Physiological Behavior of the Aquatic Plant Azolla sp. in Response to Organic and Inorganic Fertilizers
Abstract
1. Introduction
2. Results
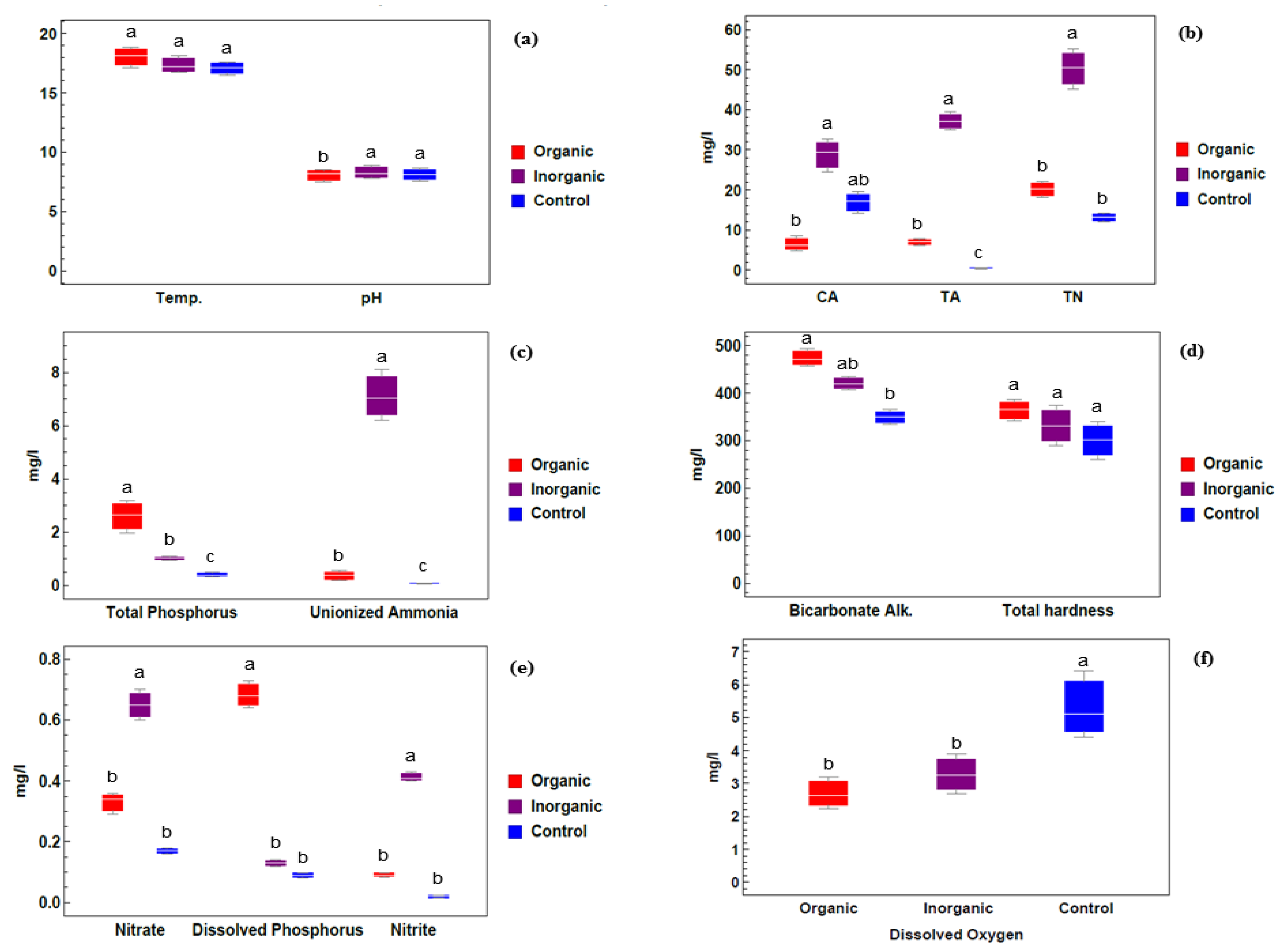
2.1. Physico-Chemical Analysis of Water
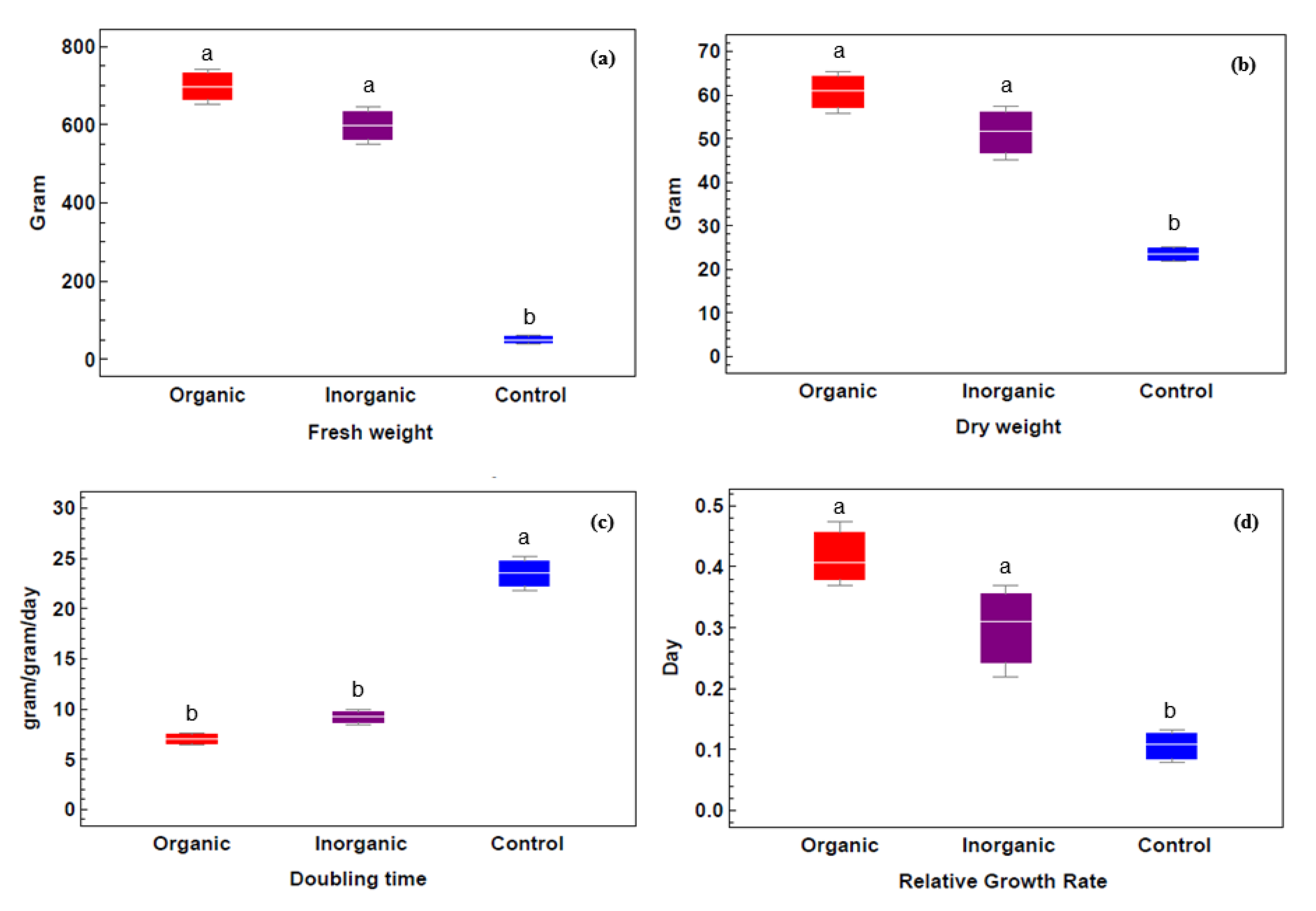
2.2. Azolla sp. Analysis
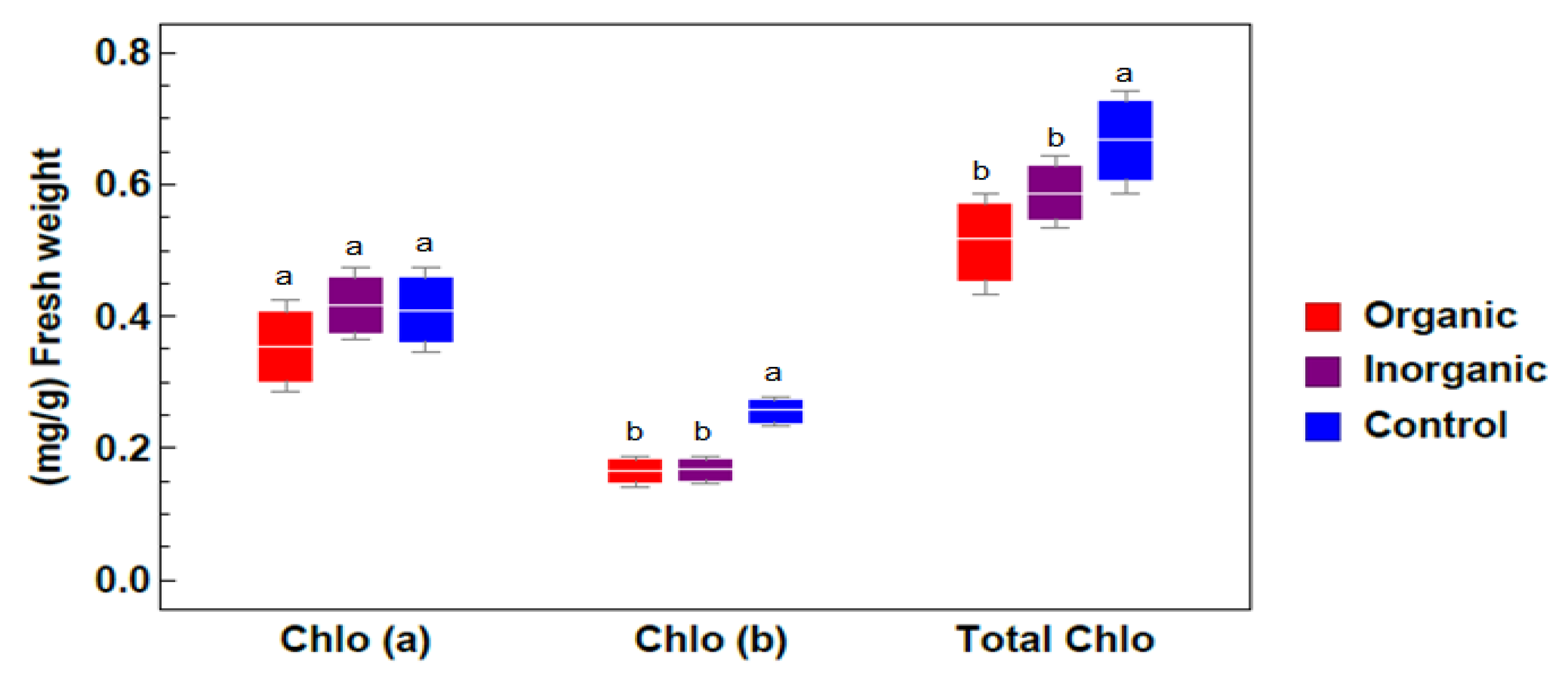
2.2.1. Chlorophyll Determination
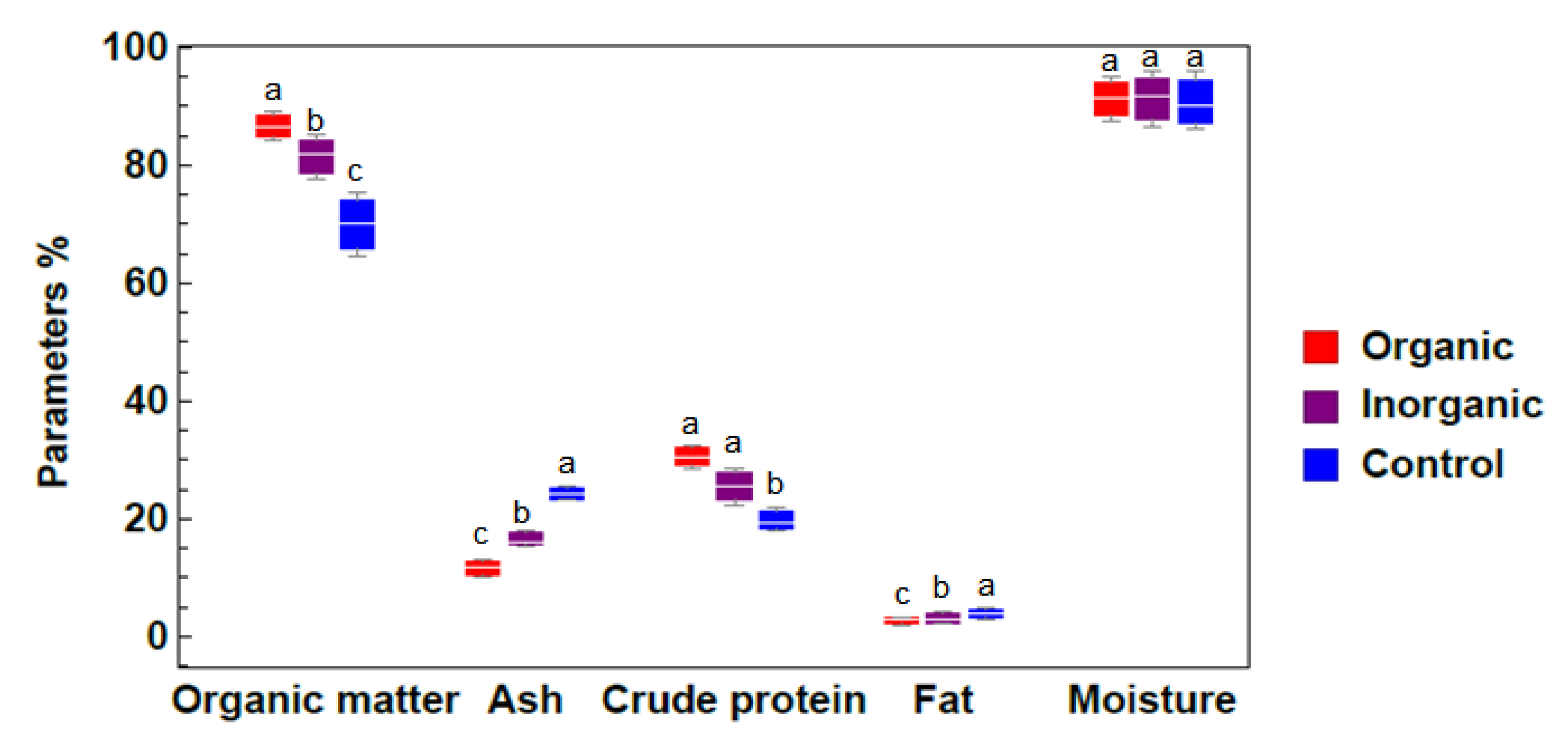
2.2.2. Biochemical composition of Azolla sp. samples subjected to different treatments
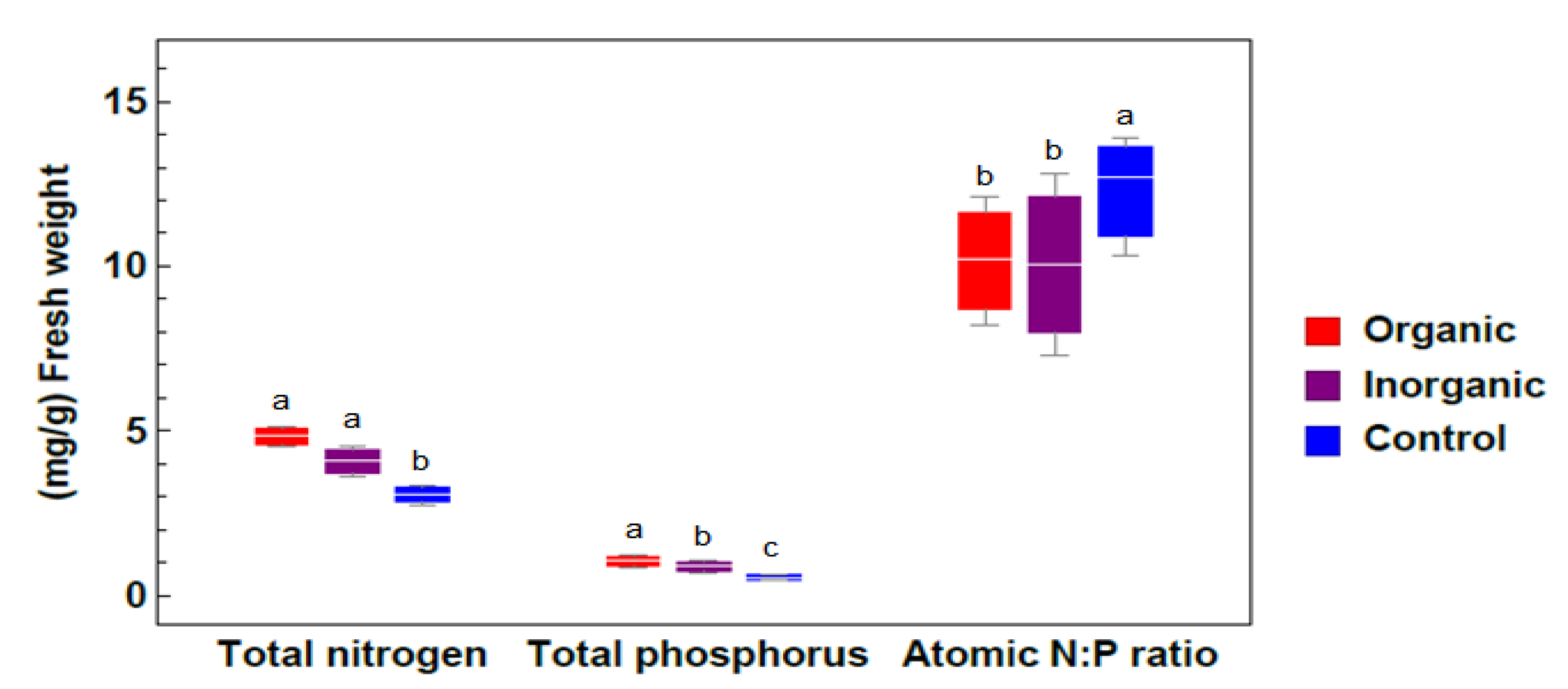
2.2.3. Total Nitrogen and Total Phosphorus Contents
2.2.4. Relative Growth Rate
2.3. Correlation Coefficients
3. Discussion
4. Materials and Methods
4.1. Sampling Points and Analysis
Physico-Chemical Analysis of Water
4.2. Azolla sp. Analysis
4.2.1. Chlorophyll Determination
4.2.2. Proximate Analysis
4.2.3. Growth Rate
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Munasinghe, J.; Dilhan, M.; Sundarabarathy, T. Utilization of aquatic plants: A method to enhance the productivity of water in seasonal tanks in the Anuradhapura District. Int. Water Manag. Inst. Conf. Pap. 2010, 1, 23. Available online: https://www.researchgate.net/publication/257653325 (accessed on 23 June 2020).
- Bindraban, P.S.; Dimkpa, C.; Nagarajan, L.; Roy, A.; Rabbinge, R. Revisiting fertilisers and fertilisation strategies for improved nutrient uptake by plants. Biol. Fertil. Soils 2015, 51, 897–911. [Google Scholar] [CrossRef]
- Boyd, C. Aquaculture pond fertilization. CAB Rev. Perspect. Agric. Vet. Sci. Nutri. Nat. Res. 2018, 13, 1–12. [Google Scholar] [CrossRef]
- Senthil Murugan, A.; Prabhahar, C.; Saleshrani, K.; Tharmaraj, K.; Ashok Raja, C. Influence of different doses of fertilizer (nitrophos) on the growth performance of major carps-labeo rohita, catla catla, cirrhinus mrigala. Int. J. Recent Sci. Res. 2012, 3, 175–178. Available online: https://recentscientific.com/sites/default/files/Download_177.pdf (accessed on 23 June 2020).
- Jana, B.B. Distribution pattern and role of phosphate solubilizing bacteria in the enhancement of fertilizer value of rock phosphate in aquaculture ponds: State-of-the-art. In First International Meeting on Microbial Phosphate Solubilization; Velázquez, E., Rodríguez-Barrueco, C., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 229–238. [Google Scholar] [CrossRef]
- Metzger, R.; Boyd, C. Liquid Ammonium Polyphosphate as a Fish Pond Fertilizer. Trans. Am. Fish. Soc. 1980, 109, 563–570. [Google Scholar] [CrossRef]
- Mischler, J.A.; Taylor, P.G.; Townsend, A.R. Nitrogen Limitation of Pond Ecosystems on the Plains of Eastern Colorado. PLoS ONE 2014, 9, e95757. [Google Scholar] [CrossRef]
- Room, P.M.; Thomas, P.A. Nitrogen, phosphorus and potassium in Salvinia molesta Mitchell in the field: Effects of weather, insect damage, fertilizers and age. Aquat. Bot. 1986, 24, 213–232. [Google Scholar] [CrossRef]
- Rascio, N.; La Rocca, N. Biological Nitrogen Fixation. In Reference Module in Earth Systems and Environmental Sciences; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar] [CrossRef]
- Tew, K.S.; Conroy, J.D.; Culver, D.A. Effects of lowered inorganic phosphorus fertilization rates on pond production of percid fingerlings. Aquaculture 2006, 255, 436–446. [Google Scholar] [CrossRef]
- Mulyani, O.; Machfud, Y.; Setiawan, A.; Joy, B. Potential of local organic matters in Jatinangor West Java Indonesia as raw materials for organic fertilizer. IOP Conf. Ser. Earth Environ. Sci. 2019, 393, 012048. [Google Scholar] [CrossRef]
- Azab, E. Effect of water stress and biological fertilization on maize growth, chemical composition and productivity in calcareous soil. Am. J. Plant Physiol. 2016, 11, 1–11. [Google Scholar] [CrossRef]
- Aubry, E.; Dinant, S.; Vilaine, F.; Bellini, C.; Le Hir, R. Lateral Transport of Organic and Inorganic Solutes. Plants 2019, 8, 20. [Google Scholar] [CrossRef]
- Reyes, T.G.; Crisosto, J.M. Characterization of Dissolved Organic Matter in River Water by Conventional Methods and Direct Sample Analysis-Time of Flight-Mass Spectrometry. J. Chem. 2016, 2016, 1537370. [Google Scholar] [CrossRef]
- Kuzucu, M. Effects of organic fertilizer application on yield, soil organic matter and porosity on kilis oil olive variety under arid conditions. Eurasian J. For. Sci. 2019, 7, 77–83. [Google Scholar] [CrossRef]
- Herold, N.; Schöning, I.; Michalzik, B.; Trumbore, S.; Schrumpf, M. Controls on soil carbon storage and turnover in German landscapes. Biogeochemistry 2014, 119, 435–451. Available online: https://www.jstor.org/stable/24716905 (accessed on 23 June 2020). [CrossRef]
- Ranalli, G.; Bottura, G.; Taddei, P.; Garavani, M.; Marchetti, R.; Sorlini, C. Composting of solid and sludge residues from agricultural and food industries. Bioindicators of monitoring and compost maturity. J. Environ. Sci. Health A 2001, 36, 415–436. [Google Scholar] [CrossRef]
- Goyal, S.; Dhull, S.K.; Kapoor, K.K. Chemical and biological changes during composting of different organic wastes and assessment of compost maturity. Bioresour. Technol. 2005, 96, 1584–1591. [Google Scholar] [CrossRef]
- Farahpour-Haghani, A.; Hassanpour, M.; Alinia, F.; Nouri-Ganbalani, G.; Razmjou, J.; Agassiz, D. Water ferns Azolla spp. (Azollaceae) as new host plants for the small China-mark moth, Cataclysta lemnata (Linnaeus, 1758) (Lepidoptera, Crambidae, Acentropinae). Nota Lepidopterol. 2017, 40, 1–13. [Google Scholar] [CrossRef]
- Hemalatha, M.; Sravan, J.S.; Min, B.; Venkata Mohan, S. Concomitant use of Azolla derived bioelectrode as anode and hydrolysate as substrate for microbial fuel cell and electro-fermentation applications. Sci. Total Environ. 2019, 707, 135851. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.; Prasanna, R.; Pabby, A.; Dominic, T.K.; Singh, P.K. Effect of urea, blue green algae and Azolla on nitrogen fixation and chlorophyll accumulation in soil under rice. Biol. Fertil. Soils 2004, 40, 67–72. [Google Scholar] [CrossRef]
- Trindade, C.R.T.; Albertoni, E.F.; Palma-Silva, C. Temporal variation in the biomass and nutrient status of Azolla filiculoides Lam. (Salviniaceae) in a small shallow dystrophic lake. Acta Limnol. Bras. 2011, 23, 368–375. [Google Scholar] [CrossRef][Green Version]
- Lumpkin, T.A.; Plucknett, D.L. Azolla as a Green Manure: Use and Management in Crop Production; Westview Press: Boulder, CO, USA, 1982; p. 230. Available online: https://www.cabdirect.org/cabdirect/abstract/19831974184 (accessed on 23 June 2020).
- Bhuvaneshwari, K.; Singh, P.K. Response of nitrogen-fixing water fern Azolla biofertilization to rice crop. 3 Biotech 2015, 5, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Arora, A.; Singh, P.K. Comparison of biomass productivity and nitrogen fixing potential of Azolla spp. Biomass Bioenergy 2003, 24, 175–178. [Google Scholar] [CrossRef]
- Bocchi, S.; Malgioglio, A. Azolla-Anabaena as a Biofertilizer for Rice Paddy Fields in the Po Valley, a Temperate Rice Area in Northern Italy. Int. J. Agron. 2010, 2010, 152158. [Google Scholar] [CrossRef]
- Setiawati, M.R.; Damayani, M.; Herdiyantoro, D.; Suryatmana, P.; Anggraini, D.; Khumairah, F.H. The application dosage of Azolla pinnata in fresh and powder form as organic fertilizer on soil chemical properties, growth and yield of rice plant. AIP Conf. Proc. 2018, 1927, 030017. [Google Scholar] [CrossRef]
- Raja, W.; Rathaur, P.; John, S.; Ramteke, P. Azolla: An aquatic pteridophyte with great potential. Int. J. Res. Biol. Sci. 2012, 2, 68–72. Available online: https://www.researchgate.net/publication/307632413_Azolla_An_aquatic_pteridophyte_with_great_potential (accessed on 23 June 2020).
- Fiogbé, E.D.; Micha, J.-C.; Van Hove, C. Use of a natural aquatic fern, Azolla microphylla, as a main component in food for the omnivorous–phytoplanktonophagous tilapia, Oreochromis niloticus L. J. Appl. Ichthyol. 2004, 20, 517–520. [Google Scholar] [CrossRef]
- Shiomi, N.; Kitoh, S. Culture of Azolla in a pond, nutrient composition, and use as fish feed. Soil Sci. Plant Nutr. 2001, 47, 27–34. [Google Scholar] [CrossRef][Green Version]
- Azab, E.; Hegazy, A.K.; El-Sharnouby, M.E.; Abd Elsalam, H.E. Phytoremediation of the organic Xenobiotic simazine by p450-1a2 transgenic Arabidopsis thaliana plants. Int. J. Phytoremediat. 2016, 18, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, A.K.; Emam, M.H.; Lovett-Doust, L.; Azab, E.; El-Khatib, A.A. Response of duckweed to lead exposure: Phytomining, bioindicators and bioremediation. Desalin. Water Treat. 2017, 70, 227–234. [Google Scholar] [CrossRef]
- Azab, E.; Kebeish, R.; Hegazy, A.K. Expression of the human gene CYP1A2 enhances tolerance and detoxification of the phenylurea herbicide linuron in Arabidopsis thaliana plants and Escherichia coli. Environ. Pollut. 2018, 238, 281–290. [Google Scholar] [CrossRef]
- Kebeish, R.; Azab, E.; Peterhaensel, C.; El-Basheer, R. Engineering the metabolism of the phenylurea herbicide chlortoluron in genetically modified Arabidopsis thaliana plants expressing the mammalian cytochrome P450 enzyme CYP1A2. Environ. Sci. Pollut. Res. Int. 2014, 21, 8224–8232. [Google Scholar] [CrossRef]
- Sood, A.; Uniyal, P.; Prasanna, R.; Ahluwalia, A. Phytoremediation Potential of Aquatic Macrophyte, Azolla. Ambio 2012, 41, 122–137. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Min, C.; Xia-shi, L.; Chungchu, L. Research on some functions of Azolla in CELSS system. Acta Astronaut. 2008, 63, 1061–1066. [Google Scholar] [CrossRef]
- Wagner, G.M. Azolla: A review of its biology and utilization. Bot. Rev. 1997, 63, 1–26. [Google Scholar] [CrossRef]
- Temmink, R.J.M.; Harpenslager, S.F.; Smolders, A.J.P.; van Dijk, G.; Peters, R.C.J.H.; Lamers, L.P.M.; van Kempen, M.M.L. Azolla along a phosphorus gradient: Biphasic growth response linked to diazotroph traits and phosphorus-induced iron chlorosis. Sci. Rep. 2018, 8, 4451. [Google Scholar] [CrossRef] [PubMed]
- Toledo, J.; Penha, J. Performance of Azolla caroliniana Willd. and Salvinia auriculata Aubl. on fish farming effluent. Braz. J. Biol. 2011, 71, 37–45. Available online: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1519-69842011000100007&nrm=iso (accessed on 23 June 2020). [CrossRef]
- Boyd, C.E. Water Quality in Ponds for Aquaculture; Alabama Agricultural Experiment Station, Auburn University: Auburn, AL, USA, 1990; Available online: https://books.google.com.sa/books?id=oVY5AQAAIAAJ (accessed on 23 June 2020).
- Utomo, R.; Noviandi, C.T.; Umami, N.; Permadi, A. Effect of Composted Animal Manure as Fertilizer on Productivity of Azolla Pinnata Grown in Earthen Ponds. OnLine J. Biol. Sci. 2019, 19, 232–236. [Google Scholar] [CrossRef][Green Version]
- Kumar, M.S.; Binh, T.T.; Burgess, S.N.; Luu, L.T. Evaluation of Optimal Species Ratio to Maximize Fish Polyculture Production. J. Appl. Aquac. 2005, 17, 35–49. [Google Scholar] [CrossRef]
- Ma, C.; Ban, T.; Yu, H.; Li, Q.; Li, X.; Jiang, W.; Xie, J. Urea Addition Promotes the Metabolism and Utilization of Nitrogen in Cucumber. Agronomy 2019, 9, 262. Available online: https://www.mdpi.com/2073-4395/9/5/262 (accessed on 23 May 2019). [CrossRef]
- Wudtisin, W.; Boyd, C.E. Determination of the phosphorus fertilization rate for bluegill ponds using regression analysis. Aquac. Res. 2005, 36, 593–599. [Google Scholar] [CrossRef]
- Das, P.C.; Ayyappan, S.; Jena, J. Comparative changes in water quality and role of pond soil after application of different levels of organic and inorganic inputs. Aquac. Res. 2005, 36, 785–798. [Google Scholar] [CrossRef]
- Kamal, S.M.; Ghanny, S.A.; Abd-El All, M.M. Effect of nutrition and fertilization on production of Nile tilapia (O. niloticus) fingerlings in concrete ponds. Aquafish Collaborative Research Support Program: Corvallis, Proceedings of The 8th International Symposium on Tilapia in Aquaculture, Cairo, Egypt, 12 October 2008; pp. 387–401. Available online: https://www.cabdirect.org/cabdirect/abstract/20133318721 (accessed on 23 June 2020).
- Brown, T.W.; Boyd, C.E.; Chappell, J.A. Organic Carbon and Dissolved Oxygen Budgets for a Commercial-Size, In-pond Raceway System. J. World Aquac. Soc. 2015, 46, 539–548. [Google Scholar] [CrossRef]
- Huang, W.; Shao, H.; Li, W.; Jiang, H.S.; Chen, Y.-Y. Effects of urea on growth and photosynthetic metabolism of two aquatic plants (Cabomba caroliniana A. Gray and Elodea nuttallii (Planch.) H. St. John). Aquat. Bot. 2017, 140, 66–77. [Google Scholar] [CrossRef]
- Alizade, A.; Nejad, T.; Rafiee, M. Effect of plant density on percent of remobilization, chlorophyll content, light penetration rate and effective grain filling period of chickpea (Cicer arietinum) in dry farming. Life Sci. J. 2011, 8, 36–39. [Google Scholar]
- Ren, B.; Liu, W.; Zhang, J.; Dong, S.; Liu, P.; Zhao, B. Effects of plant density on the photosynthetic and chloroplast characteristics of maize under high-yielding conditions. Sci. Nat. 2017, 104. [Google Scholar] [CrossRef]
- Hazary, M. Effect of Nitrogen and Phosphorus Fertilizer on Yield and Nutritional Quality of Jumbo Grass (Sorghum Grass x Sudan Grass). Adv. Anim. Vet. Sci. 2015, 3, 444–450. [Google Scholar] [CrossRef]
- Handajani, H. Optimation of Nitrogen and Phosphorus in Azolla Growth as Biofertilizer. Makara J. Technol. 2012, 15, 142–146. Available online: http://journal.ui.ac.id/technology/journal/article/view/931 (accessed on 23 June 2020). [CrossRef]
- Sanginga, N.; Van Hove, C. Amino acid composition of azolla as affected by strains and population density. Plant Soil 1989, 117, 263–267. [Google Scholar] [CrossRef]
- Sadeghi, J.; Zarkami, R.; Sabetraftar, K.; Van Damme, P. A review of some ecological factors affecting the growth of Azolla spp. Casp. J. Environ. Sci. 2013, 11, 65–76. Available online: http://hdl.handle.net/1854/LU-4104609 (accessed on 23 June 2020).
- Kösesakal, T. Effects of Seasonal Changes on Pigment Composition of Azolla filiculoides Lam. Am. Fern J. 2014, 104, 58–66. [Google Scholar] [CrossRef]
- Wheeler, P.A.; Björnsäter, B.R. Seasonal fluctuations in tissue nitrogen, phosphorus, and n:P for five macroalgal species common to the pacific northwest coast1. J. Phycol. 1992, 28, 1–6. [Google Scholar] [CrossRef]
- Lourenço, S.O.; Barbarino, E.; Nascimento, A.; Freitas, J.N.P.; Diniz, G.S. Tissue Nitrogen and Phosphorus in Seaweeds in a Tropical Eutrophic Environment: What a Long-Term Study Tells Us. J. Appl. Phycol. 2006, 18, 389–398. [Google Scholar] [CrossRef]
- Costa, M.L.; Santos, M.C.; Carrapiço, F. Biomass Characterization of Azolla Filiculoides Grown in Natural Ecosystems and Wastewater; Springer: Dordrecht, The Netherlands, 1999; pp. 323–327. [Google Scholar] [CrossRef]
- Richmond, A.E. Handbook of Microalgal Mass Culture; CRC Press: Boca Raton, FL, USA, 1986. [Google Scholar] [CrossRef]
- Menéndez, M.; Herrera, J.; Comín, F.A. Effect of nitrogen and phosphorus supply on growth, chlorophyll content and tissue composition of the macroalga Chaetomorpha linum (O.F. Müll), Kütz, in a Mediterranean Coastal Lagoon. Sci. Mar. 2002, 66, 355–364. [Google Scholar] [CrossRef]
- Chainark, S.; Boyd, C.E. Water and Sediment Quality, Phytoplankton Communities, and Channel Catfish Production in Sodium Nitrate-Treated Ponds. J. Appl. Aquac. 2010, 22, 171–185. [Google Scholar] [CrossRef]
- Boyd, C.; Thunjai, T. Aquaculture Pond Bottom Soil Quality Management; Pond Dynamics/Aquaculture Collaborative Research Support Program: Corvallis, OR, USA, 2002. [Google Scholar]
- Greenberg, A.E.; Trussell, R.R.; Clesceri, L.S. Standard Methods for the Examination of Water and Wastewater; American Public Health Association; American Water Works Association; Water Pollution Control Federation; APHA: Washington, DC, USA, 1985; Available online: https://agris.fao.org/agris-search/search.do?recordID=US19890081964 (accessed on 23 June 2020).
- Gross, A.; Boyd, C.E. A Digestion Procedure for the Simultaneous Determination of Total Nitrogen and Total Phosphorus in Pond Water. J. World Aquac. Soc. 1998, 29, 300–303. [Google Scholar] [CrossRef]
- Radke, M.G.; Schneider, M.D.; Houghtaling, D.G. Dry weight, nitrogen and phosphorus content of Schistosoma mansoni. Exp. Parasitol. 1957, 6, 202–207. [Google Scholar] [CrossRef]
- El-Sharnouby, M.E.; Azab, E.; Alotaibi, S.S.; Saleh, D. Influence of air temperature and soil moisture on growth and chemical composition of geranium plants. Pak. J. Bot. 2019, 51, 97–102. [Google Scholar] [CrossRef]
- Association of Official Analytical, C.; Helrich, K. Official Methods of Analysis of the Association of Official Analytical Chemists; The Association: Arlington, VA, USA, 1990. [Google Scholar]
- Badayos, R.B. Azolla: Its Culture, Management and Utilization in the Philippines. In Philippine Azolla Extension Program; National Azolla Action Program: Los Baños, Philippines, 1989; pp. 275–282. [Google Scholar]
- Hechler, W.D.; Dawson, J.O. Factors affecting nitrogen fixation in Azolla caroliniana. Trans. Ill. State Acad. Sci. 1995, 88, 97–107. Available online: http://ilacadofsci.com/wp-content/uploads/2013/08/088-11MS9419-print.pdf (accessed on 23 June 2020).
- Hoffmann, W.; Poorter, H. Avoiding Bias in Calculations of Relative Growth Rate. Ann. Bot. 2002, 90, 37–42. [Google Scholar] [CrossRef]
- Duncan, D.B. Multiple range and multiple F tests. Biometrics 1955, 11, 1–41. Available online: https://www.jstor.org/stable/pdf/3001478.pdf?seq=1 (accessed on 23 June 2020). [CrossRef]
| Item | r | p |
|---|---|---|
| NH4+ & NH3 | 0.996 | 0.000 |
| NO2− & NO3− | 0.982 | 0.000 |
| pH & CO3−2 | 0.978 | 0.000 |
| TP & TN | −0.266 | 0.851 |
| DO & NH3 | −0.365 | 0.762 |
| DO & TN | −0.451 | 0.702 |
| DO & NO2− | −0.439 | 0.711 |
| RGR & Dt | −0.982 | 0.000 |
| Fresh & dry weight | 1.000 | 0.000 |
| TN & protein | 1.000 | 0.000 |
| Organic Fertilizer Contents | Macronutrients | Micronutrients | |||||
| N% | P% | K% | Fe (ppm) | Zn (ppm) | Cu (ppm) | Mn (ppm) | |
| 1.93 | 0.90 | 1.23 | 1150.6 | 185 | 33.3 | 222 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azab, E.; Soror, A.-f.S. Physiological Behavior of the Aquatic Plant Azolla sp. in Response to Organic and Inorganic Fertilizers. Plants 2020, 9, 924. https://doi.org/10.3390/plants9070924
Azab E, Soror A-fS. Physiological Behavior of the Aquatic Plant Azolla sp. in Response to Organic and Inorganic Fertilizers. Plants. 2020; 9(7):924. https://doi.org/10.3390/plants9070924
Chicago/Turabian StyleAzab, Ehab, and Abdel-fatah Salah Soror. 2020. "Physiological Behavior of the Aquatic Plant Azolla sp. in Response to Organic and Inorganic Fertilizers" Plants 9, no. 7: 924. https://doi.org/10.3390/plants9070924
APA StyleAzab, E., & Soror, A.-f. S. (2020). Physiological Behavior of the Aquatic Plant Azolla sp. in Response to Organic and Inorganic Fertilizers. Plants, 9(7), 924. https://doi.org/10.3390/plants9070924






