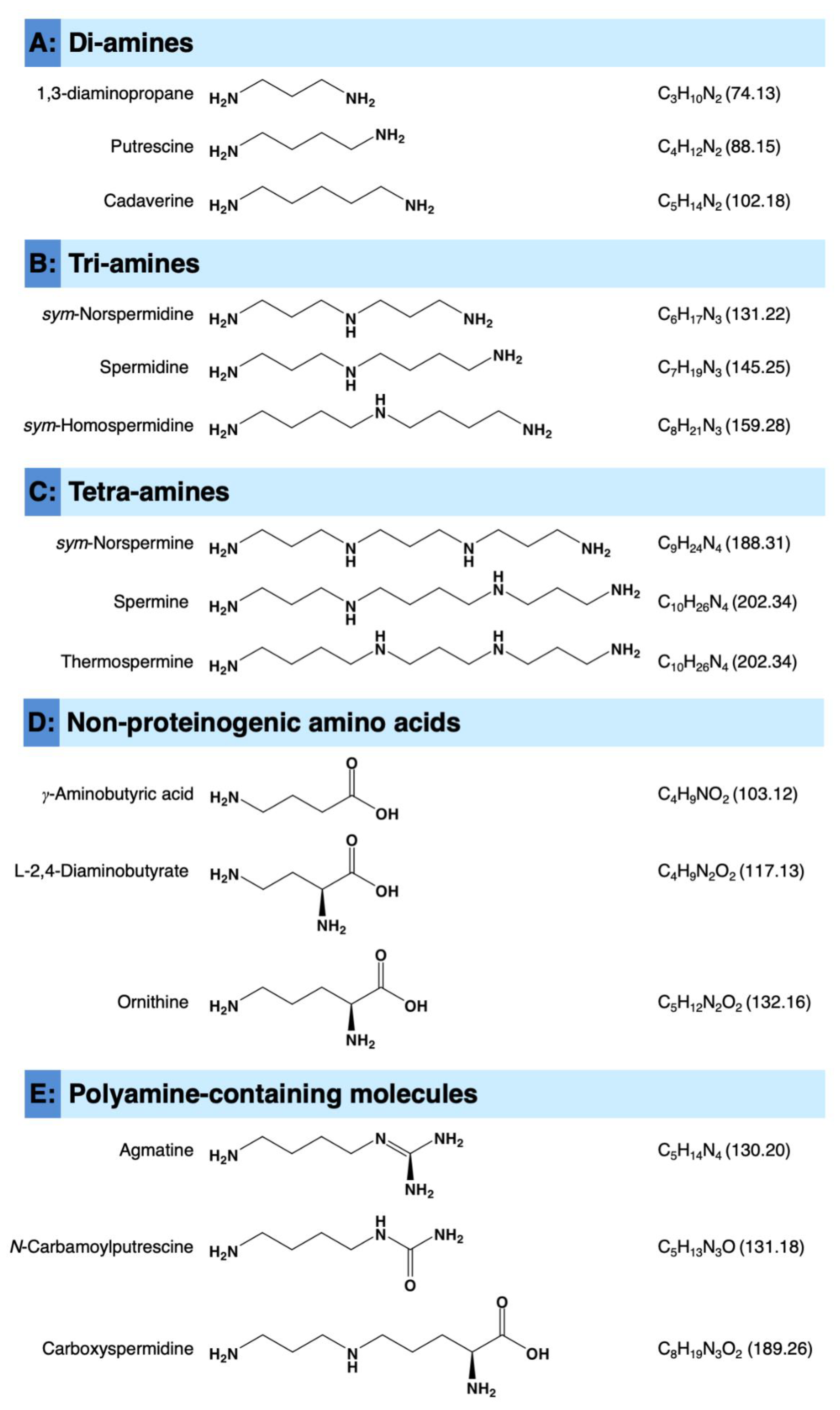
Citrus Polyamines: Structure, Biosynthesis, and Physiological Functions
Abstract
:1. Introduction
2. Biosynthesis of Polyamines (PAs) in Citrus Plants
3. Physiological Roles of PAs in Citrus
4. Role of PAs in Somatic Embryogenesis of Citrus Plants
5. Role of PAs in the Root System Architecture of Citrus Plants
6. Role of PAs in the Plant Growth and Shoot System Architecture of Citrus Plants
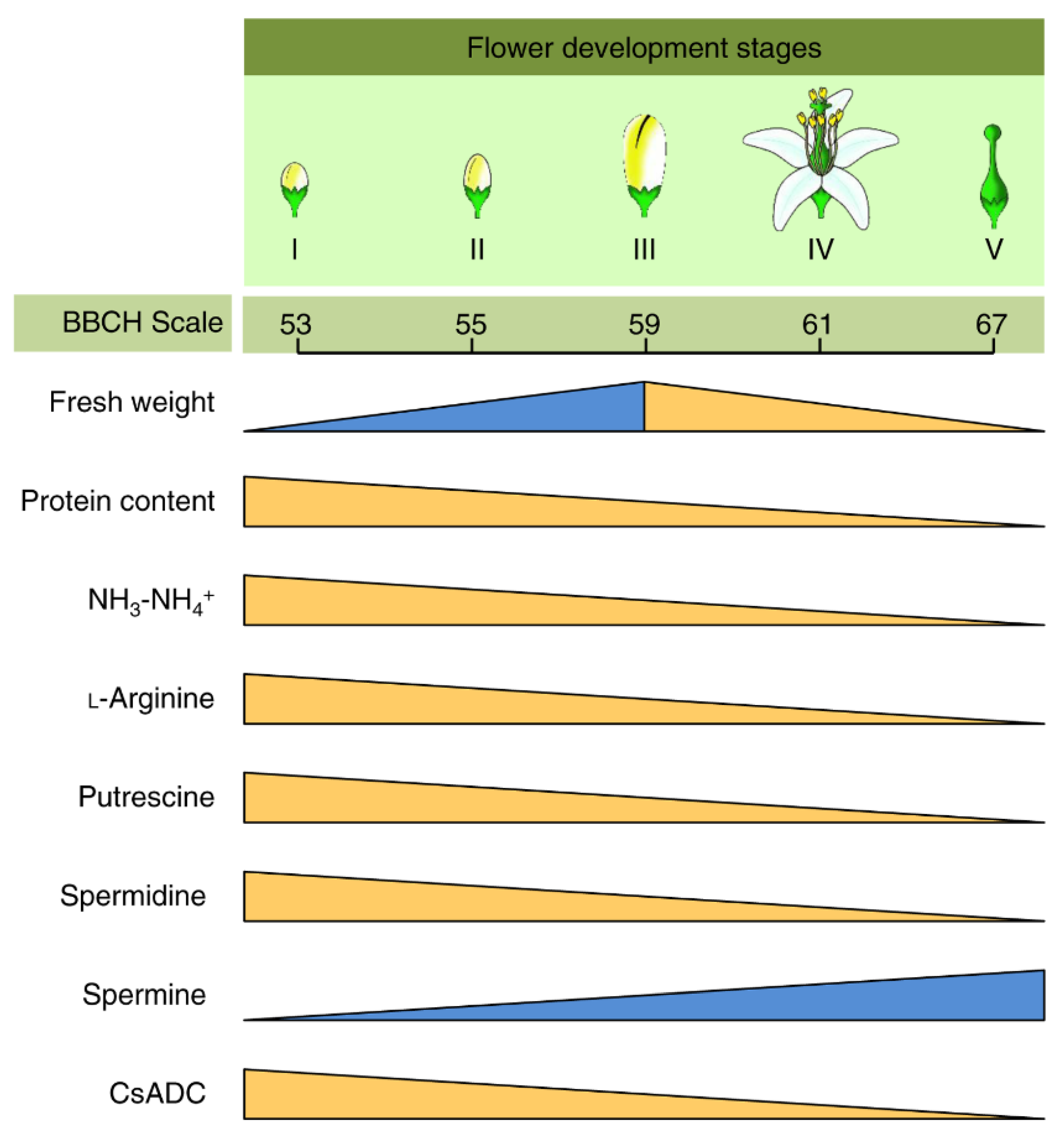
7. Role of PAs in the Flowering and Inflorescence of Citrus Plants
8. Role of PAs in the Fruit Set, Development, and Quality of Citrus Plants
9. Role of PAs in the Stomatal Closure and Gas-Exchange of Citrus Plants
10. Role of PAs in the Photosynthesis and Chlorophyll Fluorescence of Citrus Plants
11. Conclusions Remarks and Future Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tabor, C.W.; Tabor, H. Polyamines. Annu. Rev. Biochem. 1984, 53, 749–790. [Google Scholar] [CrossRef] [PubMed]
- Tabor, C.W.; Tabor, H. Polyamines in microorganisms. Microbiol. Rev. 1985, 49, 81–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, S.S. A Guide to the Polyamines; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Groppa, M.D.; Benavides, M.P. Polyamines and abiotic stress: Recent advances. Amino Acids 2008, 34, 35–45. [Google Scholar] [CrossRef]
- Hussain, S.S.; Ali, M.; Ahmad, M.; Siddique, K.H.M. Polyamines: Natural and engineered abiotic and biotic stress tolerance in plants. Biotechnol. Adv. 2011, 29, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Basu, S.; Kumar, G. Polyamines Metabolism: A Way Ahead for Abiotic Stress Tolerance in Crop Plants. In Biochemical, Physiological and Molecular Avenues for Combating Abiotic Stress Tolerance in Plants; Wani, S.H., Ed.; Academic Press: London, UK, 2018; pp. 39–55. [Google Scholar]
- Wallace, H.M.; Fraser, A.V.; Hughes, A. A perspective of polyamine metabolism. Biochem. J. 2003, 376, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Vickery, H.B.; Schmidt, C.L.A. The History of the Discovery of the Amino Acids. Chem. Rev. 1931, 9, 169–318. [Google Scholar] [CrossRef]
- Bachrach, U. The early history of polyamine research. Plant Physiol. Biochem. 2010, 48, 490–495. [Google Scholar] [CrossRef]
- Van Leeuwenhoek, A.; Observationes, D. Anthonii Lewenhoeck, De Natis E Semine Genitali Animalculis. Trans. R. Soc. Lond. 1678, 12, 1040–1046. [Google Scholar]
- Schreiner, P. Ueber eine neue organische Basis in thierischen Organismen. Justus Liebigs Annalen der Chemie 1878, 194, 68–84. [Google Scholar] [CrossRef]
- Ladenburg, A.; Abel, J. Ueber das Aethylenimin (Spermin?). Berichte der Deutschen Chemischen Gesellschaft 1888, 21, 758–766. [Google Scholar] [CrossRef] [Green Version]
- Poehl, A. Die Physiologisch-Chemischen Grundlagen der Spermintheorie Nebst Klinischem Material zur Therapeutischen Verwendung des Sperminum-Poehl; Wiencke: St. Petersburg, Russia, 1898. [Google Scholar]
- Rosenheim, O. The Isolation of Spermine Phosphate from Semen and Testis. Biochem. J. 1924, 18, 1253–1262. [Google Scholar] [CrossRef] [Green Version]
- Richards, F.J.; Coleman, R.G. Occurrence of putrescine in potassium-deficient barley. Nature 1952, 170, 460. [Google Scholar] [CrossRef] [PubMed]
- Wheaton, T.A.; Stewart, I. Feruloylputrescine: Isolation and Identification from Citrus Leaves and Fruit. Nature 1965, 206, 620–621. [Google Scholar] [CrossRef] [PubMed]
- Alcázar, R.; Marco, F.; Cuevas, J.C.; Patron, M.; Ferrando, A.; Carrasco, P.; Tiburcio, A.F.; Altabella, T. Involvement of polyamines in plant response to abiotic stress. Biotechnol. Lett. 2006, 28, 1867–1876. [Google Scholar] [CrossRef] [PubMed]
- Michael, A.J. Polyamines in eukaryotes, bacteria, and archaea. J. Biol. Chem. 2016, 291, 14896–14903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valdés-Santiago, L.; Cervantes-Chávez, J.A.; León-Ramírez, C.G.; Ruiz-Herrera, J. Polyamine metabolism in fungi with emphasis on phytopathogenic species. J. Amino Acids 2012, 2012, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Valdés-Santiago, L.; Ruiz-Herrera, J. Stress and polyamine metabolism in fungi. Front. Chem. 2014, 1, 42. [Google Scholar] [CrossRef] [Green Version]
- Auling, G.; Busse, H.-J.; Pilz, F.; Webb, L.; Kneifel, H.; Claus, D. Rapid differentiation, by polyamine analysis, of Xanthomonas strains from phytopathogenic pseudomonads and other members of the class proteobacteria interacting with plants. Int. J. Syst. Bacteriol. 1991, 41, 223–228. [Google Scholar] [CrossRef]
- Auling, G. Polyamines, biomarker for taxonomy and ecology of phytopathogenic and other bacteria belonging to the Proteobacteria. Belgian J. Bot. 1992, 125, 203–209. [Google Scholar]
- Hamana, K. Polyamine distribution patterns within the families Aeromonadaceae, Vibrionaceae, Pasteurellaceae, and Halomonadaceae, and related genera of the gamma subclass of the Proteobacteria. J. Gen. Appl. Microbiol. 1997, 43, 49–59. [Google Scholar] [CrossRef] [Green Version]
- Wortham, B.W.; Oliveira, M.A.; Patel, C.N. Polyamines in Bacteria: Pleiotropic Effects Yet Specific Mechanisms. In The Genus Yersinia: Part of the Advances in Experimental Medicine and Biology Book Series; Perry, R.D., Fetherston, J.D., Eds.; AEMB: Ruston, LA, USA, 2007; Volume 603, pp. 106–115. [Google Scholar]
- Li, B.; Kim, S.H.; Zhang, Y.; Hanfrey, C.C.; Elliott, K.A.; Ealick, S.E.; Michael, A.J. Different polyamine pathways from bacteria have replaced eukaryotic spermidine biosynthesis in ciliates Tetrahymena thermophila and Paramecium tetaurelia. Mol. Microbiol. 2015, 97, 791–807. [Google Scholar] [CrossRef] [PubMed]
- Hershey, A.D. Some minor components of bacteriophage T2 particles. Virology 1957, 4, 237–264. [Google Scholar] [CrossRef]
- Ames, B.N.; Dubin, D.T.; Rosenthal, S.M. Presence of polyamines in certain bacterial viruses. Science 1958, 127, 814–816. [Google Scholar] [CrossRef] [PubMed]
- Kaur-Sawhney, R.; Tiburcio, A.F.; Altabella, T.; Galston, A.W. Polyamines in plants: An overview. J. Cell Mol. Biol. 2003, 2, 1–12. [Google Scholar]
- Liu, J.-H.; Wang, W.; Wu, H.; Gong, X.; Moriguchi, T. Polyamines function in stress tolerance: From synthesis to regulation. Front. Plant Sci. 2015, 6, 827. [Google Scholar] [CrossRef] [Green Version]
- Michael, A.J. Biosynthesis of polyamines and polyamine-containing molecules. Biochem. J. 2016, 473, 2315–2329. [Google Scholar] [CrossRef]
- Liu, Y.; Heying, E.; Tanumihardjo, S.A. History, global distribution, and nutritional importance of citrus fruits. Compr. Rev. Food Sci. Food Saf. 2012, 11, 530–545. [Google Scholar] [CrossRef]
- Gottwald, T.R. Current epidemiological understanding of citrus Huanglongbing. Annu. Rev. Phytopathol. 2010, 48, 119–139. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.-Y.; Xiao, L.-T.; Lu, X.-D.; Hu, J.-J.; Wu, S.; He, C.-Z.; Deng, X.-X. Changes in polyamine levels in Citrus sinensis Osb. cv. Valencia callus during somatic embryogenesis. J. Plant Physiol. Mol. Biol. 2005, 31, 275–280. [Google Scholar]
- Wu, X.-B.; Wang, J.; Liu, J.-H.; Deng, X.-X. Involvement of polyamine biosynthesis in somatic embryogenesis of Valencia sweet orange (Citrus sinensis) induced by glycerol. J. Plant Physiol. 2009, 166, 52–62. [Google Scholar] [CrossRef]
- Yao, Q.; Wang, L.R.; Chen, J.Z.; Zhu, H.H. The effects of polyamines on root morphology and arbuscular mycorrhiza of citrus seedlings. Acta Hortic. 2008, 774, 151–158. [Google Scholar] [CrossRef]
- Mendes, A.F.S.; Cidade, L.C.; Otoni, W.C.; Soares-Filho, W.S.; Costa, M.G.C. Role of auxins, polyamines and ethylene in root formation and growth in sweet orange. Biol. Plant. 2011, 55, 375–378. [Google Scholar] [CrossRef]
- Wu, Q.-S.; Zou, Y.-N.; Liu, M.; Cheng, K. Effects of exogenous putrescine on mycorrhiza, root system architecture, and physiological traits of Glomus mosseae-colonized trifoliate orange seedlings. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 2012, 40, 80–85. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.S.; Zou, Y.N.; Liu, C.Y.; Lu, T. Interacted effect of arbuscular mycorrhizal fungi and polyamines on root system architecture of citrus seedlings. J. Integr. Agric. 2012, 11, 1675–1681. [Google Scholar] [CrossRef]
- Wu, Q.S.; Zou, Y.N.; Zhan, T.T.; Liu, C.Y. Polyamines participate in mycorrhizal and root development of citrus (Citrus tangerine) seedlings. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 2010, 38, 25–31. [Google Scholar]
- Yao, Q.; Wang, L.-R.; Xing, Q.-X.; Chen, J.-Z.; Zhu, H.-H. Exogenous polyamines influence root morphogenesis and arbuscular mycorrhizal development of Citrus limonia seedlings. Plant Growth Regul. 2010, 60, 27–33. [Google Scholar] [CrossRef]
- Wu, Q.S.; Zou, Y.N.; Peng, Y.H.; Liu, C.Y. Root morphological modification of mycorrhizal citrus (Citrus tangerine) seedlings after application with exogenous polyamines. J. Anim. Plant Sci. 2011, 21, 20–25. [Google Scholar]
- Ishii, T.; Aikawa, J.; Kirino, S.; Kitabayashi, H.; Matsumoto, I.; Kadoya, K. Effects of Alginate Oligosaccharide and Polyamines on Hyphal Growth of Vesicular-Arbuscular Mycorrhizal Fungi and Their Infectivity of Citrus Roots. In Proceedings of the 9th International Society of Citriculture Congress, Orlando, FL, USA, 3–7 December 2000; pp. 1030–1032. [Google Scholar]
- Anjum, M.A. Effect of NaCl concentrations in irrigation water on growth and polyamine metabolism in two citrus rootstocks with different levels of salinity tolerance. Acta Physiol. Plant. 2007, 30, 43–52. [Google Scholar] [CrossRef]
- Wu, Q.-S.; Zou, Y.-N. The effect of dual application of arbuscular mycorrhizal fungi and polyamines upon growth and nutrient uptake on trifoliate orange (Poncirus trifoliata) Seedlings. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 2009, 37, 95–98. [Google Scholar]
- Anjum, M.A. Effect of exogenously applied spermidine on growth and physiology of citrus rootstock Troyer citrange under saline conditions. Turk. J. Agric. For. 2011, 35, 43–53. [Google Scholar]
- Tanou, G.; Ziogas, V.; Belghazi, M.; Christou, A.; Filippou, P.; Job, D.; Fotopoulos, V.; Molassiotis, A. Polyamines reprogram oxidative and nitrosative status and the proteome of citrus plants exposed to salinity stress. Plant Cell Environ. 2014, 37, 864–885. [Google Scholar] [CrossRef] [PubMed]
- Khoshbakht, D.; Asghari, M.R.; Haghighi, M. Influence of foliar application of polyamines on growth, gas-exchange characteristics, and chlorophyll fluorescence in Bakraii citrus under saline conditions. Photosynthetica 2018, 56, 731–742. [Google Scholar] [CrossRef]
- Lovatt, C.J.; Zheng, Y.; Hake, K.D. Demonstration of a change in nitrogen metabolism influencing flower initiation in Citrus. Isr. J. Bot. 1988, 37, 181–188. [Google Scholar]
- Kushad, M.M.; Orvos, A.R.; Yelenosky, G. Relative changes in polyamines during citrus flower development. HortScience 1990, 25, 946–948. [Google Scholar] [CrossRef] [Green Version]
- Sagee, O.; Lovatt, C.J. Putrescine content parallels ammonia and arginine metabolism in developing flowers of the ‘Washington’ Navel Orange. J. Am. Soc. Hortic. Sci. 1991, 116, 280–285. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.G.; Zheng, Y.; Sagee, O.; Protacio, C.; Lovatt, C.J. Ammonia And/Or Its Metabolites Influence Flowering, Fruit Set, And Yield Of The ‘Washington’ Navel Orange. HortScience 1993, 28, 559. [Google Scholar]
- Ali, A.G.; Lovatt, C.J. Relationship of polyamines to low-temperature stress-induced flowering of the ‘Washington’ navel orange (Citrus sinensis L. Osbeck). J. Hortic. Sci. 1995, 70, 491–498. [Google Scholar] [CrossRef]
- Krajewski, A.J.; Rabe, E. Citrus flowering: A critical evaluation. J. Hortic. Sci. 1995, 70, 357–374. [Google Scholar] [CrossRef]
- Gentile, A.; Antognoni, F.; Iorio, R.A.; Distefano, G.; Las Casas, G.; La Malfa, S.; Serafini-Fracassini, D.; Del Duca, S. Polyamines and transglutaminase activity are involved in compatible and self-incompatible pollination of Citrus grandis. Amino Acids 2012, 42, 1025–1035. [Google Scholar] [CrossRef]
- Arias, M.; Carbonell, J.; Agustí, M. Endogenous free polyamines and their role in fruit set of low and high parthenocarpic ability citrus cultivars. J. Plant Physiol. 2005, 162, 845–853. [Google Scholar] [CrossRef]
- Trénor, M.; Perez-Amador, M.A.; Carbonell, J.; Blázquez, M.A. Expression of polyamine biosynthesis genes during parthenocarpic fruit development in Citrus clementina. Planta 2010, 231, 1401–1411. [Google Scholar] [CrossRef] [PubMed]
- Anjum, M.A. Response of Cleopatra mandarin seedlings to a polyamine-biosynthesis inhibitor under salt stress. Acta Physiol. Plant. 2010, 32, 951–959. [Google Scholar] [CrossRef]
- Shi, J.; Fu, X.-Z.; Peng, T.; Huang, X.-S.; Fan, Q.-J.; Liu, J.-H. Spermine pretreatment confers dehydration tolerance of citrus in vitro plants via modulation of antioxidative capacity and stomatal response. Tree Physiol. 2010, 30, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, M.; Hu, J.; Wang, W.; Fu, X.; Liu, J.-H. PtrABF of Poncirus trifoliata functions in dehydration tolerance by reducing stomatal density and maintaining reactive oxygen species homeostasis. J. Exp. Bot. 2015, 66, 5911–5927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, D.K.; Dubey, A.K.; Srivastav, M.; Singh, A.K.; Sairam, R.K.; Pandey, R.N.; Dahuja, A.; Kaur, C. Effect of putrescine and paclobutrazol on growth, physiochemical parameters, and nutrient acquisition of salt-sensitive citrus rootstock Karna Khatta (Citrus karna Raf.) under NaCl Stress. J. Plant Growth Regul. 2011, 30, 301–311. [Google Scholar] [CrossRef]
- Shu, S.; Guo, S.-R.; Yu, L.-Y. A Review: Polyamines and Photosynthesis. In Advances in Photosynthesis—Fundamental Aspects; Najafpour, M., Ed.; In Tech: Beijing, China, 2012; pp. 439–464. [Google Scholar]
- Lu, Y.-B.; Yang, L.-T.; Li, Y.; Xu, J.; Liao, T.-T.; Chen, Y.-B.; Chen, L.-S. Effects of boron deficiency on major metabolites, key enzymes and gas exchange in leaves and roots of Citrus sinensis seedlings. Tree Physiol. 2014, 34, 608–618. [Google Scholar] [CrossRef] [Green Version]
- Kusano, T.; Berberich, T.; Tateda, C.; Takahashi, Y. Polyamines: Essential factors for growth and survival. Planta 2008, 228, 367–381. [Google Scholar] [CrossRef]
- Carbonell, J.; Blázquez, M.A. Regulatory mechanisms of polyamine biosynthesis in plants. Genes Genomics 2009, 31, 107–118. [Google Scholar] [CrossRef]
- Vera-Sirera, F.; Minguet, E.G.; Singh, S.K.; Ljung, K.; Tuominen, H.; Blázquez, M.A.; Carbonell, J. Role of polyamines in plant vascular development. Plant Physiol. Biochem. 2010, 48, 534–539. [Google Scholar] [CrossRef]
- Pegg, A.E.; Casero, R.A. Current status of the polyamine research field. Methods Mol. Biol. 2011, 720, 3–35. [Google Scholar] [PubMed] [Green Version]
- Marina, M.; Sirera, F.V.; Rambla, J.L.; Gonzalez, M.E.; Blázquez, M.A.; Carbonell, J.; Pieckenstain, F.L.; Ruiz, O.A. Thermospermine catabolism increases Arabidopsis thaliana resistance to Pseudomonas viridiflava. J. Exp. Bot. 2013, 64, 1393–1402. [Google Scholar] [CrossRef] [Green Version]
- Gupta, K.; Dey, A.; Gupta, B. Plant polyamines in abiotic stress responses. Acta Physiol. Plant. 2013, 35, 2015–2036. [Google Scholar] [CrossRef]
- Mattoo, A.K.; Fatima, T.; Upadhyay, R.K.; Handa, A.K. Polyamines in Plants: Biosynthesis from Arginine, and Metabolic, Physiological and Stress-Response Roles. In Amino Acids in Higher Plants; D’Mello, J.P.F., Ed.; CABI: Wallingford, UK, 2015; pp. 177–194. [Google Scholar]
- Handa, A.K.; Fatima, T.; Mattoo, A.K. Polyamines: Bio-molecules with diverse functions in plant and human health and disease. Front. Chem. 2018, 6, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nathan, R.; Altman, A.; Monselise, S.P. Changes in activity of polyamine biosynthetic enzymes and in polyamine contents in developing fruit tissues of ‘Murcott’ mandarin. Sci. Hortic. 1984, 22, 359–364. [Google Scholar] [CrossRef]
- Petrocelli, S.; Pizarro, M.D.; Alet, A.; De Ollas, C.; Talón, M.; Tadeo, F.R.; Gómez-Cadenas, A.; Arbona, V.; Orellano, E.G.; Daurelio, L.D. Phytohormone participation during Citrus sinensis non-host response to Xanthomonas campestris pv. vesicatoria. Plant Gene 2018, 15, 28–36. [Google Scholar] [CrossRef]
- Hanfrey, C.; Sommer, S.; Mayer, M.J.; Burtin, D.; Michael, A.J. Arabidopsis polyamine biosynthesis: Absence of ornithine decarboxylase and the mechanism of arginine decarboxylase activity. Plant J. 2001, 27, 551–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Sun, P.-P.; Chen, C.-L.; Wang, Y.; Fu, X.-Z.; Liu, J.-H. An arginine decarboxylase gene PtADC from Poncirus trifoliata confers abiotic stress tolerance and promotes primary root growth in Arabidopsis. J. Exp. Bot. 2011, 62, 2899–2914. [Google Scholar] [CrossRef]
- Daurelio, L.D.; Petrocelli, S.; Blanco, F.; Holuigue, L.; Ottado, J.; Orellano, E.G. Transcriptome analysis reveals novel genes involved in nonhost response to bacterial infection in tobacco. J. Plant Physiol. 2011, 168, 382–391. [Google Scholar] [CrossRef]
- Hanzawa, Y.; Takahashi, T.; Michael, A.J.; Burtin, D.; Long, D.; Pineiro, M.; Coupland, G.; Komeda, Y. ACAULIS5, an Arabidopsis gene required for stem elongation, encodes a spermine synthase. EMBO J. 2000, 19, 4248–4256. [Google Scholar] [CrossRef] [Green Version]
- Panicot, M.; Minguet, E.G.; Ferrando, A.; Alcázar, R.; Blázquez, M.A.; Carbonell, J.; Altabella, T.; Koncz, C.; Tiburcio, A.F. A polyamine metabolon involving aminopropyl transferase complexes in Arabidopsis. Plant Cell 2002, 14, 2539–2551. [Google Scholar] [CrossRef] [Green Version]
- Knott, J.M.; Römer, P.; Sumper, M. Putative spermine synthases from Thalassiosira pseudonana and Arabidopsis thaliana synthesize thermospermine rather than spermine. FEBS Lett. 2007, 581, 3081–3086. [Google Scholar] [CrossRef] [Green Version]
- Fu, X.-Z.; Chen, C.-W.; Wang, Y.; Liu, J.-H.; Moriguchi, T. Ectopic expression of MdSPDS1 in sweet orange (Citrus sinensis Osbeck) reduces canker susceptibility: Involvement of H2O2 production and transcriptional alteration. BMC Plant Biol. 2011, 11, 55. [Google Scholar] [CrossRef] [Green Version]
- Even-Chen, Z.; Mattoo, A.K.; Goren, R. Inhibition of ethylene biosynthesis by aminoethoxyvinylglycine and by polyamines shunts label from 3,4-[14C]methionine into spermidine in aged orange peel discs. Plant Physiol. 1982, 69, 385–388. [Google Scholar] [CrossRef] [Green Version]
- Han, K.R.; Kim, M.W.; Kim, H.Y.; Riu, K.Z.; Stadelmann, Y.O.K. Endogeneous Levels of Polyamines in Citrus and Related Species: Their Role in In Vitro Organogenesis, Somatic Embrogenesis and Recalcitrance. In Abstract no 118 in Plant Biology Conference; American Society of Plant Biologists: San Diego, CA, USA, 2000. [Google Scholar]
- Tassoni, A.; Germanà, M.A.; Bagni, N. Free and conjugated polyamine content in Citrus sinensis Osbeck, cultivar Brasiliano N.L. 92, a Navel orange, at different maturation stages. Food Chem. 2004, 87, 537–541. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Singh, S. Biochemical markers and nutrient constraints diagnosis in citrus: A perspective. J. Plant Nutr. 2006, 29, 827–855. [Google Scholar] [CrossRef]
- Shahid, M.A.; Balal, R.M.; Khan, N.; Rossi, L.; Rathinasabapathi, B.; Liu, G.; Khan, J.; Cámara-Zapata, J.M.; Martínez-Nicolas, J.J.; Garcia-Sanchez, F. Polyamines provide new insights into the biochemical basis of Cr-tolerance in Kinnow mandarin grafted on diploid and double-diploid rootstocks. Environ. Exp. Bot. 2018, 156, 248–260. [Google Scholar] [CrossRef]
- Galston, A.W. Polyamines as modulators of plant development. Bioscience 1983, 33, 382–388. [Google Scholar] [CrossRef]
- Martin-Tanguy, J. Metabolism and function of polyamines in plants: Recent development (new approaches). Plant Growth Regul. 2001, 34, 135–148. [Google Scholar] [CrossRef]
- Couée, I.; Hummel, I.; Sulmon, C.; Gouesbet, G.; El Amrani, A. Involvement of polyamines in root development. Plant Cell. Tissue Organ Cult. 2004, 76, 1–10. [Google Scholar] [CrossRef]
- Liu, J.-H.; Honda, C.; Moriguchi, T. Involvement of polyamine in floral and fruit development. JARQ 2006, 40, 51–58. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, T.; Kakehi, J.-I. Polyamines: Ubiquitous polycations with unique roles in growth and stress responses. Ann. Bot. 2010, 105, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Tiburcio, A.F.; Altabella, T.; Bitrián, M.; Alcázar, R. The roles of polyamines during the lifespan of plants: From development to stress. Planta 2014, 240, 1–18. [Google Scholar] [CrossRef]
- Masson, P.H.; Takahashi, T.; Angelini, R. Editorial: Molecular mechanisms underlying polyamine functions in plants. Front. Plant Sci. 2017, 8, 14. [Google Scholar] [CrossRef] [Green Version]
- Tiburcio, A.F.; Alcázar, R. Potential Applications of Polyamines in Agriculture and Plant Biotechnology. In Polyamines: Methods and Protocols, Methods in Molecular Biology Volume1694; Alcázar, R., Tiburcio, A.F., Eds.; Humana Press: New York, NY, USA, 2018; pp. 489–508. [Google Scholar]
- Galston, A.W.; Sawhney, R.K. Polyamines in plant physiology. Plant Physiol. 1990, 94, 406–410. [Google Scholar] [CrossRef] [Green Version]
- Bagni, N.; Torrigiani, P. Polyamines: A New Class of Growth Substances. In Progress in Plant Growth Regulation; Springer: Dordrecht, The Netherlands, 1992; pp. 264–275. [Google Scholar]
- El-Otmani, M.; Lovatt, C.J.; Coggins, C.W.; Agustí, M. Plant growth regulators in citriculture: Factors regulating endogenous levels in citrus tissues. Crit. Rev. Plant Sci. 1995, 14, 367–412. [Google Scholar] [CrossRef]
- Pan, Z.; Guan, R.; Zhu, S.; Deng, X. Proteomic analysis of somatic embryogenesis in Valencia sweet orange (Citrus sinensis Osbeck). Plant Cell Rep. 2009, 28, 281–289. [Google Scholar] [CrossRef]
- Nieves, N.; Martinez, M.E.; Blanco, M.A.; Gonzalez, J.L.; Borroto, E.; Lorenzo, J.C.; Portilla, Y. Changes in soluble proteins and polyamines during citrus seed germination. Fruits 1998, 53, 27–33. [Google Scholar]
- Saleem, B.A.; Malik, A.U.; Anwar, R.; Farooq, M. Exogenous application of polyamines improves fruit set, yield and quality of sweet oranges. Acta Hortic. 2008, 774, 187–194. [Google Scholar] [CrossRef]
- Kawahara, R.; Komamine, A. Gene expression during somatic embryogenesis. Protein Nucleic Acid Enzym. 1992, 37, 1249–1256. [Google Scholar]
- Chugh, A.; Khurana, P. Gene expression during somatic embryogenesis—Recent advances. Curr. Sci. 2002, 83, 715–730. [Google Scholar]
- Ikeuchi, M.; Ogawa, Y.; Iwase, A.; Sugimoto, K. Plant regeneration: Cellular origins and molecular mechanisms. Development 2016, 143, 1442–1451. [Google Scholar] [CrossRef] [Green Version]
- Kochba, J.; Spiegel-Roy, P. Embryogenesis in gamma-irradiated habituated ovular callus of the “Shamouti” orange as affected by auxin and by tissue age. Environ. Exp. Bot. 1977, 17, 151–159. [Google Scholar] [CrossRef]
- Kochba, J.; Spiegel-Roy, P. The effects of auxins, cytokinins and inhibitors on embryogenesis in habituated ovular callus of the Shamouti orange (Citrus sinensis). Zeitschrift für Pflanzenphysiologie 1977, 81, 283–288. [Google Scholar] [CrossRef]
- Spiegel-Roy, P.; Kochba, J. Embryogenesis in Citrus Tissue Cultures. In Advances in Biochemical Engineering; Springer: Berlin/Heidelberg, Germany, 1980; pp. 27–48. [Google Scholar]
- Fiore, S.; De Pasquale, F.; Carimi, F.; Sajeva, M. Effect of 2,4-D and 4-CPPU on somatic embryogenesis from stigma and style transverse thin cell layers of Citrus. Plant Cell. Tissue Organ Cult. 2002, 68, 57–63. [Google Scholar] [CrossRef]
- Tao, H.; Shaolin, P.; Gaofeng, D.; Lanying, Z.; Gengguang, L. Plant regeneration from leaf-derived callus in Citrus grandis (pummelo): Effects of auxins in callus induction medium. Plant Cell. Tissue Organ Cult. 2002, 69, 141–146. [Google Scholar] [CrossRef]
- Hao, Y.J.; Wen, X.P.; Deng, X.X. Genetic and epigenetic evaluations of citrus calluses recovered from slow-growth culture. J. Plant Physiol. 2004, 161, 479–484. [Google Scholar] [CrossRef]
- Ramdan, R.; Handaji, N.; Beyahia, H.; Ibriz, M. Influence of growth regulators on callus induction from embryos of five citrus rootstocks. J. Appl. Biosci. 2014, 73, 5959–5965. [Google Scholar]
- Minocha, R.; Smith, D.R.; Reeves, C.; Steele, K.D.; Minocha, S.C. Polyamine levels during the development of zygotic and somatic embryos of Pinus radiata. Physiol. Plant. 1999, 105, 155–164. [Google Scholar] [CrossRef] [Green Version]
- Kevers, C.; Le Gal, N.; Monteiro, M.; Dommes, J.; Gaspar, T. Somatic embryogenesis of Panax ginseng in liquid cultures: A role for polyamines and their metabolic pathways. Plant Growth Regul. 2000, 31, 209–214. [Google Scholar] [CrossRef]
- Minocha, R.; Minocha, S.C.; Long, S. Polyamines and their biosynthetic enzymes during somatic embryo development in red spruce (Picea rubens Sarg.). Vitr. Cell. Dev. Biol. Plant 2004, 40, 572–580. [Google Scholar] [CrossRef]
- Wang, W.; Liu, J.-H. CsPAO4 of Citrus sinensis functions in polyamine terminal catabolism and inhibits plant growth under salt stress. Sci. Rep. 2016, 6, 31384. [Google Scholar] [CrossRef] [Green Version]
- Silveira, V.; Iochevet Segal Floh, E.; Handro, W.; Pedro Guerra, M. Effect of plant growth regulators on the cellular growth and levels of intracellular protein, starch and polyamines in embryogenic suspension cultures of Pinus taeda. Plant Cell. Tissue Organ Cult. 2004, 76, 53–60. [Google Scholar] [CrossRef]
- Silveira, V.; Santa-Catarina, C.; Tun, N.N.; Scherer, G.F.E.; Handro, W.; Guerra, M.P.; Floh, E.I.S. Polyamine effects on the endogenous polyamine contents, nitric oxide release, growth and differentiation of embryogenic suspension cultures of Araucaria angustifolia (Bert.) O. Ktze. Plant Sci. 2006, 171, 91–98. [Google Scholar] [CrossRef]
- Santa-Catarina, C.; Silveira, V.; Scherer, G.F.E.; Floh, E.I.S. Polyamine and nitric oxide levels relate with morphogenetic evolution in somatic embryogenesis of Ocotea catharinensis. Plant Cell Tissue Organ Cult. 2007, 90, 93–101. [Google Scholar] [CrossRef]
- Dutra, N.T.; Silveira, V.; de Azevedo, I.G.; Gomes-Neto, L.R.; Façanha, A.R.; Steiner, N.; Guerra, M.P.; Floh, E.I.S.; Santa-Catarina, C. Polyamines affect the cellular growth and structure of pro-embryogenic masses in Araucaria angustifolia embryogenic cultures through the modulation of proton pump activities and endogenous levels of polyamines. Physiol. Plant. 2013, 148, 121–132. [Google Scholar] [CrossRef]
- Wimalasekera, R.; Tebartz, F.; Scherer, G.F.E. Polyamines, polyamine oxidases and nitric oxide in development, abiotic and biotic stresses. Plant Sci. 2011, 181, 593–603. [Google Scholar] [CrossRef]
- Bagni, N.; Tassoni, A. Biosynthesis, oxidation and conjugation of aliphatic polyamines in higher plants. Amino Acids 2001, 20, 301–317. [Google Scholar] [CrossRef]
- Bais, H.P.; Ravishankar, G.A. Role of polyamines in the ontogeny of plants and their biotechnological applications. Plant Cell Tissue Organ Cult. 2002, 69, 1–34. [Google Scholar] [CrossRef]
- Anwar, R.; Mattoo, A.K.; Handa, A.K. Polyamine Interactions with Plant Hormones: Crosstalk at Several Levels. In Polyamines; Springer: Tokyo, Japan, 2015; pp. 267–302. [Google Scholar]
- Podlešáková, K.; Ugena, L.; Spíchal, L.; Doležal, K.; De Diego, N. Phytohormones and polyamines regulate plant stress responses by altering GABA pathway. New Biotechnol. 2019, 48, 53–65. [Google Scholar] [CrossRef]
- Smith, S.; De Smet, I. Root system architecture: Insights from Arabidopsis and cereal crops. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 1441–1452. [Google Scholar] [CrossRef] [Green Version]
- Ongaro, V.; Leyser, O. Hormonal control of shoot branching. J. Exp. Bot. 2007, 59, 67–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimizu-Sato, S.; Tanaka, M.; Mori, H. Auxin–cytokinin interactions in the control of shoot branching. Plant Mol. Biol. 2009, 69, 429–435. [Google Scholar] [CrossRef] [Green Version]
- Martín-Trillo, M.; Cubas, P. TCP genes: A family snapshot ten years later. Trends Plant Sci. 2010, 15, 31–39. [Google Scholar] [CrossRef]
- Müller, D.; Leyser, O. Auxin, cytokinin and the control of shoot branching. Ann. Bot. 2011, 107, 1203–1212. [Google Scholar] [CrossRef] [Green Version]
- Evans, P.T.; Malmberg, R.L. Do polyamines have roles in plant development? Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 235–269. [Google Scholar] [CrossRef]
- Walden, R.; Cordeiro, A.; Tiburcio, A.F. Polyamines: Small molecules triggering pathways in plant growth and development. Plant Physiol. 1997, 113, 1009–1013. [Google Scholar] [CrossRef] [Green Version]
- Galston, A.W.; Kaur-Sawhney, R. Polyamines as Endogenous Growth Regulators. In Plant Hormones; Springer: Dordrecht, The Netherlands, 1995; pp. 158–178. [Google Scholar]
- Reinhardt, D.; Kuhlemeier, C. Plant architecture. EMBO Rep. 2002, 3, 846–851. [Google Scholar] [CrossRef]
- Cui, X.; Ge, C.; Wang, R.; Wang, H.; Chen, W.; Fu, Z.; Jiang, X.; Li, J.; Wang, Y. The BUD2 mutation affects plant architecture through altering cytokinin and auxin responses in Arabidopsis. Cell Res. 2010, 20, 576–586. [Google Scholar] [CrossRef] [Green Version]
- Scholten, H.J. Effect of polyamines on the growth and development of some horticultural crops in micropropagation. Sci. Hortic. 1998, 77, 83–88. [Google Scholar] [CrossRef]
- Movahed, N.; Eshghi, S.; Tafazoli, E.; Jamali, B. Effects of polyamines on vegetative characteristics, growth, flowering and yield of strawberry (“Paros” and ’Selva’). Acta Hortic. 2012, 926, 287–293. [Google Scholar] [CrossRef]
- Steiner, N.; Santa-Catarina, C.; Silveira, V.; Floh, E.I.S.; Guerra, M.P. Polyamine effects on growth and endogenous hormones levels in Araucaria angustifolia embryogenic cultures. Plant Cell. Tissue Organ Cult. 2007, 89, 55–62. [Google Scholar] [CrossRef]
- Geuns, J.M.; Smets, R.; Struyf, T.; Prinsen, E.; Valcke, R.; Van Onckelen, H. Apical dominance in Pssu-ipt-transformed tobacco. Phytochemistry 2001, 58, 911–921. [Google Scholar] [CrossRef]
- Chen, S.; Wang, S.; Hüttermann, A.; Altman, A. Xylem abscisic acid accelerates leaf abscission by modulating polyamine and ethylene synthesis in water-stressed intact poplar. Trees 2002, 16, 16–22. [Google Scholar] [CrossRef]
- Kovács, Z.; Simon-Sarkadi, L.; Szűcs, A.; Kocsy, G. Differential effects of cold, osmotic stress and abscisic acid on polyamine accumulation in wheat. Amino Acids 2010, 38, 623–631. [Google Scholar] [CrossRef]
- Alcázar, R.; Cuevas, J.C.; Patron, M.; Altabella, T.; Tiburcio, A.F. Abscisic acid modulates polyamine metabolism under water stress in Arabidopsis thaliana. Physiol. Plant. 2006, 128, 448–455. [Google Scholar] [CrossRef]
- Urano, K.; Maruyama, K.; Ogata, Y.; Morishita, Y.; Takeda, M.; Sakurai, N.; Suzuki, H.; Saito, K.; Shibata, D.; Kobayashi, M.; et al. Characterization of the ABA-regulated global responses to dehydration in Arabidopsis by metabolomics. Plant J. 2009, 57, 1065–1078. [Google Scholar] [CrossRef]
- Friedman, R.; Levin, N.; Altman, A. Presence and identification of polyamines in xylem and Phloem exudates of plants. Plant Physiol. 1986, 82, 1154–1157. [Google Scholar] [CrossRef] [Green Version]
- Kakehi, J.-I.; Kuwashiro, Y.; Niitsu, M.; Takahashi, T. Thermospermine is Required for Stem Elongation in Arabidopsis thaliana. Plant Cell Physiol. 2008, 49, 1342–1349. [Google Scholar] [CrossRef] [Green Version]
- Miyamoto, M.; Shimao, S.; Tong, W.; Motose, H.; Takahashi, T. Effect of thermospermine on the growth and expression of polyamine-related genes in rice seedlings. Plants 2019, 8, 269. [Google Scholar] [CrossRef] [Green Version]
- Vuosku, J.; Muilu-Mäkelä, R.; Avia, K.; Suokas, M.; Kestilä, J.; Läärä, E.; Häggman, H.; Savolainen, O.; Sarjala, T. Thermospermine synthase (ACL5) and diamine oxidase (DAO) Expression is needed for zygotic embryogenesis and vascular development in Scots Pine. Front. Plant Sci. 2019, 10, 1600. [Google Scholar] [CrossRef]
- Milhinhos, A.; Prestele, J.; Bollhöner, B.; Matos, A.; Vera-Sirera, F.; Rambla, J.L.; Ljung, K.; Carbonell, J.; Blázquez, M.A.; Tuominen, H.; et al. Thermospermine levels are controlled by an auxin-dependent feedback loop mechanism in Populus xylem. Plant J. 2013, 75, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, D.J.; Cercós, M.; Colmenero-Flores, J.M.; Naranjo, M.A.; Ríos, G.; Carrera, E.; Ruiz-Rivero, O.; Lliso, I.; Morillon, R.; Tadeo, F.R.; et al. Physiology of citrus fruiting. Brazilian J. Plant Physiol. 2007, 19, 333–362. [Google Scholar] [CrossRef]
- Huchche, A.D.; Ladaniya, M.S. Citrus Flowering and Fruiting—Recent Research Advances. In Souvenir, National Seminar-cum-Workshop on Physiology of Flowering in Perennial Fruit Crops; Ravishankar, H., Singh, V.K., Misr, A.K., Eds.; Central Institute for Subtropical Horticulture (ICAR): Lucknow, India, 2014; pp. 74–88. [Google Scholar]
- Goldschmidt, E.E.; Huberman, M. The coordination of organ growth in developing citrus flowers: A possibility for sink type regulation. J. Exp. Bot. 1974, 25, 534–541. [Google Scholar] [CrossRef]
- Davis, S.J. Integrating hormones into the floral-transition pathway of Arabidopsis thaliana. Plant Cell Environ. 2009, 32, 1201–1210. [Google Scholar] [CrossRef]
- Agustí, M.; Zaragoza, S.; Bleiholder, H.; Buhr, L.; Hack, H.; Klose, R.; Staub, Y.R. Escala BBCH para la descripción de los estadios fenológicos del desarrollo de los agrios (Gén. Citrus). Levante Agrícola 1995, 3, 189–199. [Google Scholar]
- Meier, U. Phenological Growth Stages and BBCH-Identification Keys of Citrus (Citrus spp. L.). In Growth Stages of Mono-and Dicotyledonous Plants (BBCH Monograph); Meier, U., Ed.; Federal Biological Research Centre for Agriculture and Forestry: Berlin, Germany, 2001; pp. 66–68. [Google Scholar]
- Qiuming, Z.; Yusheng, Z.; Kunyu, L.; Shengxi, X.; Dazhi, L. Studies on the annual cycle of endogenous free polyamines in citrus. J. Fruit Sci. 2003, 3. [Google Scholar]
- Ali, A.G.; Lovatt, C.J. Winter application of low-biuret urea to the foliage of ‘Washington’ navel orange increased yield. J. Am. Soc. Hortic. Sci. 1994, 119, 1144–1150. [Google Scholar] [CrossRef]
- Ali, A.G.; Lovatt, C.J. Low-Temperature Stressed-Induced Flowering of the ‘Washington’ Navel Orange (Citrus sinensis l. osbeck) was Increased by Application of Putrescine or Spermidine to the Foliage. In HortScience; American Society for Horticultural Science: Alexandria, VA, USA, 1994; Volume 29. [Google Scholar]
- Kaur-Sawhney, R.; Tiburcio, A.F.; Galston, A.W. Spermidine and flower-bud differentiation in thin-layer explants of tobacco. Planta 1988, 173, 282–284. [Google Scholar] [CrossRef]
- De Cantú, L.B.; Kandeler, R. Significance of polyamines for flowering in Spirodela punctata. Plant Cell Physiol. 1989, 30, 455–458. [Google Scholar]
- Tiburcio, A.F.; Kaur-Sawhney, R.; Galston, A.W. Polyamine biosynthesis during vegetative and floral bud differentiation in thin layer tobacco tissue cultures. Plant Cell Physiol. 1988, 29, 1241–1249. [Google Scholar]
- Davies, F.S. Fruit Drop Problems of Citrus; CAB International: Wallingford, UK, 2002. [Google Scholar]
- Huchche, A.; Dass, H.C.; Kohli, R.R.; Srivastava, A.K. Leaf NH3—NH4+ content and flowering response in Nagpur Mandarin. Indian J. Hortic. 1994, 53, 163–165. [Google Scholar]
- Fraga, M.F.; Berdasco, M.; Diego, L.B.; Rodríguez, R.; Cañal, M.J. Changes in polyamine concentration associated with aging in Pinus radiata and Prunus persica. Tree Physiol. 2004, 24, 1221–1226. [Google Scholar] [CrossRef] [Green Version]
- Hussain, Z.; Singh, Z. Involvement of polyamines in creasing of sweet orange Citrus sinensis (L.) Osbeck fruit. Sci. Hortic. 2015, 190, 203–210. [Google Scholar] [CrossRef]
- Mirsoleimani, A.; Shahsavar, A.-R. Changes of free polyamines in the leaves and stems of ‘Kinnow’ mandarin tree as affected by alternate bearing. J. Plant Process Funct. 2018, 6, 29–35. [Google Scholar]
- Zheng, Y.; Zhang, Q. Effects of polyamines and salicylic acid on postharvest storage of “Ponkan” mandarin. Acta Hortic. 2004, 632, 317–320. [Google Scholar] [CrossRef]
- Liu, K.; Fu, H.; Bei, Q.; Luan, S. Inward potassium channel in guard cells as a target for polyamine regulation of stomatal movements. Plant Physiol. 2000, 124, 1315–1326. [Google Scholar] [CrossRef] [Green Version]
- Kusano, T.; Yamaguchi, K.; Berberich, T.; Takahashi, Y. The polyamine spermine rescues Arabidopsis from salinity and drought stresses. Plant Signal. Behav. 2007, 2, 251–252. [Google Scholar] [CrossRef] [Green Version]
- Agurla, S.; Gayatri, G.; Raghavendra, A.S. Polyamines increase nitric oxide and reactive oxygen species in guard cells of Arabidopsis thaliana during stomatal closure. Protoplasma 2018, 255, 153–162. [Google Scholar] [CrossRef]
- Camacho-Cristóbal, J.J.; Lunar, L.; Lafont, F.; Baumert, A.; González-Fontes, A. Boron deficiency causes accumulation of chlorogenic acid and caffeoyl polyamine conjugates in tobacco leaves. J. Plant Physiol. 2004, 161, 879–881. [Google Scholar] [CrossRef]
- Kotzabasis, K.; Fotinou, C.; Roubelakis-Angelakis, K.A.; Ghanotakis, D. Polyamines in the photosynthetic apparatus. Photosynth. Res. 1993, 38, 83–88. [Google Scholar] [CrossRef]
- Ioannidis, N.E.; Kotzabasis, K. Effects of polyamines on the functionality of photosynthetic membrane in vivo and in vitro. Biochim. Biophys. Acta Bioenerg. 2007, 1767, 1372–1382. [Google Scholar] [CrossRef] [Green Version]
- Mackay, C.E.; Christopher Hall, J.; Hofstra, G.; Fletcher, R.A. Uniconazole-induced changes in abscisic acid, total amino acids, and proline in Phaseolus vulgaris. Pestic. Biochem. Physiol. 1990, 37, 74–82. [Google Scholar] [CrossRef]
- Soumya, P.R.; Kumar, P.; Pal, M. Paclobutrazol: A novel plant growth regulator and multi-stress ameliorant. Indian J. Plant Physiol. 2017, 22, 267–278. [Google Scholar] [CrossRef]
- Armstrong, G.A.; Hearst, J.E. Carotenoids 2: Genetics and molecular biology of carotenoid pigment biosynthesis. FASEB J. 1996, 10, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Vershinin, A. Biological functions of carotenoids-diversity and evolution. Biofactors 1999, 10, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, J.-H.; Kurosawa, T.; Nada, K.; Ban, Y.; Moriguchi, T. Cloning, biochemical identification, and expression analysis of a gene encoding S-adenosylmethionine decarboxylase in navel orange (Citrus sinensis Osbeck). J. Hortic. Sci. Biotechnol. 2010, 85, 219–226. [Google Scholar] [CrossRef]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Killiny, N.; Nehela, Y. Citrus Polyamines: Structure, Biosynthesis, and Physiological Functions. Plants 2020, 9, 426. https://doi.org/10.3390/plants9040426
Killiny N, Nehela Y. Citrus Polyamines: Structure, Biosynthesis, and Physiological Functions. Plants. 2020; 9(4):426. https://doi.org/10.3390/plants9040426
Chicago/Turabian StyleKilliny, Nabil, and Yasser Nehela. 2020. "Citrus Polyamines: Structure, Biosynthesis, and Physiological Functions" Plants 9, no. 4: 426. https://doi.org/10.3390/plants9040426
APA StyleKilliny, N., & Nehela, Y. (2020). Citrus Polyamines: Structure, Biosynthesis, and Physiological Functions. Plants, 9(4), 426. https://doi.org/10.3390/plants9040426





