Effects of GABA and Vigabatrin on the Germination of Chinese Chestnut Recalcitrant Seeds and Its Implications for Seed Dormancy and Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatments
2.2. Calculation of the Seed Germination Rate
2.3. Measurement of Reactive Oxygen Species
2.4. Measurement of Soluble Sugars and Starch
2.5. Measurement of Soluble Proteins and Total Amino Acids
2.6. Measurement of Organic Acids and Amino Acids
2.7. Statistical Analysis
3. Results
3.1. Effects of GABA and VGB on Seed Germination Characteristics
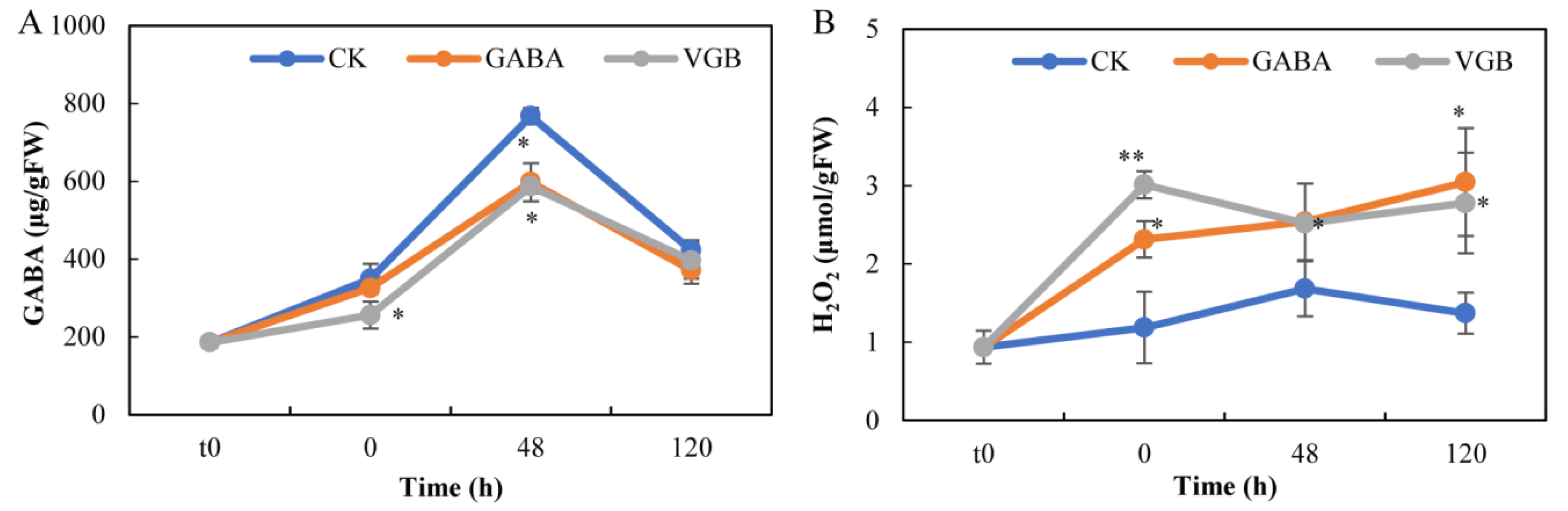
3.2. Changes to Endogenous GABA and H2O2 Contents
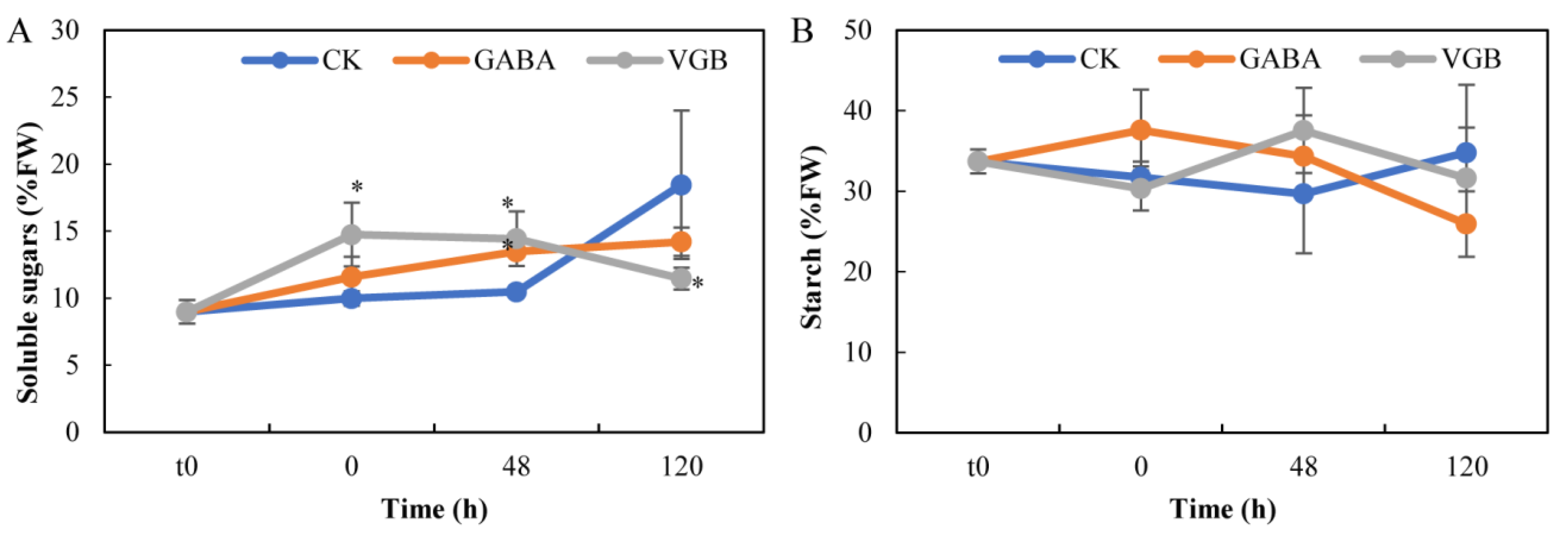
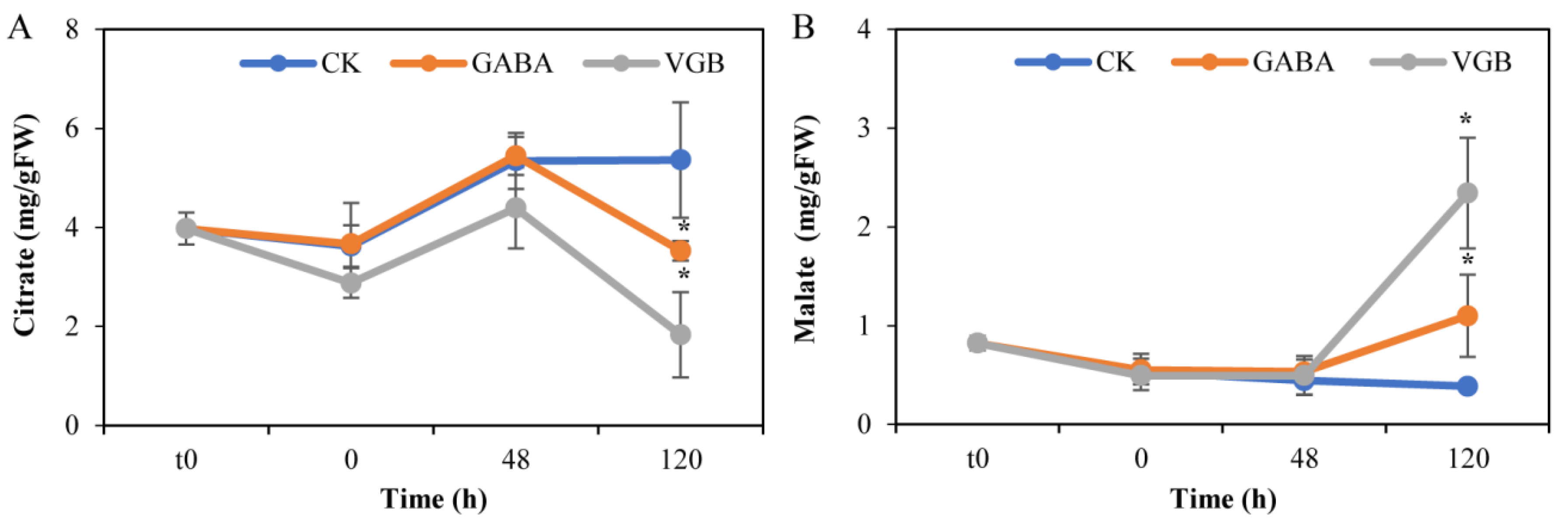
3.3. Effects of GABA and VGB on Carbon Metabolism
3.4. Effects of GABA and VGB on Nitrogen Metabolism
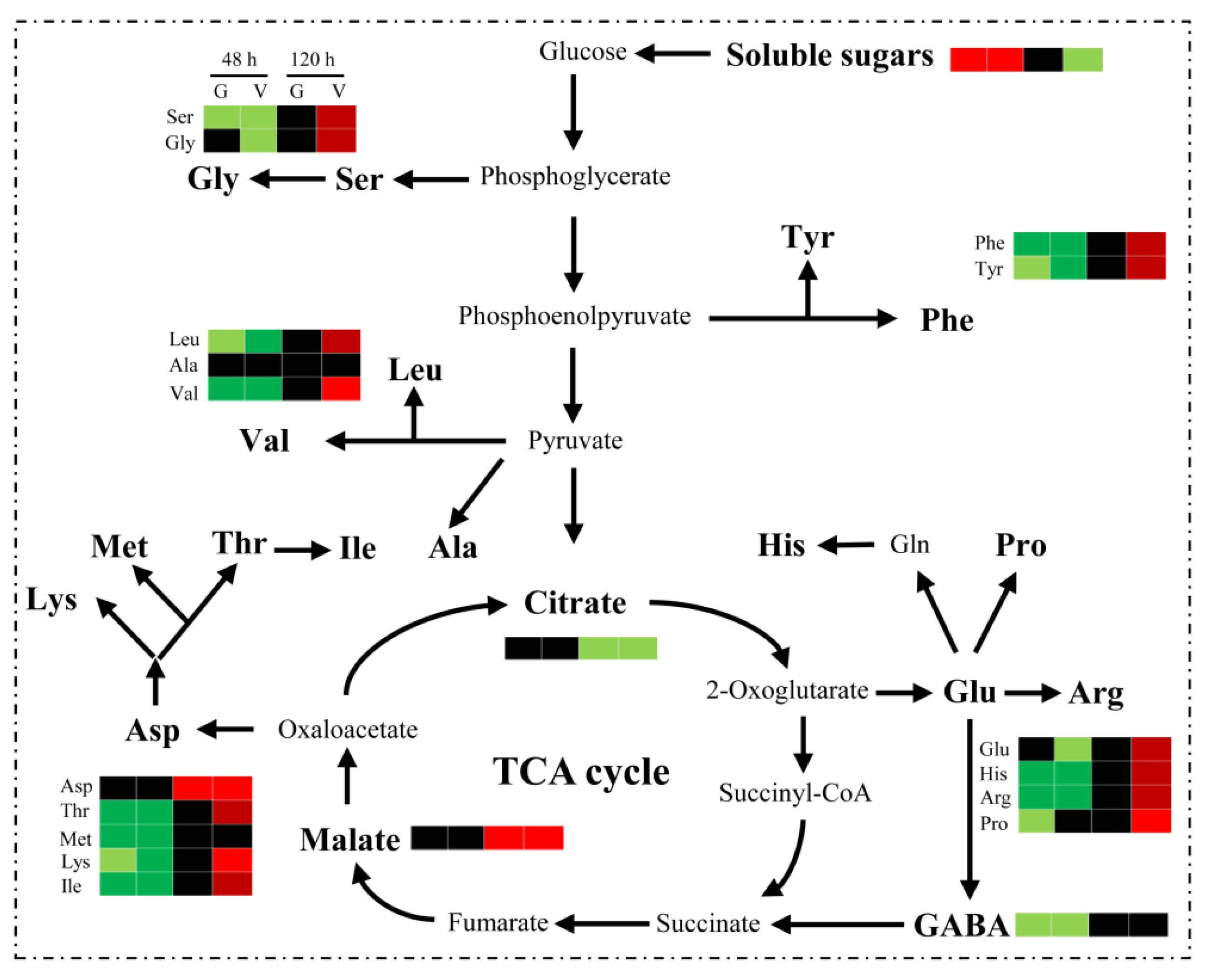
3.5. Analysis of the Physiological Response to Germination Following the GABA and VGB Treatments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- LaBonte, N.R.; Zhao, P.; Woeste, K. Signatures of selection in the genomes of Chinese chestnut (Castanea mollissima Blume): The roots of nut tree domestication. Front. Plant Sci. 2018, 9, 810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, Y.; Liu, Y.; Zhang, Q.; Nie, X.; Sun, Y.; Zhang, Z.; Li, H.; Fang, K.; Wang, G.; Huang, H.; et al. Hybrid de novo genome assembly of Chinese chestnut (Castanea mollissima). GigaScience 2019, 8, pii:giz112. [Google Scholar] [CrossRef] [PubMed]
- Staton, M.; Zhebentyayeva, T.; Olukolu, B.; Fang, G.C.; Nelson, D.; Carlson, J.E.; Abbott, A.G. Substantial genome synteny preservation among woody angiosperm species: Comparative genomics of Chinese chestnut (Castanea mollissima) and plant reference genomes. BMC Genomics 2015, 16, 744. [Google Scholar] [CrossRef] [Green Version]
- Rigling, D.; Prospero, S. Cryphonectria parasitica, the causal agent of chestnut blight: Invasion history, population biology and disease control. Mol. Plant Pathol. 2018, 19, 7–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Vasconcelos, M.C.; Bennett, R.N.; Rosa, E.A.; Ferreira-Cardoso, J.V. Composition of European chestnut (Castanea sativa Mill.) and association with health effects: Fresh and processed products. J. Sci. Food Agric. 2010, 90, 1578–1589. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lin, Q.; Feng, Y.; Fan, X.; Zou, F.; Yuan, D.Y.; Zeng, X.; Cao, H. Transcriptomic identification and expression of starch and sucrose metabolism genes in the seeds of Chinese chestnut (Castanea mollissima). J. Agric. Food Chem. 2015, 63, 929–942. [Google Scholar] [CrossRef] [PubMed]
- Desmaison, A.M.; Tixier, M. Acides amines libres de chataignes provenant de Castanea sativa Mill., Castanea crenata Sieb et Zucc.; Castanea molissima Bl. et d’hybrides: Castanea crenata × Castanea sativa. Ann. Pharm. Fr. 1984, 42, 353–357. [Google Scholar]
- Michaeli, S.; Fromm, H. Closing the loop on the GABA shunt in plants: Are GABA metabolism and signaling entwined? Front. Plant Sci. 2015, 6, 419. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Shah, N.P. High γ-aminobutyric acid production from lactic acid bacteria: Emphasis on Lactobacillus brevis as a functional dairy starter. Crit. Rev. Food Sci. Nutr. 2017, 57, 3661–3672. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Liu, K.; Gu, Z.; Yang, R. Enhanced γ-aminobutyric acid accumulation, alleviated componential deterioration and technofunctionality loss of germinated wheat by hypoxia stress. Food Chem. 2018, 269, 473–479. [Google Scholar] [CrossRef]
- Greggains, V.; Finch-Savage, W.; Quick, W.; Atherton, N. Putative desiccation tolerance mechanisms in orthodox and recalcitrant seeds of the genus Acer. Seed Sci. Res. 2000, 10, 317–332. [Google Scholar] [CrossRef]
- Fait, A.; Angelovici, R.; Less, H.; Ohad, I.; Urbanczyk-Wochniak, E.; Fernie, A.R.; Galili, G. Arabidopsis seed development and germination is associated with temporally distinct metabolic switches. Plant Physiol. 2006, 142, 839–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angelovici, R.; Fait, A.; Fernie, A.R.; Galili, G. A seed high-lysine trait is negatively associated with the TCA cycle and slows down Arabidopsis seed germination. New Phytol. 2011, 189, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Peter, K.; Georg, H.; Herbert, W.; Michael, R. Changes of folates, dietary fiber, and proteins in wheat as affected by germination. J. Agric. Food Chem. 2007, 55, 4678–4683. [Google Scholar]
- Joshi, P.; Varma, K. Effect of germination and dehulling on the nutritive value of soybean. Nutr. Food Sci. 2016, 46, 595–603. [Google Scholar] [CrossRef]
- Sharma, S.; Saxena, D.C.; Riar, C.S. Analysing the effect of germination on phenolics, dietary fibres, minerals and γ-aminobutyric acid contents of barnyard millet (Echinochloa frumentaceae). Food Biosci. 2016, 13, 60–68. [Google Scholar] [CrossRef]
- Kim, M.; Kwak, H.; Kim, S. Effects of germination on protein, γ-aminobutyric acid, phenolic acids, and antioxidant capacity in wheat. Molecules 2018, 23, 2244. [Google Scholar] [CrossRef] [Green Version]
- AL-Quraan, N.A.; AL-Ajlouni, Z.I.; Obedat, D.I. The GABA shunt pathway in germinating seeds of wheat (Triticum aestivum L.) and barley (Hordeum vulgare L.) under salt stress. Seed Sci. Res. 2019, 29, 1–11. [Google Scholar] [CrossRef]
- Zhao, G.C.; Xie, M.X.; Wang, Y.C.; Li, J.Y. Molecular mechanisms underlying γ-aminobutyric acid (GABA) accumulation in giant embryo rice seeds. J. Agric. Food Chem. 2017, 65, 4883–4889. [Google Scholar] [CrossRef]
- Sheng, Y.; Xiao, H.; Guo, C.; Wu, H.; Wang, X. Effects of exogenous gamma-aminobutyric acid on α-amylase activity in the aleurone of barley seeds. Plant Physiol. Biochem. 2018, 127, 39. [Google Scholar] [CrossRef]
- Shi, Z.; Shi, S.Q.; Zhong, C.F.; Yao, H.J.; Gao, R.F. Effect on germination and respiration of haloxylon ammodendron seeds by the application of exogenous Glu and GABA. Acta Bot. Boreal. Occident. Sin. 2008, 28, 1455–1460. [Google Scholar]
- Shi, S.Q.; Zheng, S.; Jiang, Z.P.; Qi, L.W.; Sun, X.M.; Li, C.X.; Liu, J.F.; Xiao, W.F.; Zhang, S.G. Effects of exogenous GABA on gene expression of Caragana intermedia roots under NaCl stress: Regulatory roles for H2O2 and ethylene production. Plant Cell Environ. 2010, 33, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Yue, J.; Xie, T.; Chen, W.; Du, C.; Chang, E.; Chen, L.; Jiang, Z.; Shi, S. Roles of γ-aminobutyric acid on salinity-responsive genes at transcriptomic level in poplar: Involving in abscisic acid and ethylene-signalling pathways. Planta 2018, 248, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Li, Z.; Liang, L.; Cao, Y.; Zeng, W.; Zhang, X.; Ma, X.; Huang, L.; Nie, G.; Liu, W. The γ-aminobutyric acid (GABA) alleviates salt stress damage during seeds germination of white clover associated with Na+/K+ transportation, dehydrins accumulation, and stress-related genes expression in white clover. Int. J. Mol. Sci. 2018, 19, 2520. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, P.; Wang, M.; Sun, M.; Gu, Z.; Yang, R. GABA mediates phenolic compounds accumulation and the antioxidant system enhancement in germinated hulless barley under NaCl stress. Food Chem. 2019, 270, 593–601. [Google Scholar] [CrossRef]
- Han, S.; Nan, Y.; Qu, W.; He, Y.; Ban, Q.; Lv, Y.; Rao, J. Exogenous γ-aminobutyric acid (GABA) treatment contributes to regulation of malate metabolism and ethylene synthesis in apple fruit during storage. J. Agric. Food Chem. 2018, 66, 13473–13482. [Google Scholar] [CrossRef]
- Leprince, O.; Buitink, J.; Hoekstra, F.A. Axes and cotyledons of recalcitrant seeds of Castanea sativa Mill. Exhibit contrasting responses of respiration to drying in relation to desiccation sensitivity. J. Exp. Bot. 1999, 388, 1515–1524. [Google Scholar] [CrossRef]
- Wang, G.; Liang, L.; Zong, Y. Controlling of dormancy and sprouting of stored chestnut by means of temperature. Sci. Silvae Sin. 1999, 35, 29–33. [Google Scholar]
- Obroucheva, N.; Sinkevich, I.; Lityagina, S. Physiological aspects of seed recalcitrance: A case study on the tree Aesculus hippocastanum. Tree Physiol. 2016, 36, 1127–1150. [Google Scholar] [CrossRef] [Green Version]
- Karadeniz, T.; Zeki Bostan, S.; Islam, A. The effects of different applications on seed germination in chestnut. Acta Hort. 2013, 981, 461–463. [Google Scholar] [CrossRef]
- Soylu, A.; Eris, A.; Özgür, M.; Dalkiliç, Z. Researches on the rootstock potentiality of chestnut types (Castanea sativa Mill.) grown in Marmara region. Acta Hort. 1999, 494, 213–222. [Google Scholar] [CrossRef]
- Soylu, A.; Serdar, U. Rootstock selection on chestnut (Castanea sativa Mill.) in the middle of black sea region in Turkey. Acta Hort. 2000, 538, 483–487. [Google Scholar] [CrossRef]
- Chen, H.; Ruan, J.; Chu, P.; Fu, W.; Liang, Z.; Li, Y.; Tong, J.; Xiao, L.; Liu, J.; Li, C.; et al. AtPER1 enhances primary seed dormancy and reduces seed germination by suppressing the ABA catabolism and GA biosynthesis in Arabidopsis seeds. Plant J. 2020, 101, 310–323. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Sun, Q.; Xiao, S. Studies on dormancy and germination of China chestnut seeds. Chin. Agric. Sci. Bull. 1998, 14, 24–25, 28. [Google Scholar]
- Roach, T.; Beckett, R.P.; Minibayeva, F.V.; Colville, L.; Whitaker, C.; Chen, H.; Bailly, C.; Kranner, I. Extracellular superoxide production, viability and redox poise in response to desiccation in recalcitrant Castanea sativa seeds. Plant Cell Environ. 2010, 33, 59–75. [Google Scholar]
- Desmaison, A.M.; Tixier, M. Amino acids content in germinating seeds and seedlings from Castanea sativa L. Plant Physiol. 1986, 81, 692–695. [Google Scholar] [CrossRef] [Green Version]
- Xie, T.; Ji, J.; Chen, W.; Yue, J.; Du, C.; Sun, J.; Chen, L.; Jiang, Z.; Shi, S. GABA negatively regulates adventitious root development. J. Exp. Bot. 2020, 71, 1459–1474. [Google Scholar] [CrossRef]
- Wang, B.; Guo, X.; Zhao, P.; Ruan, M.; Yu, X.; Zou, L.; Yang, Y.; Li, X.; Deng, D.; Xiao, J.; et al. Molecular diversity analysis, drought related marker-traits association mapping and discovery of excellent alleles for 100-day old plants by EST-SSRs in cassava germplasms (Manihot esculenta Cranz). PLoS ONE 2017, 12, e0177456. [Google Scholar] [CrossRef]
- Yemm, E.W.; Willis, A.J. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Delgado, H.; Zavaleta-Mancera, H.A.; Mora-Herrera, M.E.; Vázquez-Rivera, M.; Flores-Gutiérrez, F.X.; Scott, I.M. Hydrogen peroxide increases potato tuber and stem starch content, stem diameter, and stem lignin content. Amer. J. Potato Res. 2005, 82, 279–285. [Google Scholar] [CrossRef]
- Chen, Y.; Fu, X.; Mei, X.; Zhou, Y.; Cheng, S.; Zeng, L.; Dong, F.; Yang, Z. Proteolysis of chloroplast proteins is responsible for accumulation of free amino acids in dark-treated tea (Camellia sinensis) leaves. J. Proteomics 2017, 157, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; Du, C.; Ji, J.; Xie, T.; Chen, W.; Chang, E.; Chen, L.; Jiang, Z.; Shi, S. Inhibition of α-ketoglutarate dehydrogenase activity affects adventitious root growth in poplar via changes in GABA shunt. Planta 2018, 248, 963–979. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Li, H.; Liu, T.; Polle, A.; Peng, C.; Luo, Z.B. Nitrogen metabolism of two contrasting poplar species during acclimation to limiting nitrogen availability. J. Exp. Bot. 2013, 64, 4207–4224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, H.; Zhang, X.; Ma, W.; Song, J.; Rahman, S.U.; Wang, J.; Zhang, Y. Morphological and physiological responses to cyclic drought in two contrasting genotypes of Catalpa bungei. Environ. Exp. Bot. 2017, 138, 77–87. [Google Scholar] [CrossRef]
- Mujić, I.; Agayn, V.; Živković, J.; Velić, D.; Jokić, S.; Alibabić, V.; Rekić, A. Chestnuts, a “comfort” healthy food? Acta Hort. 2010, 866, 659–665. [Google Scholar] [CrossRef]
- Bao, H.; Chen, X.; Lv, S.; Jiang, P.; Feng, J.; Fan, P.; Nie, L.; Li, Y. Virus-induced gene silencing reveals control of reactive oxygen species accumulation and salt tolerance in tomato by γ-aminobutyric acid metabolic pathway. Plant Cell Environ. 2015, 38, 600–613. [Google Scholar] [CrossRef]
- Che-Othman, M.H.; Jacoby, R.P.; Millar, A.H.; Taylor, N.L. Wheat mitochondrial respiration shifts from the tricarboxylic acid cycle to the GABA shunt under salt stress. New Phytol. 2019, 225, 1166–1180. [Google Scholar] [CrossRef]
- Palanivelu, R.; Brass, L.; Edlund, A.F.; Preuss, D. Pollen tube growth and guidance is regulated by POP2, an Arabidopsis gene that controls GABA levels. Cell 2003, 114, 47–59. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, J.M.; Singh, N.K.; Cherry, J.H.; Locy, R.D. Nitrate uptake and utilization is modulated by exogenous γ-aminobutyric acid in Arabidopsis thaliana seedlings. Plant Physiol. Biochem. 2010, 48, 443–450. [Google Scholar] [CrossRef]
- Ramesh, S.A.; Tyerman, S.D.; Xu, B.; Bose, J.; Kaur, S.; Conn, V.; Domingos, P.; Ullah, S.; Wege, S.; Shabala, S.; et al. GABA signalling modulates plant growth by directly regulating the activity of plant-specific anion transporters. Nat. Commun. 2015, 6, 7879. [Google Scholar] [CrossRef] [Green Version]
- Bown, A.W.; Shelp, B.J. Plant GABA: Not just a metabolite. Trend Plant Sci. 2016, 21, 811–813. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Favela, M.A.; Gutiérrez-Dorado, R.; Cuevas-Rodríguez, E.O.; Canizalez-Román, V.A.; Del Rosario León-Sicairos, C.; Milán-Carrillo, J.; Reyes-Moreno, C. Improvement of chia seeds with antioxidant activity, GABA, essential amino acids, and dietary fiber by controlled germination bioprocess. Plant Foods Hum. Nutr. 2017, 72, 345–352. [Google Scholar] [CrossRef]
- Sun, J.; Jia, H.; Wang, P.; Zhou, T.; Wu, Y.; Liu, Z. Exogenous gibberellin weakens lipid breakdown by increasing soluble sugars levels in early germination of zanthoxylum seeds. Plant Sci. 2019, 280, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Boter, M.; Calleja-Cabrera, J.; Carrera-Castaño, G.; Wagner, G.; Hatzig, S.V.; Snowdon, R.J.; Legoahec, L.; Bianchetti, G.; Bouchereau, A.; Nesi, N.; et al. An integrative approach to analyze seed germination in Brassica napus. Front. Plant Sci. 2019, 10, 1342. [Google Scholar] [CrossRef] [Green Version]
- Ramesh, S.A.; Tyerman, S.D.; Gilliham, M.; Xu, B. γ-Aminobutyric acid (GABA) signalling in plants. Cell Mol. Life Sci. 2017, 74, 1577–1603. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, S.A.; Kamran, M.; Sullivan, W.; Chirkova, L.; Okamoto, M.; Degryse, F.; Mclauchlin, M.; Gilliham, M.; Tyerman, S.D. Aluminium-activated malate transporters can facilitate GABA transport. Plant Cell 2018, 30, 1147–1164. [Google Scholar] [CrossRef] [Green Version]
- Pandey, S.; Kumari, A.; Shree, M.; Kumar, V.; Singh, P.; Bharadwaj, C.; Loake, G.J.; Parida, S.K.; Masakapalli, S.K.; Gupta, K.J. Nitric oxide accelerates germination via the regulation of respiration in chickpea. J. Exp. Bot. 2019, 70, 4539–4555. [Google Scholar] [CrossRef]
- Dufková, H.; Berka, M.; Luklová, M.; Rashotte, A.M.; Brzobohatý, B.; Černý, M. Eggplant germination is promoted by hydrogen peroxide and temperature in an independent but overlapping manner. Molecules 2019, 24, 4270. [Google Scholar] [CrossRef] [Green Version]
- Han, R.; Khalid, M.; Juan, J.; Huang, D. Exogenous glycine inhibits root elongation and reduces nitrate-N uptake in pak choi (Brassica campestris ssp. Chinensis L.). PLoS ONE 2018, 13, e0204488. [Google Scholar] [CrossRef] [Green Version]
- Deleu, C.; Faes, P.; Niogret, M.F.; Bouchereau, A. Effects of the inhibitor of the γ-aminobutyrate-transaminase, vinyl-γ-aminobutyrate, on development and nitrogen metabolism in Brassica napus seedlings. Plant Physiol. Biochem. 2013, 64, 60–69. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, H.; Yan, H.; Qiu, L.; Baskin, C.C. Mobilization and role of starch, protein, and fat reserves during seed germination of six wild grassland species. Front. Plant Sci. 2018, 9, 234. [Google Scholar] [CrossRef] [PubMed] [Green Version]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, C.; Chen, W.; Wu, Y.; Wang, G.; Zhao, J.; Sun, J.; Ji, J.; Yan, D.; Jiang, Z.; Shi, S. Effects of GABA and Vigabatrin on the Germination of Chinese Chestnut Recalcitrant Seeds and Its Implications for Seed Dormancy and Storage. Plants 2020, 9, 449. https://doi.org/10.3390/plants9040449
Du C, Chen W, Wu Y, Wang G, Zhao J, Sun J, Ji J, Yan D, Jiang Z, Shi S. Effects of GABA and Vigabatrin on the Germination of Chinese Chestnut Recalcitrant Seeds and Its Implications for Seed Dormancy and Storage. Plants. 2020; 9(4):449. https://doi.org/10.3390/plants9040449
Chicago/Turabian StyleDu, Changjian, Wei Chen, Yanyan Wu, Guangpeng Wang, Jiabing Zhao, Jiacheng Sun, Jing Ji, Donghui Yan, Zeping Jiang, and Shengqing Shi. 2020. "Effects of GABA and Vigabatrin on the Germination of Chinese Chestnut Recalcitrant Seeds and Its Implications for Seed Dormancy and Storage" Plants 9, no. 4: 449. https://doi.org/10.3390/plants9040449



