Exogenous Ascorbic Acid Induced Chilling Tolerance in Tomato Plants Through Modulating Metabolism, Osmolytes, Antioxidants, and Transcriptional Regulation of Catalase and Heat Shock Proteins
Abstract
:1. Introduction
2. Results
2.1. Phenotypic Remarks
2.2. Oxidative Damage
2.3. Antioxidant Capacity and Ascorbic Acid Content
2.4. Proline Content
2.5. Mineral Nutrition (NPK, Na, Mg)
2.6. Photosynthetic Pigments (Chl-a, Chl-b) and Anthocyanins
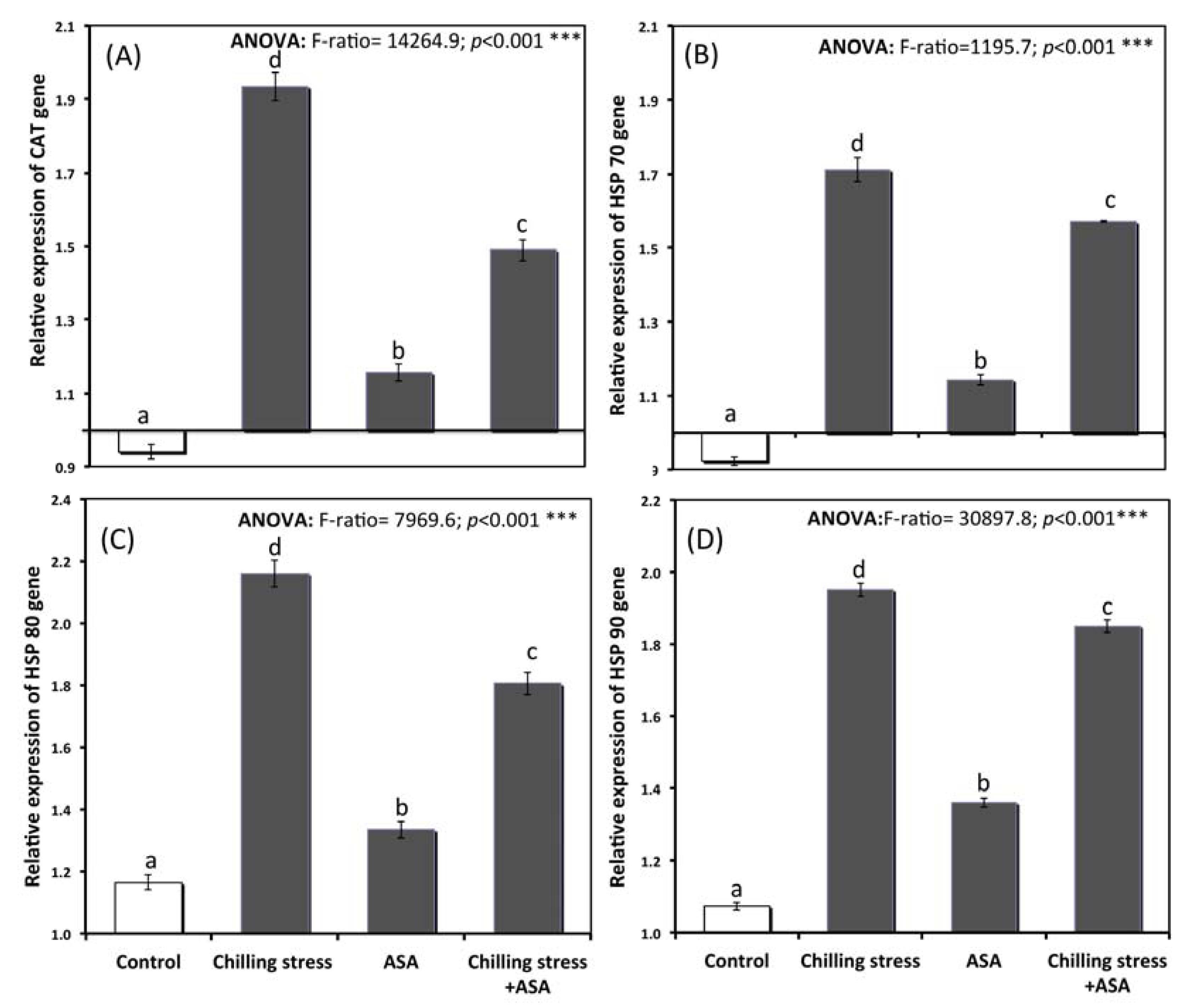
2.7. Gene Expression of Heat Shock Protein and Antioxident Genes
3. Discussion
3.1. Phenotypic Observations and Leaf Morphology
3.2. Oxidative Damage and Antioxidant Capacity
3.3. Ascorbic Acid and Proline Contents
3.4. Mineral Nutrition (NPK, Na, Mg)
3.5. Photosynthetic Pigments (Chl-a, Chl-b) and Anthocyanins
3.6. Relative Gene Expression
4. Conclusions
5. Materials and Methods
5.1. Experimental Design and Stress Condition
5.2. Leaf Measurements and Image Analysis
5.3. Determination of Chlorophyll (a, b) and Anthocyanins Content
5.4. Determination of Electrolyte Leakage Lipid Peroxidation, and Hydrogen Peroxide
5.5. Determination of Total Antioxidant Capacity, Total Oxidant Capacity, and Oxidative Stress Index
5.6. Determination of Ascorbic Acid and Proline
5.7. Mineral Ions Content (N, P, K, Na, and Mg)
5.8. RNA Extraction and Real-Time PCR Analysis
5.9. Data Analyses
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Legave, J.M.; Farrera, I.; Almeras, T.; Calleja, M. Selecting models of apple flowering time and understanding how global warming has had an impact on this trait. J. Hortic. Sci. Biotechnol. 2008, 83, 76–84. [Google Scholar] [CrossRef]
- Pereira, A. Plant Abiotic Stress Challenges from the Changing Environment. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Onaga, G.; Wydra, K. Advances in Plant Tolerance to Abiotic Stresses. In Plant Genomics; Abdurakhmonov, I.Y., Ed.; InTech: London, UK, 2016; ISBN 978-953-51-2455-9. [Google Scholar]
- Nooghabi, S.N.; Burkart, S.; Mahmoudi, H.; Taheri, F.; Damghani, A.M.; Yazdanpanah, M.; Hosseininia, G.; Azadi, H. More food or better distribution? Reviewing food policy options in developing countries. Food Rev. Int. 2018, 34, 566–580. [Google Scholar] [CrossRef]
- Newton, D.E. Spotlight On Current Events: Essays on Contemporary World Issues; ABC-CLIO: Santa Barbara, CA, USA, 2019; ISBN 978-1-4408-7063-7. [Google Scholar]
- Mabhaudhi, T.; Chimonyo, V.G.P.; Hlahla, S.; Massawe, F.; Mayes, S.; Nhamo, L.; Modi, A.T. Prospects of orphan crops in climate change. Planta 2019, 250, 695–708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kratsch, H.A.; Wise, R.R. The ultrastructure of chilling stress. Plant Cell Environ. 2000, 23, 337–350. [Google Scholar] [CrossRef]
- Soliman, M.H.; Alayafi, A.A.M.; El Kelish, A.A.; Abu-Elsaoud, A.M. Acetylsalicylic acid enhance tolerance of Phaseolus vulgaris L. to chilling stress, improving photosynthesis, antioxidants and expression of cold stress responsive genes. Bot. Stud. 2018, 59. [Google Scholar] [CrossRef] [Green Version]
- Hussain, S.; Khan, F.; Hussain, H.A.; Nie, L. Physiological and Biochemical Mechanisms of Seed Priming-Induced Chilling Tolerance in Rice Cultivars. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Saibo, N.J.M.; Lourenço, T.; Oliveira, M.M. Transcription factors and regulation of photosynthetic and related metabolism under environmental stresses. Ann. Bot. 2009, 103, 609–623. [Google Scholar] [CrossRef] [Green Version]
- Elkeilsh, A.; Awad, Y.M.; Soliman, M.H.; Abu-Elsaoud, A.; Abdelhamid, M.T.; El-Metwally, I.M. Exogenous application of β-sitosterol mediated growth and yield improvement in water-stressed wheat (Triticum aestivum) involves up-regulated antioxidant system. J. Plant Res. 2019. [Google Scholar] [CrossRef]
- Von Koskull-Döring, P.; Scharf, K.-D.; Nover, L. The diversity of plant heat stress transcription factors. Trends Plant Sci. 2007, 12, 452–457. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Sevillano, L.; Flores, F.B.; Bodbodak, S. Heat shock proteins as biochemical markers for postharvest chilling stress in fruits and vegetables. Sci. Hortic. 2013, 160, 54–64. [Google Scholar] [CrossRef] [Green Version]
- Neta-Sharir, I.; Isaacson, T.; Lurie, S.; Weiss, D. Dual Role for Tomato Heat Shock Protein 21: Protecting Photosystem II from Oxidative Stress and Promoting Color Changes during Fruit Maturation. Plant Cell 2005, 17, 1829–1838. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Matas, M.-A.; Nuñez, P.; Soto, A.; Allona, I.; Casado, R.; Collada, C.; Guevara, M.-A.; Aragoncillo, C.; Gomez, L. Protein Cryoprotective Activity of a Cytosolic Small Heat Shock Protein That Accumulates Constitutively in Chestnut Stems and Is Up-Regulated by Low and High Temperatures. Plant Physiol. 2004, 134, 1708–1717. [Google Scholar] [CrossRef] [Green Version]
- Apostolova, P.; Yordanova, R.; Popova, L. Response of antioxidative defence system to low temperature stress in two wheat cultivars. Gen Appl Plant Physiol 2008, 34, 281–294. [Google Scholar]
- Jofré, A.; Molinas, M.; Pla, M. A 10-kDa class-CI sHsp protects E. coli from oxidative and high-temperature stress. Planta 2003, 217, 813–819. [Google Scholar] [CrossRef]
- Saleh, A.A.H.; Abdel-Kader, D.; El Kelish, A. Role of Heat Shock and Salicylic Acid in Antioxidant Homeostasis in Mungbean (Vigna radiata L.) Plant Subjected to Heat Stress. Am. J. Plant Physiol. 2007, 2, 344–355. [Google Scholar]
- El-Esawi, M.A.; Elkelish, A.; Soliman, M.; Elansary, H.O.; Zaid, A.; Wani, S.H. Serratia marcescens BM1 Enhances Cadmium Stress Tolerance and Phytoremediation Potential of Soybean Through Modulation of Osmolytes, Leaf Gas Exchange, Antioxidant Machinery, and Stress-Responsive Genes Expression. Antioxidants 2020, 9, 43. [Google Scholar] [CrossRef] [Green Version]
- Farooq, M.; Hussain, M.; Nawaz, A.; Lee, D.-J.; Alghamdi, S.S.; Siddique, K.H.M. Seed priming improves chilling tolerance in chickpea by modulating germination metabolism, trehalose accumulation and carbon assimilation. Plant Physiol. Biochem. 2017, 111, 274–283. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Fotopoulos, V. (Eds.) Priming and Pretreatment of Seeds and Seedlings: Implication in Plant Stress Tolerance and Enhancing Productivity in Crop Plants; Springer: Singapore, 2019; ISBN 9789811386244. [Google Scholar]
- Hasanuzzaman, M.; Nahar, K.; Rohman, M.M.; Anee, T.I.; Huang, Y.; Fujita, M. Exogenous Silicon Protects Brassica napus Plants from Salinity-Induced Oxidative Stress Through the Modulation of AsA-GSH Pathway, Thiol-Dependent Antioxidant Enzymes and Glyoxalase Systems. Gesunde Pflanz. 2018, 70, 185–194. [Google Scholar] [CrossRef]
- Climate Change, Agriculture and Food Security. FAO (Ed.) In The State of Food and Agriculture; FAO: Rome, Italy, 2016; ISBN 978-92-5-109374-0. [Google Scholar]
- Sharma, N.; Acharya, S.; Kumar, K.; Singh, N.; Chaurasia, O.P. Hydroponics as an advanced technique for vegetable production: An overview. J. Soil Water Conserv. 2018, 17, 364. [Google Scholar] [CrossRef]
- Asaduzzaman, M.; Saifullah, M.; Mollick, A.S.R.; Hossain, M.M.; Halim, G.M.A.; Asao, T. Influence of Soilless Culture Substrate on Improvement of Yield and Produce Quality of Horticultural Crops. In Soilless Culture—Use of Substrates for the Production of Quality Horticultural Crops; Asaduzzaman, M., Ed.; InTech: London, UK, 2015; ISBN 978-953-51-1739-1. [Google Scholar]
- Müller-Moulé, P.; Havaux, M.; Niyogi, K.K. Zeaxanthin deficiency enhances the high light sensitivity of an ascorbate-deficient mutant of Arabidopsis. Plant Physiol. 2003, 133, 748–760. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Gallie, D.R. The ascorbic acid redox state controls guard cell signaling and stomatal movement. Plant Cell 2004, 16, 1143–1162. [Google Scholar] [CrossRef] [Green Version]
- Alayafi, A.A.M. Exogenous ascorbic acid induces systemic heat stress tolerance in tomato seedlings: Transcriptional regulation mechanism. Environ. Sci. Pollut. Res. 2019. [Google Scholar] [CrossRef]
- Kader, D.Z.A.; Saleh, A.A.H.; Elmeleigy, S.A.; Dosoky, N.S. Chilling-induced oxidative stress and polyamines regulatory role in two wheat varieties. J. Taibah Univ. Sci. 2011, 5, 14–24. [Google Scholar] [CrossRef] [Green Version]
- Hussain, H.A.; Hussain, S.; Khaliq, A.; Ashraf, U.; Anjum, S.A.; Men, S.; Wang, L. Chilling and Drought Stresses in Crop Plants: Implications, Cross Talk, and Potential Management Opportunities. Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef]
- Aroca, R.; Vernieri, P.; Irigoyen, J.J.; Sánchez-Díaz, M.; Tognoni, F.; Pardossi, A. Involvement of abscisic acid in leaf and root of maize (Zea mays L.) in avoiding chilling-induced water stress. Plant Sci. 2003, 165, 671–679. [Google Scholar] [CrossRef]
- Ecophysiology, Abiotic Stress Responses and Utilization of Halophytes; Hasanuzzaman, M.; Nahar, K.; Öztürk, M. (Eds.) Springer: Singapore, 2019; ISBN 9789811337611. [Google Scholar]
- Yang, J.; Cao, W.; Rui, Y. Interactions between nanoparticles and plants: phytotoxicity and defense mechanisms. J. Plant Interact. 2017, 12, 158–169. [Google Scholar] [CrossRef]
- DeRidder, B.P.; Crafts-Brandner, S.J. Chilling stress response of postemergent cotton seedlings. Physiol. Plant. 2008, 134, 430–439. [Google Scholar] [CrossRef]
- Bai, J.; Baldwin, E.A.; Imahori, Y.; Kostenyuk, I.; Burns, J.; Brecht, J.K. Chilling and heating may regulate C6 volatile aroma production by different mechanisms in tomato (Solanum lycopersicum) fruit. Postharvest Biol. Technol. 2011, 60, 111–120. [Google Scholar] [CrossRef]
- Xu, S.; Hu, J.; Li, Y.; Ma, W.; Zheng, Y.; Zhu, S. Chilling tolerance in Nicotiana tabacum induced by seed priming with putrescine. Plant Growth Regul. 2011, 63, 279–290. [Google Scholar] [CrossRef]
- Elkelish, A.A.; Alnusaire, T.S.; Soliman, M.H.; Gowayed, S.; Senousy, H.H.; Fahad, S. Calcium availability regulates antioxidant system, physio-biochemical activities and alleviates salinity stress mediated oxidative damage in soybean seedlings. J. Appl. Bot. Food Qual. 2019, 258–266. [Google Scholar]
- Elkelish, A.A.; Alhaithloul, H.A.S.; Qari, S.H.; Soliman, M.H.; Hasanuzzaman, M. Pretreatment with Trichoderma harzianum alleviates waterlogging-induced growth alterations in tomato seedlings by modulating physiological, biochemical, and molecular mechanisms. Environ. Exp. Bot. 2019, 103946. [Google Scholar] [CrossRef]
- Saltveit, M.E. The rate of ion leakage from chilling-sensitive tissue does not immediately increase upon exposure to chilling temperatures. Postharvest Biol. Technol. 2002, 26, 295–304. [Google Scholar] [CrossRef]
- Valenzuela, J.L.; Manzano, S.; Palma, F.; Carvajal, F.; Garrido, D.; Jamilena, M. Oxidative Stress Associated with Chilling Injury in Immature Fruit: Postharvest Technological and Biotechnological Solutions. Int. J. Mol. Sci. 2017, 18, 1467. [Google Scholar] [CrossRef]
- Albornoz, K.; Cantwell, M.I.; Zhang, L.; Beckles, D.M. Integrative analysis of postharvest chilling injury in cherry tomato fruit reveals contrapuntal spatio-temporal responses to ripening and cold stress. Sci. Rep. 2019, 9, 2795. [Google Scholar] [CrossRef] [Green Version]
- Furmanski, R.J.; Buescher, R.W. Influence of Chilling on Electrolyte Leakage and Internal Conductivity of Peach Fruits. Available online: https://eurekamag.com/research/000/680/000680703.php (accessed on 2 January 2020).
- El-Esawi, M.A.; Elkelish, A.; Elansary, H.O.; Ali, H.M.; Elshikh, M.; Witczak, J.; Ahmad, M. Genetic Transformation and Hairy Root Induction Enhance the Antioxidant Potential of Lactuca serriola L. Available online: https://www.hindawi.com/journals/omcl/2017/5604746/ (accessed on 9 January 2018).
- Al Mahmud, J.; Bhuyan, M.H.M.B.; Anee, T.I.; Nahar, K.; Fujita, M.; Hasanuzzaman, M. Reactive Oxygen Species Metabolism and Antioxidant Defense in Plants Under Metal/Metalloid Stress. In Plant Abiotic Stress Tolerance; Hasanuzzaman, M., Hakeem, K.R., Nahar, K., Alharby, H.F., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 221–257. ISBN 978-3-030-06117-3. [Google Scholar]
- Cao, S.; Cai, Y.; Yang, Z.; Zheng, Y. MeJA induces chilling tolerance in loquat fruit by regulating proline and γ-aminobutyric acid contents. Food Chem. 2012, 133, 1466–1470. [Google Scholar] [CrossRef]
- Sivaci, A.; Kaya, A.; Duman, S. Effects of ascorbic acid on some physiological changes of pepino (Solanum muricatum Ait.) under chilling stress. Acta Biol. Hung. 2014, 65, 305–318. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, O.R.; Opabode, J.T. Pre-treatment of two contrasting water-stressed genotypes of cassava (Manihot esculenta Crantz) with ascorbic acid. I. Growth, physiological and antioxidant responses. Physiol. Mol. Biol. Plants Int. J. Funct. Plant Biol. 2019, 25, 1385–1394. [Google Scholar] [CrossRef]
- Abdul Jaleel, C.; Manivannan, P.; Wahid, A.; Farooq, M.; Jasim Al-Juburi, H.; Somasundaram, R.; Panneerselvam, R. Drought Stress in Plants: A Review on Morphological Characteristics and Pigments Composition. Int. J. Agric. Biol. 2009, 11, 100–105. [Google Scholar]
- Abrantes, F.D.L.; Ribas, A.F.; Vieira, L.G.E.; Machado-Neto, N.B.; Custódio, C.C. Seed germination and seedling vigour of transgenic tobacco (Nicotiana tabacum L.) with increased proline accumulation under osmotic stress. J. Hortic. Sci. Biotechnol. 2018, 1–9. [Google Scholar] [CrossRef]
- Akram, N.A.; Shafiq, F.; Ashraf, M. Ascorbic Acid-A Potential Oxidant Scavenger and Its Role in Plant Development and Abiotic Stress Tolerance. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Alhaithloul, H.A.; Soliman, M.H.; Ameta, K.L.; El-Esawi, M.A.; Elkelish, A. Changes in Ecophysiology, Osmolytes, and Secondary Metabolites of the Medicinal Plants of Mentha piperita and Catharanthus roseus Subjected to Drought and Heat Stress. Biomolecules 2019, 10, 43. [Google Scholar] [CrossRef] [Green Version]
- Hasanuzzaman, M.; Alam, M.M.; Rahman, A.; Hasanuzzaman, M.; Nahar, K.; Fujita, M. Exogenous Proline and Glycine Betaine Mediated Upregulation of Antioxidant Defense and Glyoxalase Systems Provides Better Protection against Salt-Induced Oxidative Stress in Two Rice (Oryza sativa L.) Varieties. Available online: https://www.hindawi.com/journals/bmri/2014/757219/ (accessed on 19 November 2019).
- Allahveran, A.; Farokhzad, A.; Asghari, M.; Sarkhosh, A. Foliar application of ascorbic and citric acids enhanced ‘Red Spur’ apple fruit quality, bioactive compounds and antioxidant activity. Physiol. Mol. Biol. Plants 2018, 24, 433–440. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, W.; Xian, Z.; Hu, N.; Lin, D.; Ren, H.; Chen, J.; Su, D.; Li, Z. Overexpression of SlGRAS40 in Tomato Enhances Tolerance to Abiotic Stresses and Influences Auxin and Gibberellin Signaling. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef]
- Erdal, S. Androsterone-induced molecular and physiological changes in maize seedlings in response to chilling stress. Plant Physiol. Biochem. PPB 2012, 57, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tambussi, E.A.; Bartoli, C.G.; Guiamet, J.J.; Beltrano, J.; Araus, J.L. Oxidative stress and photodamage at low temperatures in soybean (Glycine max L. Merr.) leaves. Plant Sci. 2004, 167, 19–26. [Google Scholar] [CrossRef]
- Alamri, S.A.; Siddiqui, M.H.; Al-Khaishany, M.Y.; Khan, M.N.; Ali, H.M.; Alaraidh, I.A.; Alsahli, A.A.; Al-Rabiah, H.; Mateen, M. Ascorbic acid improves the tolerance of wheat plants to lead toxicity. J. Plant Interact. 2018, 13, 409–419. [Google Scholar] [CrossRef] [Green Version]
- Bista, D.; Heckathorn, S.; Jayawardena, D.; Mishra, S.; Boldt, J. Effects of Drought on Nutrient Uptake and the Levels of Nutrient-Uptake Proteins in Roots of Drought-Sensitive and -Tolerant Grasses. Plants 2018, 7, 28. [Google Scholar] [CrossRef] [Green Version]
- Pregitzer, K.S.; King, J.S. Effects of Soil Temperature on Nutrient Uptake. In Nutrient Acquisition by Plants; BassiriRad, H., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; Volume 181, pp. 277–310. ISBN 978-3-540-24186-7. [Google Scholar]
- Tränkner, M.; Tavakol, E.; Jákli, B. Functioning of potassium and magnesium in photosynthesis, photosynthate translocation and photoprotection. Physiol. Plant. 2018, 163, 414–431. [Google Scholar] [CrossRef] [Green Version]
- Steinhorst, L.; Kudla, J. Signaling in cells and organisms—calcium holds the line. Curr. Opin. Plant Biol. 2014, 22, 14–21. [Google Scholar] [CrossRef]
- Athar, H.-R.; Khan, A.; Ashraf, M. Inducing Salt Tolerance in Wheat by Exogenously Applied Ascorbic Acid through Different Modes. J. Plant Nutr. 2009, 32, 1799–1817. [Google Scholar] [CrossRef]
- Zhu, J.K. Regulation of ion homeostasis under salt stress. Curr. Opin. Plant Biol. 2003, 6, 441–445. [Google Scholar] [CrossRef]
- Farooq, M.; Irfan, M.; Aziz, T.; Ahmad, I.; Cheema, S.A. Seed Priming with Ascorbic Acid Improves Drought Resistance of Wheat. J. Agron. Crop Sci. 2013, 199, 12–22. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, Y.; Xiao, J.; Bao, F. Effects of Chilling on the Structure, Function and Development of Chloroplasts. Front. Plant Sci. 2018, 9, 1715. [Google Scholar] [CrossRef] [Green Version]
- Hendrickson, L.; Ball, M.C.; Wood, J.T.; Chow, W.S.; Furbank, R.T. Low temperature effects on photosynthesis and growth of grapevine. Plant Cell Environ. 2004, 27, 795–809. [Google Scholar] [CrossRef]
- Meng, C.; Sui, N. Overexpression of maize MYB-IF35 increases chilling tolerance in Arabidopsis. Plant Physiol. Biochem. 2019, 135, 167–173. [Google Scholar] [CrossRef]
- Kaur, G.; Kumar, S.; Thakur, P.; Malik, J.A.; Bhandhari, K.; Sharma, K.D.; Nayyar, H. Involvement of proline in response of chickpea (Cicer arietinum L.) to chilling stress at reproductive stage. Sci. Hortic. 2011, 128, 174–181. [Google Scholar] [CrossRef]
- Kalisz, A.; Jezdinský, A.; Pokluda, R.; Sękara, A.; Grabowska, A.; Gil, J. Impacts of chilling on photosynthesis and chlorophyll pigment content in juvenile basil cultivars. Hortic. Environ. Biotechnol. 2016, 57, 330–339. [Google Scholar] [CrossRef]
- Batista-Santos, P.; Lidon, F.C.; Fortunato, A.; Leitão, A.E.; Lopes, E.; Partelli, F.; Ribeiro, A.I.; Ramalho, J.C. The impact of cold on photosynthesis in genotypes of Coffea spp.—photosystem sensitivity, photoprotective mechanisms and gene expression. J. Plant Physiol. 2011, 168, 792–806. [Google Scholar] [CrossRef]
- Hwang, H.J.; Kim, J.-H.; Eu, Y.-J.; Moon, B.Y.; Cho, S.H.; Lee, C.-H. Photoinhibition of photosystem I is accelerated by dimethyldithiocarbamate, an inhibitor of superoxide dismutase, during light-chilling of spinach leaves. J. Photochem. Photobiol. B 2004, 73, 79–85. [Google Scholar] [CrossRef]
- Hussein, M.M.; Alva, A.K. Effects of Zinc and Ascorbic Acid Application on the Growth and Photosynthetic Pigments of Millet Plants Grown under Different Salinity. Agric. Sci. 2014, 05, 1253–1260. [Google Scholar] [CrossRef] [Green Version]
- Gould, K.S.; Markham, K.R.; Smith, R.H.; Goris, J.J. Functional role of anthocyanins in the leaves of Quintinia serrata A. Cunn. J. Exp. Bot. 2000, 51, 1107–1115. [Google Scholar] [CrossRef] [Green Version]
- Ebrahimian, E.; Bybordi, A. Influence of ascorbic acid foliar application on chlorophyll, flavonoids, anthocyanin and soluble sugar contents of sunflower under conditions of water deficit stress. J. Food Agric. Environ. 2012, 10, 1026–1030. [Google Scholar]
- Batiha, G.E.-S.; Magdy Beshbishy, A.; Adeyemi, O.S.; Nadwa, E.H.; Rashwan, E.; kadry, M.; Alkazmi, L.M.; Elkelish, A.A.; Igarashi, I. Phytochemical Screening and Antiprotozoal Effects of the Methanolic Berberis vulgaris and Acetonic Rhus coriaria Extracts. Molecules 2020, 25, 550. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.H.; Lee, C.B. Chilling stress-induced changes of antioxidant enzymes in the leaves of cucumber: in gel enzyme activity assays. Plant Sci. Int. J. Exp. Plant Biol. 2000, 159, 75–85. [Google Scholar]
- Anjum, S.A.; Tanveer, M.; Hussain, S.; Bao, M.; Wang, L.; Khan, I.; Ullah, E.; Tung, S.A.; Samad, R.A.; Shahzad, B. Cadmium toxicity in Maize (Zea mays L.): consequences on antioxidative systems, reactive oxygen species and cadmium accumulation. Environ. Sci. Pollut. Res. Int. 2015, 22, 17022–17030. [Google Scholar] [CrossRef]
- Khaliq, A.; Aslam, F.; Matloob, A.; Hussain, S.; Geng, M.; Wahid, A.; ur Rehman, H. Seed Priming with Selenium: Consequences for Emergence, Seedling Growth, and Biochemical Attributes of Rice. Biol. Trace Elem. Res. 2015, 166, 236–244. [Google Scholar] [CrossRef]
- Al-Whaibi, M.H. Plant heat-shock proteins: A mini review. J. King Saud Univ. Sci. 2011, 23, 139–150. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Vinocur, B.; Altman, A. Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta 2003, 218, 1–14. [Google Scholar] [CrossRef]
- Ungelenk, S.; Moayed, F.; Ho, C.-T.; Grousl, T.; Scharf, A.; Mashaghi, A.; Tans, S.; Mayer, M.P.; Mogk, A.; Bukau, B. Small heat shock proteins sequester misfolding proteins in near-native conformation for cellular protection and efficient refolding. Nat. Commun. 2016, 7, 13673. [Google Scholar] [CrossRef]
- Fowler, S.; Thomashow, M.F. Arabidopsis transcriptome profiling indicates that multiple regulatory pathways are activated during cold acclimation in addition to the CBF cold response pathway. Plant Cell 2002, 14, 1675–1690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volkov, R.A.; Panchuk, I.I.; Mullineaux, P.M.; Schöffl, F. Heat stress-induced H2O2 is required for effective expression of heat shock genes in Arabidopsis. Plant Mol. Biol. 2006, 61, 733–746. [Google Scholar] [CrossRef] [PubMed]
- Ohama, N.; Sato, H.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Transcriptional Regulatory Network of Plant Heat Stress Response. Trends Plant Sci. 2017, 22, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Iki, T.; Yoshikawa, M.; Nishikiori, M.; Jaudal, M.C.; Matsumoto-Yokoyama, E.; Mitsuhara, I.; Meshi, T.; Ishikawa, M. In vitro assembly of plant RNA-induced silencing complexes facilitated by molecular chaperone HSP90. Mol. Cell 2010, 39, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q.; Lu, X.; Zeng, H.; Zhang, Y.; Zhu, J. Heat stress induction of miR398 triggers a regulatory loop that is critical for thermotolerance in Arabidopsis. Plant J. 2013, 74, 840–851. [Google Scholar] [CrossRef] [PubMed]
- Stief, A.; Altmann, S.; Hoffmann, K.; Pant, B.D.; Scheible, W.-R.; Bäurle, I. Arabidopsis miR156 Regulates Tolerance to Recurring Environmental Stress through SPL Transcription Factors. Plant Cell 2014, 26, 1792–1807. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Zhao, H.; Cao, K.; Hu, L.; Du, T.; Baluška, F.; Zou, Z. Beneficial Roles of Melatonin on Redox Regulation of Photosynthetic Electron Transport and Synthesis of D1 Protein in Tomato Seedlings under Salt Stress. Front. Plant Sci. 2016, 7, 1823. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.; Xu, S.; Mu, D.; Sadeghnezhad, E.; Li, Q.; Ma, Z.; Zhao, L.; Zhang, Q.; Wang, L. Exogenous Melatonin Delays Dark-Induced Grape Leaf Senescence by Regulation of Antioxidant System and Senescence Associated Genes (SAGs). Plants 2019, 8, 366. [Google Scholar] [CrossRef] [Green Version]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Roychowdhury, R.; Fujita, M. Physiological, Biochemical, and Molecular Mechanisms of Heat Stress Tolerance in Plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, C.-M.; Wang, Y.-J.; Liu, J. Overexpression of chloroplast-localized small molecular heat-shock protein enhances chilling tolerance in tomato plant. Zhi Wu Sheng Li Yu Fen Zi Sheng Wu Xue Xue Bao 2005, 31, 167–174. [Google Scholar]
- Pandey, S.; Muthamilarasan, M.; Sharma, N.; Chaudhry, V.; Dulani, P.; Shweta, S.; Jha, S.; Mathur, S.; Prasad, M. Characterization of DEAD-box family of RNA helicases in tomato provides insights into their roles in biotic and abiotic stresses. Environ. Exp. Bot. 2019, 158, 107–116. [Google Scholar] [CrossRef]
- Kosová, K.; Vítámvás, P.; Urban, M.O.; Prášil, I.T.; Renaut, J. Plant Abiotic Stress Proteomics: The Major Factors Determining Alterations in Cellular Proteome. Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Park, C.-J.; Park, J.M. Endoplasmic Reticulum Plays a Critical Role in Integrating Signals Generated by Both Biotic and Abiotic Stress in Plants. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoagland, D.R.; Dennis, R.; Arnon, D.I.; Daniel, I. The Water-Culture Method for Growing Plants without Soil; College of Agriculture, University of California: Berkeley, CA, USA, 1950. [Google Scholar]
- Aboukarima, A.; Zayed, M.; Minyawi, M.; Elsoury, H.; Tarabye, H. Image Analysis-based System for Estimating Cotton Leaf Area. Asian Res. J. Agric. 2017, 5, 1–8. [Google Scholar] [CrossRef] [Green Version]
- O’Neal, M.E.; Landis, D.A.; Isaacs, R. An Inexpensive, Accurate Method for Measuring Leaf Area and Defoliation Through Digital Image Analysis. J. Econ. Entomol. 2002, 95, 1190–1194. [Google Scholar] [CrossRef]
- Holder, M. Chlorophylls: Chemistry and biochemistry of plant pigments. In Chemistry and Biochemistry of Plant Pigments; Goodwin, T.W., Ed.; Academic Press: London, UK, 1965. [Google Scholar]
- Mancinelli, A.L. Photoregulation of Anthocyanin Synthesis: VIII. Effect of Light Pretreatments. Plant Physiol. 1984, 75, 447–453. [Google Scholar] [CrossRef] [Green Version]
- Dionisio-Sese, M.L.; Tobita, S. Antioxidant responses of rice seedlings to salinity stress. Plant Sci. 1998, 135, 1–9. [Google Scholar] [CrossRef]
- De Vos, C.H.R.; Schat, H.; De Waal, M.A.M.; Vooijs, R.; Ernst, W.H.O. Increased resistance to copper-induced damage of the root cell plasmalemma in copper tolerant Silene cucubalus. Physiol. Plant. 1991, 82, 523–528. [Google Scholar] [CrossRef]
- Sergiev, I.; Alexieva, V.; Yanev, S.; Karanov, E. Effect of Atrazine and Spermine on Free Proline Content and Some Antioxidants in Pea (Pisum sativum L.) Plants. Comp Ren Acad. Bul Sci 1997, 51, 121:124. [Google Scholar]
- Erel, O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin. Biochem. 2004, 37, 112–119. [Google Scholar] [CrossRef]
- Erel, O. A new automated colorimetric method for measuring total oxidant status. Clin. Biochem. 2005, 38, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Nweze, C.C.; Abdulganiyu, M.G.; Erhabor, O.G. COMPARATIVE ANALYSIS OF VITAMIN C IN FRESH FRUITS JUICE OF Malus domestica, Citrus sinensi, Ananas comosus AND Citrullus lanatus BY IODOMETRIC TITRATION. Int. J. Sci. Environ. Technol. 2015, 4, 17–22. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Lennox, L.J.; Flanagan, M.J. An automated procedure for the determination of total Kjeldahl nitrogen. Water Res. 1982, 16, 1127–1133. [Google Scholar] [CrossRef]
- Fogg, D.N.; Wilkinson, N.T. The colorimetric determination of phosphorus. Analyst 1958, 83, 406–414. [Google Scholar] [CrossRef]
- Rowell, D.L. Soil science: methods and applications; Longman Scientific & Technical; Pearson Education Ltd.: Harlow, UK, 1994; ISBN 978-0-582-08784-2. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods San Diego Calif 2001, 25, 402–408. [Google Scholar] [CrossRef]





| Variable | Length (cm) Mean ± SE | FW (g plant−1) ) Mean ± SE |
|---|---|---|
| Shoot system | 12.86 a ± 0.40 | 3.57 b ± 0.16 |
| Root system | 11.81 a ± 0.39 | 1.32 a ± 0.09 |
| Whole Plant | 24.67 b ± 0.40 | 4.90 c ± 0.24 |
| ANOVA | ||
| F-ratio | 331.712 | 106.503 |
| p-value | < 0.001 | < 0.001 |
| Treatments | Chlorophyll-a | Chlorophyll-b | Anthocyanin |
|---|---|---|---|
| µg g−1 FW | µg g−1 FW | Unit g−1 FW | |
| Control | 74.17 ± 1.00 a | 6.76 ± 0.03 a | 0.59 ± 0.01 a |
| Chilling stress | 14.64 ± 0.03 b | 0.61 ± 0.02 b | 1.08 ± 0.03 b |
| AsA | 80.61 ± 0.73 d | 7.17 ± 0.07 d | 0.68 ± 0.01 d |
| AsA + Chilling Stress | 26.54 ± 0.03 c | 1.13 ± 0.14 c | 1.23 ± 0.02 c |
| One-way ANOVA | |||
| F-ratio | 2893.17 | 1947.63 | 249.58 |
| p-value | p < 0.001 | p < 0.001 | p < 0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elkelish, A.; Qari, S.H.; Mazrou, Y.S.A.; Abdelaal, K.A.A.; Hafez, Y.M.; Abu-Elsaoud, A.M.; Batiha, G.E.-S.; El-Esawi, M.A.; El Nahhas, N. Exogenous Ascorbic Acid Induced Chilling Tolerance in Tomato Plants Through Modulating Metabolism, Osmolytes, Antioxidants, and Transcriptional Regulation of Catalase and Heat Shock Proteins. Plants 2020, 9, 431. https://doi.org/10.3390/plants9040431
Elkelish A, Qari SH, Mazrou YSA, Abdelaal KAA, Hafez YM, Abu-Elsaoud AM, Batiha GE-S, El-Esawi MA, El Nahhas N. Exogenous Ascorbic Acid Induced Chilling Tolerance in Tomato Plants Through Modulating Metabolism, Osmolytes, Antioxidants, and Transcriptional Regulation of Catalase and Heat Shock Proteins. Plants. 2020; 9(4):431. https://doi.org/10.3390/plants9040431
Chicago/Turabian StyleElkelish, Amr, Sameer H. Qari, Yasser S. A. Mazrou, Khaled A. A. Abdelaal, Yaser M. Hafez, Abdelghafar M. Abu-Elsaoud, Gaber El-Saber Batiha, Mohamed A. El-Esawi, and Nihal El Nahhas. 2020. "Exogenous Ascorbic Acid Induced Chilling Tolerance in Tomato Plants Through Modulating Metabolism, Osmolytes, Antioxidants, and Transcriptional Regulation of Catalase and Heat Shock Proteins" Plants 9, no. 4: 431. https://doi.org/10.3390/plants9040431
APA StyleElkelish, A., Qari, S. H., Mazrou, Y. S. A., Abdelaal, K. A. A., Hafez, Y. M., Abu-Elsaoud, A. M., Batiha, G. E.-S., El-Esawi, M. A., & El Nahhas, N. (2020). Exogenous Ascorbic Acid Induced Chilling Tolerance in Tomato Plants Through Modulating Metabolism, Osmolytes, Antioxidants, and Transcriptional Regulation of Catalase and Heat Shock Proteins. Plants, 9(4), 431. https://doi.org/10.3390/plants9040431









