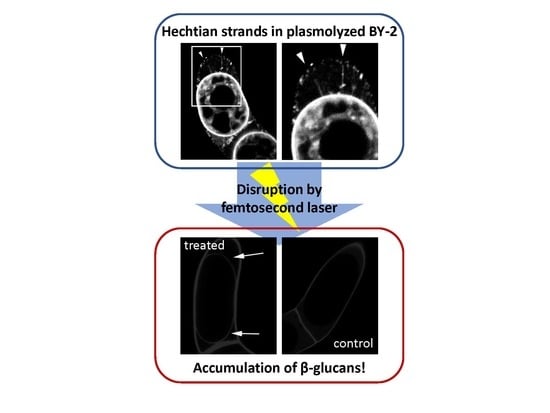
Hechtian Strands Transmit Cell Wall Integrity Signals in Plant Cells
Abstract
:1. Introduction
2. Results and Discussion
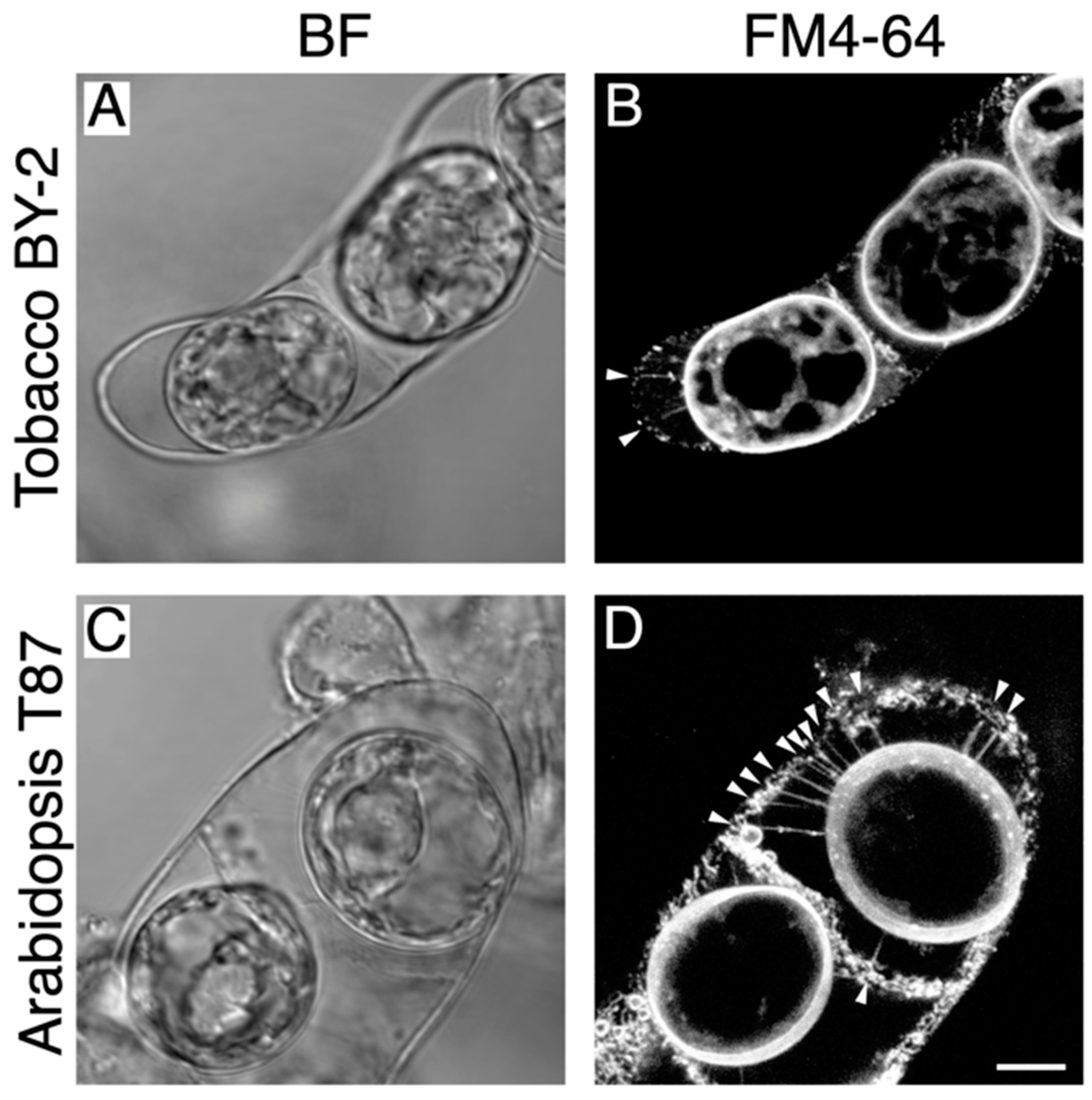
2.1. Detection of Hechtian Strands in Plasmolyzed Tobacco BY-2 Cells and Arabidopsis T87 Cells
2.2. Targeted Disruption of Hechtian Strands with a Femtosecond Laser
2.3. The Destruction of Hechtian Strands Enhances Cell Wall Damage Response
3. Materials and Methods
3.1. Plant Cell Materials and Growth Conditions
3.2. Plasmolysis Treatment
3.3. Fluorescent Dye Labeling of Cells
3.4. Confocal Laser-Scanning Microscopy and Image Processing
3.5. Intracellular Microdissection with an fs Laser Amplifier
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hecht, K. Studien über den Vorgang der Plasmolyse. Beitr. Biol. Pflanz. 1912, 11, 137–192. [Google Scholar]
- Pont-Lezica, R.F.; McNally, J.G.; Pickard, B.G. Wall-to-membrane linkers in onion epidermis: Some hypotheses. Plant Cell Environ. 1993, 16, 111–123. [Google Scholar] [CrossRef]
- Oparka, K.J.; Prior, D.A.M.; Crawford, J.W. Behaviour of plasma membrane, cortical ER and plasmodesmata during plasmolysis of onion epidermal cells. Plant Cell Environ. 1994, 44, 163–171. [Google Scholar] [CrossRef]
- Lang, I.; Barton, D.A.; Overall, R.L. Membrane-wall attachments in plasmolysed plant cells. Protoplasma 2004, 224, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Lang-Pauluzzi, I. The behaviour of the plasma membrane during plasmolysis: A study by UV microscopy. J. Microsc. 2000, 198, 188–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bachewich, C.L.; Heath, B. Differential cytoplasm-plasma membrane-cell wall adhesion patterns and their relationships to hyphal tip growth and organelle motility. Protoplasma 1997, 200, 71–86. [Google Scholar] [CrossRef]
- Volgger, M.; Lang, I.; Ovecka, M.; Lichtscheidl, I. Plasmolysis and cell wall deposition in wheat root hairs under osmotic stress. Protoplasma 2010, 243, 51–62. [Google Scholar] [CrossRef]
- Lamport, D.T.A.; Tan, L.; Held, M.A.; Kieliszewski, M.J. Pollen tube growth and guidance: Occam’s razor sharpened on a molecular arabinogalactan glycoprotein Rosetta Stone. New Phytol. 2018, 217, 491–500. [Google Scholar] [CrossRef] [Green Version]
- Johnson-Flanagan, A.M.; Singh, J. Membrane deletion during plasmolysis in hardened and non-hardened plants. Plant Cell Environ. 1986, 9, 299–305. [Google Scholar]
- Buer, C.S.; Weathers, P.J.; Swartzlander, G.A., Jr. Changes in Hechtian strands in cold-hardened cells measured by optical microsurgery. Plant Physiol. 2000, 122, 1365–1377. [Google Scholar] [CrossRef] [Green Version]
- Drake, G.; Carr, D.J.; Anderson, W.P. Plasmolysis, plasmodesmata, and the electrical coupling of oat coleoptile cells. J. Exp. Bot. 1978, 29, 1205–1214. [Google Scholar] [CrossRef]
- Cheng, X.; Lang, I.; Adeniji, O.S.; Griffing, L. Plasmolysis-deplasmolysis causes changes in endoplasmic reticulum form, movement, flow, and cytoskeletal association. J. Exp. Bot. 2017, 68, 4075–4087. [Google Scholar] [CrossRef] [Green Version]
- Knox, K.; Wang, P.; Kriechbaumer, V.; Tilsner, J.; Frigerio, L.; Sparkes, I.; Hawes, C.; Oparka, K. Putting the squeeze on plasmodesmata: A role for reticulons in primary plasmodesmata formation. Plant Physiol. 2015, 168, 1563–1572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinière, A.; Gayral, P.; Hawes, C.; Runions, J. Building bridges: formin1 of Arabidopsis forms a connection between the cell wall and the actin cytoskeleton. Plant J. 2011, 66, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Sardar, H.S.; Yang, J.; Showalter, A.M. Molecular interactions of arabinogalactan proteins with cortical microtubules and F-actin in Bright Yellow-2 tobacco cultured cells. Plant Physiol. 2006, 142, 1469–1479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, W.; Zhao, Z.D.; Hare, M.C.; Kieliszewski, M.J.; Showalter, A.M. Tomato LeAGP-1 is a plasma membrane-bound, glycosylphosphatidylinositol-anchored arabinogalactan-protein. Physiol. Plant. 2004, 120, 319–327. [Google Scholar] [CrossRef]
- Hématy, K.; Sado, P.E.; Van Tuinen, A.; Rochange, S.; Desnos, T.; Balzergue, S.; Pelletier, S.; Renou, J.P.; Höfte, H. A receptor-like kinase mediates the response of Arabidopsis cells to the inhibition of cellulose synthesis. Curr. Biol. 2007, 17, 922–931. [Google Scholar] [CrossRef] [Green Version]
- Canut, H.; Carrasco, A.; Galaud, J.P.; Cassan, C.; Bouyssou, H.; Vita, N.; Ferrara, P.; Pont-Lezica, R. High affinity RGD-binding sites at the plasma membrane of Arabidopsis thaliana links the cell wall. Plant J. 1998, 16, 63–71. [Google Scholar] [CrossRef]
- Mellersh, D.G.; Heath, M.C. Plasma membrane-cell wall adhesion is required for expression of plant defense responses during fungal penetration. Plant Cell 2001, 13, 413–424. [Google Scholar]
- Liu, Z.; Persson, S.; Sánchez-Rodríguez, C. At the border: The plasma membrane-cell wall continuum. J. Exp. Bot. 2015, 66, 1553–1563. [Google Scholar] [CrossRef]
- Lamport, D.T.A.; Tan, L.; Held, M.; Kieliszewski, M.J. The role of the primary cell wall in plant morphogenesis. Int. J. Mol. Sci. 2018, 19, 2674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cross, B.; Ronzon, F.; Roux, B.; Rieu, J.P. Measurement of the anchorage force between GPI-anchored alkaline phosphatase and supported membranes by AFM force spectroscopy. Langmuir 2005, 21, 5149–5153. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, W.; Matsunaga, S.; Fukui, K.; Itoh, K. Intracellular manipulation by femtosecond lasers: Review. J. Innov. Opt. Health Sci. 2009, 2, 1–8. [Google Scholar] [CrossRef]
- Du, X.; Wang, J.; Zhou, Q.; Zhang, L.; Wang, S.; Zhang, Z.; Yao, C. Advanced physical techniques for gene delivery based on membrane perforation. Drug Deliv. 2018, 25, 1516–1525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosokawa, Y. Applications of the femtosecond laser-induced impulse to cell research. Jpn. J. Appl. Phys. 2019, 58, 110102. [Google Scholar] [CrossRef]
- Oikawa, K.; Matsunaga, S.; Mano, S.; Kondo, M.; Yamada, K.; Hayashi, M.; Kagawa, T.; Kadota, A.; Sakamoto, W.; Higashi, S.; et al. Physical interaction between peroxisomes and chloroplasts elucidated by in situ laser analysis. Nat. Plants 2015, 1, 15035. [Google Scholar] [CrossRef]
- Rukmana, T.I.; Moran, G.; Méallet-Renault, R.; Ohtani, M.; Demura, T.; Yasukuni, R.; Hosokawa, Y. Enzyme-assisted photoinjection of megadalton molecules into intact plant cells using femtosecond laser amplifier. Sci. Rep. 2019, 9, 17530. [Google Scholar] [CrossRef]
- Guerriero, G.; Hausman, J.F.; Cai, G. No stress! relax! mechanisms governing growth and shape in plant cells. Int. J. Mol. Sci. 2014, 15, 5094–5114. [Google Scholar] [CrossRef] [Green Version]
- Yanagisawa, N.; Sugimoto, N.; Arata, H.; Higashiyama, T.; Sato, Y. Capability of tip-growing plant cells to penetrate into extremely narrow gaps. Sci. Rep. 2017, 7, 1403. [Google Scholar] [CrossRef] [Green Version]
- Manfield, I.W.; Orfila, C.; McCartney, L.; Harholt, J.; Bernal, A.J.; Scheller, H.V.; Gilmartin, P.M.; Mikkelsen, J.D.; Knox, J.P.; Willats, W.G.T. Novel cell wall architecture of isoxaben-habituated Arabidopsis suspension-cultured cells: Global transcript profiling and cellular analysis. Plant J. 2004, 40, 260–275. [Google Scholar] [CrossRef]
- Hamann, T.; Bennett, M.; Mansfield, J.; Somerville, C. Identification of cell-wall stress as a hexose-dependent and osmosensitive regulator of plant responses. Plant J. 2009, 57, 1015–1026. [Google Scholar] [CrossRef]
- Vaahtera, L.; Schulz, J.; Hamann, T. Cell wall integrity maintenance during plant development and interaction with the environment. Nat. Plants 2019, 5, 924–932. [Google Scholar] [CrossRef] [PubMed]
- Rui, Y.; Dinneny, J.R. A wall with integrity: Surveillance and maintenance of the plant cell wall under stress. New Phytol. 2020, 225, 1428–1439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohtani, M.; Morisaki, K.; Sawada, Y.; Sano, R.; Uy, A.L.T.; Yamamoto, A.; Kurata, T.; Nakano, Y.; Suzuki, S.; Matsuda, M.; et al. Primary metabolism during biosynthesis of secondary wall polymers of protoxylem vessel elements. Plant Physiol. 2006, 172, 1612–1624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawabe, H.; Ohtani, M.; Kurata, T.; Sakamoto, T.; Demura, T. Protein S-nitrosylation regulates xylem vessel cell differentiation in Arabidopsis. Plant Cell Physiol. 2018, 59, 17–29. [Google Scholar] [CrossRef]
- Abramoff, M.D.; Magalhães, P.J.; Ram, S.J. Image processing with ImageJ. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoneda, A.; Ohtani, M.; Katagiri, D.; Hosokawa, Y.; Demura, T. Hechtian Strands Transmit Cell Wall Integrity Signals in Plant Cells. Plants 2020, 9, 604. https://doi.org/10.3390/plants9050604
Yoneda A, Ohtani M, Katagiri D, Hosokawa Y, Demura T. Hechtian Strands Transmit Cell Wall Integrity Signals in Plant Cells. Plants. 2020; 9(5):604. https://doi.org/10.3390/plants9050604
Chicago/Turabian StyleYoneda, Arata, Misato Ohtani, Daisuke Katagiri, Yoichiroh Hosokawa, and Taku Demura. 2020. "Hechtian Strands Transmit Cell Wall Integrity Signals in Plant Cells" Plants 9, no. 5: 604. https://doi.org/10.3390/plants9050604
APA StyleYoneda, A., Ohtani, M., Katagiri, D., Hosokawa, Y., & Demura, T. (2020). Hechtian Strands Transmit Cell Wall Integrity Signals in Plant Cells. Plants, 9(5), 604. https://doi.org/10.3390/plants9050604