The Effect of Single and Combined Use of Gamma Radiation and Ethylmethane Sulfonate on Early Growth Parameters in Sorghum
Abstract
1. Introduction
2. Results
2.1. Experiment I
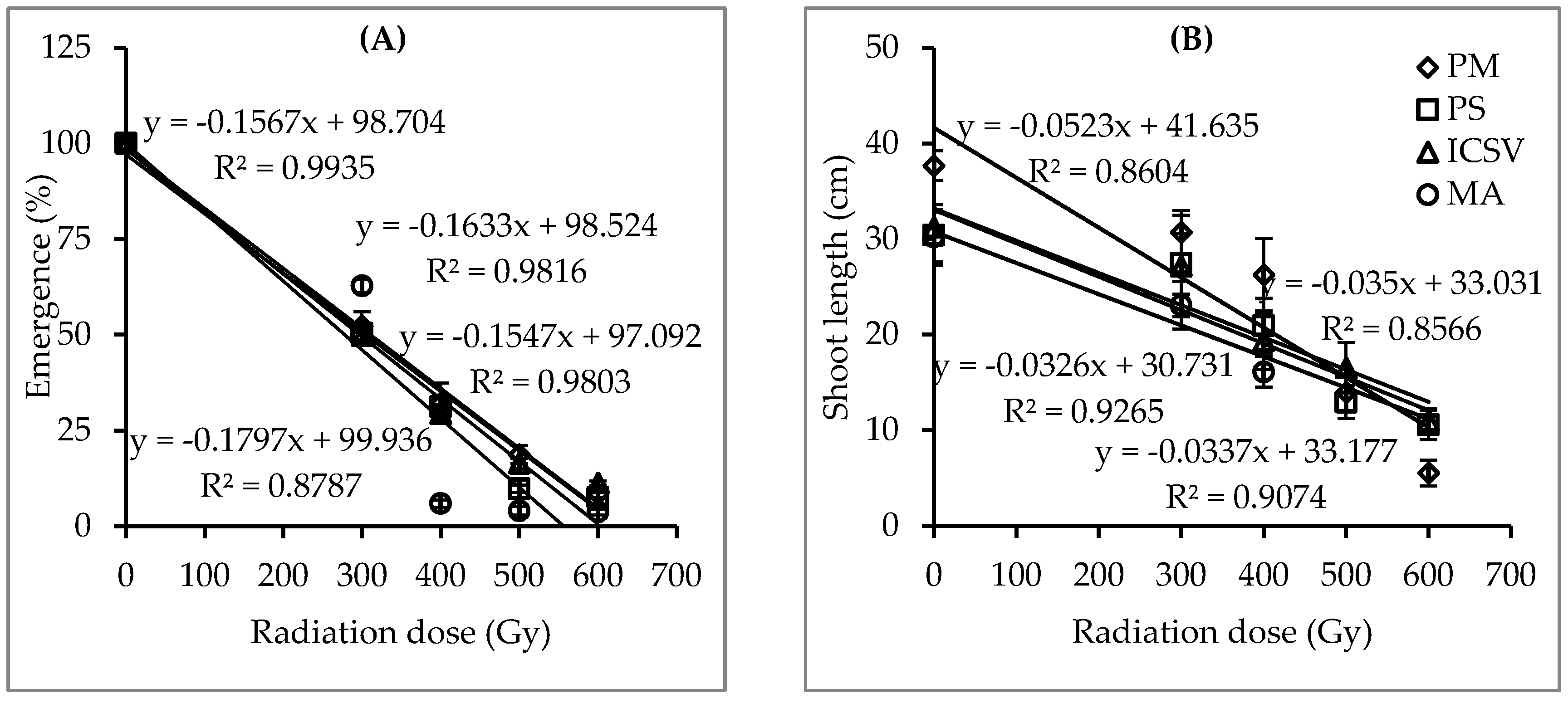
2.1.1. Effect of Single Doses of Gamma Radiation
2.1.2. Effect of Single Doses of EMS
2.1.3. Effect of Combined Doses of Gamma Radiation and EMS
2.2. Experiment II
3. Discussion
3.1. Effect of Single Doses of Gamma Radiation
3.2. Effect of Single Doses of EMS
3.3. Effect of Combined Doses of Gamma Radiation and EMS
4. Materials and Methods
4.1. Study Site and Plant Material
4.2. Seed Treatment, Planting and Experimental Design
4.2.1. Experiment I
4.2.2. Experiment II
4.3. Data Collection
4.4. Data Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lobell, D.B. Climate change adaptation in crop production: Beware of illusions. Glob. Food Sec. 2014, 3, 72–76. [Google Scholar] [CrossRef]
- FAOSTAT Statistical Database. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 29 September 2019).
- Jiang, S.Y.; Ramachandran, S. Natural and artificial mutants as valuable resources for functional genomics and molecular breeding. Int. J. Biol. Sci. 2010, 6, 228–251. [Google Scholar] [CrossRef] [PubMed]
- Shu, Q.Y.; Forster, B.P.; Nakagawa, H. Principles and applications of mutation breeding. In Plant Mutation Breeding and Biotechnology; CABI: Wallingford, CT, USA, 2012; pp. 301–325. [Google Scholar]
- Kim, Y.S.; Schumaker, K.S.; Zhu, J.K. EMS mutagenesis of arabidopsis. Methods Mol. Biol. 2006, 323, 101–103. [Google Scholar] [PubMed]
- Sikder, S.; Biswas, P.; Hazra, P.; Akhtar, S.; Chattopadhyay, A.; Badigannavar, A.M.; D’Souza, S.F. Induction of mutation in tomato (Solanum Lycopersicum L.) by gamma irradiation and EMS. Indian J. Genet. Plant Breed. 2013, 73, 392. [Google Scholar] [CrossRef]
- Davison, J.; Ammann, K. New GMO regulations for old: Determining a new future for EU crop biotechnology. GM Crops Food. 2017, 8, 13–34. [Google Scholar] [CrossRef]
- Gruère, G.P.; Sengupta, D. Biosafety and Perceived Commercial Risks: The Role of GM-Free Private Standards; IFPRI Discussion Paper 847; International Food Policy Research Institute: Washington, DC, USA, 2009. [Google Scholar]
- Gruère, G.P.; Rosegrant, M.W. Assessing the implementation effects of the biosafety protocol’s proposed stringent information requirements for genetically modified commodities in countries of the Asia Pacific economic cooperation. Rev. Agric. Econ. 2008, 30, 214–232. [Google Scholar] [CrossRef]
- Mba, C.; Afza, R.; Bado, S.; Jain, S.M. Induced mutagenesis in plants using physical and chemical agents. In Plant Cell Culture: Essential Methods; Davey, M.R., Anthony, P., Eds.; John Wiley and Sons, Ltd.: Chichester, UK, 2010; pp. 111–130. [Google Scholar]
- Parry, M.A.J.; Madgwick, P.J.; Bayon, C.; Tearall, K.; Hernandez-Lopez, A.; Baudo, M.; Rakszegi, M.; Hamada, W.; Al-Yassin, A.; Ouabbou, H.; et al. Mutation discovery for crop improvement. J. Exp. Bot. 2009, 60, 2817–2825. [Google Scholar] [CrossRef]
- Wolabu, T.W.; Tadege, M. Photoperiod response and floral transition in sorghum. Plant Signal. Behav. 2016, 11, 1261232. [Google Scholar] [CrossRef]
- Chiurugwi, T.; Kemp, S.; Powell, W.; Hickey, L.T. Speed breeding orphan crops. Theor. Appl. Genet. 2019, 132, 607–616. [Google Scholar] [CrossRef]
- Jankowicz-Cieslak, J.; Mba, C.; Till, B.J. Mutagenesis for crop breeding and functional genomics. In Biotechnologies for Plant Mutation Breeding: Protocols; Jankowicz-Cieslak, J., Tai, T., Kumlehn, J., Till, B., Eds.; Springer: Cham, Switzerland, 2017; pp. 3–18. [Google Scholar]
- Watson, A.; Ghosh, S.; Williams, M.J.; Cuddy, W.S.; Simmonds, J.; Rey, M.D.; Asyraf Md Hatta, M.; Hinchliffe, A.; Steed, A.; Reynolds, D.; et al. Speed breeding is a powerful tool to accelerate crop research and breeding. Nat. Plants 2018, 4, 23–29. [Google Scholar] [CrossRef]
- Deshmukh, S.B.; Bagade, A.B.; Choudhari, A.K. Induced mutagenesis in rabi sorghum. Int. J. Curr. Microbiol. App. Sci. 2018, 6, 766–771. [Google Scholar]
- Mani, N.S. EMS–Induced mutagenesis in Sorghum Bicolor (L.) Moench. Proc. Indian Natl. Sci. Acad. 1989, B55, 477–482. [Google Scholar]
- Suthakar, V.; Mullainathan, L.; Elangvoan, M. Mutagenic effect of gamma rays and EMS on yield attributes of sorghum (Sorghum Bicolour (L.) Moench) in M1 generation. Int. J. Adv. Res. 2014, 2, 457–465. [Google Scholar]
- Kodym, A.; Afza, R. Physical and chemical mutagenesis. Methods Mol. Biol. 2003, 236, 189–204. [Google Scholar] [PubMed]
- Acquaah, G. Principles of Plant Genetics and Breeding, 2nd ed.; Wiley: Hoboken, NJ, USA, 2012. [Google Scholar]
- Oladosu, Y.; Rafii, M.Y.; Abdullah, N.; Hussin, G.; Ramli, A.; Rahim, H.A.; Miah, G.; Usman, M. Principle and application of plant mutagenesis in crop improvement: A review. Biotechnol. Biotechnol. Equip. 2016, 30, 1–16. [Google Scholar] [CrossRef]
- Kenga, R.; Alabi, S.; Gupta, S. Heterosis and combining ability for grain yield and its components in induced sorghum mutants. Afr. Crop Sci. J. 2005, 13, 143–152. [Google Scholar] [CrossRef]
- Bretaudeau, A. Radiation induced mutations for breeding of sorghum (IAEA-TECDOC—951). International Atomic Energy Agency (IAEA): Vienna, Austria, 1997; pp. 25–29. [Google Scholar]
- Mizuno, H.; Kawahigashi, H.; Ogata, J.; Minami, H.; Kanamori, H.; Nakagawa, H.; Matsumoto, T. Genomic inversion caused by gamma irradiation contributes to downregulation of a WBC11 homolog in bloomless sorghum. Theor. Appl. Genet. 2013, 126, 1513–1520. [Google Scholar] [CrossRef]
- Xin, Z.; Huang, J.; Smith, A.R.; Chen, J.; Burke, J.; Sattler, S.E.; Zhao, D. Morphological characterization of a new and easily recognizable nuclear male sterile mutant of sorghum (Sorghum bicolor). PLoS ONE 2017, 12, e0165195. [Google Scholar] [CrossRef]
- Xin, Z.; Li Wang, M.; Barkley, N.A.; Burow, G.; Franks, C.; Pederson, G.; Burke, J. Applying genotyping (TILLING) and phenotyping analyses to elucidate gene function in a chemically induced sorghum mutant population. BMC Plant Biol. 2008, 8, 103. [Google Scholar] [CrossRef]
- Jiao, Y.; Burow, G.; Gladman, N.; Acosta-Martinez, V.; Chen, J.; Burke, J.; Ware, D.; Xin, Z. Efficient identification of causal mutations through sequencing of bulked F2 from two allelic bloomless mutants of Sorghum bicolor. Front. Plant Sci. 2017, 8, 2267. [Google Scholar] [CrossRef]
- MVD–Home. Available online: https://mvd.iaea.org/#!Home (accessed on 10 February 2020).
- Human, S.; Andreani, S.; Sihono, S.; Indriatama, W.M. Stability test for sorghum mutant lines derived from induced mutations with gamma-ray irradiation. Atom Indones. 2011, 37, 102. [Google Scholar] [CrossRef]
- Sree-Ramulu, K. Induced structural changes and meiotic abberrations in sorghum. Cytologia (Tokyo) 1971, 36, 229–236. [Google Scholar] [CrossRef]
- Reddy, C.S.; Smith, J.D. Mutagenic effects of combination treatments of hadrazine, ethyl methanesulphonate and gamma rays in Sorghum bicolor (L.) Moench. Indian J. Bot. 1981, 4, 5–14. [Google Scholar]
- Sree-Ramulu, K. Effectiveness and efficiency of single and combined treatments of radiations and ethyl methane sulphonate in sorghum. Proc. Indian Acad. Sci. 1971, 74, 147–154. [Google Scholar]
- Omar, S.R.; Ahmed, O.H.; Saamin, S.; Majid, N.M.A. Gamma radiosensitivity study on chili (Capsicum annuum). Am. J. Appl. Sci. 2008, 5, 67–70. [Google Scholar] [CrossRef]
- Mudibu, J.; Nkongolo, K.C.K.; Kalonji-Mbuyi, A.; Kizungu, V.R. Effect of gamma irradiation on morpho-agronomic characteristics of soybeans (Glycine max L.). Am. J. Plant Sci. 2012, 3, 331–337. [Google Scholar] [CrossRef]
- Golubinova, I.; Gecheff, K. M1 cytogenetic and physiological effects of gamma-rays in sudan grass (Sorghum sudanense (piper.) stapf). Bulg. J. Agric. Sci. 2011, 17, 417–423. [Google Scholar]
- Dhamayanthi, K.P.M.; Reddy, V.R.K. Cytogenetic effects of gamma rays and ethyl methane sulphonate in chilli pepper (Capsicum annuum L.). Cytologia (Tokyo) 2000, 65, 129–133. [Google Scholar] [CrossRef]
- Ojiewo, C.O.; Agong, S.G.; Murakami, K.; Tanaka, A.; Hase, Y.; Masuda, M. Male-sterility induced by gamma-ray irradiation of African nightshade (Solanum nigrum L. ssp. villosum) seed. J. Hortic. Sci. Biotechnol. 2005, 80, 699–704. [Google Scholar] [CrossRef]
- Tadele, Z. Mutagenesis and TILLING to dissect gene function in plants. Curr. Genom. 2016, 17, 499–508. [Google Scholar] [CrossRef]
- Manova, V.; Gruszka, D. DNA damage and repair in plants—From models to crops. Front. Plant Sci. 2015, 6, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Rajib, R.; Jagatpati, T. Assessment of chemical mutagenic effects in mutation breeding programme for M1 generation of carnation (Dianthus caryophyllus). Res. Plant Biol. 2011, 1, 1–14. [Google Scholar]
- Talebi, A.B.; Talebi, A.B.; Shahrokhifar, B. Ethyl methane sulphonate (EMS) induced mutagenesis in malaysian rice (Cv. MR219) for lethal dose determination. Am. J. Plant Sci. 2012, 3, 1661–1665. [Google Scholar] [CrossRef]
- Olaolorun, B.M.; Shimelis, H.A.; Mathew, I.; Laing, M.D. Optimising the dosage of ethyl methanesulphonate mutagenesis in selected wheat genotypes. S. Afr. J. Plant Soil. 2019, 36, 357–366. [Google Scholar] [CrossRef]
- ICRISAT. Sorghum variety Macia released in Tanzania. Int. Sorghum Millets Newsl. 2000, 41, 7. [Google Scholar]
- Wanga, M.A.; Kumar, A.A.; Kangueehi, G.N.; Shimelis, H.; Horn, L.N.; Sarsu, F.; Andowa, J.F.N. Breeding sorghum using induced mutations: Future prospect for Namibia. Am. J. Plant Sci. 2018, 9, 2696–2707. [Google Scholar] [CrossRef]
- Human, S.; Sihono, S.; Parno, P. Application of mutation techniques in sorghum breeding for improved drought tolerance. Atom Indones. 2006, 1, 35–43. [Google Scholar] [CrossRef]
- Jiao, Y.; Burke, J.; Chopra, R.; Burow, G.; Chen, J.; Wang, B.; Hayes, C.; Emendack, Y.; Ware, D.; Xin, Z. A sorghum mutant resource as an efficient platform for gene discovery in grasses. Plant Cell 2016, 28, 1551–1562. [Google Scholar] [CrossRef]
- Mba, C.; Afza, R.; Jain, S.M.; Gregorio, G.B.; Zapata-Arias, F.J. Induced mutations for enhancing salinity tolerance in rice. In Advances in Molecular Breeding toward Drought and Salt Tolerant Crops; Jenks, M.A., Hasegawa, P.M., Jain, S.M., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2007; pp. 413–454. [Google Scholar]
- Ndou, V.N.; Shimelis, H.; Odindo, A.; Modi, A.T. Response of selected wheat genotypes to ethylmethanesulphonate concentration, treatment temperature and duration. Sci. Res. Essays 2012, 8, 189–196. [Google Scholar]
- FAO/IAEA. Manual on Mutation Breeding, 3rd ed.; Pencer-Lopes, M.M., Forster, B.P., Jankuloski, L., Eds.; Food and Agriculture Organization of the United Nations: Rome, Italy, 2018. [Google Scholar]
- Horn, L.; Shimelis, H. Radio-sensitivity of selected cowpea (Vigna unguiculata) genotypes to varying gamma irradiation doses. Sci. Res. Essays 2013, 8, 1991–1997. [Google Scholar]
- Sako, Y.; Mcdonald, M.B.; Fujimura, K.; Evans, A.F.; Bennett, M.A. A system for automated seed vigor assessment. Seed Sci. Technol. 2001, 29, 625–636. [Google Scholar]
- VSN International. Genstat for Windows, 18th ed.; VSN International: Hemel Hempstead, UK, 2015. [Google Scholar]



| Mutagenic Treatments | Source of Variation | df | %E | %SS | SLT (cm) | |||
|---|---|---|---|---|---|---|---|---|
| Gamma radiation | Replication | 4 | 94.6 | 57.7 | 30.0 | |||
| Genotype (G) | 3 | 126.7 | *** | 268.2 | *** | 110.5 | *** | |
| Dose (D) | 4 | 9415.7 | *** | 6512.8 | *** | 1460.6 | *** | |
| Genotype x dose | 12 | 202.7 | *** | 326.9 | *** | 40.8 | ||
| Error | 76 | 16.4 | 22.8 | 35.3 | ||||
| EMS | Replication | 4 | 62.9 | 62.4 | 38.1 | |||
| Genotype (G) | 3 | 339 | *** | 275.7 | *** | 173.2 | *** | |
| Dose (D) | 2 | 11,831.3 | *** | 9367.7 | *** | 1391.4 | *** | |
| Genotype x dose | 6 | 148.8 | *** | 460.2 | *** | 26.3 | ||
| Error | 44 | 22.8 | 23.5 | 35.5 | ||||
| Gamma radiation followed by EMS | Replication | 4 | 49.7 | 53.5 | 45.7 | |||
| Genotype (G) | 3 | 185.4 | *** | 431.7 | *** | 58.2 | ||
| Dose (D) | 2 | 15,731.7 | *** | 11,334.6 | *** | 1805.3 | *** | |
| Genotype x dose | 6 | 168.9 | *** | 379.4 | *** | 30.2 | ||
| Error | 44 | 11.2 | 19.3 | 25 | ||||
| Mutagen | Dose | Variety | %E | %SS | SLT (cm) | |||
|---|---|---|---|---|---|---|---|---|
| Gamma radiation | 300 Gy | Parbhani Moti | 27.40 | ±3.63 | 22.20 | ±3.76 | 30.70 | ±2.26 |
| Parbhani Shakti | 31.00 | ±1.76 | 24.20 | ±1.69 | 27.42 | ±3.18 | ||
| ICSV 15013 | 23.00 | ±1.79 | 14.00 | ±2.17 | 27.16 | ±5.31 | ||
| Macia | 40.80 | ±1.66 | 32.20 | ±1.83 | 23.06 | ±2.48 | ||
| 400 Gy | Parbhani Moti | 16.80 | ±5.24 | 14.20 | ±4.65 | 26.26 | ±3.79 | |
| Parbhani Shakti | 19.20 | ±0.58 | 10.60 | ±1.60 | 20.92 | ±2.88 | ||
| ICSV 15013 | 13.60 | ±1.47 | 8.60 | ±1.44 | 19.34 | ±2.99 | ||
| Macia | 3.80 | ±1.02 | 0.80 | ±0.49 | 16.08 | ±1.60 | ||
| 500 Gy | Parbhani Moti | 9.80 | ±2.35 | 2.00 | ±0.32 | 13.94 | ±1.92 | |
| Parbhani Shakti | 6.00 | ±0.95 | 1.60 | ±0.40 | 12.93 | ±1.69 | ||
| ICSV 15013 | 7.60 | ±1.33 | 1.80 | ±0.86 | 16.50 | ±2.64 | ||
| Macia | 2.60 | ±1.12 | 0.00 | ±0.00 | - | |||
| 600 Gy | Parbhani Moti | 4.40 | ±0.81 | 1.60 | ±0.24 | 5.50 | ±1.35 | |
| Parbhani Shakti | 4.60 | ±1.94 | 1.20 | ±0.49 | 10.53 | ±1.53 | ||
| ICSV 15013 | 5.20 | ±0.58 | 1.80 | ±0.58 | 11.00 | ±1.22 | ||
| Macia | 2.40 | ±0.81 | 0.00 | ±0.00 | - | |||
| EMS | 0.50% | Parbhani Moti | 15.60 | ±3.01 | 11.20 | ±2.03 | 21.96 | ±1.33 |
| Parbhani Shakti | 25.80 | ±2.42 | 7.80 | ±2.18 | 17.26 | ±2.94 | ||
| ICSV 15013 | 21.00 | ±0.71 | 10.60 | ±1.17 | 22.82 | ±4.51 | ||
| Macia | 16.00 | ±0.71 | 4.20 | ±0.49 | 15.60 | ±1.88 | ||
| 1% | Parbhani Moti | 4.60 | ±0.87 | 3.00 | ±0.32 | 18.46 | ±3.86 | |
| Parbhani Shakti | 17.60 | ±4.62 | 6.40 | ±2.18 | 15.67 | ±1.05 | ||
| ICSV 15013 | 11.00 | ±0.63 | 4.80 | ±1.07 | 20.40 | ±3.15 | ||
| Macia | 8.00 | ±3.35 | 2.40 | ±1.69 | 9.80 | ±0.60 | ||
| Gamma radiation followed by EMS | 300 Gy + 0.1% EMS | Parbhani Moti | 8.80 | ±1.36 | 4.40 | ±0.68 | 20.00 | ±1.74 |
| Parbhani Shakti | 17.00 | ±2.00 | 9.00 | ±2.49 | 19.64 | ±2.04 | ||
| ICSV 15013 | 6.60 | ±2.82 | 1.20 | ±0.73 | 17.90 | ±4.67 | ||
| Macia | 12.80 | ±0.86 | 2.20 | ±0.37 | 13.56 | ±1.88 | ||
| 400 Gy + 0.05% EMS | Parbhani Moti | 8.40 | ±1.72 | 1.40 | ±0.51 | 10.28 | ±2.64 | |
| Parbhani Shakti | 3.80 | ±0.66 | 0.60 | ±0.40 | 12.43 | ±1.97 | ||
| ICSV 15013 | 6.00 | ±1.82 | 1.00 | ±0.77 | 12.83 | ±3.61 | ||
| Macia | 1.00 | ±0.45 | 0.00 | ±0.00 | - | |||
| Control | 0 | Parbhani Moti | 52.40 | ±1.12 | 27.60 | ±4.80 | 37.68 | ±1.54 |
| Parbhani Shakti | 61.60 | ±1.36 | 55.60 | ±2.94 | 30.40 | ±3.15 | ||
| ICSV 15013 | 46.20 | ±2.58 | 34.00 | ±2.76 | 31.22 | ±1.85 | ||
| Macia | 65.00 | ±1.82 | 57.20 | ±2.35 | 30.20 | ±2.63 | ||
| Grand Mean | 17.4 | 10.6 | 19.4 | |||||
| LSD (5%) | 5.3 | 4.9 | 2.7 | |||||
| CV (%) | 24.3 | 37.3 | 29.9 | |||||
| R2 (%) | 83.6 | 77.5 | 53.8 | |||||
| Source of Variation | df | %G | %E | %SS | SLT (cm) | NP | |||||
| Replication | 2 | 13.0 | 3.3 | 4.5 | 66.1 | 1.2 | |||||
| Genotype (G) | 1 | 3498.7 | *** | 92.7 | *** | 398.7 | *** | 7238.2 | *** | 151.2 | *** |
| Dose (D) | 6 | 205.8 | *** | 1515.2 | *** | 1174.9 | *** | 1494 | *** | 56.1 | *** |
| G x D | 6 | 109.2 | *** | 132.3 | *** | 53 | *** | 18.6 | 12.3 | *** | |
| Error | 26 | 13.49 | 3.3 | 3.8 | 36 | 0.9 | |||||
| Source of variation | df | NPP | PLT (cm) | PHT (cm) | %SV | ||||||
| Replication | 2 | 0.6 | 6.8 | 77.7 | 25.9 | ||||||
| Genotype (G) | 1 | 105.4 | *** | 3.3 | 25834.6 | *** | 5208 | *** | |||
| Dose (D) | 6 | 50.4 | *** | 10.5 | *** | 1252.1 | *** | 1187.3 | *** | ||
| G x D | 6 | 4.6 | *** | 13 | *** | 78.1 | 456 | *** | |||
| Error | 26 | 0.8 | 2.4 | 42 | 76.3 | ||||||
| Genotype | Dose (Gy) | %G | %E | %SS | SLT (cm) | PHT (cm) | NP | NPP | PLT (cm) | %SV | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Macia | 0 | 91.1 | ±2.9 | 46.0 | ±4.4 | 41.5 | ±5.2 | 63.2 | ±3.8 | 137.8 | ±3.2 | 8.6 | ±4.4 | 5.2 | ±2.6 | 17.6 | ±1.2 | 80.0 | ±2.9 |
| 100 | 91.1 | ±1.1 | 43.5 | ±2.0 | 38.9 | ±5.2 | 61.6 | ±2.3 | 133.4 | ±4.1 | 8.5 | ±4.4 | 7.1 | ±2.1 | 16.7 | ±0.1 | 71.7 | ±1.7 | |
| 200 | 88.9 | ±1.1 | 41.6 | ±4.4 | 27.7 | ±4.4 | 62.2 | ±3.4 | 132.1 | ±3.3 | 8.2 | ±2.4 | 6.8 | ±3.3 | 17.2 | ±2.2 | 70.0 | ±2.9 | |
| 300 | 74.4 | ±2.9 | 40.4 | ±7.4 | 16.1 | ±7.3 | 59.4 | ±2.5 | 129.4 | ±3.8 | 7.6 | ±5.5 | 5.3 | ±4.9 | 17.7 | ±1.7 | 58.3 | ±6.0 | |
| 400 | 72.2 | ±2.2 | 16.7 | ±4.9 | 12.3 | ±2.8 | 44.4 | ±5.3 | 112.5 | ±4.4 | 3.3 | ±1.7 | 1.2 | ±0.3 | 20.1 | ±0.5 | 51.7 | ±7.3 | |
| 500 | 72.2 | ±4.0 | 4.7 | ±3.8 | 2.2 | ±1.5 | 35.1 | ±3.2 | 96.2 | ±4.8 | 1.0 | ±1.7 | 0.2 | ±0.9 | 23.3 | ±0.5 | 14.0 | ±8.3 | |
| 600 | 68.9 | ±4.0 | 0.7 | ±0.9 | 0.0 | ±0.0 | - | - | - | - | - | - | |||||||
| Red sorghum | 0 | 98.9 | ±1.1 | 47.3 | ±4.1 | 42.3 | ±8.5 | 90.3 | ±1.7 | 197.7 | ±2.8 | 8.5 | ±6.0 | 7.0 | ±14.8 | 20.2 | ±0.1 | 88.3 | ±4.4 |
| 100 | 100.0 | ±0.0 | 41.7 | ±6.5 | 35.8 | ±7.9 | 92.0 | ±0.8 | 184.2 | ±2.7 | 14.8 | ±4.3 | 12.1 | ±31.0 | 16.8 | ±0.6 | 88.3 | ±3.3 | |
| 200 | 100.0 | ±0.0 | 40.6 | ±5.1 | 31.9 | ±5.6 | 86.3 | ±6.0 | 176.6 | ±1.7 | 10.0 | ±3.2 | 8.4 | ±19.7 | 17.6 | ±0.8 | 85.0 | ±2.9 | |
| 300 | 98.9 | ±1.1 | 27.7 | ±8.4 | 24.0 | ±7.2 | 85.0 | ±2.4 | 176.4 | ±3.5 | 8.5 | ±5.8 | 6.5 | ±16.8 | 19.4 | ±0.4 | 80.0 | ±2.9 | |
| 400 | 98.9 | ±1.1 | 25.1 | ±3.3 | 22.1 | ±3.3 | 78.2 | ±6.0 | 169.6 | ±6.2 | 8.6 | ±6.1 | 6.3 | ±16.6 | 16.7 | ±0.7 | 76.7 | ±1.7 | |
| 500 | 95.6 | ±1.1 | 18.1 | ±6.1 | 16.6 | ±7.4 | 64.2 | ±2.6 | 158.4 | ±5.1 | 8.1 | ±1.0 | 4.8 | ±11.9 | 18.4 | ±0.8 | 71.7 | ±8.8 | |
| 600 | 94.4 | ±1.1 | 13.9 | ±6.8 | 9.2 | ±5.3 | 36.8 | ±1.3 | 153.7 | ±2.0 | 5.2 | ±4.3 | 2.8 | ±3.0 | 19.1 | ±0.1 | 70.0 | ±2.9 | |
| Grand Mean | 89.0 | 29.1 | 22.9 | 66.1 | 150.6 | 7.8 | 5.7 | 18.5 | 69.7 | ||||||||||
| LSD (5%) | 6.2 | 3.0 | 3.3 | 4.0 | 4.3 | 0.6 | 0.6 | 1.0 | 5.8 | ||||||||||
| CV (%) | 4.1 | 6.2 | 8.5 | 9.1 | 4.3 | 12.5 | 16.1 | 8.4 | 12.5 | ||||||||||
| R2 (%) | 87.8 | 88.9 | 96.3 | 77.6 | 93.7 | 62.9 | 62.5 | 48.4 | 83.0 | ||||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wanga, M.A.; Shimelis, H.; Horn, L.N.; Sarsu, F. The Effect of Single and Combined Use of Gamma Radiation and Ethylmethane Sulfonate on Early Growth Parameters in Sorghum. Plants 2020, 9, 827. https://doi.org/10.3390/plants9070827
Wanga MA, Shimelis H, Horn LN, Sarsu F. The Effect of Single and Combined Use of Gamma Radiation and Ethylmethane Sulfonate on Early Growth Parameters in Sorghum. Plants. 2020; 9(7):827. https://doi.org/10.3390/plants9070827
Chicago/Turabian StyleWanga, Maliata Athon, Hussein Shimelis, Lydia N. Horn, and Fatma Sarsu. 2020. "The Effect of Single and Combined Use of Gamma Radiation and Ethylmethane Sulfonate on Early Growth Parameters in Sorghum" Plants 9, no. 7: 827. https://doi.org/10.3390/plants9070827
APA StyleWanga, M. A., Shimelis, H., Horn, L. N., & Sarsu, F. (2020). The Effect of Single and Combined Use of Gamma Radiation and Ethylmethane Sulfonate on Early Growth Parameters in Sorghum. Plants, 9(7), 827. https://doi.org/10.3390/plants9070827







