Abstract
Plant posture is controlled by various environmental cues, such as light, temperature, and gravity. The overall architecture is determined by the growth angles of lateral organs, such as roots and branches. The branch growth angle affected by gravity is known as the gravitropic setpoint angle (GSA), and it has been proposed that the GSA is determined by balancing two opposing growth components: gravitropism and anti-gravitropic offset (AGO). The molecular mechanisms underlying gravitropism have been studied extensively, but little is known about the nature of the AGO. Recent studies reported the importance of LAZY1-LIKE (LZY) family genes in the signaling process for gravitropism, such that loss-of-function mutants of LZY family genes resulted in reversed gravitropism, which we term it here as the “anti-gravitropic” phenotype. We assume that this peculiar phenotype manifests as the AGO due to the loss of gravitropism, we characterized the “anti-gravitropic” phenotype of Arabidopsis lzy multiple mutant genetically and physiologically. Our genetic interaction analyses strongly suggested that gravity-sensing cells are required for the “anti-gravitropic” phenotype in roots and lateral branches. We also show that starch-filled amyloplasts play a significant role in the “anti-gravitropic” phenotype, especially in the root of the lzy multiple mutant.
1. Introduction
Plants use various signals, such as light and gravity signals, to optimize shoot and root architecture to achieve directional organ growth. Previous studies have suggested that amyloplasts, plastids that accumulate starch, move in the direction of gravity to trigger a signaling process, known as gravitropism [1,2,3]. This starch-statolith hypothesis is supported by the reduced gravitropism of the starchless phosphoglucomutase (pgm) mutant [1,3,4]. The cell ablation studies demonstrated that root cap columella cells containing sedimentable amyloplasts are important for root gravitropism [5,6]. Thus, columella cells are considered to be major gravity sensing cells in roots. Meanwhile, additional genetic studies with a series of shoot gravitropism (sgr) mutants provided evidence for gravity-sensing cells in the Arabidopsis shoot [7]. It has been reported that sgr1 (also known as scarecrow (scr)) and sgr7 (also known as short-root (shr)) are agravitropic in the shoot, and these mutants lack endodermal cell layers in inflorescence stems [8]. The endodermal cells in Arabidopsis shoots contain amyloplasts that can fall due to gravity. Furthermore, the endodermal-amyloplast less 1 (eal1) mutant was reported as an agravitropic mutant in inflorescence stem [9]. The eal1 was mapped on the At4g37650 locus which is also known as SHR locus. The eal1 carries three nucleotides deletion which correspond to glutamate 230 and results in a single amino acid deletion of SHR [10]. The eal1 develops endodermal-like tissues without gravity sensing ability [10], while the sgr7 completely loses endodermis [8]. Thus, the eal1 is thought to be a hypomorphic mutant allele of sgr7/shr. These results indicate that endodermal cells are gravity-sensing cells in the Arabidopsis shoot [11].
Recently, key genes for gravitropism, including LAZY1 and its orthologous LAZY1-LIKE (LZY) family genes, were identified in rice and Arabidopsis [12,13]. The loss-of-function mutants of LAZY1 in rice and LZY1 in Arabidopsis resulted in reduced shoot gravitropism [12,13]. In Arabidopsis, LZY1, LZY2, and LZY3 genes are expressed in the endodermis and function redundantly in shoot gravitropism [14,15]. LZY2 and LZY3 are localized to the plasma membrane, while LZY1 is localized to the nucleus and plasma membrane [13,14,15]. Although a mutated LZY1 does not localize in the nucleus, it remains associated with shoot gravitropism, suggesting that plasma membrane-localized LZY1 is functionally important for gravitropism [13]. In root, LZY2, LZY3, and LZY4 are expressed in columella cells and function redundantly in gravitropism [14,15,16]. Recently, we reported that LZY3 is localized to the plasma membrane and changed its polar localization in response to gravi-stimulation by reorienting its roots [17]. It has been suggested that polar localized LZY3 recruits RCC1-LIKE DOMAIN (RLD) proteins to control polar auxin transport via auxin efflux carrier PIN proteins [17]. Taken together, LZY proteins likely play a central role in gravity-sensing cells to connect signals from physical amyloplast sedimentation to polar auxin transport. Asymmetrically distributed auxin then induces differential organ growth to achieve the gravitropic response.
Lateral roots and shoot branches maintain specific growth angles relative to the direction of gravity, which is known as the gravitropic setpoint angle (GSA). It has been proposed that the GSA is determined by a balance between gravitropism and anti-gravitropic offset (AGO), which is a growth component that counteracts gravitropic growth [18]. Physiological and genetic analyses suggest that auxin signaling, transport, and its regulatory control of gene expression are all involved in AGO and gravitropism [19,20]. In addition, sgr5 was identified as a gravitropism-deficient mutant, and its lateral branches tend to grow horizontally [21]. The SGR5 gene encodes a C2H2-type zinc finger protein, and it has been demonstrated that SGR5/INDETERMINATE DOMAIN15 (IDD15), IDD14, and IDD16 control auxin distribution via transcriptional control of auxin biosynthetic genes and the auxin transporter PIN1 [21,22]. Loss-of-function mutants of LZY genes have reduced gravitropism, and their shoot branches and lateral roots tend to grow horizontally [13,14,15]. Therefore, LZY family proteins appear to contribute to GSA control through gravity signaling that regulates polar auxin transport. However, in contrast to this understanding of the molecular mechanism underlying gravitropism, little is known about the mechanisms controlling AGO.
It has been reported that LZY family genes control growth direction of the primary root in Medicago truncatula and Arabidopsis [14,16,23]. Loss of function of three LZY genes, namely, LZY2, LZY3, and LZY4, reverses the growth direction of primary roots in response to gravi-stimulation [14,16,23]. This phenotype is termed “negative gravitropism” [14,16,23], but the “negative gravitropic” phenotype is not simply a mirror image of the normal positive gravitropism. Rather, the responsiveness to gravi-stimulation of primary roots of the lzy triple mutant is considerably weak [14]. Meanwhile, we reported that primary shoots of the lzy1;2;3 triple mutant are agravitropic, while its lateral branches curl downward [15]. We refer to this phenotype of reversed growth direction observed in both the primary root of the lzy2;3;4 mutant and the lateral branches of the lzy1;2;3 mutant as “anti-gravitropic.” Therefore, with respect to GSA, both “anti-gravitropic” phenotypes of primary roots and lateral branches lead us to hypothesize that the loss of LZY function results in manifestation of AGO [24].
In this study, we characterized the “anti-gravitropic” phenotype by analyzing lzy multiple mutants and gravitropism-deficient mutants in primary roots and lateral branches. Our genetic and physiological studies highlight the importance of columella and endodermal cells in roots and shoots for GSA determination. In this process, we demonstrated that starch-accumulated amyloplasts in columella cells are required for the recognition of the gravity vector to achieve both gravitropism and “anti-gravitropic” phenotypes in primary root. In shoots, while there is a marginal contribution of amyloplasts, endodermal cells remain functionally important for gravitropism and “anti-gravitropic” phenotypes.
2. Results
2.1. Amyloplast Sedimentation Leads to Directional Growth of the Primary Root
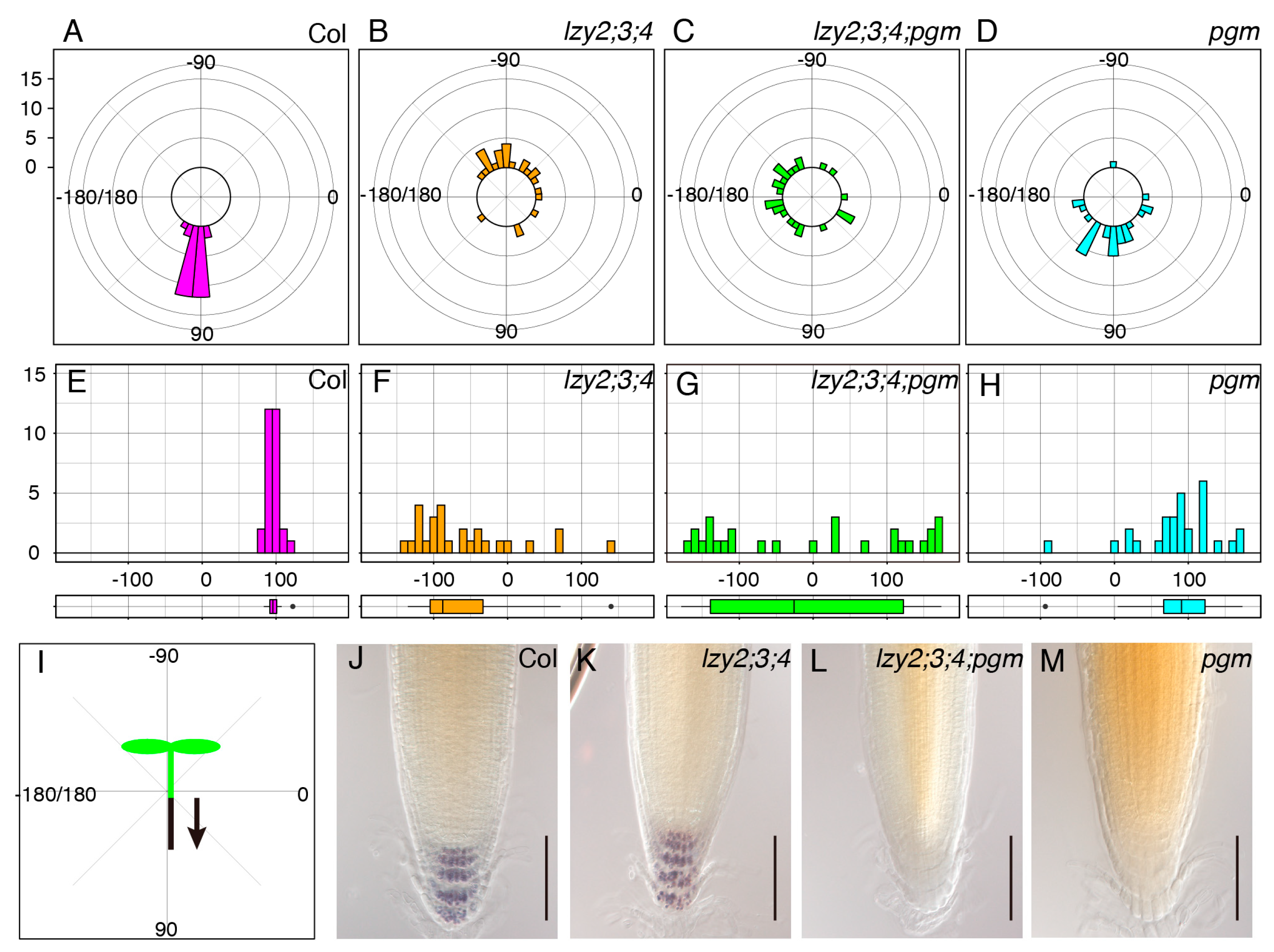
We assessed the growth direction of primary roots of 5-day-old seedlings on a vertically standing plate (Figure 1). Wild-type roots grew in the direction of gravity, but roots of the lzy2;3;4 triple mutant tended to grow upward in an “anti-gravitropic” direction, as previously reported [14,23] (Figure 1A,B,E,F,I). For gravitropism, amyloplast sedimentation in gravity-sensing cells is important to recognize the direction of gravity [2,11]. The pgm mutant is starch synthesis deficient with “light” starchless amyloplasts and partially complete root gravitropism (Figure 1D,H) [4]. To examine whether amyloplasts that accumulate starch were important for the “anti-gravitropic” phenotype of the lzy2;3;4 triple mutant, we analyzed the genetic interaction between lzy2;3;4 and pgm by generating the lzy2;3;4; pgm quadruple mutant and testing the growth direction of primary roots. Since pgm carries a mutation in the phosphoglucomutase gene which is involved in starch biosynthesis and results in starchless “light” amyloplasts development, it is an ideal mutant to investigate the contribution of starch-filled amyloplasts to “anti-gravitropic” phenotype. Interestingly, directional growth against gravity was eliminated by adding the pgm mutation in lzy2;3;4 plants (Figure 1C,G). As reported previously, starch accumulation was not observed in pgm and lzy2;3;4;pgm (Figure 1L,M), while wild-type and lzy2;3;4 accumulated starch (Figure 1J,K). These results demonstrate that pgm mutation is epistatic to the lzy2;3;4 mutant, and starch accumulation in amyloplasts is necessary for the “anti-gravitropic” phenotype of the lzy2;3;4. Therefore, gravitropic and “anti-gravitropic” growth is likely to share a similar gravity-sensing mechanism in primary roots.
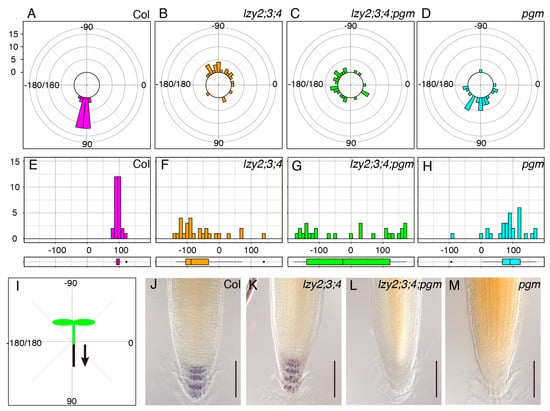
Figure 1.
Genetic analysis of growth direction of primary roots. Polar distribution of growth direction of primary root in wild-type Col (A), lzy2;3;4 (B), lzy2;3;4;pgm (C), and pgm (D). Growth angles were plotted on histograms and distributions were evaluated with boxplots from wild-type Col (E), lzy2;3;4 (F), lzy2;3;4;pgm (G), and pgm (H). Schematic representation of growth angle quantification (I). Each bar indicates the number of roots. Lugoal’s staining of primary roots of wild-type Col (J), lzy2;3;4 (K), lzy2;3;4;pgm (L), and pgm (M) at 7 days after transferring to phytocabinet. Bars indicate 100 µm.
2.2. Lateral Branch Responses to Gravi-Stimulation in the lzy1;2;3 Mutant
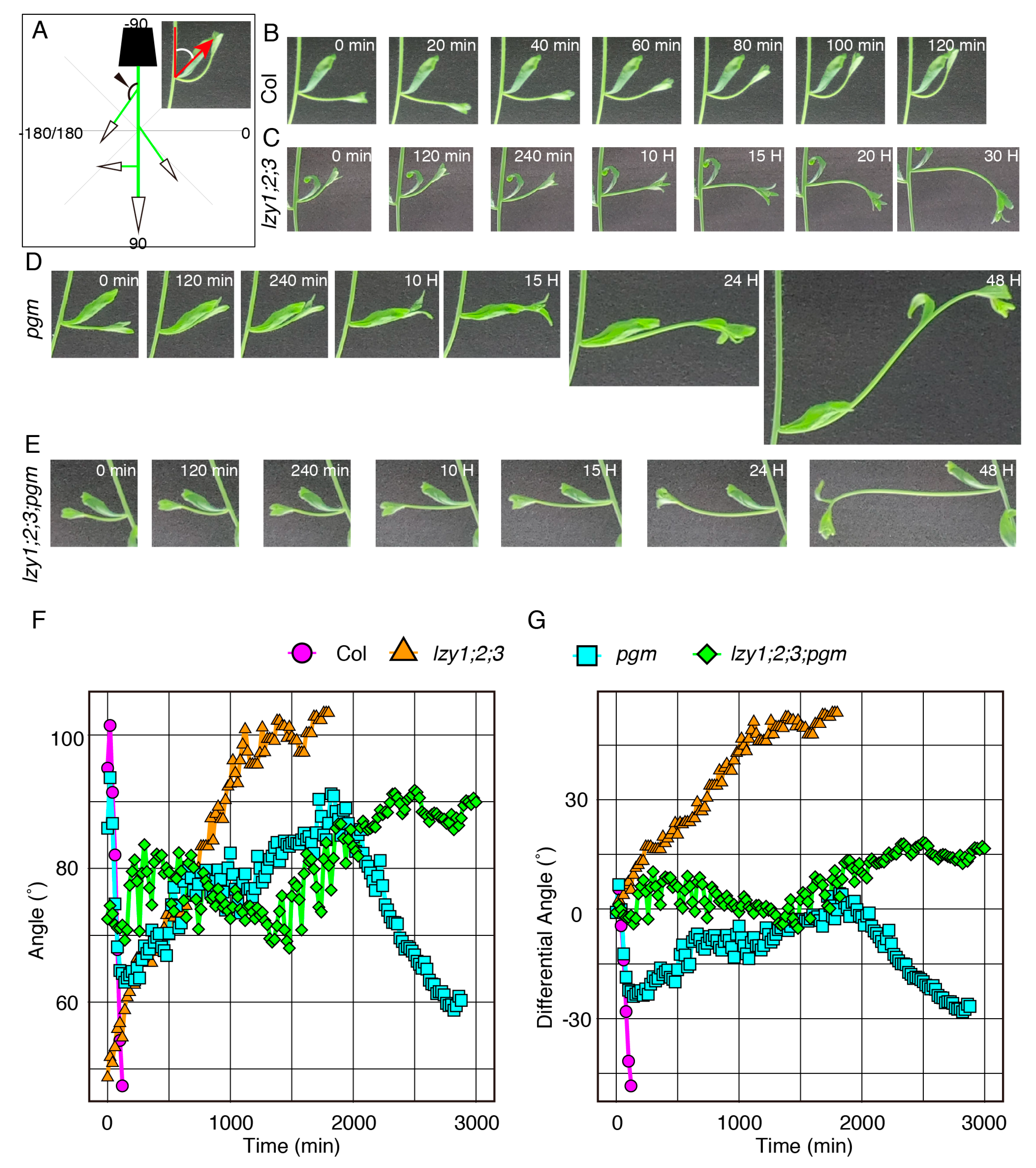
As reported previously, the lzy1;2;3 triple mutant displayed severe defects in gravitropism in the primary shoot and downward curling of lateral branches [14,15]. However, it remains unclear whether the downward-curling phenotype of the lateral branch is the result of a response to gravi-stimulation or simple epinastic growth. To address this question, we inverted 4-week-old plants containing 1.5–2 cm lateral branches and measured the growth angle of lateral branches (Figure 2A). In wild-type plants, lateral branches began growing upward within 60 min after inversion and completely changed their posture within 120 min (Figure 2B,F,G, Video S1). By contrast, the lateral branches of lzy1;2;3 gradually grew downward after inversion, and it took considerably longer to complete the posture change (Figure 2C,F,G, Video S2). This result indicates that the lateral branches of lzy1;2;3 respond to gravi-stimulation and exhibit the “anti-gravitropic” phenotype. This result also suggests that the loss of function of LZYs resulted in the “anti-gravitropic” phenotype in lateral branches, resulting in the downward curling of branches.
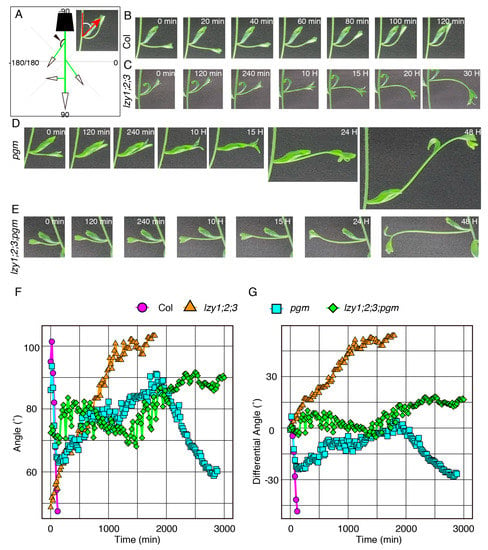
Figure 2.
The gravitropic responses of lateral branches. (A) Graphical image of the quantification of growth angles of lateral branches. Time-lapse images of lateral branches from wild-type Col (B), lzy1;2;3 (C), pgm (D), lzy1;2;3;pgm (E) plants after inversion. Immediately after inverting plants, images were obtained at 5 min intervals. Quantification of growth posture change of lateral branches during the gravitropic responses (F) and differences of growth angle from initial angles (G).
2.3. Endodermal Cells are Required for the “Anti-Gravitropic” Phenotype in Shoots
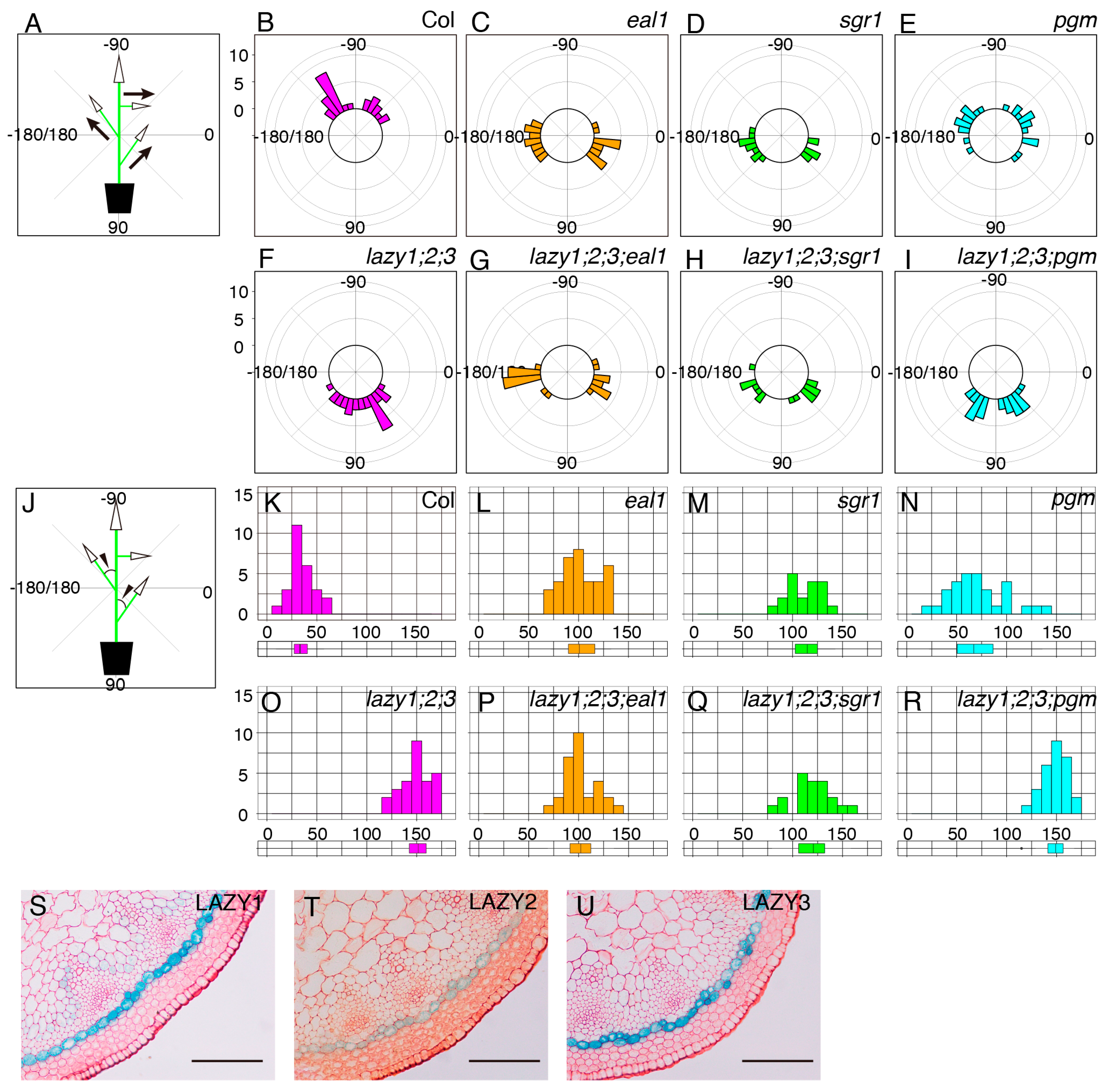
In addition to the roots, amyloplast sedimentation is thought to be important for gravity sensing in shoot gravitropism [2,9]. Therefore, we examined whether starch-accumulated amyloplasts were required for the “anti-gravitropic” phenotype of lateral branches in the lzy1;2;3 mutant. First, using the lzy1;2;3;pgm quadruple mutant, we analyzed the lateral branch phenotype. When lateral branches reached 10 cm, plants were photographed and growth angles of axillary branches were calculated (Figure 3A). Differences from vertical axis were calculated as growth angles and plotted as a histogram (Figure 3J). Lateral branches of wild-type plants tended to grow at a 30° angle (Figure 3B,K), while those of lzy1;2;3 mutants grew at an angle of around 150° (Figure 3F,O). The growth angle of lateral branches of the pgm mutant was significantly wider than the wild type, but the phenotype was much weaker than that of the lzy1;2;3 mutant (Figure 3E,N). Based on our results from the primary roots, we expected that pgm also would be epistatic to lzy1;2;3 in lateral branches. However, this does not appear to be the case with regard to the “anti-gravitropic” phenotype of lateral branches (Figure 3E,F,I,N,O,R).
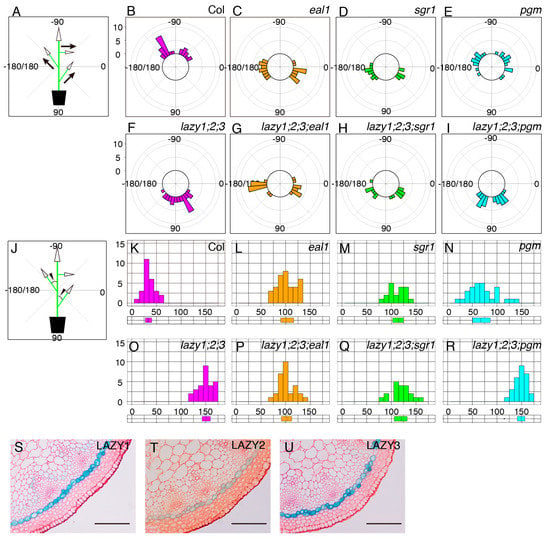
Figure 3.
Growth angles of lateral branches. Schematic representation of growth angle evaluation for posture of lateral branches (A) and differences from the vertical main axes (J). Growth angles of lateral branches from wild-type Col (B), eal1 (C), sgr1 (D), pgm (E), lzy1;2;3 (F), lzy1;2;3;eal1 (G), lzy1;2;3;sgr1 (H), and lzy1;2;3;pgm (I). Distribution of differential growth angles from the primary flower stem from wild-type Col (K), eal1 (L), sgr1 (M), pgm (N), lzy1;2;3 (O), lzy1;2;3;eal1 (P), lzy1;2;3;sgr1 (Q), and lzy1;2;3;pgm (R). Distributions were evaluated as boxplots. Expression patterns of LZY1, LZY2, and LZY3 in lateral branches. GUS staining of lateral branches expressing pLZY1:GUS (S), pLZY2:GUS (T), and pLZY3:GUS (U). Bars indicate 100 µm.
To investigate the contribution of pgm in more detail, we examined the gravitropic response of pgm and lzy1;2;3;pgm plants upon gravi-stimulation by reorientation. Lateral branches of the pgm mutant were able to respond to a new gravity vector, but it took longer to complete the gravitropic response (Figure 2D,F,G and Video S3). Although no clear difference was observed in the growth angles of lateral branches between lzy1;2;3 and lzy1;2;3;pgm under normal growth conditions (Figure 3E,F,I,N,O,R), the pgm mutation delayed the “anti-gravitropic” response in lzy1;2;3 plants upon gravi-stimulation (Figure 2E–G and Video S4). Even if the pgm has a minor impact on the lateral branch phenotype of lzy1;2;3 plants, our results suggest that pgm is an epistatic mutation to lzy1;2;3.
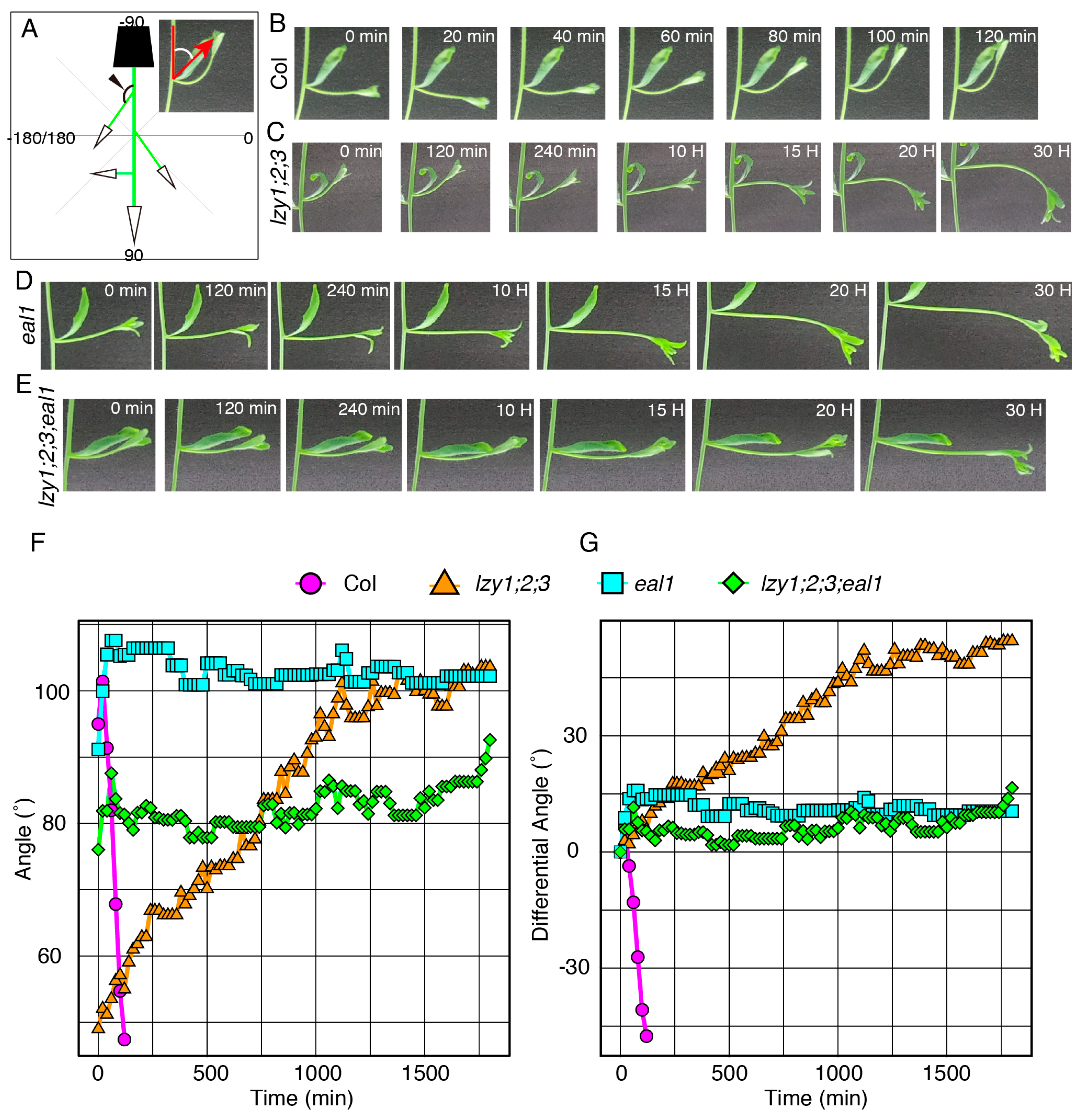
We then investigated the relationship between the endodermis and the “anti-gravitropic” phenotype in lzy1;2;3 plants. Endodermal cells where LZY genes are expressed and function are gravity-sensing cells for shoot gravitropism [8]. As has been shown in primary shoots [15], promoter activity of LZY1, LZY2, and LZY3 were detected in the endodermis of lateral branches (Figure 3S–U) and affect the growth angle phenotype. Two key transcription factors, SCR and SHR, function during development of the endodermis in Arabidopsis [25,26,27,28]. LZY genes were downregulated in the eal1 mutant, a hypomorphic allele of sgr7/shr, as well as in sgr1/scr [15]. The eal1 mutant expressed SHR with an amino acid deletion form endodermal-like tissue which lacked the ability as a gravity-sensing tissue [9,10]. In contrast to eal1, sgr1 completely lost its endodermis in the inflorescence stem [8]. Both endodermis-deficient mutants display almost completely horizontal lateral branches, with 100°–110° growth angles (Figure 3C,D,L,M). To investigate their genetic relationship, we generated quadruple mutants, lzy1;2;3;eal1 and lzy1;2;3;sgr1, and analyzed the growth angles of the lateral branches. The downward-curling phenotype observed in the lzy1;2;3 mutant was clearly suppressed by eal1 and sgr1, resulting in straight lateral branches growing horizontally (Figure 3G,H,P,Q). Moreover, lateral branches of eal1 and lzy1;2;3;eal1 plants failed to respond to gravi-stimulation by inversion (Figure 4D–G, Supplementary Movie S5,6). While eal1 and lzy1;2;3;eal1 failed to develop endodermal tissues, they developed properly in lateral branches of wild-type and lzy1;2;3 plants. Taken together, the endodermal tissue appears crucial for the normal gravitropism and “anti-gravitropic” phenotypes of lateral branches in lzy1;2;3 triple mutants, but it remains unclear whether gravitropism and “anti-gravitropic” phenotypes share a similar gravity-sensing mechanism.
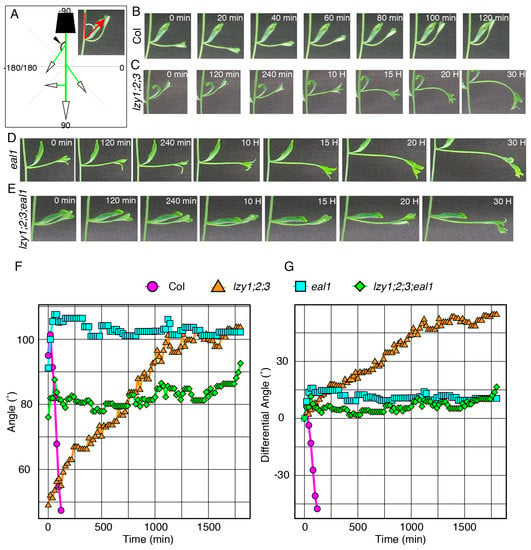
Figure 4.
The gravitropic responses of lateral branches. (A) Graphical image of the quantification of growth angles of lateral branches. Time-lapse images of lateral branches from wild-type Col (B), lzy1;2;3 (C), eal1 (D), lzy1;2;3;eal1 (E) plants after inversion. Immediately after inverting plants, images were obtained at 5 min intervals. Quantification of growth posture change of lateral branches during the gravitropic responses (F) and differences of growth angle from initial angles (G). Images of Col and lzy1;2;3 are identical to Figure 2.
2.4. Suppressor Screening of lzy2;3;4 in Primary Roots
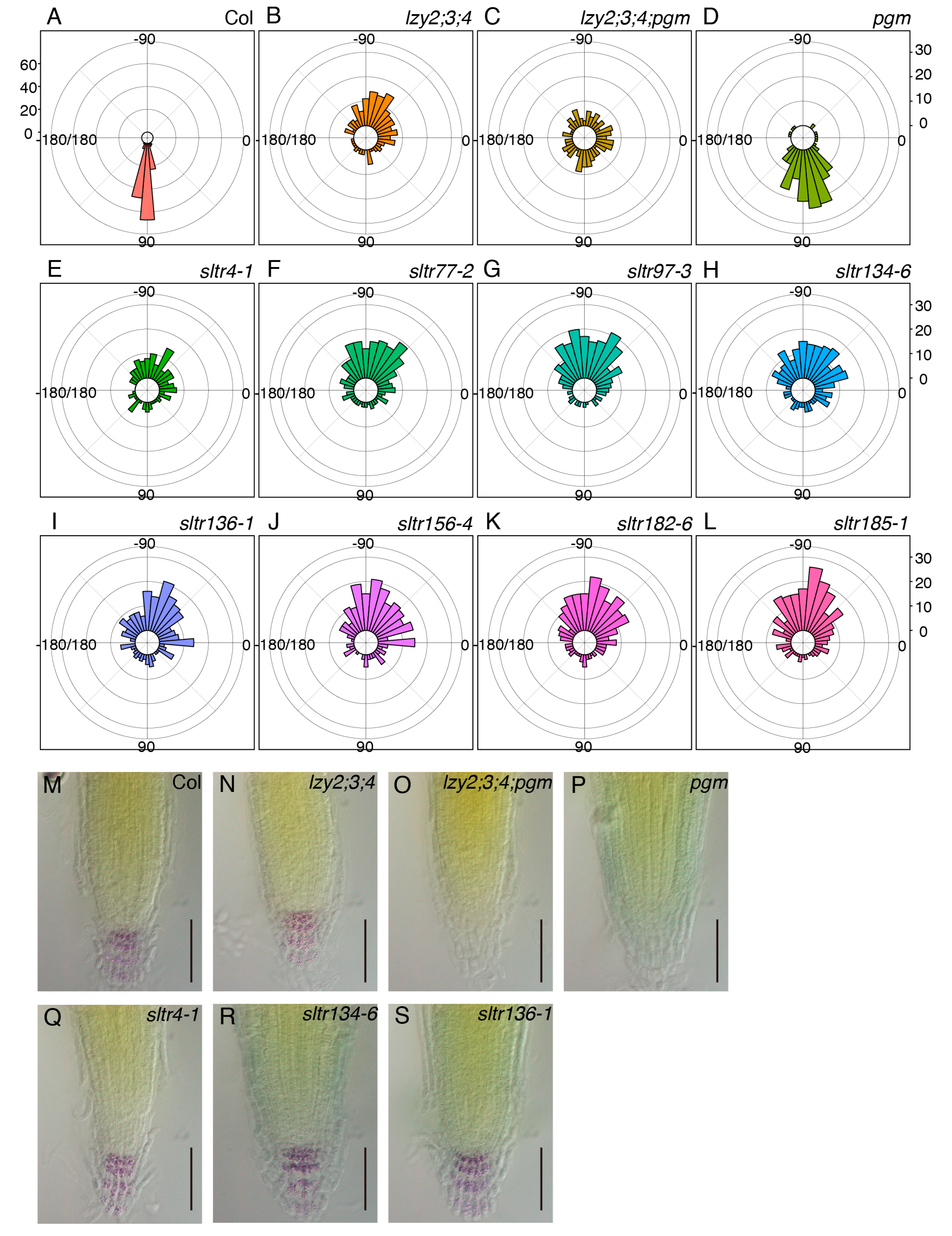
To investigate the molecular nature of the “anti-gravitropic” phenotype in the primary roots of the lzy2;3;4 mutant, plants were mutagenized and screened to isolate suppressor mutants. M2 seeds were obtained from mutagenized M1 populations and sown on 1× MS medium. In the first screening, plants that showed a primary root growing below the horizontal line (between 0° and −180°) were selected as suppressor of lazy triple in root (sltr) candidates (Supplemental Figure S1). We isolated 103 sltr candidates and obtained M3 seeds. For the second screening, we assessed the growth direction of primary roots and growth angles between the hypocotyl/root joint and root tip. Eight out of 103 sltr candidates changed their growth direction of the primary root. To test the reproducibility with larger populations, eight lines of M4 sltr seeds were sown, and we quantified their growth direction (Figure 5A–L). Although more than 50% of lzy2;3;4 roots grew between −45° and −135° (Figure 5B), the growth direction of lzy2;3;4;pgm roots occurred randomly as a consequence of the pgm mutation, with only 25% of roots between −45° and −135° (Figure 5C). Among the eight candidates, less than 50% of sltr4-1, sltr134-6, and sltr136-1 roots grew between −45° and −135° (Figure 5E,H,I). Since pgm is a sltr (Figure 1), the mutation causing starchless amyloplasts also may be a sltr. Thus, root tips of these sltr mutants were subjected to Lugoal’s staining to test whether sltr candidates were starchless. While pgm and lazy2;3;4;pgm do not accumulate starch, starch accumulation in all tested sltr mutants was observed (Figure 5M–S, Supplemental Figure S2). These results indicate that sltr4-1, sltr134-6, and sltr136-1 provide new directions for future studies on the nature of the “anti-gravitropic” phenotype in primary roots.
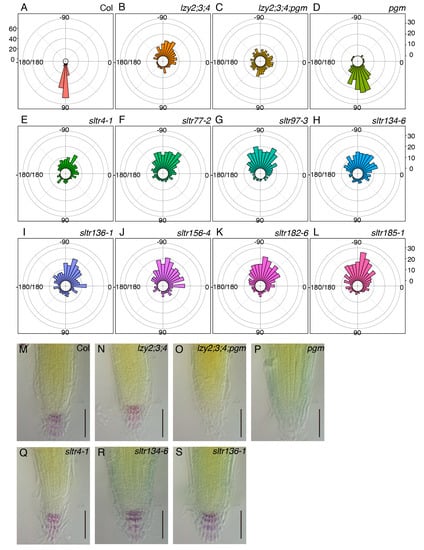
Figure 5.
Genetic screening of lzy2;3;4 suppressors. Growth direction of primary roots in wild-type Col (A), lzy2;3;4 (B), lzy2;3;4;pgm (C), and pgm (D) and sltr candidates, sltr4-1 (E), sltr77-2 (F), sltr97-3 (G), sltr134-6 (H), sltr136-1 (I), sltr156-4 (J), sltr182-6 (K), and sltr185-1 (L). More than 150 plants were evaluated for all genotypes. Bars indicate the number of plants. Lugoal’s staining of primary roots of wild-type Col (M), lzy2;3;4 (N), lzy2;3;4;pgm (O), pgm (P), sltr134-6 (Q), sltr136-1 (R), and sltr156-4 (S) at 7 days after transferring to phytocabinet. Bars indicate 100 µm.
3. Discussion
Overall plant architecture is determined by various environmental cues, such as light, temperature, and gravity. Previous studies have demonstrated that LAZY1 and LAZY1-like family genes are involved in gravitropism in rice, Arabidopsis, Medicago, and maize [12,13,14,15,29]. LZY1, LZY2, LZY3, and LZY4 facilitate gravitropism in shoots and roots, such that loss of their function is expected to cause non-responsiveness to gravi-stimulation. However, in addition to the lzy2;3;4 and lzy1;2;3 triple mutants losing normal gravitropism, they also exhibited “anti-gravitropic” phenotypes [14,15,16,23]. While LAZY1 family genes have been characterized extensively in various plant species due to their effects on gravitropism, the nature of “anti-gravitropic” phenotypes of lzy multiple mutant had not been characterized. Here, we focused on the “anti-gravitropic” phenotypes of the lzy1;2;3 mutant in lateral branches and the lzy2;3;4 mutant in the primary root.
Lateral branches and lateral roots develop at a specific angle called the gravitropic setpoint angle, GSA [30]. It has been proposed that the GSA is determined a result of the balance between gravitropism and the AGO [18,19]. Thus, we assumed that the balance between gravitropism and the AGO was disrupted in lzy1;2;3 and lzy2;3;4 mutants, and the AGO manifested as the “anti-gravitropic” phenotypes [24]. In the primary root, the AGO may share a similar gravity-sensing mechanism with gravitropism. Since sedimentation of starch-filled amyloplasts in collumela cells triggers gravitropsim in primary root, we investigated the contribution of amyloplasts in the “anti-gravitropic” phenotype of lzy2;3;4 by introducing pgm mutation. The epistasis analysis between lzy2;3;4 and pgm resulted in the suppression of “anti-gravitropic” phenotypes of lazy2;3;4 plants by the pgm mutation (Figure 1B,C,F,G). Thus, based on previous studies and our results, we deduce that the gravitropism and AGO share a similar gravity-sensing mechanism in the primary root. It has been reported that a reversed asymmetric auxin distribution is formed upon gravi-stimulation in the root tip of lzy2;3;4 [16], suggesting that this reversed auxin flow is triggered by amyloplast sedimentation. In contrast, the effect of the pgm mutation on lateral branches in lzy1;2;3 plants was subtle (Figure 2C–G). Thus, it is possible that gravity-sensing mechanisms or dependency of sensing mechanisms underlying the AGO might differ between roots and shoots. Moreover, other mechanisms for gravity sensing, such as the protoplast presser hypothesis, have been proposed [31], suggesting that other mechanisms besides the starch statolith might be involved in AGO in shoots.
It is widely accepted and supported that the endodermal cells take place in gravity sensing through the amyloplasts sedimentation in shoot. The eal1 and sgr1 are agravitropic mutants and commonly have defects in formation of the endodermis [8,9,10]. Thus, we introduced eal1 and sgr1 mutations into lzy1;2;3 triple mutant to investigate the relation between endodermis and “anti-gravitropic” phenotype of lzy1;2;3. Our genetic interaction analysis demonstrates that eal1 and sgr1 mutations are epistatic to lzy1;2;3, and quadruple mutants resulted in eal1 or sgr1 like phenotypes (Figure 3G,H,P,Q). These results clearly indicate that functions of the SHR and SCR are required for the “anti-gravitropic” phenotype of lzy1;2;3. It is known that SHR and SCR proteins function together as a protein complex in endodermis development [32]. Therefore, we deduce that the endodermis plays a crucial role for AGO as well as gravitropism in shoots.
Although LZY1, LZY2, and LZY3 were downregulated in eal1 and sgr1, a phenotypic difference between lzy1;2;3 plants and eal1 or sgr1 plants was observed (Figure 3C,D,F,L,M,O). It is hard to explain the eal1 and sgr1 phenotypes only by downregulation of LZY1, LZY2, and LZY3. Thus, besides LZY genes, it is expected that the key component genes in AGO are altered in eal1 and sgr1 mutants. We have reported the difference in gene expression between wild type and eal1 or sgr1 in inflorescence stems and showed that TILLER ANGLE CONTROL 1 (TAC1) as a markedly downregulated gene in eal1 and sgr1 plants [15,17]. The TAC1 encodes a protein that shares similarity with LAZY1 family proteins but lacks the CCL domain which is essential for interacting with RLD proteins. TAC1 was identified in rice as a regulator of tiller angle [33], and in peach tree (Prunus persica), PpeTAC1 was identified as a causal gene of the broomy mutant that leads to vertical growth of branches [34]. In Arabidopsis, lateral branches of the attac1 mutant exhibited vertical growth habits [34]. Thus, AtTAC1 might coordinate growth angle control in response to gravity signals with LZYs. However, based on the public expression database eFP browser, AtTAC1 expression is barely detected in roots, making its contribution in AGO limited.
To deepen our understanding of AGO in root, we isolated lzy2;3;4 suppressors and identified three sltr mutants (Figure 5E,H,I). We showed that these lzy suppressor genes are not related to the starch biosynthetic pathway (Figure 5M–S, Supplementary Figure S2). Although further genetic and physiological analyses will be necessary, the three sltr mutants are promising candidates to elucidate the nature of AGO in primary roots.
4. Materials and Methods
4.1. Plant Materials and Growth Condition
Arabidopsis thaliana Columbia-0 (Col) was used as the wild type. lzy1;2;3, pLZY1:GUS, pLZY2:GUS, pLZY3:GUS [15], eal1 [9], sgr1 [7], and pgm [4] were previously described. lzy2;3;4, lzy2;3;4;pgm, lzy1;2;3;pgm, lzy1;2;3;eal1, and lzy1;2;3;sgr1 were generated in this study by genetic crossing. lzy2 and lzy3 were previously described [15], and lzy4 (GABI_479C08) was used. For the phenotypic analysis of primary roots, surface-sterilized seeds were sown on Murashige and Skoog medium supplemented with 1% sucrose, 0.01% myo-inositol, 0.05% MES-KOH (pH5.7), and 0.5% Gellan Gum. For germination induction, plates were kept in the dark at 4 °C for 3 days and then transferred to 22 °C in a growth chamber under continuous light (defined as Day 0). For the phenotypic analysis of axillary branches, seeds were sterilized and kept in the dark at 4 °C for 3 days, sown on soil directly, and grown under continuous light.
4.2. Gene Nomenclature
In this manuscript, the following genes were mentioned: EAL1/SHR/SGR7 (At4g37650), SGR1/SCR (At3g54220), PGM (At5g51820), LZY1 (At5g14090), LZY2 (At1g17400), LZY3 (At1g72490), LZY4 (At1g19115), AtTAC1 (At2g46640), SGR5/IDD15 (At2g01940), IDD14 (At1g68130), IDD16 (At1g25250), PIN1 (At1g73590).
4.3. Lugoal’s Staining
Root tips of 7-day-old seedlings were collected and incubated with 5 mM iodine solution for 1 min and rinsed with deionized water. Stained root tips were mounted in chloral hydrate solution and photographed.
4.4. Shoot Inversion
After flowering, when lateral branches reached 1.5–2.0 cm, plants were inverted and photographed at 5 min intervals using the GoPro HERO Black 7. Growth angles were quantified manually from time-lapse images using ImageJ software. Experiments were repeated more than three times and representative results were used for quantification.
4.5. GUS Staining
Tissues were fixed in 80% ice-cold acetone for 15 min and incubated in GUS staining solution (100 mM sodium phosphate (pH7.0), 10 mM ferricyanide, 10 mM ferrocyanide, 0.1% Triton X-100, 2 mM 5-bromo-4-chloro-3-indolyl-β-D-glucuronic acid) at 37 °C. For thin section preparation, samples were fixed with 4% paraformaldehyde, dehydrated in an ethanol series, and embedded in plastic resin (Technovit 7100). Embedded samples were sectioned in 10 µm thickness with a microtome. Thin sections were stained with neutral red (0.01%) and mounted with Entellan for microscopy.
4.6. Data Visualization and Statistical Analysis
R (version 3.5.1) was used for data visualization and statistical analysis. The following statistical tests were used to calculate the corresponding p-values. The F-test was used to compare the distribution of two data sets. Tukey–Kramer’s test was used for multiple comparisons of all possible data pairs.
Supplementary Materials
The following are available online at https://www.mdpi.com/2223-7747/9/5/615/s1, Figure S1: Schematic representation of genetic screening of lzy2;3;4 suppressors. Figure S2: Lugoal’s staining of primary roots from all the sltr candidates. Video S1. Gravitropic response of lateral branch from wild-type Col plant; Video S2. Gravitropic response of lateral branch from lzy1;2;3 plant; Video S3. Gravitropic response of lateral branch from pgm plant; Video S4. Gravitropic response of lateral branch from lzy1;2;3;pgm; Video S5. Gravitropic response of lateral branch from eal1 plant; Video S6. Gravitropic response of lateral branch from lzy1;2;3;eal1 plant.
Author Contributions
Conceptualization, N.K. and M.T.M. Investigation, N.K.; Y.K.; M.N.; A.M. Formal analysis, N.K. Visualization, N.K. Validation, N.K. and M.T.M. Writing-original draft, N.K. and M.T.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by JSPS KAKENHI Grant-in-Aid for Scientific Research on Innovative Areas 18H05488, by Exploratory Research 17K19388, and by a Core Research for Evolutional Science and Technology (CREST) award from the Japan Science and Technology Agency (JST) JPMJCR14M5 to M.T.M.
Acknowledgments
We thank to Yasuko Hashiguchi, Wakana Takase, Kumiko Mizoguchi, Kayoko Hirano, Yuri Komada and Haruko Izuchi for techinical assistance, the Salk Institute Genomic Analysis Laboratory for providing the sequence-indexed Arabidopsis T-DNA insertion mutants; and the Arabidopsis Biological Resource Center and GABI-Kat for providing seeds of the Arabidopsis T-DNA insertion mutants.
Conflicts of Interest
The authors declare no competing interests.
References
- Kiss, J.Z.; Hertel, R.; Sack, F.D. Amyloplasts are necessary for full gravitropic sensitivity in roots of Arabidopsis thaliana. Planta 1989, 177, 198–206. [Google Scholar] [CrossRef]
- Sack, F.D. Plant Gravity Sensing. Int. Rev. Cytol. 1991, 127, 193–252. [Google Scholar]
- Caspar, T.; Pickard, B. Gravitropism in a starchless mutant of Arabidopsis. Planta Implications for the starch-statolith theory of gravity sensing. Planta 1989, 177, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Kiss, J.Z.; Guisinger, M.M.; Miller, A.J.; Stackhouse, K.S. Reduced Gravitropism in Hypocotyls of Starch-Deficient Mutants of Arabidopsis. Plant Cell Physiol. 1997, 38, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Blancaflor, E.B.; Fasano, J.M.; Gilroy, S. Mapping the functional roles of cap cells in the response of arabidopsis primary roots to gravity. Plant Physiol. 1998, 116, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Tsugeki, R.; Fedoroff, N.V. Genetic ablation of root cap cells in Arabidopsis. Proc. Natl. Acad. Sci. USA 1999, 96, 12941–12946. [Google Scholar] [CrossRef] [PubMed]
- Fukaki, H.; Fujisawa, H.; Tasaka, M. Shoot Gravitropism in Arabidopsis thaliana. Plant Physiol. 1996, 110, 945–955. [Google Scholar] [CrossRef] [PubMed]
- Fukaki, H.; Wysocka-Diller, J.; Kato, T.; Fujisawa, H.; Benfey, P.N.; Tasaka, M. Genetic evidence that the endodermis is essential for shoot gravitropism in Arabidopsis thaliana. Plant J. 1998, 14, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Fujihira, K.; Kurata, T.; Watahiki, M.K.; Karahara, I.; Yamamoto, K.T. An agravitropic mutant of Arabidopsis, endodermal-amyloplast less 1, that lacks amyloplasts in hypocotyl endodermal cell layer. Plant Cell Physiol. 2000, 41, 1193–1199. [Google Scholar] [CrossRef]
- Morita, M.T.; Saito, C.; Nakano, A.; Tasaka, M. endodermal-amyloplast less 1 is a novel allele of SHORT-ROOT. Adv. Sp. Res. 2007, 39, 1127–1133. [Google Scholar] [CrossRef]
- Morita, M.T. Directional Gravity Sensing in Gravitropism. Annu. Rev. Plant Biol. 2010, 61, 705–720. [Google Scholar] [CrossRef]
- Yoshihara, T.; Iino, M. Identification of the gravitropism-related rice gene LAZY1 and elucidation of LAZY1-dependent and -independent gravity signaling pathways. Plant Cell Physiol. 2007, 48, 678–688. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, T.; Spalding, E.P.; Iino, M. AtLAZY1 is a signaling component required for gravitropism of the Arabidopsis thaliana inflorescence. Plant J. 2013, 74, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, T.; Spalding, E.P. LAZY genes mediate the effects of gravity on auxin gradients and plant architecture. Plant Physiol. 2017, 175, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, M.; Furutani, M.; Nishimura, T.; Nakamura, M.; Fushita, T.; Iijima, K.; Baba, K.; Tanaka, H.; Toyota, M.; Tasaka, M.; et al. The arabidopsis LAZY1 family plays a key role in gravity signaling within statocytes and in branch angle control of roots and shoots. Plant Cell 2017, 29, 1984–1999. [Google Scholar] [CrossRef]
- Ge, L.; Chen, R. Negative gravitropic response of roots directs auxin flow to control root gravitropism. Plant Cell Environ. 2019, 42, 2372–2383. [Google Scholar] [CrossRef]
- Furutani, M.; Hirano, Y.; Nishimura, T.; Nakamura, M.; Taniguchi, M.; Suzuki, K.; Oshida, R.; Kondo, C.; Sun, S.; Kato, K.; et al. Polar recruitment of RLD by LAZY1-like protein during gravity signaling in root branch angle control. Nat. Commun. 2020, 11, 76. [Google Scholar] [CrossRef]
- Roychoudhry, S.; Kepinski, S. Shoot and root branch growth angle control-the wonderfulness of lateralness. Curr. Opin. Plant Biol. 2015, 23, 124–131. [Google Scholar] [CrossRef]
- Roychoudhry, S.; Del Bianco, M.; Kieffer, M.; Kepinski, S. Auxin controls gravitropic setpoint angle in higher plant lateral branches. Curr. Biol. 2013, 23, 1497–1504. [Google Scholar] [CrossRef]
- Rosquete, M.R.; Von Wangenheim, D.; Marhavý, P.; Barbez, E.; Stelzer, E.H.K.; Benková, E.; Maizel, A.; Kleine-Vehn, J. An auxin transport mechanism restricts positive orthogravitropism in lateral roots. Curr. Biol. 2013, 23, 817–822. [Google Scholar] [CrossRef]
- Morita, M.T.; Sakaguchi, K.; Kiyose, S.I.; Taira, K.; Kato, T.; Nakamura, M.; Tasaka, M. A C2H2-type zinc finger protein, SGR5, is involved in early events of gravitropism in Arabidopsis inflorescence stems. Plant J. 2006, 47, 619–628. [Google Scholar] [CrossRef]
- Cui, D.; Zhao, J.; Jing, Y.; Fan, M.; Liu, J.; Wang, Z.; Xin, W.; Hu, Y. The Arabidopsis IDD14, IDD15, and IDD16 Cooperatively Regulate Lateral Organ Morphogenesis and Gravitropism by Promoting Auxin Biosynthesis and Transport. PLoS Genet. 2013, 9. [Google Scholar] [CrossRef]
- Ge, L.; Chen, R. Negative gravitropism in plant roots. Nat. Plants 2016, 2, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Nishimura, T.; Morita, M.T. Gravity sensing and signal conversion in plant gravitropism. J. Exp. Bot. 2019, 70, 3495–3506. [Google Scholar] [CrossRef] [PubMed]
- Benfey, P.N.; Linstead, P.J.; Roberts, K.; Schiefelbein, J.W.; Hauser, M.T.; Aeschbacher, R.A. Root development in Arabidopsis: Four mutants with dramatically altered root morphogenesis. Development 1993, 119, 57–70. [Google Scholar]
- Scheres, B.; Di Laurenzio, L.; Willemsen, V.; Hauser, M.T.; Janmaat, K.; Weisbeek, P.; Benfey, P.N. Mutations affecting the radial organisation of the Arabidopsis root display specific defects throughout the embryonic axis. Development 1995, 121, 53–62. [Google Scholar]
- Di Laurenzio, L.; Wysocka-Diller, J.; Malamy, J.E.; Pysh, L.; Helariutta, Y.; Freshour, G.; Hahn, M.G.; Feldmann, K.A.; Benfey, P.N. The SCARECROW gene regulates an asymmetric cell division that is essential for generating the radial organization of the Arabidopsis root. Cell 1996, 86, 423–433. [Google Scholar] [CrossRef]
- Helariutta, Y.; Fukaki, H.; Wysocka-Diller, J.; Nakajima, K.; Jung, J.; Sena, G.; Hauser, M.T.; Benfey, P.N. The SHORT-ROOT gene controls radial patterning of the Arabidopsis root through radial signaling. Cell 2000, 101, 555–567. [Google Scholar] [CrossRef]
- Dong, Z.; Jiang, C.; Chen, X.; Zhang, T.; Ding, L.; Song, W.; Luo, H.; Lai, J.; Chen, H.; Liu, R.; et al. Maize LAZY1 mediates shoot gravitropism and inflorescence development through regulating auxin transport, auxin signaling, and light response. Plant Physiol. 2013, 163, 1306–1322. [Google Scholar] [CrossRef]
- Digby, J.; Firn, R.D. The gravitropic set-point angle (GSA): The identification of an important developmentally controlled variable governing plant architecture. Plant. Cell Environ. 1995, 18, 1434–1440. [Google Scholar] [CrossRef] [PubMed]
- Strohm, A.K.; Baldwin, K.L.; Masson, P.H. Molecular mechanisms of root gravity sensing and signal transduction. Wiley Interdiscip. Rev. Dev. Biol. 2012, 1, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Levesque, M.P.; Vernoux, T.; Jung, J.W.; Paquette, A.J.; Gallagher, K.L.; Wang, J.Y.; Blilou, I.; Scheres, B.; Benfey, P.N. An evolutionarily conserved mechanism delimiting SHR movement defines a single layer of endodermis in plants. Science 2007, 316, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Lin, Z.; Li, H.; Li, X.; Li, J.; Wang, Y.; Zhang, X.; Zhu, Z.; Zhai, W.; Wang, X.; et al. TAC1, a major quantitative trait locus controlling tiller angle in rice. Plant J. 2007, 52, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Dardick, C.; Callahan, A.; Horn, R.; Ruiz, K.B.; Zhebentyayeva, T.; Hollender, C.; Whitaker, M.; Abbott, A.; Scorza, R. PpeTAC1 promotes the horizontal growth of branches in peach trees and is a member of a functionally conserved gene family found in diverse plants species. Plant J. 2013, 75, 618–630. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).