Mechanisms of Copper Tolerance, Accumulation, and Detoxification in the Marine Macroalga Ulva compressa (Chlorophyta): 20 Years of Research
Abstract
:1. Introduction
1.1. Mechanisms of Copper Tolerance in Plants and Marine Macroalgae
1.2. Mechanisms of Copper Accumulation in Plants and Marine Macroalgae
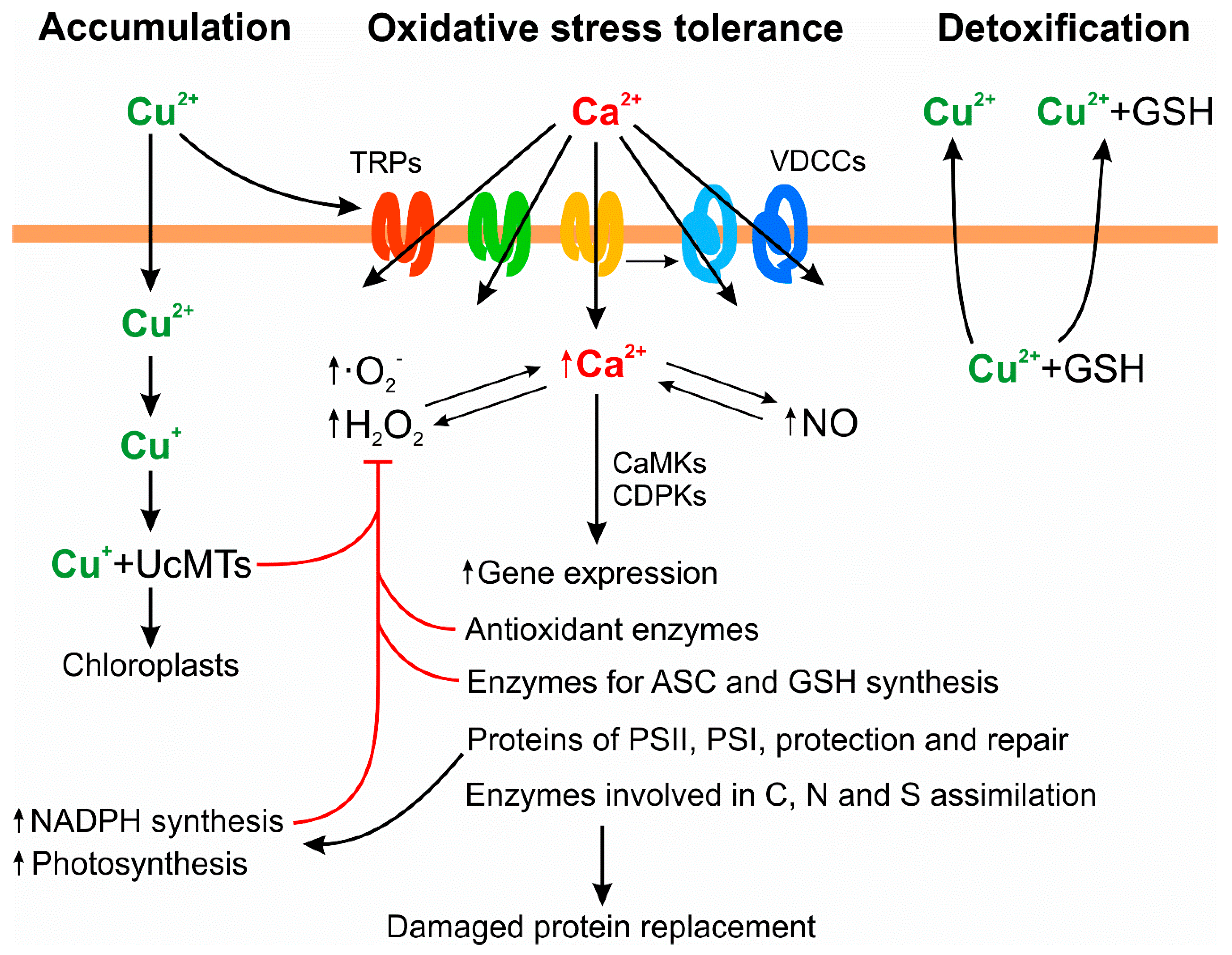
2. Mechanisms of Copper Tolerance in the Marine Macroalga U. compressa
3. Mechanisms of Copper Accumulation in the Marine Macroalga U. compressa
4. Mechanisms of Copper Detoxification in the Marine Macroalga U. compressa
5. Additional Mechanisms of Copper Tolerance in the Marine Macroalga U. compressa
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ROS: | reactive oxygen species |
| AP: | ascorbate peroxidase |
| DHAR: | dehydroascotbate reductase |
| GR: | glutathione reductase |
| CAT: | catalase |
| GP: | glutathione peroxidase |
| PRX: | peroxiredoxin |
| ASC: | ascorbate |
| GSH: | reduced glutathione |
| GSSG: | oxidized glutathione |
| NADPH: | nicotinamide adenine dinucleotide phosphate |
| PC: | phytochelatin |
| MT: | metallothionein |
| TRP: | transient receptor potencial |
| VDCC: | voltage-dependent calcium channel |
| CaM: | calmodulin |
| CaMK: | calmodulin-dependent protein kinase |
| CDPK: | calcium-dependent protein kinase |
| MAPK: | mitogen-activated protein kinase |
References
- Yadav, S.K. Heavy metal toxicity in plants: An overview on the role of glutathione and phytochelatins in heavy metal stress in plants. S. Afr. J. Bot. 2010, 76, 167–179. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.A.; Fahad, S.; Haider, I.; Ahmed, N.; Ahmad, S.; Hussain, S.; Arshad, M. Oxidative stress and antioxidant defenses in plants exposed to metal/metalloid toxicity. In Reactive Oxygen, Nitrogen and Sulfur Species in Plants: Production, Metabolism, Signaling and Defense Mechanisms; Wiley and Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Xu, Y.; Morel, F.M. Cadmium in marine phytoplankton. Met. Ions Life Sci. 2013, 11, 509–528. [Google Scholar] [PubMed]
- Foyer, C.; Noctor, G. Ascorbate and glutathione: The heart of the redox hub. Plant Physiol. 2011, 155, 2–8. [Google Scholar] [CrossRef] [Green Version]
- Valpuesta, V.; Botella, M.A. Biosynthesis of L-ascorbic acid in plants: New pathways for an old antioxidant. Trends Plant Sci. 2004, 9, 573–577. [Google Scholar] [CrossRef]
- Smirnoff, N. Vitamin C: The metabolism and function of ascorbic acid in plants. Adv. Bot. Res. 2011, 59, 107–177. [Google Scholar]
- Wheeler, G.; Jones, M.A.; Smirnoff, N. The biosynthetic pathway of vitamin C in higher plants. Nature 1998, 393, 365–369. [Google Scholar] [CrossRef]
- Shelly, C.; Lu, M.D. Glutathione synthesis. Biochim. Biophys. Acta 2013, 1830, 3143–3153. [Google Scholar]
- Zamek-Gliszczynsnki, M.J.; Hoffmaster, K.A.; Nezasa, K.I.; Tallman, M.N.; Brouwer, L.R. Integration of hepatic drug transporters and Phase II metabolizing enzymes: Mechanisms of hepatic excretion of sulfate, glucuronide, and glutathione metabolites. Eur. J. Pharmac. Sci. 2006, 27, 447–486. [Google Scholar] [CrossRef]
- Devi, S.R.; Prasad, M.N.V. Copper toxicity in Ceratophyllum demersum L. (Coontail), a free floting macrophyte: Response of antioxidant enzymes and antioxidants. Plant Sci. 1998, 138, 157–165. [Google Scholar] [CrossRef]
- Drazkiewicz, M.; Skorynska-Polit, E.; Krupa, Z. Response of the ascorbate-glutathione cycle to excess copper in Arabidopsis thaliana. Plant Sci. 2003, 164, 195–202. [Google Scholar] [CrossRef]
- Drazkiewicz, M.; Skorzynska-Polit, E.; Krupa, Z. Copper-induced oxidative stress and antioxidant defence in Arabidopsis thaliana. Biometals 2004, 17, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, J.; Lee, S.; Wen, R. Copper-caused oxidative stress triggers the activation of antioxidant enzymes via ZmMPK3 in maize leaves. PLoS ONE 2018, 13, e0203612. [Google Scholar] [CrossRef] [PubMed]
- Collén, J.; Pedersén, M.; Colepicolo, P. Oxidative stress in red macroalgae by pollutant metals. J. Phycol. 2003, 45, 337–342. [Google Scholar]
- Sáez, C.A.; Roncarati, F.; Moenne, A.; Moody, A.J.; Brown, M.T. Copper-induced intra-specific oxidative damage and antioxidant responses in strain of the brown alga Ectocarpus siliculosus with different pollution histories. Aquat. Toxicol. 2015, 159, 81–89. [Google Scholar] [CrossRef] [Green Version]
- Roncarati, F.; Sáez, C.A.; Greco, M.; Gledhill, M.; Bitonti, M.B.; Brown, M.T. Response differences between Ectocarpus siliculosus populations to copper stress involve cellular exclusion and induction of the phytochelatin biosynthetic pathway. Aquat. Toxicol. 2015, 159, 167–175. [Google Scholar] [CrossRef] [Green Version]
- Ritter, A.; Dittami, S.; Goultiquer, S.; Correa, J.A.; Boyen, C.; Potin, P.; Thonon, T. Transcriptomic and metabolomic analysis of copper stress acclimation in Ectocarpus siliculosus highlights signaling and tolerance mechanisms in brown algae. BMC Plant Biol. 2014, 14, 116. [Google Scholar] [CrossRef] [Green Version]
- Cobbett, C.; Goldsbrough, P. Phytochelatins and metallothioneins: Role in heavy metal detoxification and homeostasis. Annu. Rev. Plant Biol. 2002, 53, 159–182. [Google Scholar] [CrossRef] [Green Version]
- Filiz, E.; Saracoglu, I.A.; Ozygit, I.I.; Yalcin, B. Comparative analysis of phytochelatin synthase (PCS) genes in higher plants. Biotechnol. Biotechnol. Equip. 2019, 33, 178–194. [Google Scholar] [CrossRef] [Green Version]
- Tennsted, P.; Peisker, D.; Böttcher, C.; Trampcynska, A.; Clemens, S. Phytochelatins synthesis is essential for the detoxification of excess zinc and contributes significantly to the accumulation of zinc. Plant Physiol. 2009, 49, 938–948. [Google Scholar] [CrossRef] [Green Version]
- Blindauer, C.A.; Leszczyszyn, O.I. Metallothioneins: Unparalleled diversity in structure and functions for metal ions homeostasis and more. Nat. Prod. Rep. 2010, 27, 720–741. [Google Scholar] [CrossRef]
- Palacios, O.; Atrian, S.; Capdevila, M. Zn- and Cu-thioneins: A functional classification for metallothioneins. J. Biol. Inorg. Chem. 2011, 16, 991–1009. [Google Scholar] [CrossRef] [PubMed]
- Leszczyszyn, O.I.; Iman, H.T.; Blindauer, C.A. Diversity and distribution of metallothioneins: A review of structure, properties and function. Metallomics 2013, 5, 1146–1169. [Google Scholar] [CrossRef]
- Morris, C.A.; Nicolaus, B.; Sampson, V.; Kille, P. Identification and characterization of a recombinant metallothionein protein from a marine alga, Fucus vesiculosus. Biochem. J. 1999, 338, 553–560. [Google Scholar] [CrossRef]
- Zúñiga, A.; Laporte, D.; González, A.; Gómez, M.; Sáez, C.A.; Moenne, A. Isolation and characterization of copper- and zinc-binding metallothioneins from the marine alga Ulva compressa (Chlorophyta). Int. J. Mol. Sci. 2020, 21, 153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srivastava, S.; Mishra, S.; Tipathi, R.D.; Dwivedi, S.; Gupta, K.G. Copper-induced oxidative stress and responses of antioxidants and phytochelatins in Hydrilla verticillata (L.F.) Royle. Aquat. Toxicol. 2005, 80, 405–415. [Google Scholar] [CrossRef]
- De Vos, R.C.H.; Vonk, M.J.; Vooijs, R.; Schat, H. Glutathione depletion due to copper-induced phytochelatin synthesis causes oxidative stress in Silene cucubalus. Plant Physiol. 1992, 98, 853–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flores-Cáceres, M.L.; Hattab, S.; Bousetta, H.; Banni, M.; Hernández, L.E. Specific mechanisms of tolerance to copper and cadmium are compromised by a limited concentration of glutathione in alfalfa plants. Plant Sci. 2015, 233, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.J.; Mettam, M.; Goldsbrough, P.B. Examining the specific contribution of individual Arabidopsis metallothioneins to copper distribution and metal tolerance. Plant Physiol. 2008, 146, 1697–1706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benatti, M.R.; Yookongkaew, N.; Meetam, M.; Guo, W.J.; Punyasuk, N.; AbuQamar, S.; Goldsbrough, P. Metallothionein deficiency impacts copper accumulation and redistribution in leaves and seeds of Arabidopsis. New Phytol. 2014, 202, 940–951. [Google Scholar] [CrossRef]
- Xia, Y.; Qi, Y.; Yuan, Y.; Wang, G.; Cui, J.; Chen, Y.; Zhang, H.; Shen, Z. Overexpression of Elsholtzia haichowensis metallothionein 1 (EhMT1) in tobacco plants enhances copper tolerance and accumulation in root cytoplasm and decreases hydrogen peroxide production. J. Haz. Mat. 2012, 233, 65–71. [Google Scholar] [CrossRef]
- Ruta, L.L.; Lin, Y.F.; Kisse, R.; Nicolau, I.; Neagoe, A.D.; Ghenea Bones, A.M.; Farcasanu, I.C. Anchoring plant metallothioneins to the inner face of the plasma membrane of Saccahromyces cervisiae cells leads to heavy metal accumulation. PLoS ONE 2017, 12, e0178393. [Google Scholar] [CrossRef] [PubMed]
- Pawlik-Skowronska, B.; Pirszel, J.; Brown, M.T. Concentration of phytochelatins and glutathione found in natural assemblages of seaweeds depend on species and metal concentration of the habitat. Aquat. Toxicol. 2007, 83, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Mulchandani, A.; Chen, W. Highly selective and rapid arsenic removal by metabolically engineered Escherichia coli cells expressing Fucus vesiculosus metallothionein. Appl. Environ. Microbiol. 2008, 74, 2924–2927. [Google Scholar] [CrossRef] [Green Version]
- Ratkevicius, N.; Correa, J.A.; Moenne, A. Copper accumulation, synthesis of ascorbate and activation of ascorbate peroxidase in Enteromorpha compressa (L.) Grev. (Chlorophyta) from heavy metal-enriched environments in northern Chile. Plant Cell Environ. 2003, 26, 1599–1608. [Google Scholar] [CrossRef]
- Moenne, A.; González, A.; Sáez, C.A. Mechanisms of metal tolerance in marine macroalgae, with emphasis on copper tolerance in Chlorophyta and Rodophyta. Aquat. Toxicol. 2016, 176, 30–37. [Google Scholar] [CrossRef] [PubMed]
- González, A.; Vera, J.; Castro, J.; Dennet, G.; Mellado, M.; Morales, B.; Correa, J.A.; Moenne, A. Co-occuring increases in calcium and organellar reactive oxygen species determine differential activation of antioxidant and defense enzymes in Ulva compressa (Chlorophyta) exposed to copper excess. Plant Cell Environ. 2010, 33, 1627–1640. [Google Scholar] [CrossRef]
- González, A.; Cabrera, M.A.; Henríquez, M.J.; Contreras, R.A.; Morales, B.; Moenne, A. Cross-talk among calcium, hydrogen peroxide, and nitric oxide and activation of gene expression involving calmodulins and calcium-dependent protein kinases in Ulva compressa exposed to copper excess. Plant Physiol. 2012, 158, 1451–1462. [Google Scholar] [CrossRef] [Green Version]
- Mellado, M.; Contreras, R.A.; González, A.; Dennett, G.; Moenne, A. Copper-induced synthesis of ascorbate, glutathione and phytochelatins in the marine alga Ulva compressa (Chlorophyta). Plant Physiol. Biochem. 2012, 51, 102–108. [Google Scholar] [CrossRef]
- Laporte, D.; Valdés, N.; González, A.; Sáez, C.A.; Zúñiga, A.; Navarrete, A.; Meneses, C.; Moenne, A. Copper-induced overexpression of genes encoding antioxidant system enzymes and metallothioneins involved the activation of CaMs, CDPKs and MEK1/2 in the marine alga Ulva compressa. Aquat. Toxicol. 2016, 177, 433–440. [Google Scholar] [CrossRef]
- González, A.; Trebotich, J.; Vergara, E.; Medina, C.; Morales, B.; Moenne, A. Copper-induced calcium release from ER involves the activation of ryanodine-sensitive and IP3-sensitive channels in Ulva compressa. Plant Signal. Behav. 2010, 5, 1647–1649. [Google Scholar] [CrossRef] [Green Version]
- González, A.; Cabrera, M.A.; Mellado, M.; Cabello, S.; Márquez, S.; Morales, B.; Moenne, A. Copper-induced intracellular calcium release requires extracellular calcium entry and activation of L-type voltage-dependent calcium channels in Ulva compressa. Plant Signal. Behav. 2012, 7, 1–5. [Google Scholar]
- Gómez, M.; González, A.; Sáez, C.A.; Morales, B.; Moenne, A. Copper-induced activation of TRP channels promotes extracellular calcium entry, activation of CaMs and CDPKs, copper entry and membrane depolarization in Ulva compressa. Front. Plant Sci. 2015, 6, 182. [Google Scholar] [PubMed] [Green Version]
- Gómez, M.; González, A.; Sáez, C.A.; Moenne, A. Copper-induced membrane depolarizations involve the induction of mosaic TRPs channels, which activate VDCC leading to calcium increases in Ulva compressa. Front. Plant Sci. 2016, 7, 754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez, M.; González, A.; Moenne, F.; Sáez, C.A.; Moenne, A. Copper-induced early responses involve the activation of Transient receptor Potential (TRP) channels, release of amino acids, serotonin and adrenalin and activation of homologs of glutamate, adrenalin and serotonin receptors in the marine alga Ulva compressa. Algal Res. 2017, 26, 115–122. [Google Scholar]
- Rodríguez-Rojas, F.; Celis-Plá, P.S.M.; Méndez, L.; Moenne, F.; Muñoz, P.T.; Lobos, M.G.; Díaz, P.; Sánchez-Lizaso, J.L.; Brown, M.T.; Moenne, A.; et al. MAPK pathways under chronic copper excess in green macroalgae (Chlorophyta): Involvement in the regulation of detoxification mechanisms. Int. J. Mol. Sci. 2019, 20, 4546. [Google Scholar]
- Celis-Plá, P.; Rodríguez-Rojas, F.; Méndez, L.; Moenne, F.; Muñoz, P.T.; Lobos, M.G.; Díaz, P.; Sánchez-Lisazo, J.L.; Brown, M.T.; Moenne, A.; et al. MAPK pathway under chronic copper excess in green macroalgae (Chlorophyta): Influence on metal exclusion/extrusion mechanisms and photosynthesis. Int. J. Mol. Sci. 2019, 20, 4547. [Google Scholar]
- Navarrete, A.; González, A.; Gómez, M.; Contreras, R.A.; Díaz, P.; Lobos, G.; Brown, M.T.; Sáez, C.A.; Moenne, A. Copper excess detoxification is mediated by a coordinated and complementary induction of glutathione, phytochelatins and metallothioneins in the green seaweed Ulva compressa. Plant Physiol. Biochem. 2019, 129, 423–431. [Google Scholar] [CrossRef] [Green Version]
- Espinoza, D.; González, A.; Pizarro, J.; Segura, R.; Laporte., D.; Rodríguez-Rojas, F.; Sáez, C.A.; Moenne, A. Copper-induced synthesis of metallothioneins and accumulation of copper-containing particles in chloroplasts of Ulva compressa (Chlorophyta). 2020; in preparation. [Google Scholar]
- Leal, M.F.C.; Vascocelos, M.; Van der Berg, C.M.G. Copper-induced release of complexing ligands similar to thiols by Emiliana huxleyi in seawater cultures. Limnol. Oceanogr. 1999, 44, 1750–1752. [Google Scholar] [CrossRef] [Green Version]
- Vasconcelos, M.; Leal, M.F.C. Seasonal variability in the kinetics of Cu, Pb, Cd and Hg accumulation by macroalgae. Mar. Chem. 2001, 75, 123–129. [Google Scholar] [CrossRef]
- Rodríguez, F.; Laporte, D.; González, A.; Méndez, K.N.; Castro-Nallar, E.; Meneses, C.; Huidobro-Toro, J.P.; Moenne, A. Copper-induced increased expression of genes involved in photosynthesis, carotenoid synthesis and C assimilation in the marine alga Ulva compressa. BMC Genomics 2018, 19, 829. [Google Scholar] [CrossRef]
- Laporte, D.; Rodríguez, F.; González, A.; Zúñiga, A.; Castro-Nallar, E.; Sáez, C.A.; Moenne, A. Copper-induced concomitant increases in photosynthesis, respiration, and C, N and S assimilation revealed by transcriptomic analyses in Ulva compressa (Chlorophyta). BMC Plant Biol. 2020, 20, 25. [Google Scholar] [CrossRef] [PubMed]
- Laporte, D.; González, A.; Moenne, A. Copper-induced activation of MAPKs, CDPKs and CaMKs induced activation of hexokinase and inhibition of pyruvate kinase leading to increased synthesis of ASC, GSH and NADPH in Ulva compressa. Front. Plant Sci. 2020. under review. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moenne, A.; Gómez, M.; Laporte, D.; Espinoza, D.; Sáez, C.A.; González, A. Mechanisms of Copper Tolerance, Accumulation, and Detoxification in the Marine Macroalga Ulva compressa (Chlorophyta): 20 Years of Research. Plants 2020, 9, 681. https://doi.org/10.3390/plants9060681
Moenne A, Gómez M, Laporte D, Espinoza D, Sáez CA, González A. Mechanisms of Copper Tolerance, Accumulation, and Detoxification in the Marine Macroalga Ulva compressa (Chlorophyta): 20 Years of Research. Plants. 2020; 9(6):681. https://doi.org/10.3390/plants9060681
Chicago/Turabian StyleMoenne, Alejandra, Melissa Gómez, Daniel Laporte, Daniela Espinoza, Claudio A. Sáez, and Alberto González. 2020. "Mechanisms of Copper Tolerance, Accumulation, and Detoxification in the Marine Macroalga Ulva compressa (Chlorophyta): 20 Years of Research" Plants 9, no. 6: 681. https://doi.org/10.3390/plants9060681





