Substrate and Plant Genotype Strongly Influence the Growth and Gene Expression Response to Trichoderma afroharzianum T22 in Sugar Beet
Abstract
:1. Introduction
2. Results
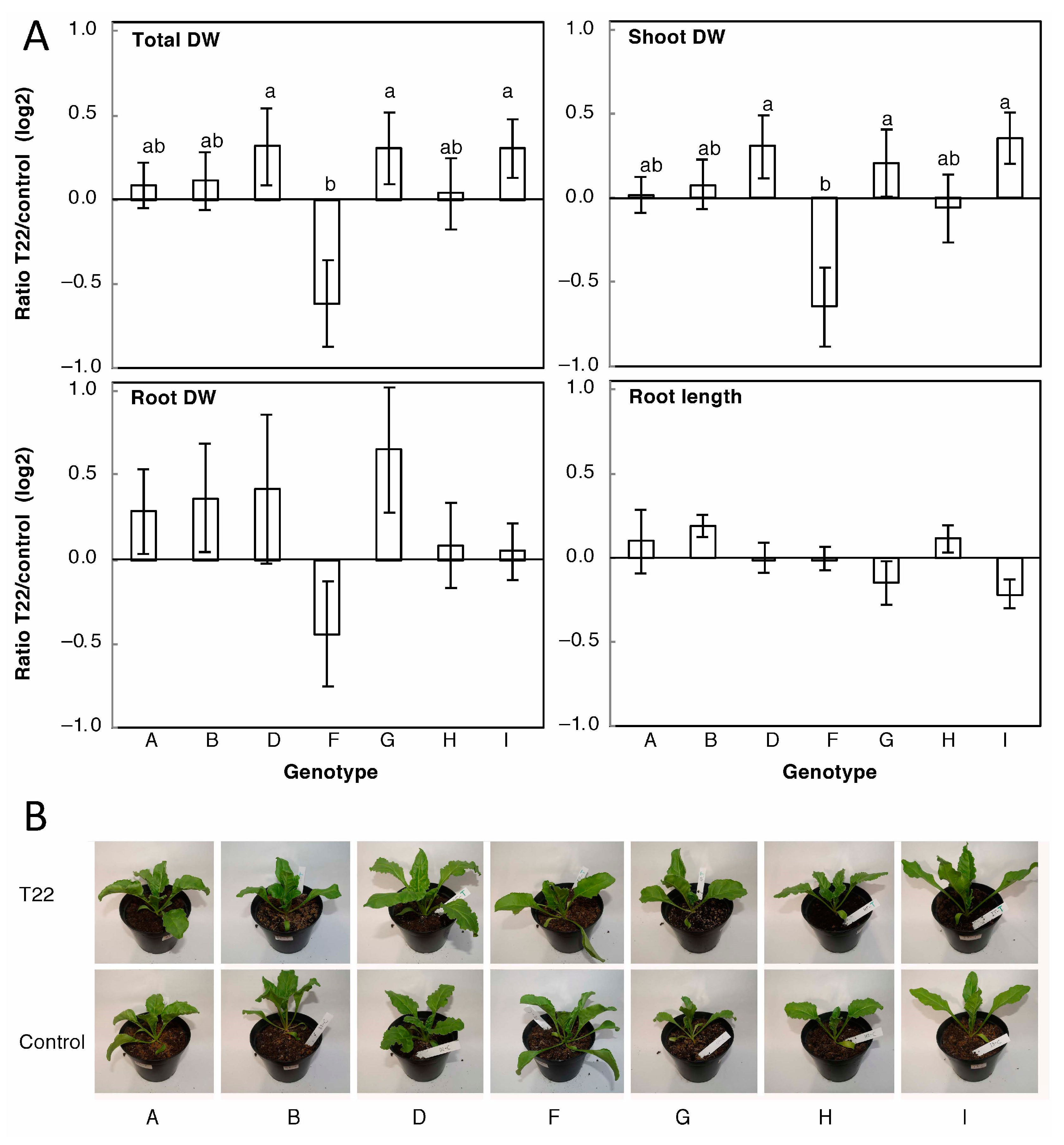
2.1. Presence of Trichoderma Promotes or Decreases Sugar Beet Growth in Soil
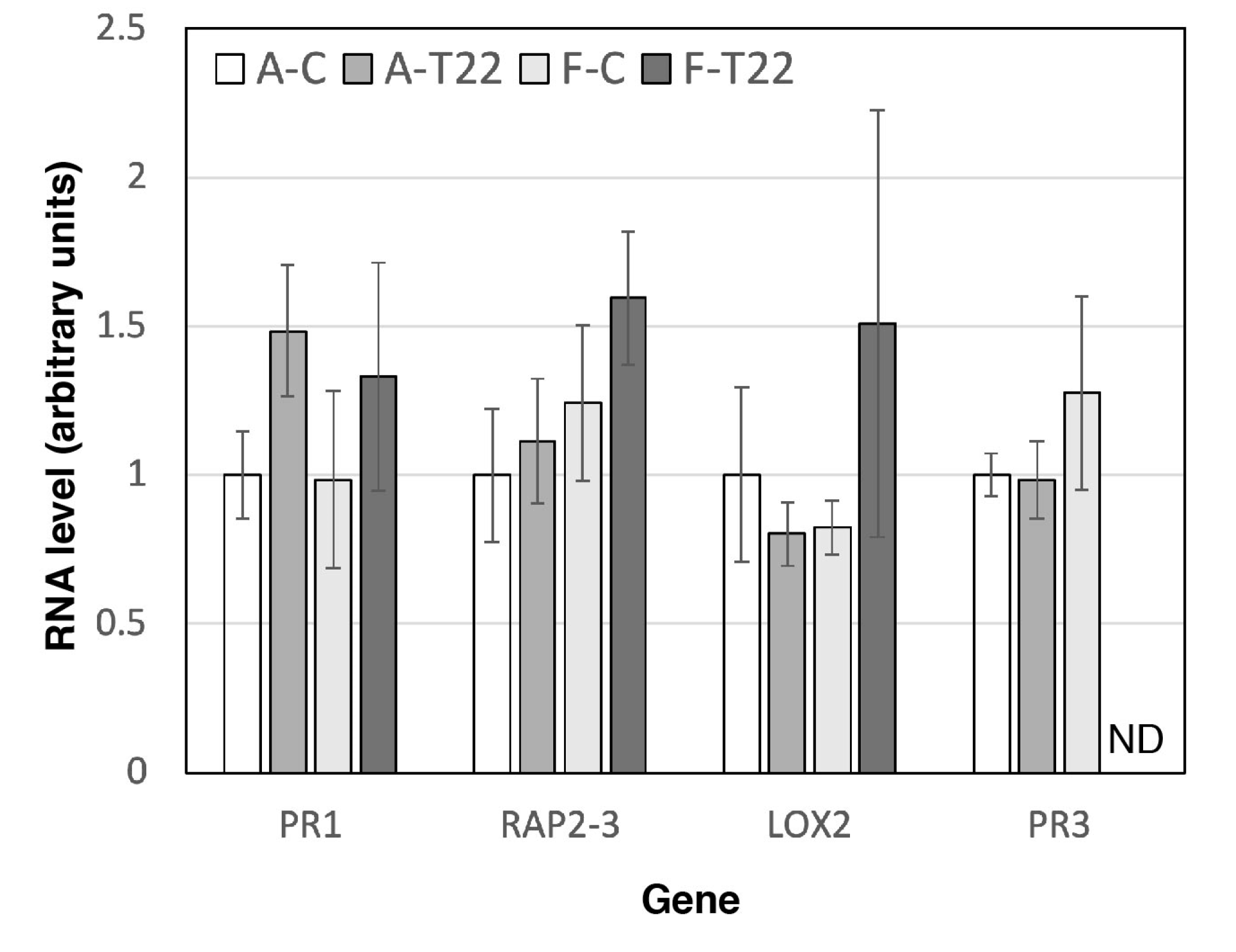
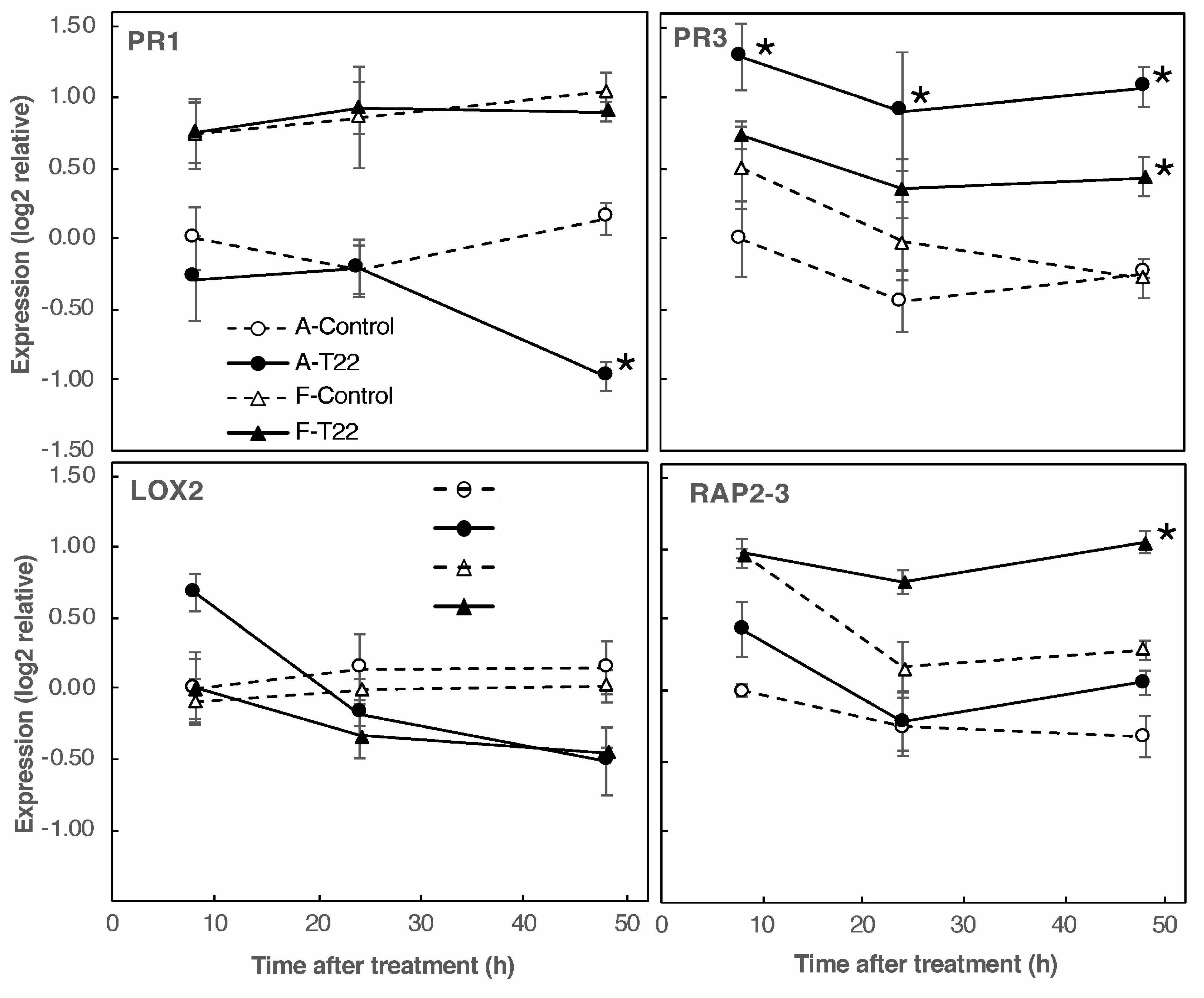
2.2. Roots of Genotype A and F Show a Difference in Gene Response to T22
2.3. T22 Decreases the Growth of Sugar Beet Seedlings in Sterile Culture
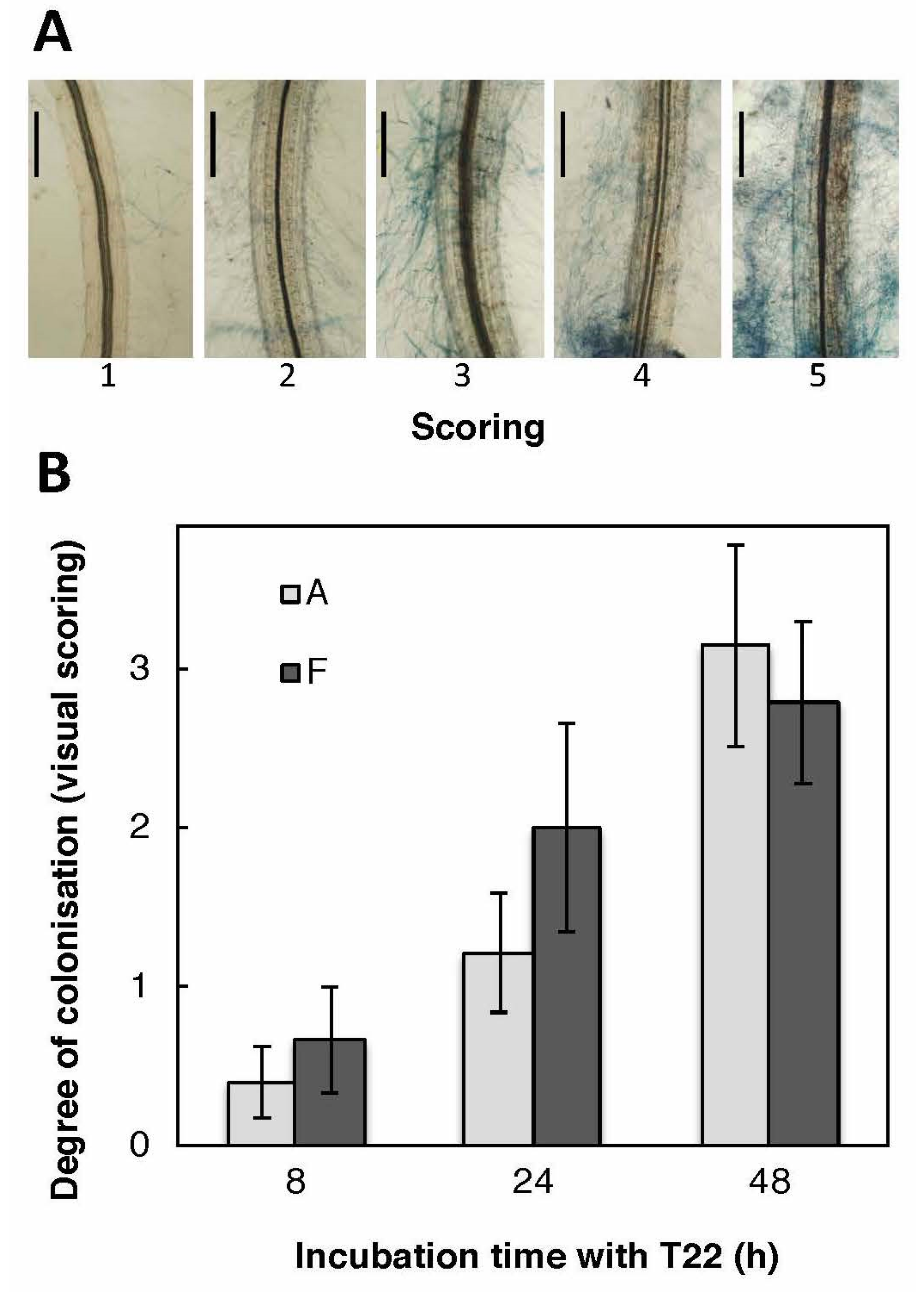
2.4. Genotypes A and F Are Similarly Colonised by T22
2.5. Sugar Beet Genotypes Show Major Differences in Gene Expression Responses Upon Sterile Co-Cultivation with T22
3. Discussion
4. Materials and Methods
4.1. Biological Material
4.2. Greenhouse Growth
4.3. Sterile Growth of Sugar Beet Seedlings
4.4. Colonization Analysis
4.5. qRT-PCR
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species-opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Druzhinina, I.S.; Seidl-Seiboth, V.; Herrera-Estrella, A.; Horwitz, B.A.; Kenerley, C.M.; Monte, E.; Mukherjee, P.K.; Zeilinger, S.; Grigoriev, I.V.; Kubicek, C.P. Trichoderma: The genomics of opportunistic success. Nat. Rev. Microbiol. 2011, 9, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Shoresh, M.; Harman, G.E.; Mastouri, F. Induced systemic resistance and plant responses to fungal biocontrol agents. Annu. Rev. Phytopathol. 2010, 48, 21–43. [Google Scholar] [CrossRef] [Green Version]
- Monte, E. Understanding Trichoderma: Between biotechnology and microbial ecology. Int. Microbiol. 2001, 4, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Harman, G.E. Overview of mechanisms and uses of Trichoderma spp. Phytopathology 2006, 96, 190–194. [Google Scholar] [CrossRef] [Green Version]
- Schuster, A.; Schmoll, M. Biology and biotechnology of Trichoderma. Appl. Microbiol. Biotechnol. 2010, 87, 787–799. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.C.; Chang, Y.C.; Baker, R.; Kleifeld, O.; Chet, I. Increased growth of plants in the presence of the biological control agent Trichoderma harzianum. Plant Dis. 1986, 70, 145–148. [Google Scholar] [CrossRef]
- Yedidia, I.; Srivastva, A.K.; Kapulnik, Y.; Chet, I. Effect of Trichoderma harzianum on microelement concentrations and increased growth of cucumber plants. Plant Soil 2001, 235, 235–242. [Google Scholar] [CrossRef]
- Harman, G.E. Trichoderma-not just for biocontrol anymore. Phytoparasitica 2011, 39, 103–108. [Google Scholar] [CrossRef] [Green Version]
- Leitgeb, B.; Szekeres, A.; Manczinger, L.; Vagvolgyi, C.; Kredics, L. The history of alamethicin: A review of the most extensively studied peptaibol. Chem. Biodivers. 2007, 4, 1027–1051. [Google Scholar] [CrossRef]
- Matic, S.; Geisler, D.A.; Møller, I.M.; Widell, S.; Rasmusson, A.G. Alamethicin permeabilizes the plasma membrane and mitochondria but not the tonoplast in tobacco (Nicotiana tabacum L. cv. Bright Yellow) suspension cells. Biochem. J. 2005, 389, 695–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Degenkolb, T.; Dieckmann, R.; Nielsen, K.F.; Grafenhan, T.; Theis, C.; Zafari, D.; Chaverri, P.; Ismaiel, A.; Bruckner, H.; von Dohren, H.; et al. The Trichoderma brevicompactum clade: A separate lineage with new species, new peptaibiotics, and mycotoxins. Mycol. Prog. 2008, 7, 177–219. [Google Scholar] [CrossRef] [Green Version]
- Aidemark, M.; Tjellström, H.; Sandelius, A.S.; Stålbrand, H.; Andreasson, E.; Rasmusson, A.G.; Widell, S. Trichoderma viride cellulase induces resistance to the antibiotic pore-forming peptide alamethicin associated with changes in the plasma membrane lipid composition of tobacco BY-2 cells. Bmc. Plant Biol. 2010, 10, 274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dotson, B.R.; Soltan, D.; Schmidt, J.; Areskoug, M.; Rabe, K.; Swart, C.; Widell, S.; Rasmusson, A.G. The antibiotic peptaibol alamethicin from Trichoderma permeabilises Arabidopsis root apical meristem and epidermis but is antagonised by cellulase-induced resistance to alamethicin. BMC Plant Biol. 2018, 18, 165. [Google Scholar] [CrossRef]
- Yedidia, I.; Shoresh, M.; Kerem, Z.; Benhamou, N.; Kapulnik, Y.; Chet, I. Concomitant induction of systemic resistance to Pseudomonas syringae pv. lachrymans in cucumber by Trichoderma asperellum (T-203) and accumulation of phytoalexins. Appl. Environ. Microbiol. 2003, 69, 7343–7353. [Google Scholar] [CrossRef] [Green Version]
- Harman, G.E.; Petzoldt, R.; Comis, A.; Chen, J. Interactions between Trichoderma harzianum strain T22 and maize inbred line Mo17 and effects of these interactions on diseases caused by Pythium ultimum and Colletotrichum graminicola. Phytopathology 2004, 94, 147–153. [Google Scholar] [CrossRef] [Green Version]
- Shoresh, M.; Yedidia, I.; Chet, I. Involvement of jasmonic acid/ethylene signaling pathway in the systemic resistance induced in cucumber by Trichoderma asperellum T203. Phytopathology 2005, 95, 76–84. [Google Scholar] [CrossRef] [Green Version]
- Samuels, G.J.; Ismaiel, A.; Bon, M.C.; De Respinis, S.; Petrini, O. Trichoderma asperellum sensu lato consists of two cryptic species. Mycologia 2010, 102, 944–966. [Google Scholar] [CrossRef]
- Chaverri, P.; Branco-Rocha, F.; Jaklitsch, W.; Gazis, R.; Degenkolb, T.; Samuels, G.J. Systematics of the Trichoderma harzianum species complex and the re-identification of commercial biocontrol strains. Mycologia 2015, 107, 558–590. [Google Scholar] [CrossRef] [Green Version]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Cortes-Penagos, C.; López-Bucio, J. Trichoderma virens, a plant beneficial fungus, enhances biomass production and promotes lateral root growth through an auxin-dependent mechanism in Arabidopsis. Plant Physiol. 2009, 149, 1579–1592. [Google Scholar] [CrossRef] [Green Version]
- Tucci, M.; Ruocco, M.; De Masi, L.; De Palma, M.; Lorito, M. The beneficial effect of Trichoderma spp. on tomato is modulated by the plant genotype. Mol. Plant Pathol. 2011, 12, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Prashar, P.; Vandenberg, A. Genotype-specific responses to the effects of commercial Trichoderma formulations in lentil (Lens culinaris ssp. culinaris) in the presence and absence of the oomycete pathogen Aphanomyces euteiches. Biocontrol Sci. Techn. 2017, 27, 1123–1144. [Google Scholar] [CrossRef]
- Draycott, A.P. Sugar Beet; Blackwell: Oxford, UK, 2006. [Google Scholar]
- Dohm, J.C.; Minoche, A.E.; Holtgräwe, D.; Capella-Gutierrez, S.; Zakrzewski, F.; Tafer, H.; Rupp, O.; Sorensen, T.; Stracke, R.; Reinhardt, R.; et al. The genome of the recently domesticated crop plant sugar beet (Beta vulgaris). Nature 2014, 505, 546–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abada, K.A. Fungi causing damping-off and root-rot on sugar-beet and their biological control with Trichoderma harzianum. Agric. Ecosyst. Environ. 1994, 51, 333–337. [Google Scholar] [CrossRef]
- Galletti, S.; Burzi, P.L.; Cerato, C.; Marinello, S.; Sala, E. Trichoderma as a potential biocontrol agent for Cercospora leaf spot of sugar beet. Biocontrol 2008, 53, 917–930. [Google Scholar] [CrossRef]
- Kakvan, N.; Heydari, A.; Zamanizadeh, H.R.; Rezaee, S.; Naraghi, L. Development of new bioformulations using Trichoderma and Talaromyces fungal antagonists for biological control of sugar beet damping-off disease. Crop Prot. 2013, 53, 80–84. [Google Scholar] [CrossRef]
- Pieterse, C.M.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.; Bakker, P.A. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef] [Green Version]
- Pieterse, C.M.; Leon-Reyes, A.; Van der Ent, S.; Van Wees, S.C. Networking by small-molecule hormones in plant immunity. Nat. Chem. Biol. 2009, 5, 308–316. [Google Scholar] [CrossRef] [Green Version]
- Pin, P.A.; Benlloch, R.; Bonnet, D.; Wremerth-Weich, E.; Kraft, T.; Gielen, J.J.; Nilsson, O. An antagonistic pair of FT homologs mediates the control of flowering time in sugar beet. Science 2010, 330, 1397–1400. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; Macias-Rodriguez, L.; Alfaro-Cuevas, R.; López-Bucio, J. Trichoderma spp. improve growth of Arabidopsis seedlings under salt stress through enhanced root development, osmolite production, and Na+ elimination through root exudates. Mol. Plant Microbe Interact. 2014, 27, 503–514. [Google Scholar] [CrossRef] [Green Version]
- Lorito, M.; Woo, S.L.; Harman, G.E.; Monte, E. Translational research on Trichoderma: From ‘omics to the field. Annu. Rev. Phytopathol. 2010, 48, 395–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kottb, M.; Gigolashvili, T.; Großkinsky, D.K.; Piechulla, B. Trichoderma volatiles effecting Arabidopsis: From inhibition to protection against phytopathogenic fungi. Front. Microbiol. 2015, 6, 995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brotman, Y.; Landau, U.; Cuadros-Inostroza, Á.; Tohge, T.; Fernie, A.R.; Chet, I.; Viterbo, A.; Willmitzer, L. Trichoderma-plant root colonization: Escaping early plant defense responses and activation of the antioxidant machinery for saline stress tolerance. PLoS Pathog. 2013, 9, e1003221. [Google Scholar] [CrossRef]
- Mathys, J.; De Cremer, K.; Timmermans, P.; Van Kerckhove, S.; Lievens, B.; Vanhaecke, M.; Cammue, B.P.; De Coninck, B. Genome-wide characterization of ISR induced in Arabidopsis thaliana by Trichoderma hamatum T382 against Botrytis cinerea infection. Front. Plant Sci. 2012, 3, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samolski, I.; Rincón, A.M.; Pinzón, L.M.; Viterbo, A.; Monte, E. The qid74 gene from Trichoderma harzianum has a role in root architecture and plant biofertilization. Microbiology 2012, 158, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Rollano-Penaloza, O.M.; Widell, S.; Mollinedo, P.; Rasmusson, A.G. Trichoderma harzianum T-22 and BOL-12QD inhibit lateral root development of Chenopodium quinoa in axenic co-culture. Cogent. Biol. 2018, 4. [Google Scholar] [CrossRef]
- Saenz-Mata, J.; Jimenez-Bremont, J.F. HR4 gene is induced in the Arabidopsis-Trichoderma atroviride beneficial interaction. Int. J. Mol. Sci. 2012, 13, 9110–9128. [Google Scholar] [CrossRef] [Green Version]
- Perazzolli, M.; Moretto, M.; Fontana, P.; Ferrarini, A.; Velasco, R.; Moser, C.; Delledonne, M.; Pertot, I. Downy mildew resistance induced by Trichoderma harzianum T39 in susceptible grapevines partially mimics transcriptional changes of resistant genotypes. BMC Genom. 2012, 13, 660. [Google Scholar] [CrossRef]
- Martinez-Medina, A.; Alguacil, M.D.; Pascual, J.A.; Van Wees, S.C.M. Phytohormone profiles induced by Trichoderma isolates correspond with their biocontrol and plant growth-promoting activity on melon plants. J. Chem. Ecol. 2014, 40, 804–815. [Google Scholar] [CrossRef]
- Ali, S.; Ganai, B.A.; Kamili, A.N.; Bhat, A.A.; Mir, Z.A.; Bhat, J.A.; Tyagi, A.; Islam, S.T.; Mushtaq, M.; Yadav, P.; et al. Pathogenesis-related proteins and peptides as promising tools for engineering plants with multiple stress tolerance. Microbiol. Res. 2018, 212, 29–37. [Google Scholar] [CrossRef]
- Norman-Setterblad, C.; Vidal, S.; Palva, E.T. Interacting signal pathways control defense gene expression in Arabidopsis in response to cell wall-degrading enzymes from Erwinia carotovora. Mol. Plant Microbe Interact. 2000, 13, 430–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, S.; Mir, Z.A.; Tyagi, A.; Bhat, J.A.; Chandrashekar, N.; Papolu, P.K.; Rawat, S.; Grover, A. Identification and comparative analysis of Brassica juncea pathogenesis-related genes in response to hormonal, biotic and abiotic stresses. Acta. Physiol. Plant 2017, 39. [Google Scholar] [CrossRef]
- Jogaiah, S.; Abdelrahman, M.; Tran, L.S.P.; Ito, S.I. Different mechanisms of Trichoderma virens-mediated resistance in tomato against Fusarium wilt involve the jasmonic and salicylic acid pathways. Mol. Plant Pathol. 2018, 19, 870–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Beltrán-Peña, E.; Herrera-Estrella, A.; López-Bucio, J. Trichoderma-induced plant immunity likely involves both hormonal- and camalexin-dependent mechanisms in Arabidopsis thaliana and confers resistance against necrotrophic fungi Botrytis cinerea. Plant Signal Behav. 2011, 6, 1554–1563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, D.D.; Liu, H.X.; Jiang, C.H.; Wang, Y.P.; Wang, Q.Y.; Jin, H.L.; Guo, J.H. The plant growth-promoting rhizobacterium Bacillus cereus AR156 induces systemic resistance in Arabidopsis thaliana by simultaneously activating salicylate- and jasmonate/ethylene-dependent signaling pathways. Mol. Plant Microbe Interact. 2011, 24, 533–542. [Google Scholar] [CrossRef] [Green Version]
- Rubio, M.B.; Quijada, N.M.; Perez, E.; Dominguez, S.; Monte, E.; Hermosa, R. Identifying beneficial qualities of Trichoderma parareesei for plants. Appl. Environ. Microbiol. 2014, 80, 1864–1873. [Google Scholar] [CrossRef] [Green Version]
- de Medeiros, H.A.; de Araujo, J.V.; de Freitas, L.G.; Castillo, P.; Rubio, M.B.; Hermosa, R.; Monte, E. Tomato progeny inherit resistance to the nematode Meloidogyne javanica linked to plant growth induced by the biocontrol fungus Trichoderma atroviride. Sci. Rep. UK 2017, 7. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Medina, A.; Fernandez, I.; Lok, G.B.; Pozo, M.J.; Pieterse, C.M.J.; Van Wees, S.C.M. Shifting from priming of salicylic acid- to jasmonic acid-regulated defences by Trichoderma protects tomato against the root knot nematode Meloidogyne incognita. New Phytol. 2017, 213, 1363–1377. [Google Scholar] [CrossRef] [Green Version]
- Tytgat, T.O.G.; Verhoeven, K.J.F.; Jansen, J.J.; Raaijmakers, C.E.; Bakx-Schotman, T.; McIntyre, L.M.; van der Putten, W.H.; Biere, A.; van Dam, N.M. Plants know where it hurts: Root and shoot jasmonic acid induction elicit differential responses in Brassica oleracea. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Yedidia, I.; Benhamou, N.; Chet, I. Induction of defense responses in cucumber plants (Cucumis sativus L.) by the biocontrol agent Trichoderma harzianum. Appl. Environ. Microbiol. 1999, 65, 1061–1070. [Google Scholar] [CrossRef] [Green Version]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Dalpé, Y.; Séguin, S.M. Microwave-assisted technology for the clearing and staining of arbuscular mycorrhizal fungi in roots. Mycorrhiza 2013, 23, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.; Pflugmacher, M.; Klages, S.; Maser, A.; Mock, A.; Stahl, D.J. Accumulation of the hormone abscisic acid (ABA) at the infection site of the fungus Cercospora beticola supports the role of ABA as a repressor of plant defence in sugar beet. Mol. Plant Pathol. 2008, 9, 661–673. [Google Scholar] [CrossRef] [PubMed]
- Wallström, S.V.; Aidemark, M.; Escobar, M.A.; Rasmusson, A.G. An alternatively spliced domain of the NDC1 NAD(P)H dehydrogenase gene strongly influences the expression of the ACTIN2 reference gene in Arabidopsis thaliana. Plant Sci. 2012, 183, 190–196. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Willems, E.; Leyns, L.; Vandesompele, J. Standardization of real-time PCR gene expression data from independent biological replicates. Anal. Biochem. 2008, 379, 127–129. [Google Scholar] [CrossRef]

| Gene | Refbeet Code 1 | A. thaliana Orthologue 2 | Primers (5′−3′) Forward/Reverse | Reference |
|---|---|---|---|---|
| GAPDH | Bv5_107870_ygnn | CATCAAGGCGGAATCAGAAGG/ACGAGCTTTGCGAAGTGGTC | This work | |
| ICDH | Bv3u_070630 | CACACCAGATGAAGGCCGT/CCCTGAAGACCGTGCCAT | Pin et al. (2010) [30] | |
| LOX2 | Bv4_072290_uwja | At3g45140 | CCAAGATGTTTGATCGGGATCG/ATTCCGTGACACGCTTGATG | This work |
| PR1 | Bv9_228910_cfgq | At2g14610 | CAAGTAGTGTGGAGAGAATCGG/TGTAATTGCCAGGAGGATCATAA | This work |
| PR3 | Bv1_008140_uzgx | At3g12500 | AAAGCCAATGTTCGCCTAGC/CAGTAGCCCATCCTCCAGTG | This work |
| RAP2–3 | Bv_33470_gcqz | At3g16770 | CCGACCTTCTCTCCTCTCATTC/CCGCCCATCCGAGTTGTG | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmidt, J.; Dotson, B.R.; Schmiderer, L.; van Tour, A.; Kumar, B.; Marttila, S.; Fredlund, K.M.; Widell, S.; Rasmusson, A.G. Substrate and Plant Genotype Strongly Influence the Growth and Gene Expression Response to Trichoderma afroharzianum T22 in Sugar Beet. Plants 2020, 9, 1005. https://doi.org/10.3390/plants9081005
Schmidt J, Dotson BR, Schmiderer L, van Tour A, Kumar B, Marttila S, Fredlund KM, Widell S, Rasmusson AG. Substrate and Plant Genotype Strongly Influence the Growth and Gene Expression Response to Trichoderma afroharzianum T22 in Sugar Beet. Plants. 2020; 9(8):1005. https://doi.org/10.3390/plants9081005
Chicago/Turabian StyleSchmidt, John, Bradley R. Dotson, Ludwig Schmiderer, Adriaan van Tour, Banushree Kumar, Salla Marttila, Kenneth M. Fredlund, Susanne Widell, and Allan G. Rasmusson. 2020. "Substrate and Plant Genotype Strongly Influence the Growth and Gene Expression Response to Trichoderma afroharzianum T22 in Sugar Beet" Plants 9, no. 8: 1005. https://doi.org/10.3390/plants9081005
APA StyleSchmidt, J., Dotson, B. R., Schmiderer, L., van Tour, A., Kumar, B., Marttila, S., Fredlund, K. M., Widell, S., & Rasmusson, A. G. (2020). Substrate and Plant Genotype Strongly Influence the Growth and Gene Expression Response to Trichoderma afroharzianum T22 in Sugar Beet. Plants, 9(8), 1005. https://doi.org/10.3390/plants9081005