Chromium Morpho-Phytotoxicity
Abstract
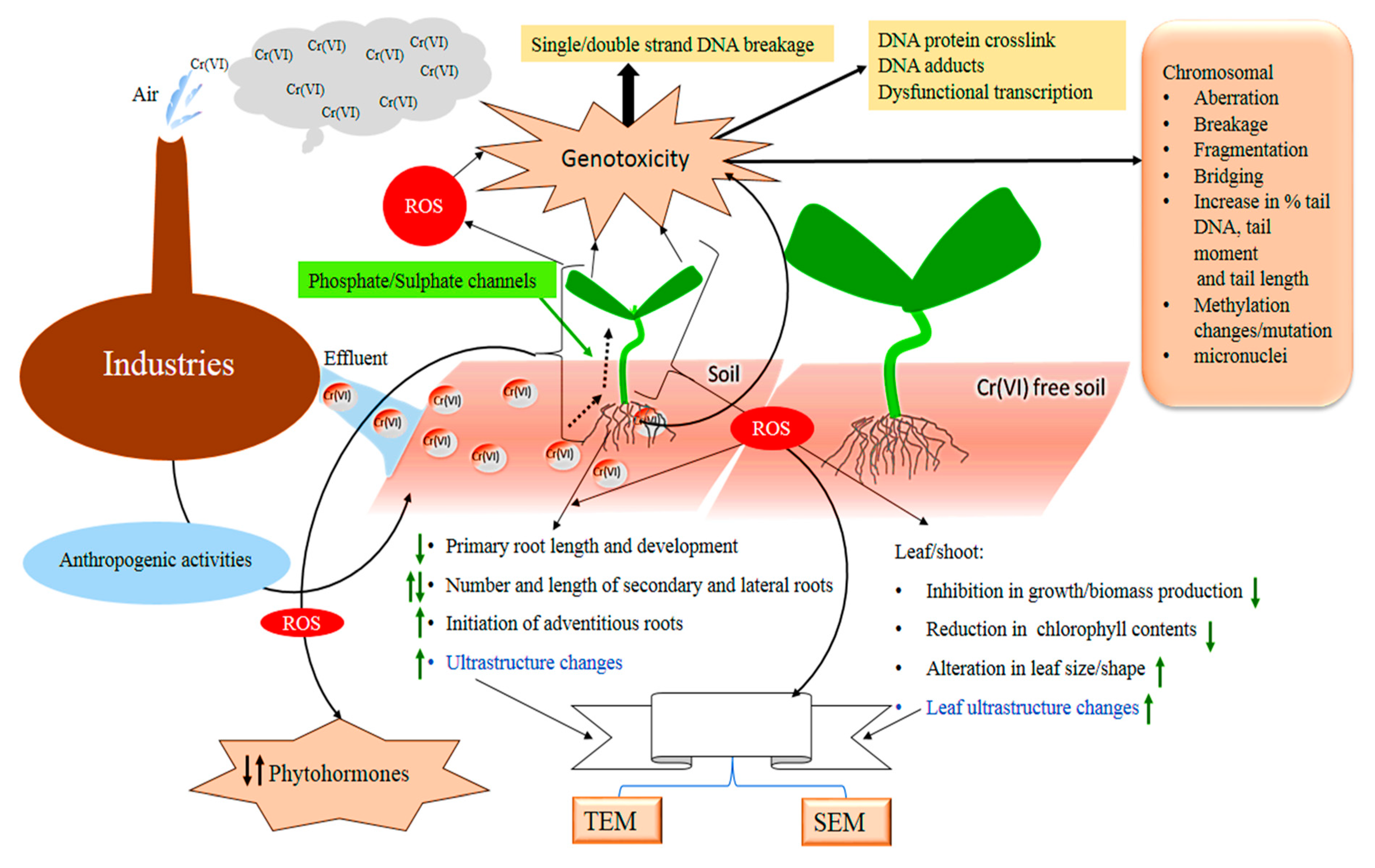
:1. Introduction
2. Chromium-Mediated Control of Seed Germination
3. Chromium-Induced Modulation of the Root Growth and Development
4. Chromium-Induced Alteration in the Shoot Growth and Development
5. Chromium Mediated Changes in Leaf Growth and Morphology
6. Chromium-Mediated Changes in Total Biomass Production in Plants
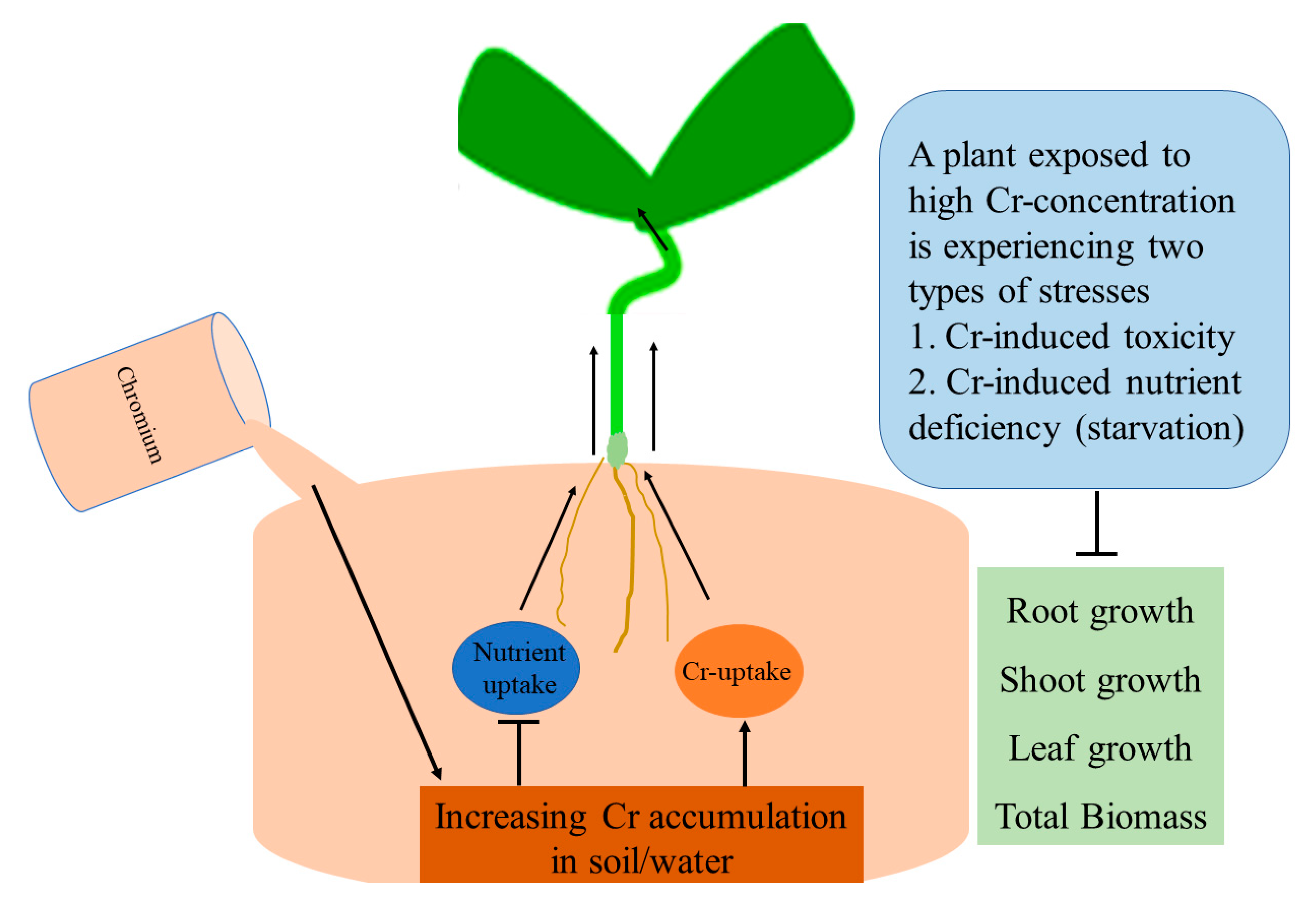
7. Chromium Interferes with the Uptake and Translocation of Macro and Micronutrients
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Oh, Y.J.; Song, H.; Shin, W.S.; Choi, S.J.; Kim, Y.-H. Effect of amorphous silica and silica sand on removal of chromium(VI) by zero-valent iron. Chemosphere 2007, 66, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Li, Y.; Dai, R.; Lan, Y. Rapid reduction of Cr(VI) coupling with efficient removal of total chromium in the coexistence of Zn(0) and silica gel. J. Hazard. Mater. 2012, 243, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Shamshad, S.; Rafiq, M.; Khalid, S.; Bibi, I.; Niazi, N.K.; Dumat, C.; Rashid, M.I. Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant system: A review. Chemosphere 2017, 178, 513–533. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, A.; Bibi, I.; Niazi, N.K.; Ok, Y.S.; Murtaza, G.; Shahid, M.; Kunhikrishnan, A.; Li, D.; Mahmood, T. Chromium(VI) sorption efficiency of acid-activated banana peel over organo-montmorillonite in aqueous solutions. Int. J. Phytoremediat. 2017, 19, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Choppala, G.; Kunhikrishnan, A.; Seshadri, B.; Park, J.H.; Bush, R.; Bolan, N. Comparative sorption of chromium species as influenced by pH, surface charge and organic matter content in contaminated soils. J. Geochem. Explor. 2018, 184, 255–260. [Google Scholar] [CrossRef]
- Pinter, I.F.; Salomon, M.V.; Gil, R.; Mastrantonio, L.; Bottini, R.; Piccoli, P. Arsenic and trace elements in soil, water, grapevine and onion in Jachal, Argentina. Sci. Total Environ. 2018, 615, 1485–1498. [Google Scholar] [CrossRef]
- Huda, A.K.M.N.; Haque, M.A.; Zaman, R.; Swaraz, A.M.; Kabir, A.H. Silicon ameliorates chromium toxicity through phytochelatin-mediated vacuolar sequestration in the roots of Oryza sativa (L.). Int. J. Phytoremediat. 2017, 19, 246–253. [Google Scholar] [CrossRef]
- Huda, A.K.M.N.; Swaraz, A.M.; Reza, M.A.; Haque, M.A.; Kabir, A.H. Remediation of Chromium Toxicity Through Exogenous Salicylic Acid in Rice (Oryza sativa L.). Water Air Soil Pollut. 2016, 227, 278. [Google Scholar] [CrossRef]
- Ertani, A.; Mietto, A.; Borin, M.; Nardi, S. Chromium in Agricultural Soils and Crops: A Review. Water Air Soil Pollut. 2017, 228, 190. [Google Scholar] [CrossRef]
- Herrero-Latorre, C.; Barciela-Garcia, J.; Garcia-Martin, S.; Pena-Crecente, R.M. Graphene and carbon nanotubes as solid phase extraction sorbents for the speciation of chromium: A review. Anal. Chim. Acta 2018, 1002, 1–17. [Google Scholar] [CrossRef]
- Jaison, S.; Muthukumar, T. Chromium Accumulation in Medicinal Plants Growing Naturally on Tannery Contaminated and Non-contaminated Soils. Biol. Trace Elem. Res. 2017, 175, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Rani, P.; Kumar, A.; Arya, R.C. Stabilization of tannery sludge amended soil using Ricinus communis, Brassica juncea and Nerium oleander. J. Soils Sediments 2017, 17, 1449–1458. [Google Scholar] [CrossRef]
- Shen, Z.J.; Xu, D.C.; Chen, Y.S.; Zhang, Z. Heavy metals translocation and accumulation from the rhizosphere soils to the edible parts of the medicinal plant Fengdan (Paeonia ostii) grown on a metal mining area, China. Ecotoxicol. Environ. Saf. 2017, 143, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Wakeel, A.; Ali, I.; Upreti, S.; Azizullah, A.; Liu, B.; Khan, A.R.; Huang, L.; Wu, M.; Gan, Y. Ethylene mediates dichromate-induced inhibition of primary root growth by altering AUX1 expression and auxin accumulation in Arabidopsis thaliana. Plant Cell Environ. 2018, 41, 1453–1467. [Google Scholar] [CrossRef]
- Wakeel, A.; Ali, I.; Wu, M.; Kkan, A.R.; Jan, M.; Ali, A.; Liu, Y.; Ge, S.; Wu, J.; Gan, Y. Ethylene mediates dichromate-induced oxidative stress and regulation of the enzymatic antioxidant system-related transcriptome in Arabidopsis thaliana. Environ. Exp. Bot. 2019, 161, 166–179. [Google Scholar] [CrossRef]
- Castro, R.O.; Trujillo, M.M.; Bucio, J.L.; Cervantes, C. Effects of dichromate on growth and root system architecture of Arabidopsis thaliana seedlings. Plant Sci. 2007, 173, 71. [Google Scholar] [CrossRef]
- Lopez-Bucio, J.; Hernandez-Madrigal, F.; Cervantes, C.; Ortiz-Castro, R.; Carreon-Abud, Y.; Martinez-Trujillo, M. Phosphate relieves chromium toxicity in Arabidopsis thaliana plants by interfering with chromate uptake. Biometals 2014, 27, 363–370. [Google Scholar] [CrossRef]
- Martinez-Trujillo, M.; Mendez-Bravo, A.; Ortiz-Castro, R.; Hernandez-Madrigal, F.; Ibarra-Laclette, E.; Ruiz-Herrera, L.F.; Long, T.A.; Cervantes, C.; Herrera-Estrella, L.; Lopez-Bucio, J. Chromate alters root system architecture and activates expression of genes involved in iron homeostasis and signaling in Arabidopsis thaliana. Plant Mol. Biol. 2014, 86, 35–50. [Google Scholar] [CrossRef]
- Eleftheriou, E.P.; Adamakis, I.-D.S.; Panteris, E.; Fatsiou, M. Chromium-Induced Ultrastructural Changes and Oxidative Stress in Roots of Arabidopsis thaliana. Int. J. Mol. Sci. 2015, 16, 15852–15871. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Bucio, J.; Ortiz-Castro, R.; Ruiz-Herrera, L.F.; Juarez, C.V.; Hernandez-Madrigal, F.; Carreon-Abud, Y.; Martinez-Trujillo, M. Chromate induces adventitious root formation via auxin signalling and SOLITARY-ROOT/IAA14 gene function in Arabidopsis thaliana. Biometals 2015, 28, 353–365. [Google Scholar] [CrossRef]
- Xu, W.; Wang, Q.; Yang, W.; Sun, J.; Dai, T. Effect of Chromium (Cr~(6+)) Stress on Seed Germination, Anti-oxidation and Osmotic Adjustment in Seedling of Different Genotypes of Wheat. J. Triticeae Crop. 2017, 37, 1112–1119. [Google Scholar]
- Ali, I.; Wakeel, A.; Upreti, S.; Liu, D.; Azizullah, A.; Jan, M.; Ullah, W.; Liu, B.; Ali, A.; Daud, M. Effect of Bisphenol A-induced Oxidative Stress on the Ultra Structure and Antioxidant Defence System of Arabidopsis thialiana Leaves. Pol. J. Environ. Stud. 2018, 27. [Google Scholar] [CrossRef]
- Wakeel, A.; Ali, I.; Khan, A.R.; Wu, M.; Upreti, S.; Liu, D.; Liu, B.; Gan, Y. Involvement of histone acetylation and deacetylation in regulating auxin responses and associated phenotypic changes in plants. Plant Cell Rep. 2018, 37, 51–59. [Google Scholar] [CrossRef]
- Zeid, I.M. Responses of Phaseolus vulgaris to chromium and cobalt treatments. Biol. Plant. 2001, 44, 111–115. [Google Scholar] [CrossRef]
- Lopez-Luna, J.; Gonzalez-Chavez, M.C.; Esparza-Garcia, F.J.; Rodriguez-Vazquez, R. Toxicity assessment of soil amended with tannery sludge, trivalent chromium and hexavalent chromium, using wheat, oat and sorghum plants. J. Hazard. Mater. 2009, 163, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Bautista, O.V.; Fischer, G.; Cárdenas, J.F. Cadmium and chromium effects on seed germination and root elongation in lettuce, spinach and Swiss chard. Agronomía Colombiana 2013, 31, 48–57. [Google Scholar]
- Handa, N.; Kohli, S.K.; Thukral, A.K.; Bhardwaj, R.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P. Protective role of selenium against chromium stress involving metabolites and essential elements in Brassica juncea L. seedlings. 3 Biotech 2018, 8, 66. [Google Scholar] [CrossRef]
- Hou, J.; Liu, G.-N.; Xue, W.; Fu, W.-J.; Liang, B.-C.; Liu, X.-H. Seed germination, root elongation, root-tip mitosis, and micronucleus induction of five crop plants exposed to chromium in fluvo-aquic soil. Environ. Toxicol. Chem. 2014, 33, 671–676. [Google Scholar] [CrossRef]
- Dotaniya, M.L.; Meena, V.D.; Das, H. Chromium toxicity on seed germination, root elongation and coleoptile growth of pigeon pea (Cajanus cajan). Legume Res. 2014, 37, 227–229. [Google Scholar] [CrossRef]
- Xu, F.; Deng, J. Effects of Salicylic Acid on Germination of Soybean Seed under Chromium Stress. Soybean Sci. 2012, 31, 852–854. [Google Scholar]
- Shinwari, K.I.; Jan, M.; Shah, G.; Khattak, S.R.; Urehman, S.; Daud, M.K.; Naeem, R.; Jamil, M. Seed priming with salicylic acid induces tolerance against chromium (VI) toxicity in rice (Oryza sativa L.). Pak. J. Bot. 2015, 47, 161–170. [Google Scholar]
- Dotaniya, M.L.; Das, H.; Meena, V.D. Assessment of chromium efficacy on germination, root elongation, and coleoptile growth of wheat (Triticum aestivum L.) at different growth periods. Environ. Monit. Assess. 2014, 186, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Mallick, S.; Sinam, G.; Mishra, R.K.; Sinha, S. Interactive effects of Cr and Fe treatments on plants growth, nutrition and oxidative status in Zea mays L. Ecotoxicol. Environ. Saf. 2010, 73, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Qing, X.; Zhao, X.; Hu, C.; Wang, P.; Zhang, Y.; Zhang, X.; Wang, P.; Shi, H.; Jia, F.; Qu, C. Selenium alleviates chromium toxicity by preventing oxidative stress in cabbage (Brassica campestris L. ssp Pekinensis) leaves. Ecotoxicol. Environ. Saf. 2015, 114, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Gill, R.A.; Zhang, N.; Ali, B.; Farooq, M.A.; Xu, J.; Gill, M.B.; Mao, B.; Zhou, W. Role of exogenous salicylic acid in regulating physio-morphic and molecular changes under chromium toxicity in black- and yellow- seeded Brassica napus L. Environ. Sci. Pollut. Res. 2016, 23, 20483–20496. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, X.; Liu, Y.; Wei, J.; Shen, W.; Shen, Z.; Cui, J. Hemin-mediated alleviation of zinc, lead and chromium toxicity is associated with elevated photosynthesis, antioxidative capacity; suppressed metal uptake and oxidative stress in rice seedlings. Plant Growth Regul. 2017, 81, 253–264. [Google Scholar] [CrossRef]
- Adrees, M.; Ali, S.; Iqbal, M.; Bharwana, S.A.; Siddiqi, Z.; Farid, M.; Ali, Q.; Saeed, R.; Rizwan, M. Mannitol alleviates chromium toxicity in wheat plants in relation to growth, yield, stimulation of anti-oxidative enzymes, oxidative stress and Cr uptake in sand and soil media. Ecotoxicol. Environ. Saf. 2015, 122, 1–8. [Google Scholar] [CrossRef]
- Lopez-Luna, J.; Silva-Silva, M.J.; Martinez-Vargas, S.; Mijangos-Ricardez, O.F.; Gonzalez-Chavez, M.C.; Solis-Dominguez, F.A.; Cuevas-Diaz, M.C. Magnetite nanoparticle (NP) uptake by wheat plants and its effect on cadmium and chromium toxicological behavior. Sci. Total Environ. 2016, 565, 941–950. [Google Scholar] [CrossRef]
- Lukina, A.O.; Boutin, C.; Rowland, O.; Carpenter, D.J. Evaluating trivalent chromium toxicity on wild terrestrial and wetland plants. Chemosphere 2016, 162, 355–364. [Google Scholar] [CrossRef]
- Ali, S.; Zeng, F.; Qiu, B.; Cai, S.; Qiu, L.; Wu, F.; Zhang, G. Interactive effects of aluminum and chromium stresses on the uptake of nutrients and the metals in barley. Soil Sci. Plant Nutr. 2011, 57, 68–79. [Google Scholar] [CrossRef]
- UdDin, I.; Bano, A.; Masood, S. Chromium toxicity tolerance of Solanum nigrum L. and Parthenium hysterophorus L. plants with reference to ion pattern, antioxidation activity and root exudation. Ecotoxicol. Environ. Saf. 2015, 113, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Jan, M.; Wakeel, A.; Azizullah, A.; Liu, B.; Islam, F.; Ali, A.; Daud, M.K.; Liu, Y.; Gan, Y. Biochemical responses and ultrastructural changes in ethylene insensitive mutants of Arabidopsis thialiana subjected to bisphenol A exposure. Ecotoxicol. Environ. Saf. 2017, 144, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, J.; Chatterjee, C. Phytotoxicity of cobalt, chromium and copper in cauliflower. Environ. Pollut. 2000, 109, 69–74. [Google Scholar] [CrossRef]
- Dubey, S.; Misra, P.; Dwivedi, S.; Chatterjee, S.; Bag, S.K.; Mantri, S.; Asif, M.H.; Rai, A.; Kumar, S.; Shri, M.; et al. Transcriptomic and metabolomic shifts in rice roots in response to Cr (VI) stress. BMC Genom. 2010, 11, 648. [Google Scholar] [CrossRef] [Green Version]
- Dube, B.K.; Tewari, K.; Chatterjee, J.; Chatterjee, C. Excess chromium alters uptake and translocation of certain nutrients in citrullus. Chemosphere 2003, 53, 1147–1153. [Google Scholar] [CrossRef]
- Mahmud, J.A.L.; Hasanuzzaman, M.; Nahar, K.; Rahman, A.; Hossain, M.S.; Fujita, M. gamma-aminobutyric acid (GABA) confers chromium stress tolerance in Brassica juncea L. by modulating the antioxidant defense and glyoxalase systems. Ecotoxicology 2017, 26, 675–690. [Google Scholar] [CrossRef]
- Shanker, A.K.; Cervantes, C.; Loza-Tavera, H.; Avudainayagam, S. Chromium toxicity in plants. Environ. Int. 2005, 31, 739–753. [Google Scholar] [CrossRef]
- Singh, M.; Kushwaha, B.K.; Singh, S.; Kumar, V.; Singh, V.P.; Prasad, S.M. Sulphur alters chromium (VI) toxicity in Solarium melongena seedlings: Role of sulphur assimilation and sulphur-containing antioxidants. Plant Physiol. Biochem. 2017, 112, 183–192. [Google Scholar] [CrossRef]
- Cervantes, C.; Campos-Garcia, J.; Devars, S.; Gutierrez-Corona, F.; Loza-Tavera, H.; Torres-Guzman, J.C.; Moreno-Sanchez, R. Interactions of chromium with microorganisms and plants. Fems Microbiol. Rev. 2001, 25, 335–347. [Google Scholar] [CrossRef]
- Singh, H.P.; Mahajan, P.; Kaur, S.; Batish, D.R.; Kohli, R.K. Chromium toxicity and tolerance in plants. Environ. Chem. Lett. 2013, 11, 229–254. [Google Scholar] [CrossRef]
- Bashri, G.; Parihar, P.; Singh, R.; Singh, S.; Singh, V.P.; Prasad, S.M. Physiological and biochemical characterization of two Amaranthus species under Cr(VI) stress differing in Cr(VI) tolerance. Plant Physiol. Biochem. 2016, 108, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Ashfaque, F.; Inam, A.; Inam, A.; Iqbal, S.; Sahay, S. Response of silicon on metal accumulation, photosynthetic inhibition and oxidative stress in chromium-induced mustard (Brassica juncea L.). S. Afr. J. Bot. 2017, 111, 153–160. [Google Scholar] [CrossRef]
- Afshan, S.; Ali, S.; Bharwana, S.A.; Rizwan, M.; Farid, M.; Abbas, F.; Ibrahim, M.; Mehmood, M.A.; Abbasi, G.H. Citric acid enhances the phytoextraction of chromium, plant growth, and photosynthesis by alleviating the oxidative damages in Brassica napus L. Environ. Sci. Pollut. Res. 2015, 22, 11679–11689. [Google Scholar] [CrossRef] [PubMed]
- Balal, R.M.; Shahid, M.A.; Vincent, C.; Zotarelli, L.; Liu, G.; Mattson, N.S.; Rathinasabapathi, B.; Martinez-Nicolas, J.J.; Garcia-Sanchez, F. Kinnow mandarin plants grafted on tetraploid rootstocks are more tolerant to Cr-toxicity than those grafted on its diploids one. Environ. Exp. Bot. 2017, 140, 8–18. [Google Scholar] [CrossRef]
- Li, S.; Huang, H.; Li, Z.; Li, Z.; He, Z.; Liang, H. Chromium removal capability and photosynthetic characteristics of Cyperus alternifolius and Coix lacryma-jobi L. in vertical flow constructed wetland treated with hexavalent chromium bearing domestic sewage. Water Sci. Technol. 2017, 76, 2203–2212. [Google Scholar] [CrossRef]
- Ali, S.; Farooq, M.A.; Yasmeen, T.; Hussain, S.; Arif, M.S.; Abbas, F.; Bharwana, S.A.; Zhang, G. The influence of silicon on barley growth, photosynthesis and ultra-structure under chromium stress. Ecotoxicol. Environ. Saf. 2013, 89, 66–72. [Google Scholar] [CrossRef]
- Reale, L.; Ferranti, F.; Mantilacci, S.; Corboli, M.; Aversa, S.; Landucci, F.; Baldisserotto, C.; Ferroni, L.; Pancaldi, S.; Venanzoni, R. Cyto-histological and morpho-physiological responses of common duckweed (Lemna minor L.) to chromium. Chemosphere 2016, 145, 98–105. [Google Scholar] [CrossRef]
- Tiwari, K.K.; Dwivedi, S.; Singh, N.K.; Rai, U.N.; Tripathi, R.D. Chromium (VI) induced phytotoxicity and oxidative stress in pea (Pisum sativum L.): Biochemical changes and translocation of essential nutrients. J. Environ. Biol. 2009, 30, 389–394. [Google Scholar]
- Tiwari, K.K.; Singh, N.K.; Rai, U.N. Chromium Phytotoxicity in Radish (Raphanus sativus): Effects on Metabolism and Nutrient Uptake. Bull. Environ. Contam. Toxicol. 2013, 91, 339–344. [Google Scholar] [CrossRef]
- Dong, J.; Wu, F.; Huang, R.; Zang, G. A chromium-tolerant plant growing in Cr-contaminated land. Int. J. Phytoremediat. 2007, 9, 167–179. [Google Scholar] [CrossRef]
- Vernay, P.; Gauthier-Moussard, C.; Hitmi, A. Interaction of bioaccumulation of heavy metal chromium with water relation, mineral nutrition and photosynthesis in developed leaves of Lolium perenne L. Chemosphere 2007, 68, 1563–1575. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.C.; Moutinho-Pereira, J.; Correia, C.; Monteiro, C.; Araujo, M.; Brueggemann, W.; Santos, C. Physiological mechanisms to cope with Cr(VI) toxicity in lettuce: Can lettuce be used in Cr phytoremediation? Environ. Sci. Pollut. Res. 2016, 23, 15627–15637. [Google Scholar] [CrossRef] [PubMed]
- Sundaramoorthy, P.; Chidambaram, A.; Ganesh, K.S.; Unnikannan, P.; Baskaran, L. Chromium stress in paddy: (i) Nutrient status of paddy under chromium stress; (ii) Phytoremediation of chromium by aquatic and terrestrial weeds. Comptes Rendus Biol. 2010, 333, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Qiu, B.; Ali, S.; Zhang, G. Genotypic differences in nutrient uptake and accumulation in rice under chromium stress. J. Plant Nutr. 2010, 33, 518–528. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Singh, V.P.; Prasad, S.M.; Chauhan, D.K.; Dubey, N.K. Silicon nanoparticles (SiNp) alleviate chromium (VI) phytotoxicity in Pisum sativum (L.) seedlings. Plant Physiol. Biochem. 2015, 96, 189–198. [Google Scholar] [CrossRef]
- Gautam, M.; Singh, A.K.; Johri, R.M. Effect of chromium toxicity on growth, chlorophyll and some macronutrients of Solanum lycopersicum and Solanum melongena. Indian J. Agric. Sci. 2014, 84, 1115–1123. [Google Scholar]
| Plant Species | Common Name | Chromium Concentration | Medium | Time of Exposure (Days) | Seed Germination (%) | References |
|---|---|---|---|---|---|---|
| Avena sativa | Oat | 500 mg/kg Cr(VI) 2000 mg/kg Cr(III) | Soil | 7 | ≈82 ≈95 | [25] |
| Beta vulgaris | Swiss chard | 50 µM Cr(III) | Distilled water | 12 | 71 | [26] |
| Brassica juncea | Mustard | 300 µM Cr(VI) | ½-strength Hoagland | 3 | 80.8 | [27] |
| Brassica oleracea | Cabbage | 300 mg/kg Cr(VI) | Distilled water | 3 | ≈65 | [28] |
| Cajanus cajan | Pigeon Pea | 100 ppm | Distilled water | 3 | 93 | [29] |
| Cucumis sativus | Cucumber | 300 mg/kg Cr(VI) | Distilled water | 3 | ≈96 | [28] |
| Glycine max | Soybean | 200 mg/L Cr(VI) | Hydroponic | - | 72.6 | [30] |
| Lactuca sativa | Lettuce | 300 mg/kg Cr(VI) | Distilled water | 3 | ≈50 | [28] |
| Lactuca sativa | Lettuce | 50 µM Cr(III) | Distilled water | 12 | 94 | [26] |
| Oryza sativa | Rice | 100 µM Cr(VI) | Distilled water | 4 | ≈50 | [31] |
| Sorghum bicolor | Sorghum | 500 mg/kg Cr(VI) 2000 mg/kg Cr(III) | Soil | 7 | ≈60 ≈10 | [25] |
| Spinacia oleracea | Spinach | 50 µM Cr(III) | Distilled water | 15 | 64 | [26] |
| Triticum aestivum | Wheat | 100 ppm 300 mg/kg Cr(VI) 500 mg/kg Cr(VI) 2000 mg/kg Cr(III) | Distilled water Distilled water Soil | 0.17 3 7 | 63 ≈90 ≈70 ≈25 | [32] [28] [25] |
| Zea mays | Corn | 300 mg/kg Cr(VI) | Distilled water | 3 | ≈99 | [28] |
| Plant Species | Common Name | Chromium Concentration | Medium | Time of Exposure (Days) | Root Growth (%) | References |
|---|---|---|---|---|---|---|
| Arabidopsis thaliana | Arabidopsis | 200 µM Cr(VI) | ½ MS | 1 | 92.8 | [14] |
| Avena sativa | Oat | 500 mg/kg Cr(VI) 2000 mg/kg Cr(III) | Soil | 7 | ≈40 ≈55 | [25] |
| Brassica campestris | Cabbage | 1 mg/L Cr(VI) | ½-strength Hoagland | 21 | ≈35 FW | [34] |
| Brassica juncea | Mustard | 300 µM Cr(VI) | ½-strength Hoagland | 15 | 43.7 | [27] |
| Brassica napus | Oilseed Rape | 400 μM Cr(VI) | Hoagland’s | 6 | ≈50 | [35] |
| Brassica oleracea | Cabbage | 300 mg/kg Cr(VI) | Distilled water | 3 | ≈25 | [28] |
| Cajanus cajan | Pigeon Pea | 100 ppm | Distilled water | 10 | 32 | [29] |
| Cucumis sativus | Cucumber | 300 mg/kg Cr(VI) | Distilled water | 3 | ≈15 | [28] |
| Lactuca sativa | Lettuce | 300 mg/kg Cr(VI) | Distilled water | 3 | <10 | [28] |
| Oryza sativa | Rice | 80 µM Cr(VI) | ¼ -strength Kimura B | 7 | 78 | [36] |
| Sorghum bicolor | Sorghum | 500 mg/kg Cr(VI) 2000 mg/kg Cr(III) | Soil | 7 | ≈10 ≈30 | [25] |
| Triticum aestivum | Wheat | 500 µM Cr(VI) 10 mg/kg Cr(VI) 300 mg/kg Cr(VI) 500 mg/kg Cr(VI) 2000 mg/kg Cr(III) | Sand Quartz sand Distilled water Soil | - 7 3 7 | ≈57 ≈20 < 10 ≈10 ≈45 | [37] [38] [28] [25] |
| Zea mays | Corn | 300 mg/kg Cr(VI) 173 µM Cr(VI) | Distilled water Hydroponic | 3 7 | ≈43% ≈70% | [28] [33] |
| Plant Species | Common Name | Chromium Concentration | Medium | Time of Exposure (Days) | Shoot Growth (%) | References |
|---|---|---|---|---|---|---|
| Arabidopsis thaliana | Arabidopsis | 800 µM Cr(VI) | ½-strength MS | 2 | ≈50 FW | [15] |
| Avena sativa | Oat | 500 mg/kg Cr(VI) 2000 mg/kg Cr(III) | Soil | 7 | Reduced | [25] |
| Brassica campestris | Cabbage | 1 mg/L Cr(VI) | ½-strength Hoagland | 21 | ≈70 FW | [34] |
| Brassica juncea | Mustard | 300 µM Cr(VI) | ½-strength Hoagland | 15 | 89.1 | [27] |
| Brassica napus | Oilseed Rape | 400 μM Cr(VI) | Hoagland | 6 | 58–67 | [35] |
| Cajanus cajan | Pigeon Pea | 100 ppm | Distilled water | 10 | Reduced | [29] |
| Hordeum vulgare | Barley | 100 μM Cr(VI) | Nutrient solution | 50 | ≈7–20 DW | [40] |
| Oryza sativa | Rice | 80 µM Cr(VI) | Hydroponic | 7 | 77 | [36] |
| Parthenium hysterophorus Solanum nigrum | Santa Maria Black Nightshade | 500 µM Cr(VI) | Soil | 21 | 43 FW 65 DW 110 FW 115 DW | [41] |
| Sorghum bicolor | Sorghum | 500 mg/kg Cr(VI) 2000 mg/kg Cr(III) | Soil | 7 | Reduced | [25] |
| Triticum aestivum | Wheat | 500 µM Cr(VI) 10 mg/kg Cr(VI) | Sand Quartz sand | 7 | ≈80% ≈80% | [37] [38] |
| Zea mays | Corn | 173 µM Cr(VI) | Hydroponic | 7 | ≈80% | [33] |
| Plant Species | Common Name | Chromium Concentration | Medium | Time of Exposure (Days) | Induced Changes in Leaf Growth and Morphology | References |
|---|---|---|---|---|---|---|
| Arabidopsis thaliana | Arabidopsis | 800 μM Cr(VI) | ½-strength MS | 2 | Reduced: growth, water content (RWC), chlorophyll (chl), cell and tissue viability | [15] |
| Brassica juncea | Mustard | 300 μM Cr(VI) | Semi-hydroponic medium | 5 | Reduced: growth, RWC, and chl content | [46] |
| Brassica napus | Oilseed Rape | 400 μM Cr(VI) | Hoagland | 6 | 61%–71% Reduced biomass | [35] |
| Hordeum vulgare | Barley | 100 μM Cr(VI) | Nutrient solution | 50 | ≈62%–67% Reduced DW | [40] |
| Oryza sativa | Rice | 80 µM Cr(VI) | Hydroponic | 7 | Chlorosis | [36] |
| Zea mays | Corn | 173 µM Cr(VI) | Hydroponic | 7 | Reduced leaf number | [33] |
| Plant Species | Common Name | Chromium Concentration | Medium | Time of Exposure (Days) | Total Biomass Production (%) | References |
|---|---|---|---|---|---|---|
| Amaranthus viridis and Amaranthus cruentus | Green and Blood Amaranth | 50 μM | ½-strength Hoagland | 7 | > 50 FW ≈80 FW | [51] |
| Arabidopsis thaliana | Arabidopsis | 800 μM Cr(VI) | ½-strength MS | 2 | 50 FW 75 DW | [15] |
| Brassica juncea | Mustard | 300 μM Cr(VI) | Semi-hydroponic medium | 5 | 80–89 growth | [46] |
| Brassica juncea | Mustard | 100 µM Cr(VI) | Soil | 20 | > 50 FW and DW | [52] |
| Brassica napus | Oilseed Rape | 400 μM Cr(VI) | Hoagland | 6 | 67 DW | [35] |
| Brassica napus | Rapeseed | 500 μM Cr | Soil | 56 | 30.6 FW 28 DW | [53] |
| Citrus reticulata | Kinnow Mandarin | 750 μM Cr(VI) | Soil | 120 | 63 DW | [54] |
| Cyperus alternifolius and Coix lacryma-jobi | Umbrella Palm and Adlay Millet | 40 mg/L Cr(VI) | Soil | 120 | 77 DW 44 DW | [55] |
| Hordeum vulgare | Barley | 100 μM Cr(VI) | Quartz sand | 60 | ≈23.7DW | [56] |
| Lemna minor | Duckweed | 500 μM Cr(VI) | SIS growth medium | 7 | 60 | [57] |
| Oryza sativa | Rice | 80 µM Cr(VI) | Hydroponic | 7 | 58 | [36] |
| Parthenium hysterophorus Solanum nigrum | Santa Maria Black Nightshade | 500 µM Cr(VI) | Soil | 21 | 65.5 FW 64.DW 110 FW 106 DW | [41] |
| Solanum melongena | Eggplant | 25 µM Cr(VI) | ½-strength Hoagland | 7 | 87 FW 83 DW | [48] |
| Triticum aestivum | Wheat | 500 µM Cr(VI) | Sand Quartz sand | 7 | ≈65% | [37] |
| Zea mays | Corn | 173 µM Cr(VI) | Hydroponic | 7 | ≈85 FW | [33] |
| Plant Species | Common Name | Nutrients | Alteration in Uptake/Translocation | Reference |
|---|---|---|---|---|
| Brassica juncea | Brown Mustard | Na, K, Ca, Mg, C, H, and N | Reduced both | [27] |
| Cocos mucifera | Coconut Palm | Fe, K, Cu, Zn, Mn, and Mg | Uptake | [3] |
| Hordeum vulgare | Barley | P, K, Mg, S, Fe, Zn, Mn, and Ca | Uptake and Translocation | [40] |
| Lactuca sativa | Lettuce | K, Mg, Fe, and Zn | Uptake/translocation | [62] |
| Oryza sativa | Rice | N, P, K, Ca, Mg, Mn, Zn, Fe, and Cu | Uptake/translocation | [63,64] |
| Pisum sativum | Pea | Decreased micro and macronutrients (except S) | Uptake/translocation | [65] |
| Raphanus sativus | Radish | Fe, S, P, Zn, Mn, Cu, and B | Translocation | [59] |
| Solanum lycopersicum and Solanum melongena | Tomato and Eggplant | Affected N, P and K content | Translocation | [66] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wakeel, A.; Xu, M. Chromium Morpho-Phytotoxicity. Plants 2020, 9, 564. https://doi.org/10.3390/plants9050564
Wakeel A, Xu M. Chromium Morpho-Phytotoxicity. Plants. 2020; 9(5):564. https://doi.org/10.3390/plants9050564
Chicago/Turabian StyleWakeel, Abdul, and Ming Xu. 2020. "Chromium Morpho-Phytotoxicity" Plants 9, no. 5: 564. https://doi.org/10.3390/plants9050564
APA StyleWakeel, A., & Xu, M. (2020). Chromium Morpho-Phytotoxicity. Plants, 9(5), 564. https://doi.org/10.3390/plants9050564





