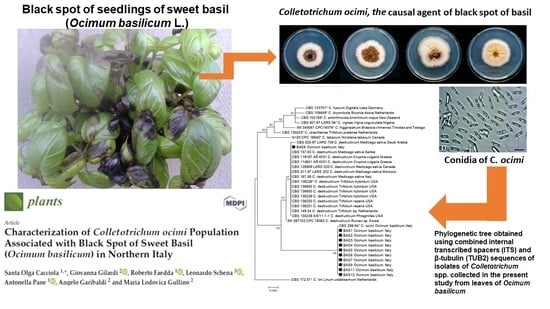
Characterization of Colletotrichum ocimi Population Associated with Black Spot of Sweet Basil (Ocimum basilicum) in Northern Italy
Abstract
:1. Introduction
2. Results
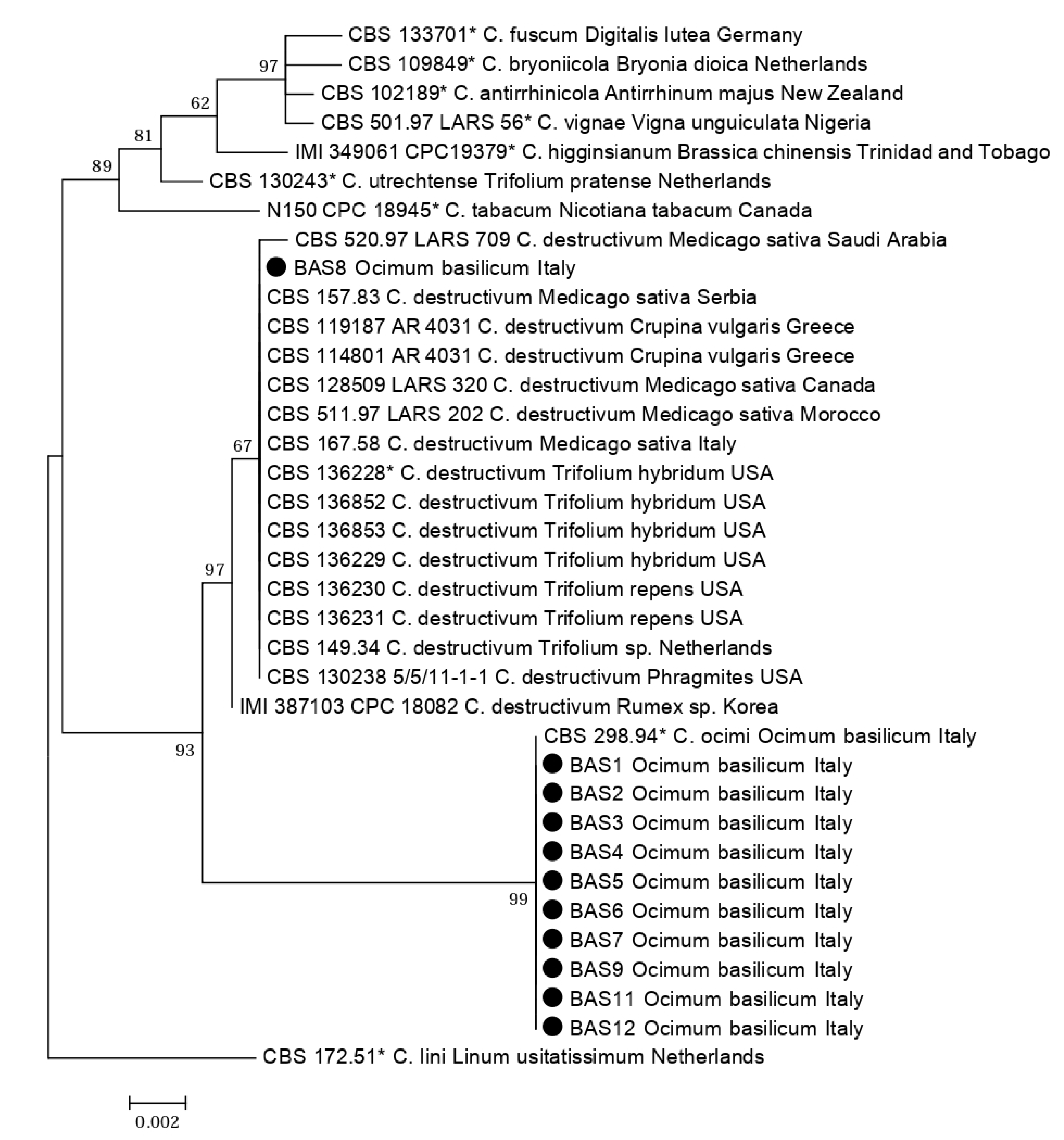
2.1. Molecular Identification of Isolates
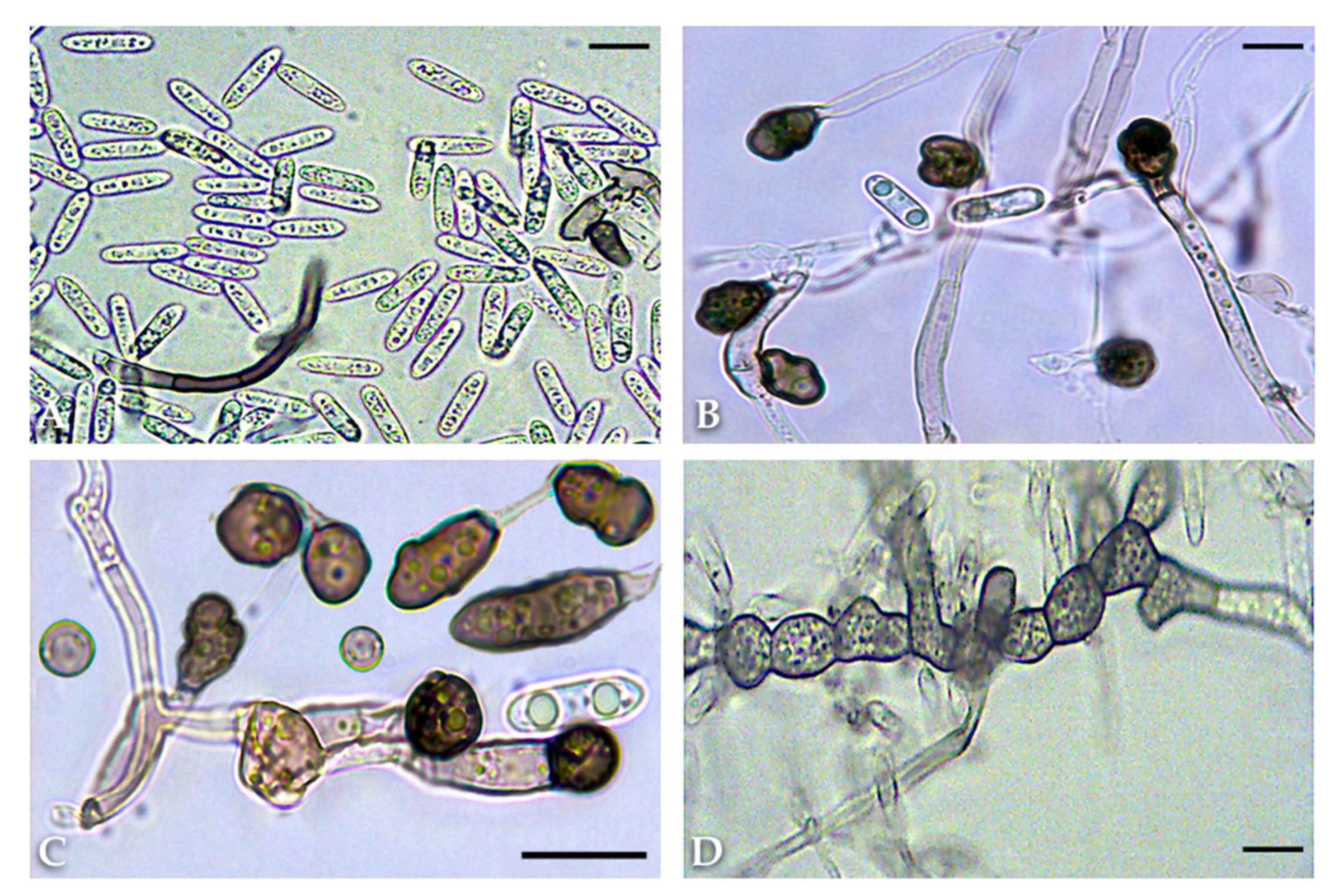
2.2. Morphological and Cultural Characteristics
2.3. Baseline Sensitivity of Isolates to Benomyl
2.4. Pathogenicity Tests
3. Discussion
4. Materials and Methods
4.1. Fungal Isolates
4.2. DNA Extraction, Amplification, and Sequencing
4.3. Morphological and Cultural Characteristics
4.4. Baseline Sensitivity of Isolates to Benomyl
4.5. Pathogenicity Tests
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- ISTAT 2019. Available online: http://dati.istat.it/Index.aspx?DataSetCode=DCSP_COLTIVAZIONI (accessed on 2 April 2020).
- Garibaldi, A.; Gullino, M.L.; Minuto, G. Diseases of basil and their management. Plant Dis. 1997, 81, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Montalti, M. Producendo basilico in coltura protetta. Colt. Protette 1995, 24, 45–49. (In Italian) [Google Scholar]
- Cogliati, E.E.; Gilardi, G.; Gullino, M.L.; Garibaldi, A. Effect of electrical conductivity and silicate on infection of basil with Colletotrichum gloeosporioides in soilless culture. J. Phytopathol. 2012, 160, 655–660. [Google Scholar] [CrossRef]
- Alfieri, S.A., Jr.; Langdon, K.R.; Wehlburg, C.; Kimbrough, J.W. Index of Plant Diseases in Florida (Revised). Florida Dept. Agric. Consum. Serv. Div. Plant Ind. Bull. 1984, 11, 1–389. [Google Scholar]
- Gullino, M.L.; Garibaldi, A.; Minuto, G. First report of “black spot” of basil incited by Colletotrichum gloeosporioides in Italy. Plant Dis. 1995, 79, 539. [Google Scholar] [CrossRef]
- Cannon, P.F.; Buddie, A.G.; Bridge, P.D. The typification of Colletotrichum gloeosporioides. Mycotaxon 2008, 104, 189–204. [Google Scholar]
- Cai, L.; Hyde, K.D.; Taylor, P.W.J.; Weir, B.S.; Waller, J.; Abang, M.M.; Zhang, J.Z.; Yang, Y.L.; Phoulivong, S.; Liu, Z.Y.; et al. A polyphasic approach for studying Colletotrichum. Fungal Divers. 2009, 39, 183–204. [Google Scholar]
- Crouch, J.A.; Clarke, B.B.; Hillman, B.I. What is the value of ITS sequence data in Colletotrichum systematics and species diagnosis? A case study using the falcate-spored graminicolous Colletotrichum group. Mycologia 2009, 101, 648–656. [Google Scholar] [CrossRef]
- Hyde, K.D.; Cai, L.; McKenzie, E.H.C.; Yang, Y.L.; Zhang, J.Z.; Prihastuti, H. Colletotrichum: A catalogue of confusion. Fungal Divers. 2009, 39, 1–17. [Google Scholar]
- Cannon, P.F.; Damm, U.; Johnston, P.R.; Weir, B.S. Colletotrichum–current status and future directions. Stud. Mycol. 2012, 73, 181–213. [Google Scholar] [CrossRef] [Green Version]
- Damm, U.; Cannon, P.F.; Woudenberg, J.H.C.; Crous, P.W. The Colletotrichum acutatum species complex. Stud. Mycol. 2012, 73, 37–113. [Google Scholar] [CrossRef] [Green Version]
- Damm, U.; Cannon, P.F.; Woudenberg, J.H.C.; Johnston, P.R.; Weir, B.S.; Tan, Y.P.; Shivas, R.G.; Crous, P.W. The Colletotrichum boninense species complex. Stud. Mycol. 2012, 73, 1–36. [Google Scholar] [CrossRef] [Green Version]
- Weir, B.S.; Johnston, P.R.; Damm, U. The Colletotrichum gloeosporioides species complex. Stud. Mycol. 2012, 73, 115–180. [Google Scholar] [CrossRef] [Green Version]
- Phoulivong, S.L.; Cai, H.; Chen, E.H.C.; McKenzie, K.; Abdelsalam, E.; Chukeatirote, E.; Hyde, K.D. Colletotrichum gloeosporioides is not a common pathogen on tropical fruits. Fungal Divers. 2010, 44, 33–43. [Google Scholar] [CrossRef]
- Wikee, S.; Cai, L.; Pairin, N.; McKenzie, E.H.C.; Su, Y.Y.; Chukeatirote, E.; Thi, H.N.; Bahkali, A.H.; Moslem, M.A.; Abdelsalam, K.; et al. Colletotrichum species from jasmine (Jasminum sambac). Fungal Divers. 2011, 46, 171–182. [Google Scholar] [CrossRef]
- Sharma, G.; Shenoy, B.D. Colletotrichum systematics: Past, present and prospects. Mycosphere 2016, 7, 1093–1102. [Google Scholar] [CrossRef]
- Damm, U.; Cannon, P.F.; Liu, F.; Barreto, R.W.; Guatimosim, E.; Crous, P.W. The Colletotrichum orbiculare species complex: Important pathogens of field crops and weeds. Fungal Divers. 2013, 61, 29–59. [Google Scholar] [CrossRef]
- Noireung, P.; Phoulivong, S.; Liu, F.; Cai, L.; Mckenzie, E.H.C.; Chukeatirote, E.; Jones, E.B.G.; Bahkali, H.; Hyde, K.D. Novel species of Colletotrichum revealed by morphology and molecular analysis. Cryptogam. Mycol. 2012, 33, 347–362. [Google Scholar] [CrossRef]
- Liu, F.; Damm, U.; Cai, L.; Crous, P.W. Species of the Colletotrichum gloeosporioides complex associated with anthracnose diseases of Proteaceae. Fungal Divers. 2013, 61, 89–105. [Google Scholar] [CrossRef]
- Lima, N.B.; Batista, M.V.A.; De Morais, M.A.; Barbosa, M.A.G. Five Colletotrichum species are responsible for mango anthracnose in northeastern Brazil. Fungal Divers. 2013, 61, 75–88. [Google Scholar] [CrossRef]
- Liu, F.; Cai, L.; Crous, P.W.; Damm, U. The Colletotrichum gigasporum species complex. Persoonia-Mol. Phylogeny Evol. Fungi 2014, 33, 83–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guarnaccia, V.; Groenewald, J.Z.; Polizzi, G.; Crous, P.W. High species diversity in Colletotrichum associated with citrus diseases in Europe. Persoonia 2017, 39, 32–50. [Google Scholar] [CrossRef] [PubMed]
- Damm, U.; O’Connell, R.J.; Groenewald, J.Z.; Crous, W. The Colletotrichum destructivum species complex-hemibiotrophic pathogens of forage and field crops. Stud. Mycol. 2014, 78, 49–84. [Google Scholar] [CrossRef]
- Liu, F.; Wang, M.; Damm, U.; Crous, P.W.; Cai, L. Species boundaries in plant pathogenic fungi: A Colletotrichum case study. BMC Evol. Biol. 2016, 16, 81. [Google Scholar] [CrossRef] [Green Version]
- Guarnaccia, V.; Gilardi, G.; Martina, I.; Garibaldi, A.; Gullino, M.L. Species diversity in Colletotrichum causing anthracnose of aromatic and ornamental Lamiaceae in Italy. Agronomy 2019, 9, 613. [Google Scholar] [CrossRef] [Green Version]
- Shivas, R.G.; Tan, Y.P.; Edwards, J.; Dinh, Q.; Maxwell, A.; Andjic, V.; Liberato, J.R.; Anderson, C.; Beasley, D.R.; Bransgrove, K.; et al. Colletotrichum species in Australia. Australas. Plant Pathol. 2016, 45, 447–464. [Google Scholar] [CrossRef]
- Jayawardena, R.S.; Hyde, K.D.; Damm, U.; Cai, L.; Liu, M.; Li, X.H.; Zhang, W.; Zhao, W.S.; Yan, J.Y. Notes on currently accepted species of Colletotrichum. Mycosphere 2016, 7, 1192–1260. [Google Scholar] [CrossRef]
- Talhinhas, P.; Sreenivasaprasad, S.; Neves-Martins, J.; Oliveira, H. Molecular and phenotypic analyses reveal association of diverse Colletotrichum acutatum groups and a low level of C. gloeosporioides with olive anthracnose. Appl. Environ. Microb. 2005, 71, 2987–2998. [Google Scholar] [CrossRef] [Green Version]
- Huang, F.; Chen, G.Q.; Hou, X.; Fu, Y.S.; Cai, L.; Hyde, K.D.; Li, H.Y. Colletotrichum species associated with cultivated citrus in China. Fungal Divers. 2013, 61, 61–74. [Google Scholar] [CrossRef]
- Abdelfattah, A.; Li Destri Nicosia, M.G.; Cacciola, S.O.; Droby, S.; Schena, L. Metabarcoding analysis of fungal diversity in the phyllosphere and carposphere of olive (Olea europaea). PLoS ONE 2015, 10, e0131069. [Google Scholar] [CrossRef] [Green Version]
- Baroncelli, R.; Zapparata, A.; Sarocco, S.; Sukno, S.A.; Lane, C.R.; Thon, M.R.; Vannacci, G.; Holub, E.; Sreenivasaprasad, S. Molecular diversity of anthracnose pathogen populations associated with UK strawberry production suggests multiple introductions of three different Colletotrichum species. PLoS ONE 2015, 10, e0129140. [Google Scholar] [CrossRef]
- Han, Y.C.; Zeng, X.G.; Xiang, F.Y.; Ren, L.; Chen, F.Y.; Gu, Y.C. Distribution and characteristics of Colletotrichum spp. associated with anthracnose of strawberry in Hubei, China. Plant Dis. 2016, 100, 996–1006. [Google Scholar] [CrossRef] [Green Version]
- De Silva, D.D.; Ades, P.K.; Crous, P.W.; Taylor, P.W.G. Colletotrichum species associated with chili anthracnose in Australia. Plant Pathol. 2017, 66, 254–267. [Google Scholar] [CrossRef]
- Tovar-Pedraza, J.M.; Mora-Aguilera, J.A.; Nava-Díaz, C.; Lima, N.B.; Michereff, S.J.; Sandoval-Islas, J.S.; Câmara, M.P.S.; Téliz-Ortiz, D.; Leyva-Mir, S.G. Distribution and pathogenicity of Colletotrichum species associated with mango anthracnose in Mexico. Plant Dis. 2020, 104, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Cacciola, S.O.; Faedda, R.; Sinatra, F.; Agosteo, G.E.; Schena, L.; Frisullo, S.; Magnano di San Lio, G. Olive anthracnose. J. Plant Pathol. 2012, 94, 29–44. [Google Scholar]
- Talhinhas, P.; Neves-Martins, J.; Oliveira, H.; Sreenivasaprasad, S. The distinctive population structure of Colletotrichum species associated with olive anthracnose in the Algarve region of Portugal reflects a host-pathogen diversity hot spot. FEMS Microbiol. Lett. 2009, 29, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Schena, L.; Mosca, S.; Cacciola, S.O.; Faedda, R.; Sanzani, S.M.; Agosteo, G.E.; Sergeeva, V.; Magnano di San Lio, G. Species of the Colletotrichum gloeosporioides and C. boninense complexes associated with olive anthracnose. Plant Pathol. 2014, 63, 437–466. [Google Scholar] [CrossRef]
- Munir, M.; Amsden, B.; Dixon, E.; Vaillancourt, L.; Gauthier, N.A.W. Characterization of Colletotrichum species causing bitter rot of apple in Kentucky orchards. Plant Dis. 2016, 100, 2194–2203. [Google Scholar] [CrossRef] [Green Version]
- Sharma, G.; Maymon, M.; Freeman, S. Epidemiology, pathology and identification of Colletotrichum including a novel species associated with avocado (Persea americana) anthracnose in Israel. Sci. Rep. 2017, 7, 15839. [Google Scholar] [CrossRef]
- Talhinhas, P.; Loureiro, A.; Oliveira, H. Olive anthracnose: A yield- and oil quality-degrading disease caused by several species of Colletotrichum that differ in virulence, host preference and geographical distribution. Mol. Plant Pathol. 2018, 19, 1797–1807. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.C.; Hao, X.Y.; Wang, L.; Bin, X.; Wang, X.C.; Yang, Y.J. Diverse Colletotrichum species cause anthracnose of tea plants (Camellia sinensis (L.) O. Kuntze) in China. Sci. Rep. 2016, 6, 35287. [Google Scholar] [CrossRef] [Green Version]
- Diao, Y.-Z.; Zhang, C.; Liu, F.; Wang, W.-Z.; Liu, L.; Cai, L.; Liu, X.-L. Colletotrichum species causing anthracnose disease of chili in China. Persoonia 2017, 38, 20–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, M.; Crous, P.W.; Bai, Q.; Zhang, P.F.; Xiang, J.; Guo, Y.S.; Zhao, F.F.; Yang, M.M.; Hong, N.; Xu, W.X.; et al. Colletotrichum species associated with anthracnose of Pyrus spp. in China. Persoonia 2019, 42, 1–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Bu, J.; Shu, J.; Yu, Z.; Tang, L.; Huang, S.; Guo, T.; Mo, J.; Luo, S.; Solangi, G.S.; et al. Colletotrichum species associated with mango in southern China. Sci. Rep. 2019, 9, 18891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos, A.P.; Talhinhas, P.; Sreenivasaprasad, S.; Oliveira, H. Characterization of Colletotrichum gloeosporioides, as the main causal agent of citrus anthracnose, and C. karstii as species preferentially associated with lemon twig dieback in Portugal. Phytoparasitica 2016, 44, 549–561. [Google Scholar] [CrossRef]
- Schena, L.; Abdelfattah, A.; Mosca, S.; Li Destri Nicosia, M.G.; Agosteo, G.E.; Cacciola, S.O. Quantitative detection of Colletotrichum godetiae and C. acutatum sensu stricto in the phyllosphere and carposphere of olive during four phenological phases. Eur. J. Plant Pathol. 2017, 149, 337–347. [Google Scholar] [CrossRef]
- De Silva, D.D.; Crous, P.W.; Ades, P.; Hyde, K.D.; Taylor, P.W.J. Life styles of Colletotrichum species and implications for plant biosecurity. Fungal Biol. Rev. 2017, 31, 155–168. [Google Scholar] [CrossRef]
- Agosteo, G.E.; Cacciola, S.O.; Pane, A.; Frisullo, S. Vegetative compatibility groups of Colletotrichum gloeosporioides from olive in Italy. In Proceedings of the 10th Congress of the Mediterranean Phytopathological Union, Le Corum-Montpellier, France; Société Francaise de Phytopathologie; Mediterranean Phytopathological Union, Florence, Italy, 1–5 June 1997; pp. 95–99. [Google Scholar]
- Silva, D.N.; Talhinhas, P.; Cai, L.; Manuel, L.; Gichuru, E.K.; Loureiro, A.; Varzea, V.; Paulo, O.S.; Batista, D. Host jump drives rapid and recent ecological speciation of the emergent fungal pathogen Colletotrichum kahawae. Mol. Ecol. 2012, 21, 2655–2670. [Google Scholar] [CrossRef]
- Baroncelli, R.; Amby, D.B.; Zapparata, A.; Sarocco, S.; Vannacci, G.; Le Floch, G.; Harrison, R.J.; Holub, E.; Sukno, S.A.; Sreenivasaprasad, S.; et al. Gene family expansions and contractions are associated with host range in plant pathogens of the genus Colletotrichum. BMC Genom. 2016, 17, 555. [Google Scholar] [CrossRef] [Green Version]
- Baroncelli, R.; Talhinhas, P.; Pensec, F.; Sukno, S.A.; Le Floch, G.; Thon, M.R. The Colletotrichum acutatum species complex as a model system to study evolution and host specialization in plant pathogens. Front. Microbiol. 2017, 8, 2001. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.; Wang, B.; Dong, Q.; Li, L.; Rollins, J.A.; Zhang, R.; Sun, G. Pathogenic adaptations of Colletotrichum fungi revealed by genome wide gene family evolutionary analyses. PLoS ONE 2018, 13, e0196303. [Google Scholar] [CrossRef] [PubMed]
- da Silva, L.L.; Moreno, H.L.A.; Correia, H.L.N.; Santana, M.F.; de Queiroz, M.V. Colletotrichum: Species complexes, lifestyle, and peculiarities of some sources of genetic variability. Appl. Microbiol. Biotechnol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Adaskaveg, J.E.; Hartin, R.J. Characterization of Colletotrichum acutatum isolates causing anthracnose of almond and peach in California. Phytopathology 1997, 87, 979–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freeman, S.; Katan, T.; Shabi, E. Characterization of Colletotrichum species responsible for anthracnose diseases of various fruits. Plant Dis. 1998, 82, 596–605. [Google Scholar] [CrossRef] [Green Version]
- Peres, N.A.R.; Souza, N.L.; Zitko, S.E.; Timmer, L.W. Activity of benomyl for control of postbloom fruit drop of citrus caused by Colletotrichum acutatum. Plant Dis. 2002, 86, 620–624. [Google Scholar] [CrossRef] [Green Version]
- Talhinhas, P.; Sreenivasaprasad, S.; Neves-Martins, J.; Oliveira, H. Genetic and morphological characterization of Colletotrichum acutatum causing anthracnose of lupins. Phytopathology 2002, 92, 986–996. [Google Scholar] [CrossRef] [Green Version]
- Faedda, R.; Agosteo, G.E.; Schena, L.; Mosca, S.; Frisullo, S.; Magnano di San Lio, G.; Cacciola, S.O. Colletotrichum clavatum sp. nov. identified as the causal agent of olive anthracnose in Italy. Phytopathol. Mediterr. 2011, 50, 283–302. [Google Scholar]
- ChromasPro v. 1.5. Available online: http://www.technelysium.com.au/ (accessed on 2 April 2020).
- TOPALi v2. Available online: http://www.topali.org/ (accessed on 2 April 2020).
- Ruocco, M.; Baroncelli, R.; Cacciola, S.O.; Pane, C.; Monti, M.M.; Firrao, G.; Vergara, M.; Magnano di San Lio, G.; Vannacci, G.; Scala, F. Polyketide synthases of Diaporthe helianthi and involvement of DhPKS1 in virulence on sunflower. BMC Genom. 2018, 19, 27. [Google Scholar] [CrossRef]
- Farr, D.F.; Rossman, A.Y. Fungal Databases, U.S. National Fungus Collections, ARS, USDA. Available online: https://nt.ars-grin.gov/fungaldatabases/ (accessed on 2 April 2020).

| Colletotrichum Species | Isolate | Mean Area a (±SD) |
|---|---|---|
| C. acutatum | OLE | - b |
| 67 | 0.26 ± 0.10 | |
| C. destructivum | BAS 8 | 82.15 ± 9.25 |
| C. fioriniae | 1409 | 7.94 ± 2.46 |
| C. gloeosporioides | CINA | 0.61 ± 0.29 |
| 1765 | - b | |
| PEP | 0.74 ± 0.22 | |
| C1 | - b | |
| C. godetiae | IMI 398854 | 1.13 ± 0.35 |
| IMI 398855 | 1.22 ± 0.25 | |
| C. karstii | CAM | - b |
| OLF38 | - b | |
| C. musae | F15 | 1.79 ± 0.85 |
| C. nymphaeae | 725 | - b |
| SPL 103 | - b | |
| CBS 231.49 | - b | |
| C. ocimi | BAS 2 | 96.65 ± 14. 42 |
| BAS 3 | 85.15 ± 8.25 | |
| BAS 5 | 88. 96 ± 10.12 | |
| BAS 6 | 86.45 ± 8.54 | |
| BAS 15 | 91.75 ± 12.25 | |
| C. queenslandicum | VMIN | 0.74 ± 0.42 |
| Control c | - b |
| Colletotrichum Species | Isolate | Mean Length a (±SD) |
|---|---|---|
| C. acutatum | OLE | 4.00 ± 0.64 |
| 67 | 5.40 ± 0.80 | |
| C. destructivum | BAS 8 | 9.82 ± 1.25 |
| C. fioriniae | 1409 | 7.89 ± 0.92 |
| C. gloeosporioides | CINA | 6.44 ± 1.03 |
| 1765 | 5.10 ± 1.50 | |
| PEP | 5.10 ± 1.50 | |
| C1 | 6.15 ± 0.82 | |
| C. godetiae | IMI 398854 | 1.89 ± 0.20 |
| IMI 398855 | 2.22 ± 0.35 | |
| C. karstii | CAM | 1.11 ± 0.11 |
| OLF38 | 1.23 ± 0.15 | |
| C. musae | F15 | 1.55 ± 0.17 |
| C. nymphaeae | 725 | 7.52 ± 0.81 |
| SP L103 | 8.67 ± 0.76 | |
| CBS 231.49 | 7.48 ± 1.01 | |
| C. ocimi | BAS 2 | 13.00 ± 2.03 |
| BAS 3 | 11.55 ± 1.08 | |
| BAS 5 | 10.22 ± 1.05 | |
| BAS 6 | 9.45 ± 1.55 | |
| BAS 15 | 12.05 ± 1.45 | |
| C. queenslandicum | VMIN | 3.33 ± 0.23 |
| Control b | - c |
| Colletotrichum Species | Isolate | Cultivar/Producer | Sourced From | Cropping System | Geographic Origin | Collection Date |
|---|---|---|---|---|---|---|
| C. ocimi | BAS 1 | Superbo/SAIS | Seedling | Greenhouse a | Grugliasco (TO) | 2011 |
| BAS 2 | Gecom FT/SAIS | Seedling | Greenhouse a | Grugliasco (TO) | 2011 | |
| BAS 3 | Italiko/ANSEME | Seedling | Greenhouse a | Grugliasco (TO) | 2011 | |
| BAS 4 | Italiano/Olter | Seedling | Greenhouse a | Grugliasco (TO) | 2011 | |
| BAS 5 | Italiano RCS/Four | Seedling | Greenhouse a | Grugliasco (TO) | 2011 | |
| BAS 6 | Aromatico/Semencoop | Seedling | Greenhouse a | Grugliasco (TO) | 2011 | |
| BAS 7 | Profumo/Semencoop | Seedling | Greenhouse a | Grugliasco (TO) | 2011 | |
| BAS 9 | Italiano classico/SAIS | Leaf | Greenhouse | Albenga (SV) | 2007 | |
| BAS 10 | Italiko/La Semiorto | Leaf | Open field | Alessandria (AL) | 2013 | |
| BAS 11 | Italiko/La Semiorto | Leaf | Soilless Greenhouse a | Moncalieri (TO) | 2013 | |
| BAS 12 | Aromatico Ligure/Semencoop | Leaf | Greenhouse a | Albenga (SV) | 2007 | |
| BAS 13 | Genovese type b | Leaf | Greenhouse a | Albenga (SV) | 2011 | |
| BAS 14 | Italiano classico/Pagano | Seedling | Open field | Nichelino (TO) | 2014 | |
| BAS 15 | Italiano classico/Pagano | Leaf | Open field | Nichelino (TO) | 2014 | |
| C. destructivum | BAS 8 | Italiano classico/Pagano | Leaf | Open field | Castagnole Piemonte (TO) | 2014 |
| Colletotrichum Species | Isolate | Origin | Host | GenBank Accession | References | |
|---|---|---|---|---|---|---|
| ITS | TUB2 | |||||
| C. antirrhinicola | CBS 102189 * | New Zealand | Antirrhinum majus | KM105180 | KM105460 | [23] |
| C. bryoniicola | CBS 109849 * | Netherlands | Bryonia dioica | KM105181 | KM105461 | [23] |
| C. destructivum | BAS 8 | Italy: Piedmont | Ocimum basilicum | MT269781 | MT326884 | This study |
| C. destructivum | CBS 520.97 LARS 709 | Saudi Arabia | Medicago sativa | KM105217 | KM105497 | [23] |
| C. destructivum | CBS 157.83 | Serbia | Medicago sativa | KM105215 | KM105495 | [23] |
| C. destructivum | CBS 119187, AR 4031 | Greece | Crupina vulgaris | KM105220 | KM105500 | [23] |
| C. destructivum | CBS 114801, AR 4031 | Greece | Crupina vulgaris | KM105219 | KM105499 | [23] |
| C. destructivum | CBS 128509, LARS 320 | Canada | Medicago sativa | KM105214 | KM105494 | [23] |
| C. destructivum | CBS 511.97, LARS 202 | Morocco | Medicago sativa | KM105216 | KM105496 | [23] |
| C. destructivum | CBS 167.58 | Italy | Medicago sativa | KM105213 | KM105493 | [23] |
| C. destructivum | CBS 136228 * | USA | Trifolium hybridum | KM105207 | KM105487 | [23] |
| C. destructivum | CBS 136852 | USA | Trifolium hybridum | KM105208 | KM105488 | [23] |
| C. destructivum | CBS 136853 | USA | Trifolium hybridum | KM105209 | KM105489 | [23] |
| C. destructivum | CBS 136229 | USA | Trifolium hybridum | KM105211 | KM105491 | [23] |
| C. destructivum | CBS 136230 | USA | Trifolium repens | KM105210 | KM105490 | [23] |
| C. destructivum | CBS 136231 | USA | Trifolium repens | KM105212 | KM105492 | [23] |
| C. destructivum | CBS 149.34 | Netherlands | Trifolium sp. | JQ005764 | JQ005848 | [23] |
| C. destructivum | CBS 130238, 5/5/11-1-1 | USA | Phragmites | KM105218 | KM105498 | [23] |
| C. destructivum | IMI 387103, CPC 18082 | Korea | Rumex sp. | KM105221 | KM105501 | [23] |
| C. fuscum | CBS 133701 * | Germany | Digitalis lutea | KM105174 | KM105454 | [23] |
| C. higginsianum | IMI 349061, CPC 19379 * | Trinidad and Tobago | Brassica chinensis | KM105184 | KM105464 | [23] |
| C. lini | CBS 172.51 * | Netherlands | Linum usitatissimum | JQ005765 | JQ005849 | [23] |
| C. ocimi | BAS 1 | Italy: Piedmont | Ocimum basilicum | MT269774 | MT319098 | This study |
| C. ocimi | BAS 2 | Italy: Piedmont | Ocimum basilicum | MT269775 | MT326875 | This study |
| C. ocimi | BAS 3 | Italy: Piedmont | Ocimum basilicum | MT269776 | MT326876 | This study |
| C. ocimi | BAS 4 | Italy: Piedmont | Ocimum basilicum | MT269777 | MT326877 | This study |
| C. ocimi | BAS 5 | Italy: Piedmont | Ocimum basilicum | MT269778 | MT326878 | This study |
| C. ocimi | BAS 6 | Italy: Piedmont | Ocimum basilicum | MT269779 | MT326879 | This study |
| C. ocimi | BAS 7 | Italy: Piedmont | Ocimum basilicum | MT269780 | MT326880 | This study |
| C. ocimi | BAS 9 | Italy: Liguria | Ocimum basilicum | MT269782 | MT326881 | This study |
| C. ocimi | BAS 11 | Italy: Piedmont | Ocimum basilicum | MT269783 | MT326882 | This study |
| C. ocimi | BAS 12 | Italy: Liguria | Ocimum basilicum | MT269784 | MT326883 | This study |
| C. ocimi | CBS 298.94 * | Italy | Ocimum basilicum | KM105222 | KM105502 | [23] |
| C. tabacum | N150, CPC 18945 * | Canada | Nicotiana tabacum | KM105204 | KM105484 | [23] |
| C. utrechtense | CBS 130243 * | Netherlands | Trifolium pratense | KM105201 | KM105481 | [23] |
| C. vignae | CBS 501.97, LARS 56 * | Nigeria | Vigna unguiculata | KM105183 | KM105463 | [23] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cacciola, S.O.; Gilardi, G.; Faedda, R.; Schena, L.; Pane, A.; Garibaldi, A.; Gullino, M.L. Characterization of Colletotrichum ocimi Population Associated with Black Spot of Sweet Basil (Ocimum basilicum) in Northern Italy. Plants 2020, 9, 654. https://doi.org/10.3390/plants9050654
Cacciola SO, Gilardi G, Faedda R, Schena L, Pane A, Garibaldi A, Gullino ML. Characterization of Colletotrichum ocimi Population Associated with Black Spot of Sweet Basil (Ocimum basilicum) in Northern Italy. Plants. 2020; 9(5):654. https://doi.org/10.3390/plants9050654
Chicago/Turabian StyleCacciola, Santa Olga, Giovanna Gilardi, Roberto Faedda, Leonardo Schena, Antonella Pane, Angelo Garibaldi, and Maria Lodovica Gullino. 2020. "Characterization of Colletotrichum ocimi Population Associated with Black Spot of Sweet Basil (Ocimum basilicum) in Northern Italy" Plants 9, no. 5: 654. https://doi.org/10.3390/plants9050654
APA StyleCacciola, S. O., Gilardi, G., Faedda, R., Schena, L., Pane, A., Garibaldi, A., & Gullino, M. L. (2020). Characterization of Colletotrichum ocimi Population Associated with Black Spot of Sweet Basil (Ocimum basilicum) in Northern Italy. Plants, 9(5), 654. https://doi.org/10.3390/plants9050654