Growth, Physiology and Nutrient Use Efficiency in Eugenia dysenterica DC under Varying Rates of Nitrogen and Phosphorus
Abstract
:1. Introduction
2. Results
2.1. Physiological Traits
2.2. Morphological Traits
2.3. Contribution of Nitrogen and Phosphorous Rates to Physiological and Morphological Changes in Eugenia dysenterica Seedlings
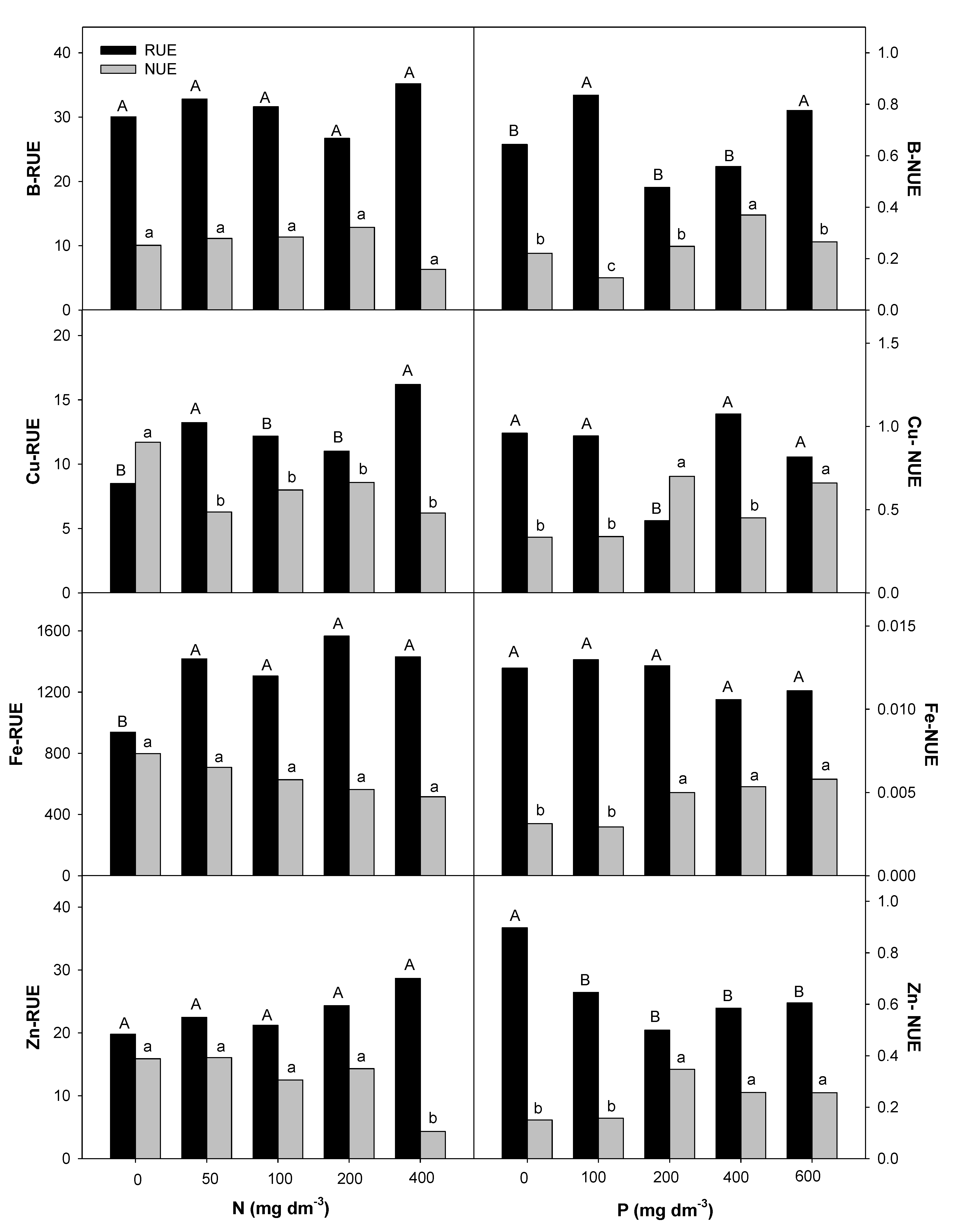
2.4. Nitrogen and Phosphorous Uptake and Use Efficiency
3. Discussion
3.1. Nitrogen Fertilization Increased Photosynthetic Rates of Eugenia dysenterica
3.2. EugeniaDysenterica Growth Was Stimulated by Phosphate Fertilization
3.3. Effect of Nitrogen and Phosphorous Rates on Root Uptake and Plant Nutrient Use Efficiencies
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Treatments and Experimental Design
4.3. Evaluations
4.3.1. Physiological Traits
4.3.2. Morphological Traits
4.3.3. Root Uptake Efficiency and Plant Nutrient Use Efficiency
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rezende-Silva, S.L.; Costa, A.C.; Dyszyb, F.H.; Batista, P.F.; Crispim-Filho, A.J.; Nascimento, K.J.T. Pouteria torta is a remarkable native plant for biomonitoring the glyphosate effects on Cerrado vegetation. Ecol. Indic. 2019, 102, 497–506. [Google Scholar] [CrossRef]
- Arruda, H.S.; Botrel, D.A.; Fernandes, R.V.B.; Almeida, M.E.F. Development and sensory evaluation of products containing the Brazilian Savannah fruits araticum (Annona crassiflora Mart.) and cagaita (Eugenia dysenterica Mart.). Braz. J. Food Technol. 2016, 19, e2015105. [Google Scholar] [CrossRef]
- Moreira, L.C.; Ávila, R.I.; Veloso, D.F.M.C.; Pedrosa, T.N.; Lima, E.S.; Couto, R.O.; Lima, E.M.; Batista, A.C.; Paula, J.R.; Valadares, M.C. In vitro safety and efficacy evaluations of a complex botanical mixture of Eugenia dysenterica DC. (Myrtaceae): Prospects for developing a new dermocosmetic product. Toxicol. In Vitro 2017, 1, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tunholi, V.P.; Ramos, M.A.; Scariot, A. Availability and use of woody plants in a agrarian reform settlement in the cerrado of the state of Goiás, Brazil. Acta Bot. Bras. 2013, 27, 604–612. [Google Scholar] [CrossRef]
- Rodrigues, A.A.; Vasconcelos Filho, S.C.V.; Müller, C.; Rodrigues, D.A.; Sales, J.F.; Zuchi, J.; Costa, A.C.; Rodrigues, C.L.; Silva, A.A.; Barbosa, D.P. Tolerance of Eugenia dysenterica to aluminum: Germination and plant growth. Plants 2019, 8, 317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes, A.S.; Guilherme, L.R.G. A career perspective on soil management in the Cerrado region of Brazil. Adv. Agron. 2016, 137, 1–72. [Google Scholar] [CrossRef]
- Souza, E.R.B.; Naves, R.V.; Oliveira, M.F. Initial fruiting of the cagaita tree (Eugenia dysenterica DC) cultivated in Goiânia, Goiás, Brazil. Rev. Bras. Frutic. 2013, 35, 906–909. [Google Scholar] [CrossRef] [Green Version]
- Camilo, Y.M.V.; Souza, E.R.B.; Vera, R.; Naves, R.V. Phenology, production and precocity of Eugenia dysenterica plants aiming breeding. Rev. Ciênc. Agrár. 2013, 36, 192–198. [Google Scholar]
- Huber, D.; Romheld, V.; Weinmann, M. Relationship between Nutrition, Plant Diseases and Pests. In Marschener’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Elsevier/Academic Press: Amsterdam, The Netherlands, 2012; pp. 283–298. [Google Scholar]
- Pallardy, S.G. Nitrogen Metabolism. In Physiology of Woody Plants, 3rd ed.; Pallardy, S.G., Ed.; Academic Press: Missouri, MO, USA, 2008; pp. 233–254. [Google Scholar]
- Pilbeam, D.J. Nitrogen. In Plant Nutrition Handbook, 2nd ed.; Pilbeam, D.J., Barker, A.V., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2015; pp. 20–64. [Google Scholar] [CrossRef]
- Hopkins, B.G. Phosphorus. In Plant Nutrition Handbook, 2nd ed.; Pilbeam, D.J., Barker, A.V., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2015; pp. 65–126. [Google Scholar] [CrossRef]
- Singh, S.K.; Reddy, V.R.; Fleisher, D.H.; Timlin, D.J. Relationship between photosynthetic pigments and chlorophyll fluorescence in soybean under varying phosphorus nutrition at ambient and elevated CO2. Photosynthetica 2017, 55, 421–433. [Google Scholar] [CrossRef]
- Cheng, L.; Fuchigami, L.H. Rubisco activation state decreases with increasing nitrogen content in apple leaves. J. Exp. Bot. 2000, 51, 1687–1694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warren, C.R.; Adams, M.A. Phosphorus affects growth and partioning of nitrogen to Rubisco in Pinus pinaster. Tree Physiol. 2002, 22, 11–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freitas, J.C.O.; Almeida, A.A.F.; Lago, M.F.; Souza, M.M.; Junior, J.O.S. Morphophysiological characteristics of clonal plants Passiflora alata grown in different doses of nitrogen and shading levels. Rev. Bras. Frutic. 2012, 34, 859–872. [Google Scholar] [CrossRef] [Green Version]
- Barreto, P.A.B.; Gama-Rodrigues, A.C.; Gama-Rodrigues, E.F.; Barros, N.F. Nitrogen balance in soil under eucalyptus plantations. Rev. Bras. Cien Solo 2012, 36, 1239–1248. [Google Scholar] [CrossRef] [Green Version]
- Carranca, C.; Brunetto, G.; Tagliavini, M. Nitrogen nutrition of fruit trees to reconcile productivity and environmental concerns. Plants 2018, 7, 4. [Google Scholar] [CrossRef] [Green Version]
- Jacob, J.; Lawlor, D.W. Stomatal and mesophyll limitations of photosynthesis in phosphate deficient sunflower, maize and wheat plants. J. Exp. Bot. 1991, 42, 1003–1011. [Google Scholar] [CrossRef]
- Seika, N.; Yano, K. Stomatal density of cowpea correlates with carbon isotope discrimination in different phosphorus, water and CO2 environments. New Phytol. 2008, 179, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Salter, W.T.; Turnbull, T.L.; Okazaki, Y.; Saito, K.; Kreuzwieser, J.; Rennenberg, H.; Adams, M.A. Plant and soil P determine functional attributes of subalpine Australian plants. Arct Antarct. Alp. Res. 2018, 50, 1. [Google Scholar] [CrossRef] [Green Version]
- Bilger, W.; Björkman, O. Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis. Photosynth. Res. 1990, 5, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Carstensen, A.; Herdean, A.; Schimidt, S.B.; Sharma, A.; Spetea, C.; Pribil, M.; Husted, S. The impacts of phosphorus deficiency on the photosynthetic electron transport chain. Plant. Physiol. 2018, 177, 271–284. [Google Scholar] [CrossRef] [Green Version]
- Duarte, T.F.; Bron, I.U.; Ribeiro, R.V.; Machado, E.C.; Mazzafera, P.; Shimizu, M.M. Effect of crop loading on quality of ‘Valencia’ orange fruit. Rev. Bras. Frutic. 2011, 33, 823–829. [Google Scholar] [CrossRef] [Green Version]
- Tosta, M.S.; Almeida, J.P.N.; Góes, G.B.; Freire, P.A.; Mendonça, V. Nitrogen fertilization in the production of seedlings of Talisia suculenta (A. ST. Hil) Radlk. R. Bras. Eng. Agríc. Ambiental. 2017, 21, 443–447. [Google Scholar] [CrossRef] [Green Version]
- Millard, P.; Grelet, G. Nitrogen storage and remobilization by trees: Ecophysiological relevance in a changing world. Tree Physiol. 2010, 30, 1083–1095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Oosten, M.J.; Dell’Aversana, E.; Ruggiero, A.; Cirillo, V.; Gibon, Y.; Woodrow, P.; Maggio, A.; Carillo, P. Omeprazole treatment enhances nitrogen use efficiency through increased nitrogen uptake and assimilation in corn. Front. Plant. Sci. 2019, 10, 1507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van de Wiel, C.C.M.; van der Linden, C.G.; Scholten, O.E. Improving phosphorus use efficiency in agriculture: Opportunities for breeding. Euphytica 2016, 207, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Tng, D.Y.; Janos, D.P.; Jordan, G.J.; Weber, E.; Bowman, D.M. Phosphorus limits Eucalyptus grandis seedling growth in an unburnt rain forest soil. Front. Plant. Sci. 2014, 5, 527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Razaq, M.; Zhang, P.; Shen, H.; Salahuddin. Influence of nitrogen and phosphorus on the growth and root morphology of Acer mono. PLoS ONE 2017, 12, e0171321. [Google Scholar] [CrossRef] [Green Version]
- Sardans, J.; Peñuelas, J. Trees increase their P : N ratio with size. Glob. Ecol. Biogeogr. 2015, 24, 147–156. [Google Scholar] [CrossRef] [Green Version]
- Freibeiger, M.B.; Guerrini, I.A.; Galetti, G.; Fernandes, D.M.; Corrêa, J.C. Early growth and nutrition of cedar (Cedrela fissilis Vell.) as affected by nitrogen rates. Rev. Árvore. 2013, 37, 385–392. [Google Scholar] [CrossRef]
- Ulas, A.; Doganci, E.; Ulas, F.; Yetisir, H. Root-growth characteristics contributing to genotypic variation in nitrogen efficiency of bottle gourd and rootstock potential for watermelon. Plants 2019, 8, 77. [Google Scholar] [CrossRef] [Green Version]
- Melo, J.T.; Haridasan, M. Response of cagaita (Eugenia dysenterica DC) seedlings to N, P, K, Ca and Mg doses, 1st ed.; EMBRAPA—Boletim de Pesquisa e Desenvolvimento: Planaltina, DF, Brazil, 2009; p. 27. (In Portuguese) [Google Scholar]
- Costa, A.M.; Carlos, L.; Silva, P.O.; Barbosa, K.P.; Rodrigues, C.R. Nitrogen and potassium fertilization in the initial growth of Annona crassiflora Mart. Florest. Ambiente. 2019, 26, 1–9. [Google Scholar] [CrossRef]
- Constantino, V.; Barbosa, J.Z.; Motta, A.V.; Dolinski, M.A.; Prior, S.A.; Zanette, F. Initial growth of Araucaria angustifolia (Bertol.) Kuntze in response to fertilization with nitrogen, phosphorus and potassium. Floresta 2019, 49, 99–108. [Google Scholar] [CrossRef]
- Bessa, L.A.; Moreira, M.A.; Silva, F.G.; Mota, C.S.; Vitorino, L. Growth, nutrient concentration and principal component analysis of Cagaita (Eugenia dysenterica DC.) seedlings grown in nutrient solution. Aust. J. Crop Sci. 2016, 10, 425–433. [Google Scholar] [CrossRef]
- Shen, H.; Chen, J.; Wang, Z.; Yang, C.; Sasaki, T.; Yamamoto, Y.; Matsumoto, H.; Yan, X. Root plasma membrane H+-ATPase is involved in the adaptation of soybean to phosphorus starvation. J. Exp. Bot. 2006, 57, 1353–1362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silveira, C.E.S.; Palhares, D.; Pereira, L.A.R.; Pereira, K.B.D.; Silva, F.A.B. Strategies of plant establishment of two Cerrado species: Byrsonima basiloba Juss. (Malpighiaceae) and Eugenia dysenterica Mart. Ex DC. (Myrtaceae). Plant. Spec. Biol. 2013, 28, 130–137. [Google Scholar] [CrossRef]
- Carnevali, N.H.S.; Marchetti, M.E.; Vieira, M.C.; Carnevali, T.O.; Ramos, D.D. Nutritional efficiency of Stryphnodendron polyphyllum seedlings in function of nitrogen and phosphorus. Ciên. Florest. 2016, 26, 449–461. [Google Scholar] [CrossRef] [Green Version]
- Pinto, J.V.; Vieira, M.C.; Zárate, N.A.H.; Formagio, A.S.N.; Cardoso, C.A.L.; Carnevali, T.O.; Souza, P.H.N. Effect of soil nitrogen and phosphorus on early development and essential oil composition of Schinus terebinthifolius Raddi. J. Essent. Oil Bear. Pl. 2016, 19, 247–257. [Google Scholar] [CrossRef]
- Fageria, N.K. Nitrogen interaction with other nutrients. In Nitrogen Management in Crop Production; Fageria, N.K., Ed.; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2014; pp. 245–274. [Google Scholar]
- Pimentel, L.D.; Bruckner, C.H.; Martinez, H.E.P.; Motoike, S.Y.; Manfio, C.E.; Santos, R.C. Effect of nitrogen and potassium rates on early development of macaw palm. Rev. Bras. Ciênc. Solo 2015, 39, 1671–1680. [Google Scholar] [CrossRef] [Green Version]
- Pavinato, P.S.; Merlin, A.; Rosolem, C.A. Phosphorus fractions in Brazilian Cerrado soils as affected by tillage. Soil Till. Res. 2009, 105, 149–155. [Google Scholar] [CrossRef]
- White, P.J. Long-distance transport in the xylem and phloem. In Marschener’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Elsevier/Academic Press: Amsterdam, The Netherlands, 2012; pp. 42–70. [Google Scholar]
- Grzebisz, W. Potassium fertilization of arable crops—The crop rotation oriented concept. Fertiliz 2005, 3, 328–341. [Google Scholar] [CrossRef] [Green Version]
- Marschner, P.; Rengel, Z. Nutrient availability in soils. In Marschener’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Elsevier/Academic Press: Amsterdam, The Netherlands, 2012; pp. 315–330. [Google Scholar]
- White, P.J.; Broadley, M.R. Calcium in plants. Ann. Bot. 2003, 92, 487–511. [Google Scholar] [CrossRef]
- Mengel, K.; Kirkby, E.A. Principles of Plant Nutrition, 5th ed.; Kluwer Academic Publisher: Dodrecht, The Netherlands, 2001; pp. 210–212. [Google Scholar]
- Andrés, Z.; Pérez-Hormaeche, J.; Leidi, E.O.; Schulucking, K.; Steinhorst, L.; Mclachlan, D.H.; Pardo, J.M. Control of vacuolar dynamics and regulation of stomatal aperture by tonoplast potassium uptake. Proc. Natl. Acad. Sci. USA 2014, 111, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alva, A.K.; Mattos-Junior, D.; Paramasivam, S.; Patil, B.; Dou, H.; Sajwan, K. Potassium management for optimizing citrus production and quality. Int. J. Fruit Sci. 2006, 6, 3–43. [Google Scholar] [CrossRef]
- Quaggio, J.A.; Mattos-Junior, D.; Boaretto, R.M. Sources and rates of potassium for sweet orange production. Sci. Agricola 2011, 68, 369–375. [Google Scholar] [CrossRef]
- Gonçalves, W.V.; Vieira, M.C.D.; Carnevali, T.O.; Zárate, N.A.H.; Aran, H.D.V.R.; Mineli, K.C.S. Nitrogen and phosphorus fertilization promotes aerial part development and affect nutrient uptake by carobinha of the Brazilian Cerrado. Am. J. Plant Sci. 2017, 8, 3377–3398. [Google Scholar] [CrossRef] [Green Version]
- Bessa, L.A.; Silva, F.G.; Moreira, M.A.; Teodoro, J.P.R.; Soares, F.A.L. Characterization of nutrient deficiency in Hancornia speciosa Gomes seedlings by omitting micronutrients from the nutrient solution. Rev. Bras. Frutic. 2013, 35, 616–624. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, I.R.; Borém, A.; Araújo, G.A.A.; Fontes, R.L.F. Manganese and zinc leaf application on common bean grown on a “Cerrado”. Soil Sci. Agric. 2004, 61, 77–81. [Google Scholar] [CrossRef]
- Carlisle, E.; Myers, S.; Raboy, V.; Bloom, A. The effects of inorganic nitrogen form and CO2 concentration on wheat yield and nutrient accumulation and distribution. Front. Plant Sci. 2012, 3, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, M.; Nakagawa, H.; Asaka, T.; Matoh, T. Boraterhamnogalacturonan II bonding reinforced by Ca2+ retains pectic polysaccharides in higher-plant cell walls. Plant Physiol. 1999, 119, 199–204. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.; Brown, P.H. Localization of boron in cell walls of squash and tobacco and its association with pectin. Plant Physiol. 1994, 105, 681–689. [Google Scholar] [CrossRef] [Green Version]
- Brun, F.G.K.; Brin, E.J.; Gerber, D.; Szymczak, D.A.; Londero, A.K.; Meyer, E.; Navrosky, M.C. Nutrition facts and limits for micronutrients in tree species used in urban forestry. An. Acad. Bras. Ciênc. 2017, 89, 1881–1893. [Google Scholar] [CrossRef]
- Silva, F.C.; Abreu, M.F.; Perez, D.V.; Eira, P.A.; Abreu, C.A.; Raij, B.; Gianello, C.; Coelho, A.M.; Quaggio, J.A.; Tedesco, M.J.; et al. Chemical Analysis Methods for Soil Fertility Evaluation, 2nd ed.; Embrapa Solos: Rio de Janeiro, Brazil, 2009; p. 627. [Google Scholar]
- Malavolta, E.; Vitti, G.C.; Oliveira, A.S. Evaluation of Plant Nutritional Status: Principles and Applications, 2nd ed.; Potafos: Piracicaba, Brazil, 1997; pp. 215–241. [Google Scholar]
- Swiader, J.M.; Chyan, Y.; Freiji, F.G. Genotypic differences in nitrate uptake and utilization efficiency in pumpkin hybrids. J. Plant Nutr. 1994, 17, 1687–1699. [Google Scholar] [CrossRef]
- Siddiqi, M.Y.; Glass, A.D.M. Utilization index: A modified approach to the estimation and comparison of nutrient utilization efficiency in plants. J. Plant Nutr. 1981, 4, 289–302. [Google Scholar] [CrossRef]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nogueira dos Reis, D.; Guimarães Silva, F.; da Costa Santana, R.; Caetano de Oliveira, T.; Brito Freiberger, M.; Barbosa da Silva, F.; Monteiro Júnior, E.; Müller, C. Growth, Physiology and Nutrient Use Efficiency in Eugenia dysenterica DC under Varying Rates of Nitrogen and Phosphorus. Plants 2020, 9, 722. https://doi.org/10.3390/plants9060722
Nogueira dos Reis D, Guimarães Silva F, da Costa Santana R, Caetano de Oliveira T, Brito Freiberger M, Barbosa da Silva F, Monteiro Júnior E, Müller C. Growth, Physiology and Nutrient Use Efficiency in Eugenia dysenterica DC under Varying Rates of Nitrogen and Phosphorus. Plants. 2020; 9(6):722. https://doi.org/10.3390/plants9060722
Chicago/Turabian StyleNogueira dos Reis, Daniele, Fabiano Guimarães Silva, Reginaldo da Costa Santana, Thales Caetano de Oliveira, Mariângela Brito Freiberger, Fábia Barbosa da Silva, Elídio Monteiro Júnior, and Caroline Müller. 2020. "Growth, Physiology and Nutrient Use Efficiency in Eugenia dysenterica DC under Varying Rates of Nitrogen and Phosphorus" Plants 9, no. 6: 722. https://doi.org/10.3390/plants9060722
APA StyleNogueira dos Reis, D., Guimarães Silva, F., da Costa Santana, R., Caetano de Oliveira, T., Brito Freiberger, M., Barbosa da Silva, F., Monteiro Júnior, E., & Müller, C. (2020). Growth, Physiology and Nutrient Use Efficiency in Eugenia dysenterica DC under Varying Rates of Nitrogen and Phosphorus. Plants, 9(6), 722. https://doi.org/10.3390/plants9060722





