Trends in Seaweed Extract Based Biostimulants: Manufacturing Process and Beneficial Effect on Soil-Plant Systems
Abstract
1. Introduction
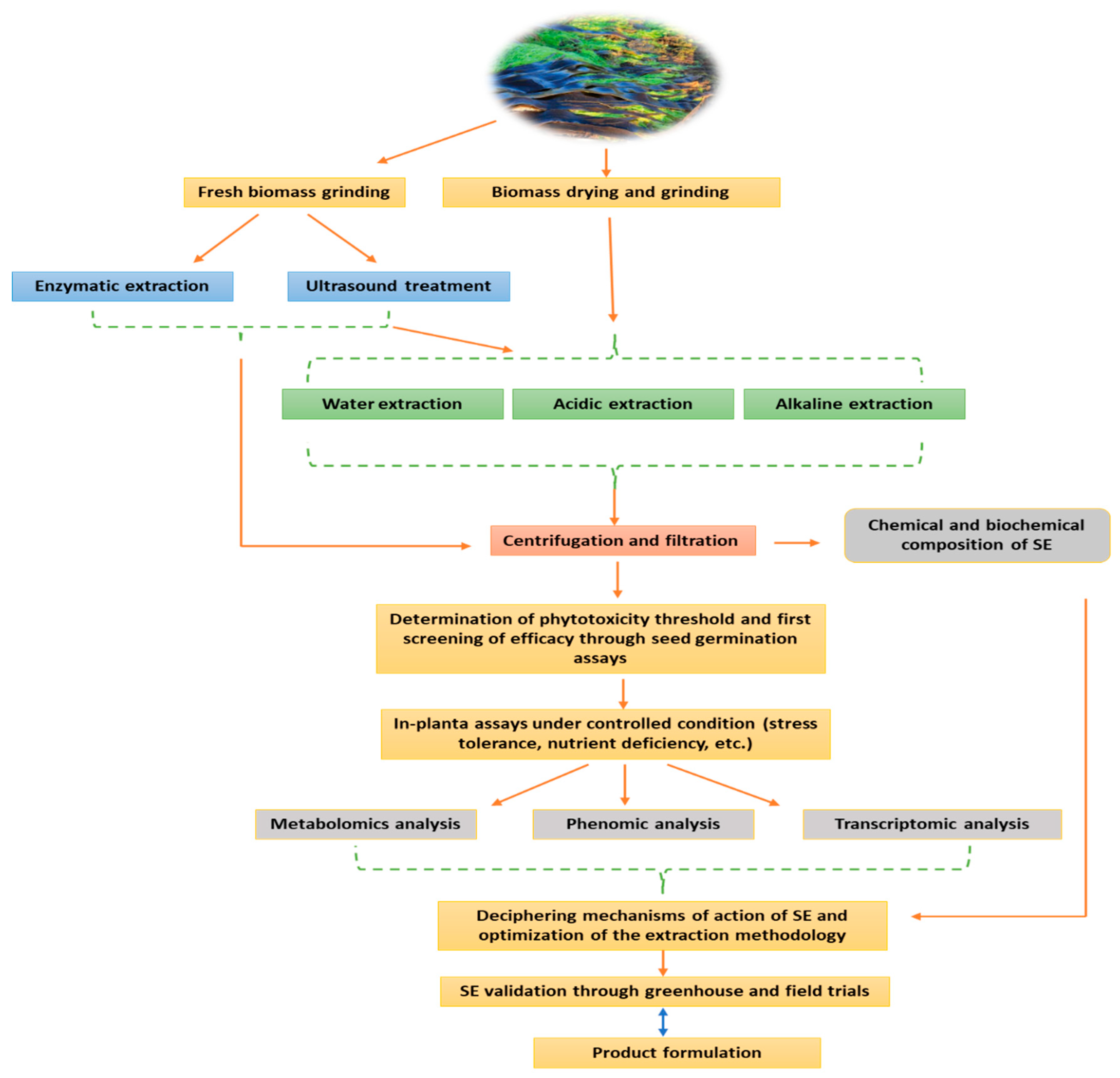
2. Manufacturing Process of Seaweed Extract Biostimulant: The Most Critical Challenge in SE Development
3. Overview on the Positive Effect of Seaweed Extracts on Plant Productivity
4. Biostimulant Properties of Seaweed Extract Are Emphasized under Abiotic Stress
5. Effect of Seaweed Extract on the Beneficial Microbiome of Plants: A Vital Component that Is Often Disregarded
6. Seaweed Extract as a Potential Solution to Enhance Nutrient Use Efficiency
| Plant | Seaweed Species | Extraction Technic | Elemental Composition | Experiment Conditions | Mode of Application | Findings | Source |
|---|---|---|---|---|---|---|---|
| Maize | Kappaphycus alvarezii (Ka)/Gracilaria edulis (Ge) (Applied separately) | Liquid filtrate from fresh seaweed | K+ 33,654; 682.1, P3+ 17.45; not detected, Ca2+ 321; 352, Mg2+ 112; 311 for Ka and Ge respectively. | Field experiment | Foliar spray | Enhanced N, P and K uptake (grain + stover) for both extracts | [78] |
| Oilseed Rape | Ecklonia maxima | Cold cell burst | N 3.6 g.kg−1, P 8.2 g.kg−1, K 7.2 g.kg−1, Ca 0.8 g.kg−1, Mg 0.2 g.kg−1, Fe 13.6 mg.kg−1, Mn 8.4 mg.kg−1, B 0.24 mg.kg−1, Zn 4.2 mg.kg−1, and Cu 0.2 mg.kg−1 | Pot experiment | Root application | Enhanced Leaf P and K concentration. | [38] |
| Tomato | Ecklonia maxima | Cold cell burst | N 3.6 g.kg−1, P 8.2 g.kg−1, K 7.2 g.kg−1, Ca 0.8 g.kg−1, Mg 0.2 g.kg−1, Fe 13.6 mg.kg−1, Mn 8.4 mg.kg−1, B 0.24 mg.kg−1, Zn 4.2 mg.kg−1, and Cu 0.2 mg.kg−1 | In soil under greenhouse | Foliar spray | Enhanced Fruit Ca concentration. | [79] |
| Tomato | Ascophyllum nodosum | Not mentioned | Fe 39.9 µg.mL−1, Mn 20.9 µg.mL−1, Cu 3.0 µg.mL−1, Zn 4.1 µg.mL−1 | Pots under growth chamber | Not mentioned | Enhanced concentration of Mn, Cu, and Zn in root and leaf. | [57] |
| Oilseed Rape | Ascophyllum nodosum | Acid extraction | Ca 0.854, Cu 0.009, Fe 0.030, K 6.630, Mg 0.919, N not detected, Na 3.102, P 0.116, S 2.660, Si 0.027, Zn 0.002% of dry weight | Hydroponic under greenhouse | In nutrient solution | Increased relative Mn, Cu, and Mg concentration in whole plant. | [80] |
| Wheat | Ascophyllum nodosum | Acid extraction | K 4442, P 78, S 1782, N 0 (concentration of 67 g DW dissolved in 1 L of water). | Pot experiment | Foliar spray | Enhanced Grain K | [81] |
| Oilseed Rape | Ascophyllum nodosum | Acid extraction | K 4442, P 78, S 1782, N 0 (concentration of 67 g DW dissolved in 1 L of water). | Pots under greenhouse conditions | In nutrient solution | Stimulation of root and shoot N and S. | [76] |
| Soybean | Kappaphycus alvarezii | Liquid filtrate from fresh seaweed | N 0.03%, P 33.99 mg.L−1, K 1.97%, S 0.06%, Ca 460.11 mg.L−1, Mg 581.2 mg.L−1, Na 0.51%, Cu 0.3 mg.L−1, Fe 10.59 mg.L−1, Mn 2.5 mg.L−1, Zn 0.62 mg.L−1 | Soil, field experiment | Foliar spray | Enhanced N, P, K, S grain uptake and N, P straw uptake. | [82] |
7. Seaweed Extract: A Complex Mixture with Multiple Mechanisms of Action
7.1. Mechanism of Action under Abiotic Stress
| Plant | Seaweed Species | Findings | Sources |
|---|---|---|---|
| Wheat | Lessonia nigrescens | Downregulation of TaHKT2; 1, a transporter implied in Na+ uptake from the soil, and the upregulation of TaSOS1 and TaNHX2 antiporters functioning in the Na+ exclusion to vacuoles. | [49] |
| Soybean | Ascophyllum nodosum | Over expression of stress responsive genes GmRD22, GmDREB, GmFIB1a, GmERD1, GmBIPD during drought stress and GmPIP1b during the recovery stage. | [91] |
| Arabidopsis | Ascophyllum nodosum | ANE A and B dysregulated the expression of 4.47% and 0.87% of the transcriptome respectively. | [96] |
| Oilseed Rape | Ascophyllum nodosum | Over expression of (BnNRT1.1; BnNRT2.1) and (BnSultr4.1; BnSultr4.2) which are genes encoding N and S root transporters respectively. | [76] |
| Arabidopsis | Ascophyllum nodosum | Up regulation of cold related genes CB73, RD29A, and COR15A. | [97] |
7.2. Mechanism of Action under Biotic Stress
8. Novel Approach in SE Development: The Promise of the Algae Bio-Refinery Concept
9. Seaweed Extract Biostimulants: Legal Acts
10. Concluding Remarks and Future Prospects
- ✓
- Gaining the farmer trust: Meeting farmer’s requirements is vital in the success of any given agri-input, which can only be achieved through adopting a farmer proximity approaches focusing on field demonstration, education as well as generating data specifically tailored for the end-customer. Furthermore, future research dealing with SE must focus on providing a manual of good farmer practices consisting of the best method of application, rates, frequencies, and time of application, etc. This would be the missing piece in the way of exploring the high potential of SE and harmonizing their final effect under different environments.
- ✓
- The transition from a niche to a broader market: SE and more generally plant biostimulant are still viewed as niche market, as they exclusively target horticulture and specialty crops. This can be explained by several factors, including the lack of consistency and rigorous regulation, their application method, and the farmers’ impressions. This latter is of high importance as SE biostimulants are still exhibited as an eco-friendly alternative for classical fertilization, which is erroneous and detrimental for the industry. Instead, SE should be aligned with mineral fertilization, thus making the most of a wider market through focusing on enhancing fertilizer eco-efficiency.
- ✓
- Breaking down SE mode of action: Biostimulants as they are formulated currently should be considered as unique cases requiring typical approaches. Indeed, additionally to their complex composition which makes it difficult to precisely pinpoint bioactive molecules inducing a specific effect, they are lacking broad-spectrum properties resulting in inconsistency, not always fully explained as the plant/soil domain is an intricate ecosystem governed by a string of variables including pedoclimatic conditions, crop types and soil microbiome distinctive features. It is mandatory to breakdown those complex mixtures to single molecule to ascertain occurring synergisms and antagonisms. The challenge may seem at first sight insurmountable, as the number of data to analyze is simply overwhelming. That said research is undoubtedly making progress through exploitation of novel high-throughput approaches (i.e., high-throughput sequencing and phenotyping, metabolomics, etc.).
- ✓
- Enhancing SE agri-economic efficiency: Several aspects need to be addressed to improve the agronomic efficiency of SE. Such issues could be mainly attributed to the peculiar nature of seaweed biomass, as its composition could radically change depending on the collection timing and the overall environmental conditions. Moreover, as described in our review the extraction process is still not fully optimized. Rising to the challenge will require the establishment of novel integrated approach as to fully control the manufacturing steps. In this regard, algal biorefinery concept integrating controlled algae cultivation is highly promising. This could resolve both the inconsistency in composition problem, as well as the product pricing. Ultimately, given the positive impact of SE beneficial plant microbes, furthering synergies between SE and microbial biostimulants could be the key to the development of next-generation plant biostimulants.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- United Nations, Department of Economic and Social Affairs, Population Division (UNDESA). World Population Prospects: The 2017 Revision, Key Findings and Advance Tables; ESA/P/WP/248; United Nations, Department of Economic and Social Affairs, Population Division (UNDESA): New York, NY, USA, 2017. [Google Scholar]
- United Nations Convention to Combat Desertification Global Land Outlook; United nations: Bonn, Germany, 2017.
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef]
- FAO. The Impact of Natural Hazards and Disasters on Agriculture, Food Security and Nutrition; Food and Agriculture Organisation: Rome, Italy, 2015. [Google Scholar]
- OECD/FAO. Agriculture in Sub-Saharan Africa: Prospects and challenges for the next decade. In OECD-FAO Agricultural Outlook 2016–2025; OECD Publishing: Paris, France, 2016. [Google Scholar]
- Timilsena, Y.P.; Adhikari, R.; Casey, P.; Muster, T.; Gill, H.; Adhikari, B. Enhanced efficiency fertilisers: A review of formulation and nutrient release patterns. J. Sci. Food Agric. 2015, 95, 1131–1142. [Google Scholar] [CrossRef]
- Wolfert, S.; Ge, L.; Verdouw, C.; Bogaardt, M.-J. Big Data in Smart Farming—A review. Agric. Syst. 2017, 153, 69–80. [Google Scholar] [CrossRef]
- Chojnacka, K.; Wieczorek, P.P.; Schroeder, G.; Michalak, I. (Eds.) Algae Biomass: Characteristics and Applications; Springer: Cham, Switzerland, 2018; ISBN 9783319747026. [Google Scholar]
- Craigie, J.S. Seaweed extract stimuli in plant science and agriculture. J. Appl. Phycol. 2011, 23, 371–393. [Google Scholar] [CrossRef]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Eef, B.; Marlies, D.; van Swam, K.; Veen, A.; Burger, L. Identification of the Seaweed Biostimulant Market (Phase 1); The North Sea Farm Foundation: AD Den Haag, The Netherlands, 2018. [Google Scholar]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed Extracts as Biostimulants of Plant Growth and Development. J. Plant Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Michalak, I.; Chojnacka, K. Algal extracts: Technology and advances. Eng. Life Sci. 2014, 14, 581–591. [Google Scholar] [CrossRef]
- U.S. DOE. National Algal Biofuels Technology Roadmap; US Department of Energy, Office of Energy Efficiency and Renewable Energy, Office of the Biomass Program: Washington, DC, USA, 2010; pp. 1–124. [Google Scholar]
- Michalak, I.; Chojnacka, K. Algae as production systems of bioactive compounds. Eng. Life Sci. 2015, 15, 160–176. [Google Scholar] [CrossRef]
- Kadam, S.U.; Tiwari, B.K.; O’Donnell, C.P. Application of Novel Extraction Technologies for Bioactives from Marine Algae. J. Agric. Food Chem. 2013, 61, 4667–4675. [Google Scholar] [CrossRef] [PubMed]
- Shukla, P.S.; Mantin, E.G.; Adil, M.; Bajpai, S.; Critchley, A.T.; Prithiviraj, B. Ascophyllum nodosum-Based Biostimulants: Sustainable Applications in Agriculture for the Stimulation of Plant Growth, Stress Tolerance, and Disease Management. Front. Plant Sci. 2019, 10, 655. [Google Scholar] [CrossRef] [PubMed]
- Ertani, A.; Francioso, O.; Tinti, A.; Schiavon, M.; Pizzeghello, D.; Nardi, S. Evaluation of Seaweed Extracts From Laminaria and Ascophyllum nodosum spp. as Biostimulants in Zea mays L. Using a Combination of Chemical, Biochemical and Morphological Approaches. Front. Plant Sci. 2018, 9, 428. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.S.S.; Fleming, C.; Selby, C.; Rao, J.R.; Martin, T. Plant biostimulants: A review on the processing of macroalgae and use of extracts for crop management to reduce abiotic and biotic stresses. J. Appl. Phycol. 2014, 26, 465–490. [Google Scholar] [CrossRef]
- Youssouf, L.; Lallemand, L.; Giraud, P.; Soule, F.; Bhaw-Luximon, A.; Meilhac, O.; D’Hellencourt, C.L.; Jhurry, D.; Couprie, J. Ultrasound-assisted extraction and structural characterization by NMR of alginates and carrageenans from seaweeds. Carbohydr. Polym. 2017, 166, 55–63. [Google Scholar] [CrossRef]
- Kadam, S.U.; Tiwari, B.K.; O’Connell, S.; O’Donnell, C.P. Effect of Ultrasound Pretreatment on the Extraction Kinetics of Bioactives from Brown Seaweed (Ascophyllum nodosum). Sep. Sci. Technol. 2015, 50, 670–675. [Google Scholar] [CrossRef]
- Alboofetileh, M.; Rezaei, M.; Tabarsa, M. Enzyme-assisted extraction of Nizamuddinia zanardinii for the recovery of sulfated polysaccharides with anticancer and immune-enhancing activities. J. Appl. Phycol. 2019, 31, 1391–1402. [Google Scholar] [CrossRef]
- Praveen, A.M.; Parvathy, K.K.R.; Jayabalan, R.; Balasubramanian, P. Dietary fiber from Indian edible seaweeds and its in-vitro prebiotic effect on the gut microbiota. Food Hydrocoll. 2019, 96, 343–353. [Google Scholar] [CrossRef]
- Rodrigues, D.; Costa-Pinto, A.R.; Sousa, S.; Vasconcelos, M.W.; Pintado, M.M.; Pereira, L.; Rocha-Santos, T.A.P.; da Costa, J.P.; Silva, A.M.S.; Duarte, A.C.; et al. Sargassum muticum and Osmundea pinnatifida Enzymatic Extracts: Chemical, Structural, and Cytotoxic Characterization. Mar. Drugs 2019, 17, 209. [Google Scholar] [CrossRef]
- Vasquez, V.; Martinez, R.; Bernal, C. Enzyme-assisted extraction of proteins from the seaweeds Macrocystis pyrifera and Chondracanthus chamissoi: Characterization of the extracts and their bioactive potential. J. Appl. Phycol. 2019, 31, 1999–2010. [Google Scholar] [CrossRef]
- Okolie, C.L.; Mason, B.; Mohan, A.; Pitts, N.; Udenigwe, C.C. The comparative influence of novel extraction technologies on in vitro prebiotic-inducing chemical properties of fucoidan extracts from Ascophyllum Nodosum. Food Hydrocoll. 2019, 90, 462–471. [Google Scholar] [CrossRef]
- Trivedi, K.; Vijay Anand, K.G.; Vaghela, P.; Ghosh, A. Differential growth, yield and biochemical responses of maize to the exogenous application of Kappaphycus alvarezii seaweed extract, at grain-filling stage under normal and drought conditions. Algal Res. 2018, 35, 236–244. [Google Scholar] [CrossRef]
- Singh, S.; Singh, M.K.; Pal, S.K.; Trivedi, K.; Yesuraj, D.; Singh, C.S.; Anand, K.G.V.; Chandramohan, M.; Patidar, R.; Kubavat, D.; et al. Sustainable enhancement in yield and quality of rain-fed maize through Gracilaria edulis and Kappaphycus alvarezii seaweed sap. J. Appl. Phycol. 2016, 28, 2099–2112. [Google Scholar] [CrossRef]
- Pramanick, B.; Brahmachari, K.; Mahapatra, B.S.; Ghosh, A.; Ghosh, D.; Kar, S. Growth, yield and quality improvement of potato tubers through the application of seaweed sap derived from the marine algae Kappaphycus alvarezii. J. Appl. Phycol. 2017, 29, 3253–3260. [Google Scholar] [CrossRef]
- Mattner, S.W.; Milinkovic, M.; Arioli, T. Increased growth response of strawberry roots to a commercial extract from Durvillaea potatorum and Ascophyllum nodosum. J. Appl. Phycol. 2018, 30, 2943–2951. [Google Scholar] [CrossRef]
- Kulkarni, M.G.; Rengasamy, K.R.R.; Pendota, S.C.; Gruz, J.; Plackova, L.; Novak, O.; Dolezal, K.; Van Staden, J. Bioactive molecules derived from smoke and seaweed Ecklonia maxima showing phytohormone-like activity in Spinacia oleracea L. N. Biotechnol. 2019, 48, 83–89. [Google Scholar] [CrossRef]
- Werner, T.; Motyka, V.; Strnad, M.; Schmülling, T. Regulation of plant growth by cytokinin. Proc. Natl. Acad. Sci. USA 2001, 98, 10487–10492. [Google Scholar] [CrossRef]
- Digruber, T.; Sass, L.; Cseri, A.; Paul, K.; Nagy, A.V.; Remenyik, J.; Molnar, I.; Vass, I.; Toldi, O.; Gyuricza, C.; et al. Stimulation of energy willow biomass with triacontanol and seaweed extract. Ind. Crops Prod. 2018, 120, 104–112. [Google Scholar] [CrossRef]
- Al-Ghamdi, A.A.; Elansary, H.O. Synergetic effects of 5-aminolevulinic acid and Ascophyllum nodosum seaweed extracts on Asparagus phenolics and stress related genes under saline irrigation. Plant Physiol. Biochem. 2018, 129, 273–284. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.H.; Srivastava, A.K.; Saber, H.; Alwaleed, E.A.; Tran, L.-S.P. Sargassum muticum and Jania rubens regulate amino acid metabolism to improve growth and alleviate salinity in chickpea. Sci. Rep. 2017, 7, 10537. [Google Scholar] [CrossRef]
- Spann, T.M.; Little, H.A. Applications of a Commercial Extract of the Brown Seaweed Ascophyllum nodosum Increases Drought Tolerance in Container-grown `Hamlin’ Sweet Orange Nursery Trees. Hortscience 2011, 46, 577–582. [Google Scholar] [CrossRef]
- Di Stasio, E.; Rouphael, Y.; Colla, G.; Raimondi, G.; Giordano, M.; Pannico, A.; El-Nakhel, C.; De Pascale, S. The influence of Ecklonia maxima seaweed extract on growth, photosynthetic activity and mineral composition of Brassica rapa L. subsp sylvestris under nutrient stress conditions. Eur. J. Hortic. Sci. 2017, 82, 286–293. [Google Scholar] [CrossRef]
- Trejo Valencia, R.; Sánchez Acosta, L.; Fortis Hernández, M.; Preciado Rangel, P.; Gallegos Robles, Á.M.; Antonio Cruz, D.R.; Vázquez Vázquez, C. Effect of Seaweed Aqueous Extracts and Compost on Vegetative Growth, Yield, and Nutraceutical Quality of Cucumber (Cucumis sativus L.) Fruit. Agronomy 2018, 8, 264. [Google Scholar] [CrossRef]
- Kapur, B.; Sarıdaş, M.A.; Çeliktopuz, E.; Kafkas, E.; Kargı, S.P. Health and taste related compounds in strawberries under various irrigation regimes and bio-stimulant application. Food Chem. 2018, 263, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Frioni, T.; Sabbatini, P.; Tombesi, S.; Norrie, J.; Poni, S.; Gatti, M.; Palliotti, A. Effects of a biostimulant derived from the brown seaweed Ascophyllum nodosum on ripening dynamics and fruit quality of grapevines. Sci. Hortic. 2018, 232, 97–106. [Google Scholar] [CrossRef]
- Augusto, A.; Simões, T.; Pedrosa, R.; Silva, S.F.J. Evaluation of seaweed extracts functionality as post-harvest treatment for minimally processed Fuji apples. Innov. Food Sci. Emerg. Technol. 2016, 33, 589–595. [Google Scholar] [CrossRef]
- Debnath, M.; Pandey, M.; Bisen, P.S. An Omics Approach to Understand the Plant Abiotic Stress. Omi. J. Integr. Biol. 2011, 15, 739–762. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Gruszka, D.; Janeczko, A.; Dziurka, M.; Pociecha, E.; Fodor, J. Non-enzymatic antioxidant accumulations in BR-deficient and BR-insensitive barley mutants under control and drought conditions. Physiol. Plant. 2018, 163, 155–169. [Google Scholar] [CrossRef]
- Sreeharsha, R.V.; Mudalkar, S.; Sengupta, D.; Unnikrishnan, D.K.; Reddy, A.R. Mitigation of drought-induced oxidative damage by enhanced carbon assimilation and an efficient antioxidative metabolism under high CO2 environment in pigeonpea (Cajanus cajan L.). Photosynth. Res. 2019, 139, 425–439. [Google Scholar] [CrossRef]
- Yadav, D.S.; Rai, R.; Mishra, A.K.; Chaudhary, N.; Mukherjee, A.; Agrawal, S.B.; Agrawal, M. ROS production and its detoxification in early and late sown cultivars of wheat under future O3 concentration. Sci. Total Environ. 2019, 659, 200–210. [Google Scholar] [CrossRef]
- Zou, P.; Lu, X.; Zhao, H.; Yuan, Y.; Meng, L.; Zhang, C.; Li, Y. Polysaccharides Derived From the Brown Algae Lessonia nigrescens Enhance Salt Stress Tolerance to Wheat Seedlings by Enhancing the Antioxidant System and Modulating Intracellular Ion Concentration. Front. Plant Sci. 2019, 10, 48. [Google Scholar] [CrossRef]
- Liu, H.; Chen, X.; Song, L.; Li, K.; Zhang, X.; Liu, S.; Qin, Y.; Li, P. Polysaccharides from Grateloupia filicina enhance tolerance of rice seeds (Oryza sativa L.) under salt stress. Int. J. Biol. Macromol. 2019, 124, 1197–1204. [Google Scholar] [CrossRef]
- Elansary, H.O.; Yessoufou, K.; Abdel-Hamid, A.M.E.; El-Esawi, M.A.; Ali, H.M.; Elshikh, M.S. Seaweed Extracts Enhance Salam Turfgrass Performance during Prolonged Irrigation Intervals and Saline Shock. Front. Plant Sci. 2017, 8, 830. [Google Scholar] [CrossRef]
- Patel, K.; Agarwal, P.; Agarwal, P.K. Kappaphycus alvarezii sap mitigates abiotic-induced stress in Triticum durum by modulating metabolic coordination and improves growth and yield. J. Appl. Phycol. 2018, 30, 2659–2673. [Google Scholar] [CrossRef]
- Sharma, S.; Chen, C.; Khatri, K.; Rathore, M.S.; Pandey, S.P. Gracilaria dura extract confers drought tolerance in wheat by modulating abscisic acid homeostasis. Plant Physiol. Biochem. 2019, 136, 143–154. [Google Scholar] [CrossRef]
- Bradacova, K.; Weber, N.F.; Morad-Talab, N.; Asim, M.; Imran, M.; Weinmann, M.; Neumann, G. Micronutrients (Zn/Mn), seaweed extracts, and plant growth-promoting bacteria as cold-stress protectants in maize. Chem. Biol. Technol. Agric. 2016, 3, 19. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Xylia, P.; Anastasiou, M.; Pantelides, I.; Tzortzakis, N. Effects of Ascophyllum nodosum seaweed extracts on lettuce growth, physiology and fresh-cut salad storage under potassium deficiency. J. Sci. Food Agric. 2018, 98, 5861–5872. [Google Scholar] [CrossRef]
- Saa, S.; Olivos-Del Rio, A.; Castro, S.; Brown, P.H. Foliar application of microbial and plant based biostimulants increases growth and potassium uptake in almond (Prunus dulcis [Mill.] D. A. Webb). Front. Plant Sci. 2015, 6, 87. [Google Scholar] [CrossRef]
- Carrasco-Gil, S.; Hernandez-Apaolaza, L.; Lucena, J.J. Effect of several commercial seaweed extracts in the mitigation of iron chlorosis of tomato plants (Solanum lycopersicum L.). Plant Growth Regul. 2018, 86, 401–411. [Google Scholar] [CrossRef]
- Jisha, K.C.; Vijayakumari, K.; Puthur, J.T. Seed priming for abiotic stress tolerance: An overview. Acta Physiol. Plant. 2013, 35, 1381–1396. [Google Scholar] [CrossRef]
- Paparella, S.; Araújo, S.S.; Rossi, G.; Wijayasinghe, M.; Carbonera, D.; Balestrazzi, A. Seed priming: State of the art and new perspectives. Plant Cell Rep. 2015, 34, 1281–1293. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Pre-Sowing Seed Treatment—A Shotgun Approach to Improve Germination, Plant Growth, and Crop Yield Under Saline and Non-Saline Conditions. Adv. Agron. 2005, 88, 223–271. [Google Scholar]
- Masondo, N.A.; Kulkarni, M.G.; Finnie, J.F.; Van Staden, J. Influence of biostimulants-seed-priming on Ceratotheca triloba germination and seedling growth under low temperatures, low osmotic potential and salinity stress. Ecotoxicol. Environ. Saf. 2018, 147, 43–48. [Google Scholar] [CrossRef]
- Kasim, W.A.E.-A.; Saad-Allah, K.M.; Hamouda, M. Seed Priming with Extracts of two Seaweeds Alleviates the Physiological and Molecular Impacts of Salinity Stress on Radish (Raphanus sativus). Int. J. Agric. Biol. 2016, 18, 653–660. [Google Scholar] [CrossRef]
- Ji, R.; Dong, G.; Shi, W.; Min, J. Effects of Liquid Organic Fertilizers on Plant Growth and Rhizosphere Soil Characteristics of Chrysanthemum. Sustainability 2017, 9, 841. [Google Scholar] [CrossRef]
- Renaut, S.; Masse, J.; Norrie, J.P.; Blal, B.; Hijri, M. A commercial seaweed extract structured microbial communities associated with tomato and pepper roots and significantly increased crop yield. Microb. Biotechnol. 2019, 12, 1346–1358. [Google Scholar] [CrossRef]
- Onet, A.; Dincua, L.C.; Grenni, P.; Laslo, V.; Teusdea, A.C.; Vasile, D.L.; Enescu, R.E.; Crisan, V.E. Biological indicators for evaluating soil quality improvement in a soil degraded by erosion processes. J. Soils Sediments 2019, 19, 2393–2404. [Google Scholar] [CrossRef]
- Wang, Y.; Fu, F.; Li, J.; Wang, G.; Wu, M.; Zhan, J.; Chen, X.; Mao, Z. Effects of seaweed fertilizer on the growth of Malus hupehensis Rehd. seedlings, soil enzyme activities and fungal communities under replant condition. Eur. J. Soil Biol. 2016, 75, 1–7. [Google Scholar] [CrossRef]
- Frankeberger, W.T.; Johanson, J.B. Method of measuring invertase activity in soils. Plant Soil 1983, 74, 301–311. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Assay of urease activity in soils. Soil Biol. Biochem. 1972, 4, 479–487. [Google Scholar] [CrossRef]
- Hayano, K. Protease activity in a paddy field soil: Origin and some properties. Soil Sci. Plant Nutr. 1993, 39, 539–546. [Google Scholar] [CrossRef]
- Margalef, O.; Sardans, J.; Fernández-Martínez, M.; Molowny-Horas, R.; Janssens, I.A.; Ciais, P.; Goll, D.; Richter, A.; Obersteiner, M.; Asensio, D.; et al. Global patterns of phosphatase activity in natural soils. Sci. Rep. 2017, 7, 1337. [Google Scholar] [CrossRef]
- Wang, Y.; Xiang, L.; Wang, S.; Wang, X.; Chen, X.; Mao, Z. Effects of seaweed fertilizer on the Malus hupehensis Rehd. seedlings growth and soil microbial numbers under continue cropping. Acta Ecol. Sin. 2017, 37, 180–186. [Google Scholar] [CrossRef]
- Alam, M.; Braun, G.; Norrie, J.; Hodges, D. Effect of Ascophyllum extract application on plant growth, fruit yield and soil microbial communities of strawberry. Can. J. Plant Sci. 2013, 93, 23–36. [Google Scholar] [CrossRef]
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S. Agricultural sustainability and intensive production practices. Nature 2002, 418, 671–677. [Google Scholar] [CrossRef]
- Fei, H.; Crouse, M.; Papadopoulos, Y.; Vessey, J.K. Enhancing the productivity of hybrid poplar (Populus x hybrid) and switchgrass (Panicum virgatum L.) by the application of beneficial soil microbes and a seaweed extract. Biomass Bioenergy 2017, 107, 122–134. [Google Scholar] [CrossRef]
- Layek, J.; Das, A.; Idapuganti, R.G.; Sarkar, D.; Ghosh, A.; Zodape, S.T.; Lal, R.; Yadav, G.S.; Panwar, A.S.; Ngachan, S.; et al. Seaweed extract as organic bio-stimulant improves productivity and quality of rice in eastern Himalayas. J. Appl. Phycol. 2018, 30, 547–558. [Google Scholar] [CrossRef]
- Jannin, L.; Arkoun, M.; Etienne, P.; Laîné, P.; Goux, D.; Garnica, M.; Fuentes, M.; Francisco, S.S.; Baigorri, R.; Cruz, F.; et al. Brassica napus Growth is Promoted by Ascophyllum nodosum (L.) Le Jol. Seaweed Extract: Microarray Analysis and Physiological Characterization of N, C, and S Metabolisms. J. Plant Growth Regul. 2013, 32, 31–52. [Google Scholar] [CrossRef]
- Mugnai, S.; Azzarello, E.; Pandolfi, C.; Salamagne, S.; Briand, X.; Mancuso, S. Enhancement of ammonium and potassium root influxes by the application of marine bioactive substances positively affects Vitis vinifera plant growth. J. Appl. Phycol. 2008, 20, 177–182. [Google Scholar] [CrossRef]
- Basavaraja, P.K.; Yogendra, N.D.; Zodape, S.T.; Prakash, R.; Ghosh, A. Effect of seaweed sap as foliar spray on growth and yield of hybrid maize. J. Plant Nutr. 2018, 41, 1851–1861. [Google Scholar] [CrossRef]
- Colla, G.; Cardarelli, M.; Bonini, P.; Rouphael, Y. Foliar Applications of Protein Hydrolysate, Plant and Seaweed Extracts Increase Yield but Differentially Modulate Fruit Quality of Greenhouse Tomato. Hortscience 2017, 52, 1214–1220. [Google Scholar] [CrossRef]
- Billard, V.; Etienne, P.; Jannin, L.; Garnica, M.; Cruz, F.; Garcia-Mina, J.-M.; Yvin, J.-C.; Ourry, A. Two Biostimulants Derived from Algae or Humic Acid Induce Similar Responses in the Mineral Content and Gene Expression of Winter Oilseed Rape (Brassica napus L.). J. Plant Growth Regul. 2014, 33, 305–316. [Google Scholar] [CrossRef]
- Stamatiadis, S.; Evangelou, L.; Yvin, J.-C.; Tsadilas, C.; Mina, J.M.G.; Cruz, F. Responses of winter wheat to Ascophyllum nodosum (L.) Le Jol. extract application under the effect of N fertilization and water supply. J. Appl. Phycol. 2015, 27, 589–600. [Google Scholar] [CrossRef]
- Rathore, S.S.; Chaudhary, D.R.; Boricha, G.N.; Ghosh, A.; Bhatt, B.P.; Zodape, S.T.; Patolia, J.S. Effect of seaweed extract on the growth, yield and nutrient uptake of soybean (Glycine max) under rainfed conditions. S. Afr. J. Bot. 2009, 75, 351–355. [Google Scholar] [CrossRef]
- Zerrifi, S.E.A.; El Khalloufi, F.; Oudra, B.; Vasconcelos, V. Seaweed bioactive compounds against pathogens and microalgae: Potential uses on pharmacology and harmful algae bloom control. Mar. Drugs 2018, 16, 55. [Google Scholar] [CrossRef]
- Rasyid, A. Evaluation of nutritional composition of the dried seaweed Ulva lactuca from Pameungpeuk waters, Indonesia. Trop. Life Sci. Res. 2017, 28, 119. [Google Scholar] [CrossRef]
- Chojnacka, K.; Saeid, A.; Witkowska, Z.; Tuhy, L. Biologically active compounds in seaweed extracts-the prospects for the application. Open Conf. Proc. J. 2012, 3, 20–28. [Google Scholar]
- Crouch, I.J.; Smith, M.T.; van Staden, J.; Lewis, M.J.; Hoad, G.V. Identification of Auxins in a Commercial Seaweed Concentrate. J. Plant Physiol. 1992, 139, 590–594. [Google Scholar] [CrossRef]
- Stirk, W.A.; Novak, O.; Hradecka, V.; Pencik, A.; Rolcik, J.; Strnad, M.; Van Staden, J. Endogenous cytokinins, auxins and abscisic acid in Ulva fasciata (Chlorophyta) and Dictyota humifusa (Phaeophyta): Towards understanding their biosynthesis and homoeostasis. Eur. J. Phycol. 2009, 44, 231–240. [Google Scholar] [CrossRef]
- Zhang, X.; Ervin, E.H.; Schmidt, R.E. Physiological Effects of Liquid Applications of a Seaweed Extract and a Humic Acid on Creeping Bentgrass. J. Am. Soc. Hortic. Sci. Jashs 2003, 128, 492–496. [Google Scholar] [CrossRef]
- Deinlein, U.; Stephan, A.B.; Horie, T.; Luo, W.; Xu, G.; Schroeder, J.I. Plant salt-tolerance mechanisms. Trends Plant Sci. 2014, 19, 371–379. [Google Scholar] [CrossRef]
- Fang, Y.; Xiong, L. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell. Mol. Life Sci. 2015, 72, 673–689. [Google Scholar] [CrossRef]
- Shukla, P.S.; Shotton, K.; Norman, E.; Neily, W.; Critchley, A.T.; Prithiviraj, B. Seaweed extract improve drought tolerance of soybean by regulating stress-response genes. AoB Plants 2018, 10, plx051. [Google Scholar] [CrossRef]
- Jithesh, M.N.; Shukla, P.S.; Kant, P.; Joshi, J.; Critchley, A.T.; Prithiviraj, B. Physiological and Transcriptomics Analyses Reveal that Ascophyllum nodosum Extracts Induce Salinity Tolerance in Arabidopsis by Regulating the Expression of Stress Responsive Genes. J. Plant Growth Regul. 2019, 38, 463–478. [Google Scholar] [CrossRef]
- Santaniello, A.; Scartazza, A.; Gresta, F.; Loreti, E.; Biasone, A.; Di Tommaso, D.; Piaggesi, A.; Perata, P. Ascophyllum nodosum Seaweed Extract Alleviates Drought Stress in Arabidopsis by Affecting Photosynthetic Performance and Related Gene Expression. Front. Plant Sci. 2017, 8, 1362. [Google Scholar] [CrossRef]
- Goni, O.; Quille, P.; O’Connell, S. Ascophyllum nodosum biostimulants and their role in enhancing tolerance to drought stress in tomato plants. Plant Physiol. Biochem. 2018, 126, 63–73. [Google Scholar] [CrossRef]
- Ali, O.; Ramsubhag, A.; Jayaraman, J. Biostimulatory activities of Ascophyllum nodosum extract in tomato and sweet pepper crops in a tropical environment. PLoS ONE 2019, 14, e0216710. [Google Scholar] [CrossRef]
- Goni, O.; Fort, A.; Quille, P.; McKeown, P.C.; Spillane, C.; O’Connell, S. Comparative Transcriptome Analysis of Two Ascophyllum nodosum Extract Biostimulants: Same Seaweed but Different. J. Agric. Food Chem. 2016, 64, 2980–2989. [Google Scholar] [CrossRef]
- Rayirath, P.; Benkel, B.; Hodges, D.M.; Allan-Wojtas, P.; MacKinnon, S.; Critchley, A.T.; Prithiviraj, B. Lipophilic components of the brown seaweed, Ascophyllum nodosum, enhance freezing tolerance in Arabidopsis thaliana. Planta 2009, 230, 135–147. [Google Scholar] [CrossRef]
- Sultana, V.; Baloch, G.N.; Ara, J.; Ehteshamul-Haque, S.; Tariq, R.M.; Athar, M. Seaweeds as an alternative to chemical pesticides for the management of root diseases of sunflower and tomato. J. Appl. Bot. Food Qual. 2012, 84, 162. [Google Scholar]
- Sahayaraj, K.; Kalidas, S. Evaluation of nymphicidal and ovicidal effect of a seaweed, Padina pavonica (Linn.)(Phaeophyceae) on cotton pest, Dysdercus cingulatus (Fab.). Indian J. Geo-Marine Sci. 2011, 40, 125–129. [Google Scholar]
- El-Ansary, M.S.M.; Hamouda, R.A. Biocontrol of root-knot nematode infected banana plants by some marine algae. Russ. J. Mar. Biol. 2014, 40, 140–146. [Google Scholar] [CrossRef]
- Machado, L.P.; Matsumoto, S.T.; Jamal, C.M.; da Silva, M.B.; da Cruz Centeno, D.; Neto, P.C.; de Carvalho, L.R.; Yokoya, N.S. Chemical analysis and toxicity of seaweed extracts with inhibitory activity against tropical fruit anthracnose fungi. J. Sci. Food Agric. 2014, 94, 1739–1744. [Google Scholar] [CrossRef] [PubMed]
- Cluzet, S.; Torregrosa, C.; Jacqket, C.; Lafitte, C.; Fournier, J.; Mercier, L.; Salamagne, S.; Briand, X.; Esquerré-Tugayé, M.-T.; Dumas, B. Gene expression profiling and protection of Medicago truncatula against a fungal infection in response to an elicitor from green algae Ulva spp. Plant. Cell Environ. 2004, 27, 917–928. [Google Scholar] [CrossRef]
- Rojas, C.; Senthil-Kumar, M.; Tzin, V.; Mysore, K. Regulation of primary plant metabolism during plant-pathogen interactions and its contribution to plant defense. Front. Plant Sci. 2014, 5, 17. [Google Scholar] [CrossRef]
- Chandra, R.; Iqbal, H.M.N.; Vishal, G.; Lee, H.-S.; Nagra, S. Algal biorefinery: A sustainable approach to valorize algal-based biomass towards multiple product recovery. Bioresour. Technol. 2019, 278, 346–359. [Google Scholar] [CrossRef]
- Chew, K.W.; Yap, J.Y.; Show, P.L.; Suan, N.H.; Juan, J.C.; Ling, T.C.; Lee, D.-J.; Chang, J.-S. Microalgae biorefinery: High value products perspectives. Bioresour. Technol. 2017, 229, 53–62. [Google Scholar] [CrossRef]
- Di Visconte, G.; Spicer, A.; Chuck, C.J.; Allen, M.J. The Microalgae Biorefinery: A Perspective on the Current Status and Future Opportunities Using Genetic Modification. Appl. Sci. 2019, 9, 4793. [Google Scholar] [CrossRef]
- Gilmour, D.J. Chapter One—Microalgae for biofuel production. Adv. Appl. Microbiol. 2019, 109, 1–30. [Google Scholar]
- Zollmann, M.; Robin, A.; Prabhu, M.; Polikovsky, M.; Gillis, A.; Greiserman, S.; Golberg, A. Green technology in green macroalgal biorefineries. Phycologia 2019, 58, 516–534. [Google Scholar] [CrossRef]
- Álvarez-Viñas, M.; Flórez-Fernández, N.; Torres, M.D.; Domínguez, H. Successful Approaches for a Red Seaweed Biorefinery. Mar. Drugs 2019, 17, 620. [Google Scholar] [CrossRef]
- Ingle, K.; Vitkin, E.; Robin, A.; Yakhini, Z.; Mishori, D.; Golberg, A. Macroalgae Biorefinery from Kappaphycus alvarezii: Conversion Modeling and Performance Prediction for India and Philippines as Examples. BioEnergy Res. 2018, 11, 22–32. [Google Scholar] [CrossRef]
- Chatzikonstantinou, L. First CE-Marked plant Biostimulants to be Placed on the Single Market on 16 July 2022. Available online: http://www.biostimulants.eu/2019/06/first-ce-marked-plant-biostimulants-to-be-placed-on-the-single-market-on-16-july-2022/ (accessed on 16 August 2019).
- European Union. Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 laying down rules on the making available on the market of EU fertilising products and amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and repealing Regula. 2019, p. 114. Available online: https://eur-lex.europa.eu/eli/reg/2019/1009/oj (accessed on 19 August 2019).
- Ricci, M.; Tilbury, L.; Daridon, B.; Sukalac, K. General Principles to Justify Plant Biostimulant Claims. Front. Plant Sci. 2019, 10, 494. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
EL Boukhari, M.E.M.; Barakate, M.; Bouhia, Y.; Lyamlouli, K. Trends in Seaweed Extract Based Biostimulants: Manufacturing Process and Beneficial Effect on Soil-Plant Systems. Plants 2020, 9, 359. https://doi.org/10.3390/plants9030359
EL Boukhari MEM, Barakate M, Bouhia Y, Lyamlouli K. Trends in Seaweed Extract Based Biostimulants: Manufacturing Process and Beneficial Effect on Soil-Plant Systems. Plants. 2020; 9(3):359. https://doi.org/10.3390/plants9030359
Chicago/Turabian StyleEL Boukhari, Mohammed EL Mehdi, Mustapha Barakate, Youness Bouhia, and Karim Lyamlouli. 2020. "Trends in Seaweed Extract Based Biostimulants: Manufacturing Process and Beneficial Effect on Soil-Plant Systems" Plants 9, no. 3: 359. https://doi.org/10.3390/plants9030359
APA StyleEL Boukhari, M. E. M., Barakate, M., Bouhia, Y., & Lyamlouli, K. (2020). Trends in Seaweed Extract Based Biostimulants: Manufacturing Process and Beneficial Effect on Soil-Plant Systems. Plants, 9(3), 359. https://doi.org/10.3390/plants9030359





