Composition, Antioxidant Potential, and Antimicrobial Activity of Helichrysum plicatum DC. Various Extracts
Abstract
:1. Introduction
2. Results
2.1. Phytochemical Profile
2.2. Antioxidant Potential
2.3. Antimicrobial Activity
3. Discussion
3.1. Phytochemical Profile
3.2. Antioxidant Potential
3.3. Antimicrobial Activity
4. Materials and Methods
4.1. Plant Material
4.2. Extraction Procedure
4.3. HPLC-DAD/ESI-ToF-MS Analyses
4.4. Antioxidant Assay
4.5. Antimicrobial Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Akaberi, M.; Sahebkar, A.; Azizi, N.; Emami, S.A. Everlasting flowers: Phytochemistry and pharmacology of the genus Helichrysum. Ind. Crops Prod. 2019, 138, 111471. [Google Scholar] [CrossRef]
- Bayer, R.J.; Breitwieser, I.; Ward, J.; Puttock, C. Tribe Gnaphalieae (Cass.) Lecoq & Juillet (1831). In Flowering Plants Eudicots: Asterales; Kadereit, J.W., Jeffrey, C., Eds.; The Families and Genera of Vascular Plants; Springer: Berlin, Heidelberg, Germany, 2007; Volume 8, pp. 246–284. [Google Scholar] [CrossRef]
- Asong, J.A.; Amoo, S.O.; McGaw, L.J.; Nkadimeng, S.M.; Aremu, A.O.; Otang-Mbeng, W. Antimicrobial Activity, Antioxidant Potential, Cytotoxicity and Phytochemical Profiling of Four Plants Locally Used against Skin Diseases. Plants 2019, 8, 350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarzycka, A.; Lewińska, A.; Gancarz, R.; Wilk, K.A. Assessment of extracts of Helichrysum arenarium, Crataegus monogyna, Sambucus nigra in photoprotective UVA and UVB; photostability in cosmetic emulsions. J. Photochem. Photobiol. B 2013, 128, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Barroso, M.R.; Barros, L.; Dueñas, M.; Carvalho, A.M.; Santos-Buelga, C.; Fernandes, I.P.; Barreiro, M.F.; Ferreira, I.C.F.R. Exploring the antioxidant potential of Helichrysum stoechas (L.) Moench phenolic compounds for cosmetic applications: Chemical characterization, microencapsulation and incorporation into a moisturizer. Ind. Crops Prod. 2014, 53, 330–336. [Google Scholar] [CrossRef] [Green Version]
- Lourens, A.C.U.; Reddy, D.; Başer, K.H.C.; Viljoen, A.M.; Van Vuuren, S.F. In vitro biological activity and essential oil composition of four indigenous South African Helichrysum species. J. Ethnopharmacol. 2004, 95, 253–258. [Google Scholar] [CrossRef]
- Süntar, I.; Küpeli Akkol, E.; Keles, H.; Yesilada, E.; Sarker, S.D. Exploration of the wound healing potential of Helichrysum graveolens (Bieb.) Sweet: Isolation of apigenin as an active component. J. Ethnopharmacol. 2013, 149, 103–110. [Google Scholar] [CrossRef]
- Yagura, T.; Motomiya, T.; Ito, M.; Honda, G.; Iida, A.; Kiuchi, F.; Tokuda, H.; Nishino, H. Anticarcinogenic compounds in the Uzbek medicinal plant, Helichrysum maracandicum. J. Nat. Med. 2008, 62, 174. [Google Scholar] [CrossRef]
- Liu, Y.-J.; Zhang, J.-L.; Li, C.; Mu, X.-G.; Liu, X.-L.; Wang, L.; Zhao, Y.-C.; Zhang, P.; Li, X.-D.; Zhang, X.-X. Antimicrobial Secondary Metabolites from the Seawater-Derived Fungus Aspergillus sydowii SW9. Molecules 2019, 24, 4596. [Google Scholar] [CrossRef] [Green Version]
- Munekata, P.E.S.; Alcántara, C.; Collado, M.C.; Garcia-Perez, J.V.; Saraiva, J.A.; Lopes, R.P.; Barba, F.J.; do Prado Silva, L.; Sant’Ana, A.S.; Fierro, E.M.; et al. Ethnopharmacology, phytochemistry and biological activity of Erodium species: A review. Food Res. Int. 2019, 126, 108659. [Google Scholar] [CrossRef]
- Donkor, S.; Larbie, C.; Komlaga, G.; Emikpe, B.O. Phytochemical, Antimicrobial, and Antioxidant Profiles of Duranta erecta L. Parts. Biochem. Res. Int. 2019, 2019, 8731595. [Google Scholar] [CrossRef] [Green Version]
- Casciaro, B.; Calcaterra, A.; Cappiello, F.; Mori, M.; Loffredo, M.R.; Ghirga, F.; Mangoni, M.L.; Botta, B.; Quaglio, D. Nigritanine as a New Potential Antimicrobial Alkaloid for the Treatment of Staphylococcus aureus-Induced Infections. Toxins 2019, 11, 511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bigović, D.J.; Stević, T.R.; Janković, T.R.; Noveski, N.B.; Radanović, D.S.; Pljevljakušić, D.S.; Djurić, Z.R. Antimicrobial activity of Helichrysum plicatum DC. Hem. Ind. 2017, 71, 337–342. [Google Scholar] [CrossRef]
- Kulevanova, S.; Stefova, M.; Stafilov, T. HPLC identification and determination of flavone aglycones in Helichrysum plicatum DC. (Asteraceae). Pharmazie 2000, 55, 391–392. [Google Scholar] [PubMed]
- Polat, R.; Cakilcioglu, U.; Satıl, F. Traditional uses of medicinal plants in Solhan (Bingöl—Turkey). J. Ethnopharmacol. 2013, 148, 951–963. [Google Scholar] [CrossRef] [PubMed]
- Tetik, F.; Civelek, S.; Cakilcioglu, U. Traditional uses of some medicinal plants in Malatya (Turkey). J. Ethnopharmacol. 2013, 146, 331–346. [Google Scholar] [CrossRef]
- Bigovic, D.; Brankovic, S.; Kitic, D.; Radenkovic, M.; Jankovic, T.; Savikin, K.; Zivanovic, S. Relaxant Effect of the Ethanol Extract of Helichrysum plicatum (Asteraceae) on Isolated Rat Ileum Contractions. Molecules 2010, 15, 3391–3401. [Google Scholar] [CrossRef] [Green Version]
- Aslan, M.; Deliorman Orhan, D.; Orhan, N.; Sezik, E.; Yesilada, E. In vivo antidiabetic and antioxidant potential of Helichrysum plicatum ssp. plicatum capitulums in streptozotocin-induced-diabetic rats. J. Ethnopharmacol. 2007, 109, 54–59. [Google Scholar] [CrossRef]
- Bayir, Y.; Halici, Z.; Keles, M.S.; Colak, S.; Cakir, A.; Kaya, Y.; Akçay, F. Helichrysum plicatum DC. subsp. plicatum extract as a preventive agent in experimentally induced urolithiasis model. J. Ethnopharmacol. 2011, 138, 408–414. [Google Scholar] [CrossRef]
- Ozbek, T.; Gulluce, M.; Adiguzel, A.; Ozkan, H.; Sahin, F.; Orhan, F. Antimutagenic Activity of the Methanol Extract of Helichrysum plicatum ssp. plicatum. Asian J. Chem. 2009, 21, 2705–2710. [Google Scholar]
- Apaydin Yildirim, B.; Kordali, S.; Terim Kapakin, K.A.; Yildirim, F.; Aktas Senocak, E.; Altun, S. Effect of Helichrysum plicatum DC. subsp. plicatum ethanol extract on gentamicin-induced nephrotoxicity in rats. J. Zhejiang Univ. Sci. B 2017, 18, 501–511. [Google Scholar] [CrossRef] [Green Version]
- Hänsel, R.; Cybulski, E.-M.; Çubukçu, B.; Meriçli, A.H.; Bohlmann, F.; Zdero, C. Neue pyron-derivate aus Helichrysum-arten. Phytochemistry 1980, 19, 639–644. [Google Scholar] [CrossRef]
- Taglialatela-Scafati, O.; Pollastro, F.; Chianese, G.; Minassi, A.; Gibbons, S.; Arunotayanun, W.; Mabebie, B.; Ballero, M.; Appendino, G. Antimicrobial Phenolics and Unusual Glycerides from Helichrysum italicum subsp. microphyllum. J. Nat. Prod. 2013, 76, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Akaberi, M.; Danton, O.; Tayarani-Najaran, Z.; Asili, J.; Iranshahi, M.; Emami, S.A.; Hamburger, M. HPLC-Based Activity Profiling for Antiprotozoal Compounds in the Endemic Iranian Medicinal Plant Helichrysum oocephalum. J. Nat. Prod. 2019, 82, 958–969. [Google Scholar] [CrossRef]
- Vrkoč, J.; Dolejš, L.; Buděšínský, M. Methylene-bis-2H-pyran-2-ones and phenolic constituents from the root of Helichrysum arenarium. Phytochemistry 1975, 14, 1383–1384. [Google Scholar] [CrossRef]
- Rios, J.L.; Recio, M.C.; Villar, A. Isolation and identification of the antibacterial compounds from Helichrysum stoechas. J. Ethnopharmacol. 1991, 33, 51–55. [Google Scholar] [CrossRef]
- Vrkoč, J.; Dolejš, L.; Sedmera, P.; Vašíčková, S.; Šorm, F. The structure of arenol and homoarenol, α-pyrone derivatives from Helichrysum arenarium (L.) Moench. Tetrahedron Lett. 1971, 12, 247–250. [Google Scholar] [CrossRef]
- Bohlmann, F.; Misra, L.N.; Jakupovic, J. Weitere Phloroglucin- und α-Pyron-Derivate aus Helichrysum-Arten. Planta Med. 1984, 50, 174–176. [Google Scholar] [CrossRef]
- Tomás-Lorente, F.; Iniesta-Sanmartín, E.; Tomás-Barberán, F.A.; Trowitzsch-Kienast, W.; Wray, V. Antifungal phloroglucinol derivatives and lipophilic flavonoids from Helichrysum decumbens. Phytochemistry 1989, 28, 1613–1615. [Google Scholar] [CrossRef]
- Jakupovic, J.; Kuhnke, J.; Schuster, A.; Metwally, M.A.; Bohlmann, F. Phloroglucinol derivatives and other constituents from South African Helichrysum species. Phytochemistry 1986, 25, 1133–1142. [Google Scholar] [CrossRef]
- Kanlayavattanakul, M.; Ruangrungsi, N.; Watanabe, T.; Ishikawa, T. Chemical Constituents of Pterocaulon redolens. Heterocycles 2003, 61, 183. [Google Scholar] [CrossRef]
- Rumbero, A.; Arriaga-Giner, F.J.; Wollenweber, E. A New Oxyprenyl Coumarin and Highly Methylated Flavones from the Exudate of Ozothamnus lycopodioides (Asteraceae). Z. Für Nat. C 2014, 55, 1–4. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Wang, M.; Gao, M.; Zhao, M.; Bai, Y.; Zhao, C. Chemical composition and biological activities of Gerbera anandria. Molecules 2014, 19, 4046–4057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maes, D.; Syngel, K.V.; Debenedetti, S.; De Kimpe, N. Synthesis of purpurasol, a highly oxygenated coumarin from Pterocaulon purpurascens. Tetrahedron 2006, 62, 4426–4429. [Google Scholar] [CrossRef]
- Medeiros-Neves, B.; De Barros, F.M.C.; Von Poser, G.L.; Teixeira, H.F. Quantification of Coumarins in Aqueous Extract of Pterocaulon balansae (Asteraceae) and Characterization of a New Compound. Molecules 2015, 20, 18083–18094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohlmann, F.; Zdero, C. Neue obliquin-derivate aus Helichrysum serpyllifolium. Phytochemistry 1980, 19, 331–332. [Google Scholar] [CrossRef]
- Bohlmann, F.; Zdero, C. Neue phloroglucin-derivate aus Helichrysum-arten. Phytochemistry 1980, 19, 153–155. [Google Scholar] [CrossRef]
- Bohlmann, F.; Zdero, C. Flavanones from Helichrysum thapsus. Phytochemistry 1983, 22, 2877–2878. [Google Scholar] [CrossRef]
- Bohlmann, F.; Zdero, C. Neue phloroglucin-derivate aus Helichrysum natalitium und Helichrysum bellum. Phytochemistry 1979, 18, 641–644. [Google Scholar] [CrossRef]
- Grinev, V.S.; Shirokov, A.A.; Navolokin, N.A.; Polukonova, N.V.; Kurchatova, M.N.; Durnova, N.A.; Bucharskaya, A.B.; Maslyakova, G.N. Polyphenolic compounds of a new biologically active extract from immortelle sandy flowers (Helichrysum arenarium (L.) Moench.). Russ. J. Bioorganic Chem. 2016, 42, 770–776. [Google Scholar] [CrossRef]
- Mao, Z.; Gan, C.; Zhu, J.; Ma, N.; Wu, L.; Wang, L.; Wang, X. Anti-atherosclerotic activities of flavonoids from the flowers of Helichrysum arenarium L. MOENCH through the pathway of anti-inflammation. Bioorg. Med. Chem. Lett. 2017, 27, 2812–2817. [Google Scholar] [CrossRef]
- Tomas-Barberán, F.A.; Msonthi, J.D.; Hostettmann, K. Antifungal epicuticular methylated flavonoids from Helichrysum nitens. Phytochemistry 1988, 27, 753–755. [Google Scholar] [CrossRef]
- Geissman, T.A.; Mukherjee, R.; Sim, K.Y. Constituents of Helichrysum viscosum var. bracteatum DC. Phytochemistry 1967, 6, 1575–1581. [Google Scholar] [CrossRef]
- Cubukcu, B. Studies on lipophilic flavonoids of Helichrysum species growing in Anatolia. Doga Bilim Derg. Seri Temel Bilim. 1982, 6, 83–90. [Google Scholar]
- Lv, H.; Li, Q.; Zhong, J.; Liao, L.-X.; Haji Akber, A. Studies on flavonoids from Helichrysum arenarium. Chin. Pharm. J. 2008, 43, 11–13. [Google Scholar]
- Wollenweber, E.; Roitman, J.N. New reports on surface flavonoids from Chamaebatiaria (Rosaceae), Dodonaea (Sapindaceae), Elsholtzia (Lamiaceae), and Silphium (Asteraceae). Nat. Prod. Commun. 2007, 2, 385–389. [Google Scholar] [CrossRef]
- Jakupovic, J.; Zdero, C.; Grenz, M.; Tsichritzis, F.; Lehmann, L.; Hashemi-Nejad, S.M.; Bohlmann, F. Twenty-one acylphloroglucinol derivatives and further constituents from south african Helichrysum species. Phytochemistry 1989, 28, 1119–1131. [Google Scholar] [CrossRef]
- Czinner, E.; Kursinszki, L.; Baumann, D.; Hamburger, M.; Kéry, Á.; Szöke, É.; Lemberkovics, É. Phytochemical Study of Phenolic Compounds from Helichrysi flos by LC-DAD-MS. In Natural Products in the New Millennium: Prospects and Industrial Application; Rauter, A.P., Palma, F.B., Justino, J., Araújo, M.E., dos Santos, S.P., Eds.; Proceedings of the Phytochemical Society of Europe; Springer: Dordrecht, The Netherlands, 2002; Volume 47, pp. 99–109. ISBN 978-94-015-9876-7. [Google Scholar] [CrossRef]
- Albayrak, S.; Aksoy, A.; Sagdic, O.; Hamzaoglu, E. Compositions, antioxidant and antimicrobial activities of Helichrysum (Asteraceae) species collected from Turkey. Food Chem. 2010, 119, 114–122. [Google Scholar] [CrossRef]
- Wang, L.-B.; Toshio, M.; Gao, H.-Y.; Huang, J.; Masayuki, Y.; Wu, L.-J. Isolation and identification of chemical constituents of flavones from Flos Helichrysi Arenarii. J. Shenyang Pharm. Univ. 2009, 792–795. [Google Scholar]
- D’Abrosca, B.; Buommino, E.; Caputo, P.; Scognamiglio, M.; Chambery, A.; Donnarumma, G.; Fiorentino, A. Phytochemical study of Helichrysum italicum (Roth) G. Don: Spectroscopic elucidation of unusual amino-phlorogucinols and antimicrobial assessment of secondary metabolites from medium-polar extract. Phytochemistry 2016, 132, 86–94. [Google Scholar] [CrossRef]
- Szadowska, A. Pharmacology of galenic preparations and flavonoids isolated from Helichrysum arenarium. Acta Pol. Pharm. 1962, 19, 465–479. [Google Scholar]
- Morikawa, T.; Ninomiya, K.; Akaki, J.; Kakihara, N.; Kuramoto, H.; Matsumoto, Y.; Hayakawa, T.; Muraoka, O.; Wang, L.-B.; Wu, L.-J.; et al. Dipeptidyl peptidase-IV inhibitory activity of dimeric dihydrochalcone glycosides from flowers of Helichrysum arenarium. J. Nat. Med. 2015, 69, 494–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohlmann, F.; Ziesche, J.; Mahanta, P.K. Neue chalkon-derivate und humulon-ähnliche verbindungen aus Helichrysum-arten. Phytochemistry 1979, 18, 1033–1036. [Google Scholar] [CrossRef]
- Spring, O.; Zipper, R.; Reeb, S.; Vogler, B.; Da Costa, F.B. Sesquiterpene lactones and a myoinositol from glandular trichomes of Viguiera quinqueremis (Heliantheae; Asteraceae). Phytochemistry 2001, 57, 267–272. [Google Scholar] [CrossRef]
- Sakamoto, H.T.; Laudares, E.P.; Crotti, A.E.M.; Lopes, N.P.; Vichnewski, W.; Lopes, J.L.C.; Heleno, V.C.G. Sesquiterpenes lactones and flavonoids from Eremanthus argenteus (Asteraceae). Nat. Prod. Commun. 2010, 5, 681–684. [Google Scholar] [CrossRef] [Green Version]
- Gousiadou, C.; Skaltsa, H. Secondary metabolites from Centaurea orphanidea. Biochem. Syst. Ecol. 2003, 31, 389–396. [Google Scholar] [CrossRef]
- Özçelik, B.; Gürbüz, I.; Karaoglu, T.; Yeşilada, E. Antiviral and antimicrobial activities of three sesquiterpene lactones from Centaurea solstitialis L. ssp. solstitialis. Microbiol. Res. 2009, 164, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Atrrog, A.A.B.; Natić, M.; Tosti, T.; Milojković-Opsenica, D.; Đorđević, I.; Tešević, V.; Jadranin, M.; Milosavljević, S.; Lazić, M.; Radulović, S.; et al. Lipophilicity of some guaianolides isolated from two endemic subspecies of Amphoricarpos neumayeri (Asteraceae) from Montenegro. Biomed. Chromatogr. 2009, 23, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Liu, Q.; Cen, Y.; Huang, J.; Zhang, X.; Guo, S.; Zhang, L.; Guo, T.; Ho, C.-T.; Bai, N. A new sesquiterpene lactone glucoside and other constituents from Inula salsoloides with insecticidal activities on striped flea beetle (Phyllotreta striolata Fabricius). Nat. Prod. Res. 2018, 32, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Perry, D.L.; Fischer, N.H. New germacranolide sesquiterpene dilactones from the genus Melampodium (Compositae). J. Org. Chem. 1975, 40, 3480–3486. [Google Scholar] [CrossRef]
- Siedle, B.; García-Piñeres, A.J.; Murillo, R.; Schulte-Mönting, J.; Castro, V.; Rüngeler, P.; Klaas, C.A.; Da Costa, F.B.; Kisiel, W.; Merfort, I. Quantitative Structure−Activity Relationship of Sesquiterpene Lactones as Inhibitors of the Transcription Factor NF-κB. J. Med. Chem. 2004, 47, 6042–6054. [Google Scholar] [CrossRef]
- Kimani, N.M.; Matasyoh, J.C.; Kaiser, M.; Brun, R.; Schmidt, T.J. Anti-Trypanosomatid Elemanolide Sesquiterpene Lactones from Vernonia lasiopus O. Hoffm. Molecules 2017, 22, 597. [Google Scholar] [CrossRef] [Green Version]
- Erasto, P.; Grierson, D.S.; Afolayan, A.J. Antioxidant Constituents in Vernonia amygdalina Leaves. Pharm. Biol. 2007, 45, 195–199. [Google Scholar] [CrossRef] [Green Version]
- Ono, M.; Masuoka, C.; Odake, Y.; Ito, Y.; Nohara, T. Eudesmane derivatives from Tessaria integrifolia. Phytochemistry 2000, 53, 479–484. [Google Scholar] [CrossRef]
- Yamada, M.; Matsuura, N.; Suzuki, H.; Kurosaka, C.; Hasegawa, N.; Ubukata, M.; Tanaka, T.; Iinuma, M. Germacranolides from Calea urticifolia. Phytochemistry 2004, 65, 3107–3111. [Google Scholar] [CrossRef]
- Torrenegra, R.D.; Tellez, A.N. Chemotaxonomic value of melampolides in Espeletia species (Asteraceae). Biochem. Syst. Ecol. 1995, 23, 449–450. [Google Scholar] [CrossRef]
- Schorr, K.; Merfort, I.; Costa, F.B.D. A Novel Dimeric Melampolide and Further Terpenoids from Smallanthus sonchifolius (Asteraceae) and the Inhibition of the Transcription Factor NF-κB. Nat. Prod. Commun. 2019. [Google Scholar] [CrossRef] [Green Version]
- Miyase, T.; Ozaki, H.; Ueno, A. Sesquiterpene Glycosides from Ainsliaea cordifolia FRANCH. et SAV. Chem. Pharm. Bull. (Tokyo) 1991, 39, 937–938. [Google Scholar] [CrossRef] [Green Version]
- Ramseyer, J.; Thuerig, B.; De Mieri, M.; Schärer, H.-J.; Oberhänsli, T.; Gupta, M.P.; Tamm, L.; Hamburger, M.; Potterat, O. Eudesmane Sesquiterpenes from Verbesina lanata with Inhibitory Activity against Grapevine Downy Mildew. J. Nat. Prod. 2017, 80, 3296–3304. [Google Scholar] [CrossRef] [PubMed]
- Pan, E.; Gorka, A.P.; Alumasa, J.N.; Slebodnick, C.; Harinantenaina, L.; Brodie, P.J.; Roepe, P.D.; Randrianaivo, R.; Birkinshaw, C.; Kingston, D.G.I. Antiplasmodial and Antiproliferative Pseudoguaianolides of Athroisma proteiforme from the Madagascar Dry Forest. J. Nat. Prod. 2011, 74, 2174–2180. [Google Scholar] [CrossRef] [Green Version]
- Jakupovic, J.; Schuster, A.; Bohlmann, F.; Ganzer, U.; King, R.M.; Robinson, H. Diterpenes and other constituents from Australian Helichrysum and related species. Phytochemistry 1989, 28, 543–551. [Google Scholar] [CrossRef]
- Coll Aráoz, M.V.; Mercado, M.I.; Grau, A.; Catalán, C.A.N. Ent-kaurane derivatives from the root cortex of yacon and other three Smallanthus species (Heliantheae, Asteraceae). Biochem. Syst. Ecol. 2010, 38, 1042–1048. [Google Scholar] [CrossRef]
- Miyakado, M.; Ohno, N.; Yoshioka, H.; Mabry, T.J.; Whiffin, T. Gymnospermin: A new labdan triol from Gymnosperma glutinosa. Phytochemistry 1974, 13, 189–190. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, H.; Lei, J.; Yu, J. Biological activity of extracts and active compounds isolated from Siegesbeckia orientalis L. Ind. Crops Prod. 2016, 94, 288–293. [Google Scholar] [CrossRef]
- Cruz, F.G.; Roque, N.F. Relative stereochemistry determination of pimaradienes through oxidative products. Quím. Nova 1997, 20, 261–266. [Google Scholar] [CrossRef]
- Jakupovic, J.; Baruah, R.N.; Zdero, C.; Eid, F.; Pathak, V.P.; Chau-thi, T.V.; Bohlmann, F.; King, R.M.; Robinson, H. Further diterpenes from plants of the Compositae, subtribe Solidagininae. Phytochemistry 1986, 25, 1873–1881. [Google Scholar] [CrossRef]
- Bohlmann, F.; Ziesche, J. Neue diterpene aus Gnaphalium-arten. Phytochemistry 1980, 19, 71–74. [Google Scholar] [CrossRef]
- Bohlmann, F.; Suwita, A. Weitere phloroglucin-derivate aus Helichrysum-arten. Phytochemistry 1979, 18, 2046–2049. [Google Scholar] [CrossRef]
- Bohlmann, F.; Zdero, C.; Ziesche, J. Neue flavone und phloroglucin-derivate aus Helichrysum herbaceum und Helichrysum chrysargyrum. Phytochemistry 1979, 18, 1375–1378. [Google Scholar] [CrossRef]
- Bohlmann, F.; Zdero, C. Neue geranylphloroglucin-derivate aus Helichrysum monticola. Phytochemistry 1980, 19, 683–684. [Google Scholar] [CrossRef]
- Wang, L.-B.; Liu, F.-Z.; Gan, C.-L.; Dong, N.-W.; Hou, Y.-L.; Wang, C. Isolation and identification of chemical constituents in the lipid-lowering fraction of Flos Helichrysum arenarium(Ⅱ). J. Shenyang Pharm. Univ. 2012, 109–112, 125. [Google Scholar]
- Vrkoč, J.; Herout, V.; Šorm, F. Über pflanzenstoffe X. Isolierung der kristallinen bestandteile der sandstrohblume (Helichrysum arenarium MCH). Collect. Czechoslov. Chem. Commun. 1959, 24, 3938–3954. [Google Scholar] [CrossRef]
- Kurkina, A.V.; Ryzhov, V.M.; Avdeeva, E.V. Assay of isosalipurposide in raw material and drugs from the dwarf everlast (Helichrysum arenarium). Pharm. Chem. J. 2012, 46, 171–176. [Google Scholar] [CrossRef]
- Vrkoč, J.; Buděšínský, M.; Dolejš, L.; Vašíčková, S. Arenophthalide A: A new phthalide glycoside from Helichrysum arenarium roots. Phytochemistry 1975, 14, 1845–1848. [Google Scholar] [CrossRef]
- Babotă, M.; Mocan, A.; Vlase, L.; Crișan, O.; Ielciu, I.; Gheldiu, A.-M.; Vodnar, D.C.; Crișan, G.; Păltinean, R. Phytochemical Analysis, Antioxidant and Antimicrobial Activities of Helichrysum arenarium (L.) Moench. and Antennaria dioica (L.) Gaertn. Flowers. Molecules 2018, 23, 409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albayrak, S.; Aksoy, A.; Sağdiç, O.; Budak, Ü. Phenolic compounds and antioxidant and antimicrobial properties of Helichrysum species collected from eastern Anatolia, Turkey. Turk. J. Biol. 2010, 34, 463–473. [Google Scholar] [CrossRef]
- Morikawa, T.; Wang, L.-B.; Ninomiya, K.; Nakamura, S.; Matsuda, H.; Muraoka, O.; Wu, L.-J.; Yoshikawa, M. Medicinal Flowers. XXX. Eight New Glycosides, Everlastosides F—M, from the Flowers of Helichrysum arenarium. Chem. Pharm. Bull. (Tokyo) 2009, 57, 853–859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yong, F.; Aisa, H.A.; Mukhamatkhanova, R.F.; Shamyanov, I.D.; Levkovich, M.G. New flavanone and other constituents of Helichrysum arenarium indigenous to China. Chem. Nat. Compd. 2011, 46, 872–875. [Google Scholar] [CrossRef]
- Jakupovic, J.; Pathak, V.P.; Bohlmann, F.; King, R.M.; Robinson, H. Obliquin derivatives and other constituents from Australian Helichrysum species. Phytochemistry 1987, 26, 803–807. [Google Scholar] [CrossRef]
- Pereira, C.G.; Barreira, L.; Bijttebier, S.; Pieters, L.; Neves, V.; Rodrigues, M.J.; Rivas, R.; Varela, J.; Custódio, L. Chemical profiling of infusions and decoctions of Helichrysum italicum subsp. picardii by UHPLC-PDA-MS and in vitro biological activities comparatively with green tea (Camellia sinensis) and rooibos tisane (Aspalathus linearis). J. Pharm. Biomed. Anal. 2017, 145, 593–603. [Google Scholar] [CrossRef]
- Zhang, F.; Su, R.-N.; Wu, H.-B.; Wang, W.-S. Jacaranone Derivatives from Senecio laetus (Compositae). Plant Divers. 2013, 35, 501–504. [Google Scholar] [CrossRef]
- de Gomes, K.S.; Tamayose, C.I.; Ferreira, M.J.P.; Murakami, C.; Young, M.C.M.; Antar, G.M.; Camilo, F.F.; Sartorelli, P.; Lago, J.H.G.; de Gomes, K.S.; et al. Isolation of antifungal quinoid derivatives from leaves of Pentacalia desiderabilis (Vell.) Cuatre. (Asteraceae) using ionic liquid in the microwave assisted extraction. Quím. Nova 2019, 42, 156–158. [Google Scholar] [CrossRef]
- Jakupovic, J.; Schuster, A.; Sun, H.; Bohlmann, F.; Bhakuni, D.S. Prenylated phthalides from Anaphalis araneosa and Helichrysum platypterum. Phytochemistry 1987, 26, 580–581. [Google Scholar] [CrossRef]
- Yang, Y.-N.; Huang, X.-Y.; Feng, Z.-M.; Jiang, J.-S.; Zhang, P.-C. Hepatoprotective Activity of Twelve Novel 7′-Hydroxy Lignan Glucosides from Arctii Fructus. J. Agric. Food Chem. 2014, 62, 9095–9102. [Google Scholar] [CrossRef]
- Ruan, J.; Li, Z.; Yan, J.; Huang, P.; Yu, H.; Han, L.; Zhang, Y.; Wang, T. Bioactive Constituents from the Aerial Parts of Pluchea indica Less. Molecules 2018, 23, 2104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Du, H.-B.; Zhu, F.-Y.; Shang, J.; Silafu, A. Chemical constituents from Artemisia rupestris L. J. China Pharm. Univ. 2011, 42, 310–313. [Google Scholar]
- Hänsel, R.; Rimpler, H.; Schwarz, R. Erstmaliger nachweis eines auronols (aroylcumaranons) im pflanzenreich. Tetrahedron Lett. 1965, 6, 1545–1548. [Google Scholar] [CrossRef]
- Li, X.; Gao, P.; Gjetvaj, B.; Westcott, N.; Gruber, M.Y. Analysis of the metabolome and transcriptome of Brassica carinata seedlings after lithium chloride exposure. Plant Sci. 2009, 177, 68–80. [Google Scholar] [CrossRef]
- Granica, S.; Lohwasser, U.; Jöhrer, K.; Zidorn, C. Qualitative and quantitative analyses of secondary metabolites in aerial and subaerial of Scorzonera hispanica L. (black salsify). Food Chem. 2015, 173, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Powell, R.G.; Smith, C.R.; Wolff, I.A. Helichrysum seed oil. I. Separation and characterization of individual acids. J. Am. Oil Chem. Soc. 1965, 42, 165–169. [Google Scholar] [CrossRef]
- Gouveia, S.C.; Castilho, P.C. Characterization of phenolic compounds in Helichrysum melaleucum by high-performance liquid chromatography with on-line ultraviolet and mass spectrometry detection. Rapid Commun. Mass Spectrom. RCM 2010, 24, 1851–1868. [Google Scholar] [CrossRef]
- Meusel, I.; Neinhuis, C.; Markstädter, C.; Barthlott, W. Chemical Composition and Recrystallization of Epicuticular Waxes: Coiled Rodlets and Tubules. Plant Biol. 2000, 2, 462–470. [Google Scholar] [CrossRef]
- Popoola, O.K.; Marnewick, J.L.; Rautenbach, F.; Iwuoha, E.I.; Hussein, A.A. Acylphloroglucinol Derivatives from the South African Helichrysum niveum and Their Biological Activities. Mol. Basel Switz. 2015, 20, 17309–17324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; He, H.; Yang, X.; Chen, M.; Hao, X. Chemical Constituents of the Flowers of Helichrysum bracteatum. Nat. Prod. Res. Dev. 2007, 19, 423–426. [Google Scholar]
- Bohlmann, F.; Mahanta, P.K.; Zdero, C. Neue chalkon-derivate aus südafrikanischen Helichrysum-arten. Phytochemistry 1978, 17, 1935–1937. [Google Scholar] [CrossRef]
- han Çubukcu, B. Helichrysum Species as Choleretic and Cholagogue Crude Drugs. ACTA Pharm. Sci. 2002, 44, 144–150. [Google Scholar]
- Bigović, D.; Šavikin, K.; Janković, T.; Menković, N.; Zdunić, G.; Stanojković, T.; Djurić, Z. Antiradical and Cytotoxic Activity of Different Helichrysum plicatum Flower Extracts. Nat. Prod. Commun. 2011, 6, 1934578X1100600617. [Google Scholar] [CrossRef] [Green Version]
- Appendino, G.; Ottino, M.; Marquez, N.; Bianchi, F.; Giana, A.; Ballero, M.; Sterner, O.; Fiebich, B.L.; Munoz, E. Arzanol, an Anti-inflammatory and Anti-HIV-1 Phloroglucinol α-Pyrone from Helichrysum italicum ssp. microphyllum. J. Nat. Prod. 2007, 70, 608–612. [Google Scholar] [CrossRef]
- Rosa, A.; Deiana, M.; Atzeri, A.; Corona, G.; Incani, A.; Melis, M.P.; Appendino, G.; Dessì, M.A. Evaluation of the antioxidant and cytotoxic activity of arzanol, a prenylated α-pyrone–phloroglucinol etherodimer from Helichrysum italicum subsp. microphyllum. Chem. Biol. Interact. 2007, 165, 117–126. [Google Scholar] [CrossRef]
- Kothavade, P.S.; Nagmoti, D.M.; Bulani, V.D.; Juvekar, A.R. Arzanol, a Potent mPGES-1 Inhibitor: Novel Anti-Inflammatory Agent. Sci. World J. 2013, 2013, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Czinner, E.; Kéry, Á.; Hagymási, K.; Blázovics, A.; Lugasi, A.; Szõke, É.; Lemberkovics, É. Biologically active compounds of Helichrysum arenarium (L.) Moench. Eur. J. Drug Metab. Pharm. 1999, 24, 309–313. [Google Scholar] [CrossRef]
- Czinner, E.; Hagymási, K.; Blázovics, A.; Kéry, A.; Szoke, E.; Lemberkovics, E. In vitro antioxidant properties of Helichrysum arenarium (L.) Moench. J. Ethnopharmacol. 2000, 73, 437–443. [Google Scholar] [CrossRef]
- Malolo, F.-A.E.; Bissoue Nouga, A.; Kakam, A.; Franke, K.; Ngah, L.; Flausino, O.; Mpondo Mpondo, E.; Ntie-Kang, F.; Ndom, J.C.; da Bolzani, V.S.; et al. Protease-inhibiting, molecular modeling and antimicrobial activities of extracts and constituents from Helichrysum foetidum and Helichrysum mechowianum (Compositae). Chem. Cent. J. 2015, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iniesta-Sanmartín, E.; Tomás-Barberán, F.A.; Guirado, A.; Tomás-Lorente, F. Antibacterial Flavonoids from Helichrysum picardii and H. italicum. Planta Med. 1990, 56, 648–649. [Google Scholar] [CrossRef]
- Legoalea, P.B.; Mashimbyeb, M.J.; van Rec, T. Antiinflammatory and antioxidant flavonoids from Helichrysum kraussii and H. odoratissimum flowers. Nat. Prod. Commun. 2013, 8, 1403–1404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pietta, P.-G. Flavonoids as Antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekara, A. Phenolic Acids. In Encyclopedia of Food Chemistry; Melton, L., Shahidi, F., Varelis, P., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 535–545. [Google Scholar] [CrossRef]
- Kadifkova Panovska, T.; Kulevanova, S. Antioxidant potential of Helichrysum plicatum DC. (Asteraceae). Maced. Pharm. Bull. 2005, 51, 29–34. [Google Scholar] [CrossRef]
- Acet, T.; Ozcan, K.; Zengin, G. An assessment of phenolic profiles, fatty acid compositions, and biological activities of two Helichrysum species: H. plicatum and H. chionophilum. J. Food Biochem. 2019, 44, e13128. [Google Scholar] [CrossRef]
- Rosa, A.; Pollastro, F.; Atzeri, A.; Appendino, G.; Melis, M.P.; Deiana, M.; Incani, A.; Loru, D.; Dessì, M.A. Protective role of arzanol against lipid peroxidation in biological systems. Chem. Phys. Lipids 2011, 164, 24–32. [Google Scholar] [CrossRef]
- Demir, A.; Taban, B.M.; Aslan, M.; Yesilada, E.; Aytac, S.A. Antimicrobial effect of Helichrysum plicatum subsp. plicatum. Pharm. Biol. 2009, 47, 289–297. [Google Scholar] [CrossRef]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef]
- Almada-Ruiz, E.; Martínez-Téllez, M.Á.; Hernández-Álamos, M.M.; Vallejo, S.; Primo-Yúfera, E.; Vargas-Arispuro, I. Fungicidal potential of methoxylated flavones from citrus for in vitro control of Colletotrichum gloeosporioides, causal agent of anthracnose disease in tropical fruits. Pest Manag. Sci. 2003, 59, 1245–1249. [Google Scholar] [CrossRef] [PubMed]
- Ortuño, A.; Báidez, A.; Gómez, P.; Arcas, M.C.; Porras, I.; García-Lidón, A.; Río, J.A.D. Citrus paradisi and Citrus sinensis flavonoids: Their influence in the defence mechanism against Penicillium digitatum. Food Chem. 2006, 98, 351–358. [Google Scholar] [CrossRef]
- Berim, A.; Gang, D.R. Methoxylated flavones: Occurrence, importance, biosynthesis. Phytochem. Rev. 2016, 15, 363–390. [Google Scholar] [CrossRef]
- Nostro, A.; Germanò, M.P.; D’Angelo, V.; Marino, A.; Cannatelli, M.A. Extraction methods and bioautography for evaluation of medicinal plant antimicrobial activity. Lett. Appl. Microbiol. 2000, 30, 379–384. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- National Committee for Clinical Laboratory Standards, 5th ed.; Methods for Dilution antimicrobial susceptibility tests for bacteria that grow aerobically: Approved standards. NCCLS document M7-A5; NCCLS: Wayne, PA, USA, 2000.
- Novaković, M.; Novaković, I.; Cvetković, M.; Sladić, D.; Tešević, V. Antimicrobial activity of the diarylheptanoids from the black and green alder. Braz. J. Bot. 2015, 38, 441–446. [Google Scholar] [CrossRef]
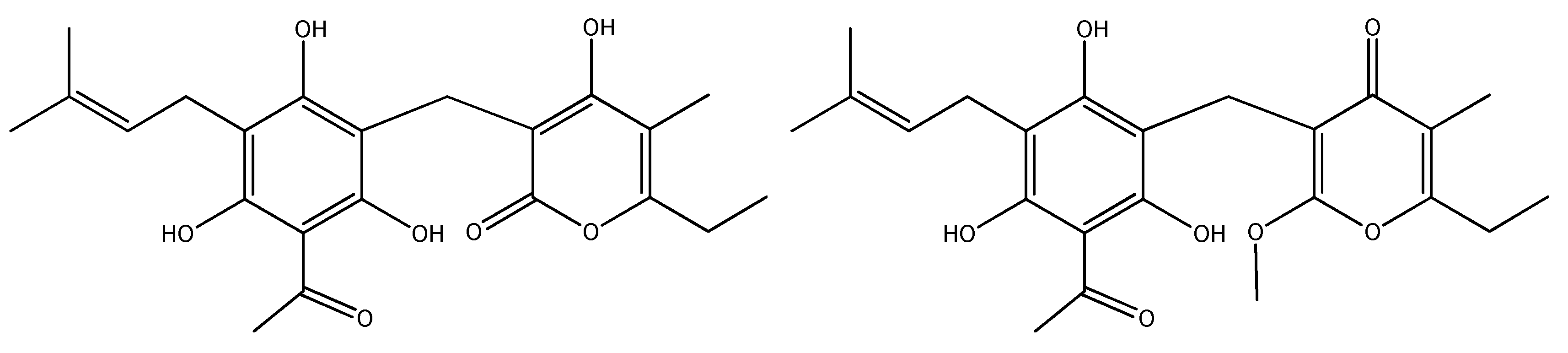
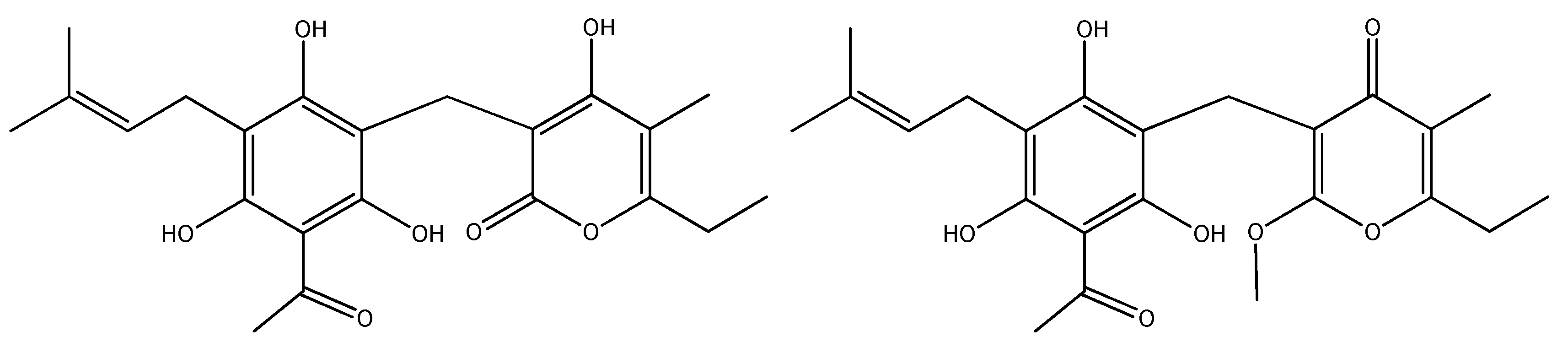
| No | Compound Class/Name | EE | DCME | ACNE |
|---|---|---|---|---|
| Pyrone | ||||
| α-pyrone | ||||
| 1 | 6-ethyl-4-hydroxy-5-methyl-3-(3-oxopentyl)-2H-pyran-2-one [22] | + | ||
| 2 | micropyrone [23] | + | ||
| 3 | micropyrone analog [19] | + | ||
| 4 | helipyrone C [20] | + | + | + |
| 5 | bisnorhelipyrone [21] | + | ||
| 6 | plicatipyrone analog [22] | + | ||
| 7 | 3,3’-methylenebis[4-(acetyloxy)-5,6-dimethyl-2H-pyran-2-one [21] | + | ||
| 8 | helipyrone B [20] | + | ||
| 9 | 3-[[3-acetyl-2,4,6-trihydroxy-5-(3-methyl-2-buten-1-yl)phenyl]methyl]-4-hydroxy-6-methyl-2H-pyran-2-one [19] | + | ||
| 10 | plicatipyrone [22] | + | + | + |
| 11 | arenol [23] | + | + | + |
| 12 | plicatipyrone analog [22] | + | ||
| 13 | arzanol [27] | + | + | + |
| 14 | heliarzanol [23] | + | + | |
| 15 | cycloarzanol [23] | + | ||
| 16 | helipyrone diacetate [25] | + | + | |
| 17 | methylarzanol [23] | + | + | |
| 18 | 3-[1-[3-acetyl-2,4,6-trihydroxy-5-(3-methyl-2-buten-1-yl)phenyl]ethyl]-6-ethyl-4-hydroxy-5-methyl-2H-pyran-2-one [23] | + | + | |
| 19 | auricepyrone [22], 23-methyl-6-O-desmethylauricepyrone [22] | + | ||
| 20 | helicerastripyrone [28] | + | ||
| 21 | 4-hydroxy-5,6-dimethyl-3-[[2,4,6-trihydroxy-3-(3-methyl-2-buten-1-yl)-5-(2-methyl-1-oxobutyl)phenyl]methyl]-2H-pyran-2-one [24] | + | ||
| 22 | 3-[[3-acetyl-5-(3,7-dimethyl-2,6-octadien-1-yl)-2,4,6-trihydroxyphenyl]methyl]-4-hydroxy-5,6-dimethyl-2H-pyran-2-one [29] | + | ||
| 23 | plicatipyrone analog [26] | + | + | |
| 24 | 6-ethyl-4-hydroxy-5-methyl-3-[[2,4,6-trihydroxy-3-(3-methyl-2-buten-1-yl)-5-(2-methyl-1-oxopropyl)phenyl]methyl]-2H-pyran-2-one [23] | + | + | |
| 25 | norauricepyrone [24] | + | ||
| 26 | 3-[[2,4-dihydroxy-6-methoxy-3-(2-methyl-1-oxobutyl)phenyl]methyl]-6-ethyl-4-hydroxy-5-methyl-2H-pyran-2-one [30] | + | ||
| 27 | 3-[1-[3-acetyl-2,4,6-trihydroxy-5-(3-methyl-2-buten-1-yl)phenyl]heptyl]-4-hydroxy-6-methyl-2H-pyran-2-one [23], 3-[[2,4-dihydroxy-6-methoxy-5-(3-methyl-2-buten-1-yl)-3-(2-methyl-1-oxobutyl)phenyl]methyl]-6-ethyl-4-hydroxy-5-methyl-2H-pyran-2-one [30], 3-[[2,4-dihydroxy-6-methoxy-3-(3-methyl-2-buten-1-yl)-5-(2-methyl-1-oxobutyl)phenyl]methyl]-6-ethyl-4-hydroxy-5-methyl-2H-pyran-2-one [22] | + | + | |
| 28 | 3-[[3-acetyl-5-(3,7-dimethyl-2,6-octadienyl)-2,4,6-trihydroxyphenyl]methyl]-4-hydroxy-5-methyl-6-propyl-2H-pyran-2-one [29], 3-[[3-(3,7-dimethyl-2,6-octadien-1-yl)-2,4,6-trihydroxy-5-(2-methyl-1-oxopropyl)phenyl]methyl]-4-hydroxy-5,6-dimethyl-2H-pyran-2-one [24] | + | ||
| 29 | 3-[[3-(3,7-dimethyl-2,6-octadien-1-yl)-2,4,6-trihydroxy-5-(2-methyl-1-oxobutyl)phenyl]methyl]-6-ethyl-4-hydroxy-5-methyl-2H-pyran-2-one [24] | + | ||
| 30 | 3-[[3,7-dimethyl-2,6-octadien-1-yl]-2,4,6-trihydroxy-5-(2-methyl-1-oxobutyl)phenyl]methyl]-4-hydroxy-5,6-dimethyl-2H-pyran-2-one [24] | + | + | |
| 31 | 3-[[2,3-dihydro-4,6-dihydroxy-2-(1-methylethenyl)-5-(2-methyl-1-oxobutyl)-7-benzofuranyl]methyl]-6-ethyl-4-hydroxy-5-methyl-2H-pyran-2-one [30], 3-[[5,7-dihydroxy-2,2-dimethyl-6-(2-methyl-1-oxobutyl)-2H-1-benzopyran-8-yl]methyl]-6-ethyl-4-hydroxy-5-methyl-2H-pyran-2-one [30] | + | ||
| α-pyrone (coumarin) | ||||
| 32 | 2’,3’-dihydroxypuberulin [31] | + | ||
| 33 | 7-(2,3-dihydroxy-3-methylbutoxy)-5-hydroxy-6-methoxy-2H-1-benzopyran-2-one [32] | + | + | |
| 34 | coumarin derivative [33,34] | + | ||
| 35 | 7-(2,3-dihydroxy-3-methylbutoxy)-5,6-dimethoxy-2H-1-benzopyran-2-one [35] | + | ||
| 36 | 2,3-dihydro-10-methoxy-2-(1-methylethenyl)-7H-pyrano[2,3-g]-1,4-benzodioxin-7-one [36] | + | ||
| γ-pyrone | ||||
| 37 | 2-methoxy-3,5-dimethyl-6-(1-methylethyl)-4H-pyran-4-one [30] | + | ||
| 38 | 3-[[2,4-dihydroxy-6-methoxy-3-(3-methyl-2-buten-1-yl)-5-(2-methyl-1-oxobutyl)phenyl]methyl]-6-ethyl-2-methoxy-5-methyl-4H-pyran-4-one [37] | + | ||
| 39 | italipyrone [22] | + | ||
| 40 | italipyrone analog [22] | + | ||
| γ-pyrone (chromone) | ||||
| 41 | 3-(acetyloxy)-2,3-dihydro-5,7-dihydroxy-6-(3-methyl-2-butenyl)-2-phenyl-4H-1-benzopyran-4-one [38] | + | ||
| 42 | 2,3-dihydro-5,7-dihydroxy-3-methyl-8-(3-methyl-2-butenyl)-2-(1-methylethyl)-4H-1-benzopyran-4-one [39] | + | ||
| Flavonoid | ||||
| flavone | ||||
| 43 | apigenin-7-O-glucoside [4] | + | ||
| 44 | apigenin-5-O-glucoside [40] | + | ||
| 45 | luteolin [41] | + | ||
| 46 | 3,5,6,7,8-pentamethoxyflavone [42] | + | ||
| 47 | 3’,4’,5,6,7-pentamethoxyflavone [43] | + | ||
| 48 | apigenin [40] | + | ||
| 49 | dihydroxy-dimethoxyflavone [44] | + | ||
| 50 | jaceosidin [45] | + | ||
| 51 | chrysoeriol [45] | + | ||
| 52 | 5,7-dihydroxy-3-methoxyflavone [44] | + | ||
| 53 | 5,7,4’-trihydroxy-3,6-dimethoxy-3’-prenylflavone [46] | + | ||
| 54 | TEDMF 1 [43] | + | ||
| 55 | TEDMF [43] | + | ||
| 56 | 6-[(6-ethyl-4-hydroxy-5-methyl-2-oxo-2H-pyran-3-yl)methyl]-2,3-dihydro-5,7-dihydroxy-8-(3-methyl-2-buten-1-yl)-2-phenyl-4H-1-benzopyran-4-one [47] | + | ||
| 57 | TEDMF [43] | + | ||
| flavanone | ||||
| 58 | naringenin-5-O-glucoside [40] | + | ||
| 59 | naringenin-4′-O-glucoside [48] | + | ||
| 60 | naringenin-7-O-glucoside [40] | + | ||
| 61 | eriodictyol [49] | + | ||
| 62 | naringenin [40] | + | + | |
| flavonol | ||||
| 63 | quercetin-3-O-glucoside [4], hyperoside (quercetin-3-O-galactoside) [4] | + | ||
| 64 | kaempferol-3-O-glucoside [50] | + | ||
| 65 | helichrysoside [51] | + | ||
| 66 | quercetin [40] | + | + | |
| 67 | tiliroside [52] | + | ||
| 68 | tiliroside analog [52] | + | ||
| 69 | kaempferol [41] | + | ||
| chalcone | ||||
| 70 | arenariumoside V [53], arenariumoside VI [53], arenariumoside VII [53] | + | ||
| 71 | tomoroside A [53] | + | ||
| 72 | isosalipurposide [40] | + | ||
| 73 | helilupolone [54] | + | ||
| Terpenoid | ||||
| sesquiterpenoid | ||||
| 74 | sesquiterpene derivative [55,56,57] | + | ||
| 75 | sesquiterpene derivative [55,56,57] | + | ||
| 76 | sesquiterpene derivative [58,59,60] | + | ||
| 77 | sesquiterpene derivative [61,62] | + | ||
| 78 | sesquiterpene derivative [62,63,64] | + | ||
| 79 | eudesmane derivative [65] | + | ||
| 80 | sesquiterpene derivative [66,67,68] | + | ||
| 81 | sesquiterpene derivative [66,67,68] | + | ||
| 82 | ainsliaside E [69] | + | ||
| 83 | cinnamoyloxy-hydroxyeudesmane [70] | + | ||
| 84 | athrolide C [71] | + | + | |
| 85 | athrolide D [71] | + | ||
| diterpenoid | ||||
| 86 | diterpene derivative [30,72] | + | ||
| 87 | 8-(acetyloxy)-3-ethenyloctahydro-10-hydroxy-3,4a,7,7,10a-pentamethyl-1H-naphtho[2,1-b]pyran-2,5(3H,4aH)-dione [72] | + | ||
| 88 | (7-ethenyl-1,2,3,4,4a,4b,5,6,7,8,10,10a-dodecahydro-1,4a,7-trimethyl-1-phenanthrenyl)methyl-butanedioic acid methyl ester [72] | + | ||
| 89 | ent-kaurane derivative [73] | + | ||
| 90 | gymnospermin [74] | + | ||
| 91 | diterpene derivative [75,76,77] | + | ||
| 92 | ent-kaurane derivative [73] | + | ||
| 93 | 5-(acetyloxy)-α-ethenyldecahydro-α,3a,5,7b-tetramethyl-1H-cyclopropa[a]naphthalene-4-propanol acetate [78] | + | ||
| 94 | ent-kaurane derivative [73] | + | + | |
| Phloroglucinol | ||||
| 95 | 4-[3,5-dihydroxy-4-(1-oxobutyl)phenoxy]-2-methyl-butanoic acid [47], 4-[3,5-dihydroxy-4-(2-methyl-1-oxopropyl)phenoxy]-2-methyl-butanoic acid [47], 1-[2,6-dihydroxy-4-[[4-hydroxy-3-(hydroxymethyl)-2-buten-1-yl]oxy]phenyl]-1-butanone [47] | + | ||
| 96 | 2,3-dihydro-4,5-dimethoxy-2,2-dimethyl-6-benzofuranol [79] | + | ||
| 97 | 4,5-dimethoxy-6-(2-methyl-1-propen-1-yl)-1,3-benzenediol-1,3-diacetate [79] | + | ||
| 98 | 1-(4,6-dihydroxy-2,3-dimethoxyphenyl)-2-methyl-1-butanone [43,79,80] | + | ||
| 99 | 4-[3,5-dihydroxy-4-(2-methyl-1-oxopropyl)phenoxy]-2-methyl-2-butenoic acid methyl ester [47] | + | ||
| 100 | acylphloroglucinol derivative [47] | + | + | |
| 101 | 2-methyl-1-[2,4,6-trihydroxy-3-(3-methyl-2-buten-1-yl)phenyl]-1-propanone [47], 1-[2,6-dihydroxy-4-[(3-methyl-2-buten-1-yl)oxy]phenyl]-2-methyl-1-propanone [47], 1-[2,6-dihydroxy-4-[(3-methyl-2-buten-1-yl)oxy]phenyl]-1-butanone [47], 1-[2,4,6-trihydroxy-3-(3-methyl-2-buten-1-yl)phenyl]-1-butanone [47], 1-(3,4-dihydro-5,7-dihydroxy-2,2-dimethyl-2H-1-benzopyran-6-yl)-2-methyl-1-propanone [30] | + | ||
| 102 | [3,4-dihydro-5,7-dihydroxy-2-(4-methyl-3-penten-1-yl)-2H-1-benzopyran-6-yl]phenyl-methanone [81] | + | + | |
| 103 | 2-(3,7-dimethyl-2,6-octadienyl)-3-hydroxy-5-methoxy-6-(2-methyl-1-oxopropyl)-2,5-cyclohexadiene-1,4-dione [30] | + | ||
| 104 | phenyl[2,4,6-trihydroxy-3-(3-methyl-2-buten-1-yl)phenyl]-methanone [47], [2,6-dihydroxy-4-[(3-methyl-2-buten-1-yl)oxy]phenyl]phenyl-methanone [47] | + | ||
| 105 | 1-[2,3-dihydro-4,6-dihydroxy-2-(1-methylethenyl)-5-benzofuranyl]-2-methyl-1-propanone [30] | + | ||
| 106 | 7-acetyl-5’-ethyl-4,6-dihydroxy-4’-methyl-5-(3-methyl-2-buten-1-yl)-spiro[benzofuran-2(3H),2’(3’H)-furan]-3’-one [24] | + | + | |
| 107 | 1-[2,6-bis(acetyloxy)-4-[[4-(acetyloxy)-3-[(acetyloxy)methyl]-2-buten-1-yl]oxy]phenyl]-1-butanone [47] | + | + | |
| 108 | 4,6-dihydroxy-4’,5’-dimethyl-5-(3-methyl-2-buten-1-yl)-7-(2-methyl-1-oxobutyl)-spiro[benzofuran-2(3H),2’(3’H)-furan]-3’-one [24], 5’-ethyl-4,6-dihydroxy-4’-methyl-5-(3-methyl-2-buten-1-yl)-7-(2-methyl-1-oxopropyl)-spiro[benzofuran-2(3H),2’(3’H)-furan]-3’-one [24] | + | ||
| 109 | 2-methyl-1-[2,4,6-trihydroxy-5-[(1S)-1-(4-hydroxy-6-methoxy-1,3-benzodioxol-5-yl)-2-methylpropyl]-3-(3-methyl-2-butenyl)phenyl]-1-propanone [30] | + | ||
| Phthalide | ||||
| 110 | 7-(β-D-glucopyranosyloxy)-5-hydroxy-1(3H)-isobenzofuranone [82] | + | ||
| 111 | 5,7-dihydroxyphthalide [83] | + | + | |
| 112 | 7-(β-D-glucopyranosyloxy)-5-methoxy-phthalide [83] | + | ||
| 113 | 5-methoxy-7-hydroxyphthalide [84] | + | + | |
| 114 | 4-(4-hydroxy-3-methylbutyl)-5,7-dimethoxy-1(3H)-isobenzofuranone [85] | + | ||
| Phenolic acid (derivative) | ||||
| 115 | chlorogenic acid [86] | + | ||
| 116 | caffeic acid [87] | + | ||
| 117 | everlastoside M [88] | + | ||
| 118 | syringic acid [87] | + | ||
| 119 | 3’,4’-methylenedioxycinnamic acid [89] | + | ||
| 120 | di-O-caffeoylquinic acid [4] | + | ||
| 121 | di-O-caffeoylquinic acid [4] | + | ||
| 122 | 4-(3-methoxy-3-oxo-1-propen-1-yl)-2-(3-methyl-2-buten-1-yl)phenyl-3-(acetyloxy)-butanoic acid ester [90] | + | ||
| Acetophenone | ||||
| 123 | 4’-hydroxy-3’-(3-methyl-2-butenyl)-acetophenone [26] | + | ||
| 124 | 1-[2-[1-[(acetyloxy)methyl]ethenyl]-2,3-dihydro-3-hydroxy-5-benzofuranyl]ethanone [51] | + | + | |
| Other | ||||
| 125 | inositol [83] | + | ||
| 126 | quinic acid [91] | + | ||
| 127 | 1,6-dihydroxy-4-oxo-2-cyclohexene-1-acetic acid methyl ester [92] | + | ||
| 128 | tetrahydrojacarone [93] | + | ||
| 129 | loliolide [94] | + | ||
| 130 | 2-[4-(1,3-dihydroxypropyl)-2-methoxyphenoxy]-3-hydroxy-1-(4-hydroxy-3-methoxyphenyl)-1-propanone [95] | + | ||
| 131 | pinellic acid [96], tianshic acid [97] | + | + | |
| 132 | (3,4,5,6,7-pentamethoxy-2-benzofuranyl)phenylmethanone [98] | + | ||
| 133 | PUFA 2 [99,100,101] | + | ||
| 134 | pinoresinol [102] | + | ||
| 135 | PUFA [99,100,101] | + | ||
| 136 | dihydro-5-(5,8-tetradecadienyl)-2(3H)-furanone [47] | + | ||
| 137 | PUFA [99,100,101] | + | + | |
| 138 | hexadecanal [103] | + | ||
| Unclassified3 | ||||
| 139 | 4-hydroxy-3,5-dimethyl-6-(1-methylethyl)-2H-pyran-2-one [30], 2-ethyl-6-methoxy-3,5-dimethyl-4H-pyran-4-one [28] | + | ||
| 140 | 3-prenyl-2,4,6-trihydroxyacetophenone [30], 4,6-dimethoxy-5-(2-methyl-1-propen-1-yl)-1,3-benzodioxole [80] | + | + | |
| 141 | helinivene A [104], piperitol [105], 1-[6-(acetyloxy)-2,3,4-trimethoxyphenyl]-3-phenyl- 2-propen-1-one [106] | + | ||
| 142 | ent-kaurane derivative [73], eudesmane derivative [65] | + |
| EE | DCME | ACNE | OE | Trolox | BHT (Methanol) | BHT (Toluene) | |
|---|---|---|---|---|---|---|---|
| EC50 1 (mg/mL) | 0.45 ± 0.04 | 0.58 ± 0.02 | 1.74 ± 0.01 | 17.61 ± 0.16 | 0.064 ± 0.01 | 0.33 ± 0.01 | 1.42 ± 0.01 |
| Escherichia coli | Pseudomonas aeruginosa | Proteus hauseri | Klebsiella pneumoniae | Salmonella enterica subsp. enterica | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| mg/mL | mg/mL | mg/mL | mg/mL | mg/mL | ||||||
| EE | 2.5 | >10 | 0.625 | 2.5 | 2.5 | >10 | 1.25 | 5 | 2.5 | 5 |
| DCME | 0.313 | 2.5 | 0.1571 | 0.625 | 0.157 | 0.625 | 0.157 | 0.625 | 1.25 | 10 |
| ACNE | 0.625 | 2.5 | 1.25 | 5 | 1.25 | 5 | 0.625 | 1.25 | 1.25 | 5 |
| Chloramphenicol | 0.062 | 0.25 | 0.125 | 0.062 | 0.125 | |||||
| Staphylococcus aureus | Bacillus subtilis | Clostridium sporogenes | ||||
|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | |
| mg/mL | mg/mL | mg/mL | ||||
| EE | 0.313 | 2.5 | 0.313 | 1.25 | 2.5 | 10 |
| DCME | 0.157 | 0.625 | 0.157 | 0.625 | 0.313 | 1.25 |
| ACNE | 0.625 | 5 | 2.5 | 10 | 2.5 | 10 |
| Chloramphenicol | 0.015 | 0.015 | 0.25 | |||
| Aspergillus brasiliensis | Saccharomyces cerevisiae | Candida albicans | ||||
|---|---|---|---|---|---|---|
| MIC | MFC | MIC | MFC | MIC | MFC | |
| mg/mL | mg/mL | mg/mL | ||||
| EE | 1.251 | >10 | 1.25 | 10 | 2.5 | 5 |
| DCME | 0.625 | 2.5 | 1.25 | 5 | 1.25 | 2.5 |
| ACNE | 1.25 | 10 | 1.25 | 10 | 2.5 | 5 |
| Nystatin | 2.5 | 1.25 | 2.5 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vujić, B.; Vidaković, V.; Jadranin, M.; Novaković, I.; Trifunović, S.; Tešević, V.; Mandić, B. Composition, Antioxidant Potential, and Antimicrobial Activity of Helichrysum plicatum DC. Various Extracts. Plants 2020, 9, 337. https://doi.org/10.3390/plants9030337
Vujić B, Vidaković V, Jadranin M, Novaković I, Trifunović S, Tešević V, Mandić B. Composition, Antioxidant Potential, and Antimicrobial Activity of Helichrysum plicatum DC. Various Extracts. Plants. 2020; 9(3):337. https://doi.org/10.3390/plants9030337
Chicago/Turabian StyleVujić, Bojan, Vera Vidaković, Milka Jadranin, Irena Novaković, Snežana Trifunović, Vele Tešević, and Boris Mandić. 2020. "Composition, Antioxidant Potential, and Antimicrobial Activity of Helichrysum plicatum DC. Various Extracts" Plants 9, no. 3: 337. https://doi.org/10.3390/plants9030337
APA StyleVujić, B., Vidaković, V., Jadranin, M., Novaković, I., Trifunović, S., Tešević, V., & Mandić, B. (2020). Composition, Antioxidant Potential, and Antimicrobial Activity of Helichrysum plicatum DC. Various Extracts. Plants, 9(3), 337. https://doi.org/10.3390/plants9030337





