in chloroplast division mutants. Plant J. 2015, 84, 428–442. [Google Scholar] [CrossRef] [Green Version]
- Yendrek, C.R.; Tomaz, T.; Montes, C.M.; Cao, Y.; Morse, A.M.; Brown, P.J.; McIntyre, L.M.; Leakey, A.D.B.; Ainsworth, E.A. High-throughput phenotyping of maize leaf physiological and biochemical traits using hyperspectral reflectance. Plant Physiol. 2017, 173, 614–626. [Google Scholar] [CrossRef] [PubMed]
- Hancock, A.M.; Brachi, B.; Faure, N.; Horton, M.W.; Jarymowycz, L.B.; Sperone, F.G.; Toomajian, C.; Roux, F.; Bergelson, J. Adaptation to climate across the Arabidopsis thaliana genome. Science (80-. ) 2011, 334, 83–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.Y.; Wah, S.C.; Hong, Y.-N. Photoinactivation of Photosystem II in leaves of Capsicum annuum. Photosynth. Res. 1999, 105, 377–384. [Google Scholar]
- Consortium, 1001 Genomes 1,135 Genomes Reveal the Global Pattern of Polymorphism in Arabidopsis thaliana. Cell 2016, 166, 481–491. [CrossRef] [PubMed] [Green Version]
- Köhl, K.; Tohge, T.; Schöttler, M.A. Performance of Arabidopsis thaliana under different light qualities: Comparison of light-emitting diodes to fluorescent lamp. Funct. Plant Biol. 2017, 44, 727–738. [Google Scholar] [CrossRef]
- Felsenstein, J. PHYLIP (Phylogeny Inference Package), version 3.698. 2019. [Google Scholar]
- Horton, M.W.; Hancock, A.M.; Huang, Y.S.; Toomajian, C.; Atwell, S.; Auton, A.; Muliyati, N.W.; Platt, A.; Sperone, F.G.; Vilhjálmsson, B.J.; et al. Genome-wide patterns of genetic variation in worldwide Arabidopsis thaliana accessions from the RegMap panel. Nat. Genet. 2012, 44, 212–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]



| Experiment | Name | Abbreviation | Country of Origin | Latitude | #Leaves Flowering |
|---|---|---|---|---|---|
| 1 | Be-1 | Be | Germany | 49.68 | nd |
| 1 | Cen-0 | Cen | France | 49.00 | nd |
| 1 & 2 | Col-0 | Col | USA | nd | 16.00 |
| 1 | Da(1)-12 | Da | Czech Republic | 49.85 | 17.50 |
| 1 | DraIV6-16 | Dra | Czech Republic | 49.41 | nd |
| 1 | Fei-0 | Fei | Portugal | 40.50 | 22.25 |
| 1 | Ge-0 | Ge0 | Switzerland | 46.50 | 60.00 |
| 1 | Hn-0 | Hn | Germany | 51.35 | 21.25 |
| 1 | Hs-0 | Hs | Germany | 52.24 | 17.50 |
| 1 | Kelsterbach-2 | Kel | Germany | 50.07 | nd |
| 1 | Ler-1 | Ler | Germany | nd | 11.25 |
| 1 | Mnz-0 | Mnz | Germany | 50.00 | 23.50 |
| 1 | PHW-34 | P34 | France | 48.61 | 43.50 |
| 1 | PHW-37 | P37 | France | 48.61 | nd |
| 1 | Pla-0 | Pla | Spain | 41.50 | 40.50 |
| 1 | Sha | Sha | Tajikistan | 38.35 | nd |
| 1 | TOU-H-13 | TOU | France | 46.67 | nd |
| 1 | Tsu-0 | Tsu | Japan | 34.43 | 36.75 |
| 1 | Ws-0 | Ws | Russia | nd | 42.25 |
| 1 | Yo-0 | Yo | USA | 37.45 | 56.33 |
| 1 | ZdrI2-24 | Zdr | Czech Republic | 49.39 | nd |
| 2 | Amel-1 | Ame | Netherlands | 53.45 | 41.50 |
| 2 | Db-0 | Db | Germany | 50.31 | nd |
| 2 | Ge-1 | Ge1 | Switzerland | 46.50 | nd |
| 2 | Gel-1 | Gel | Netherlands | 51.02 | 20.0 |
| 2 | HSm | HSm | Czech Republic | 49.33 | 41.66 |
| 2 | Kin-0 | Kin | USA | 44.46 | nd |
| 2 | LDV-58 | LDV | France | 48.52 | nd |
| 2 | MNF-Che-2 | MNF | USA | 43.53 | 46.00 |
| 2 | PAR-3 | PAR | France | 46.65 | nd |
| 2 | Sapporo-0 | Sap | Japan | 43.06 | nd |
| 2 | Ta-0 | Ta | Czech Republic | 49.50 | 35.75 |
| 2 | Tad01 | Tad | Sweden | 62.87 | nd |
| 2 | TDr-3 | TDr | Sweden | 55.77 | nd |
| 2 | UKNW06-060 | UKN | UK | 54.40 | nd |
| 2 | VOU-2 | VOU | France | 46.65 | nd |
| Trait | #Leaves at Flowering | ΦPSII | NPQ | Fv/Fm | PLA | #Leaves | Leaf Size |
|---|---|---|---|---|---|---|---|
| Lat. | −0.51 | −0.03 | −0.09 | −0.05 | −0.18 | −0.16 | −0.17 |
| #leaves at flowering | −0.13 | 0.16 | −0.41 * | 0.16 | 0.10 | 0.10 | |
| ΦPSII | −0.64 *** | 0.38 *** | 0.52 *** | 0.12 | 0.58 *** | ||
| NPQ | 0.00 | −0.41 *** | −0.08 | −0.47 *** | |||
| Fv/Fm | 0.31 ** | 0.38 ** | 0.27 * | ||||
| PLA | 0.72 *** | 0.98 *** | |||||
| #leaves | 0.59 *** |
| Trait | #Leaves Flowering | ΔΦPSII | ΔNPQ | ΔFv/Fm | ΔPLA | Δ#Leaves | ΔLeaf Size |
|---|---|---|---|---|---|---|---|
| Lat. | −0.51 | −0.36 * | 0.04 | −0.03 | −0.17 | −0.07 | −0.16 |
| #leaves at flowering | 0.07 | 0.05 | 0.13 | −0.01 | −0.01 | 0.06 | |
| ΔΦPSII | −0.16 | 0.57 *** | 0.17 | 0.16 | 0.14 | ||
| ΔNPQ | 0.05 | −0.29 | −0.19 | −0.35 * | |||
| ΔFv/Fm | 0.42 * | 0.48 ** | 0.38 * | ||||
| ΔPLA | 0.90 *** | 0.98 *** | |||||
| Δ#leaves | 0.84 *** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaiser, E.; Walther, D.; Armbruster, U. Growth under Fluctuating Light Reveals Large Trait Variation in a Panel of Arabidopsis Accessions. Plants 2020, 9, 316. https://doi.org/10.3390/plants9030316
Kaiser E, Walther D, Armbruster U. Growth under Fluctuating Light Reveals Large Trait Variation in a Panel of Arabidopsis Accessions. Plants. 2020; 9(3):316. https://doi.org/10.3390/plants9030316
Chicago/Turabian StyleKaiser, Elias, Dirk Walther, and Ute Armbruster. 2020. "Growth under Fluctuating Light Reveals Large Trait Variation in a Panel of Arabidopsis Accessions" Plants 9, no. 3: 316. https://doi.org/10.3390/plants9030316





