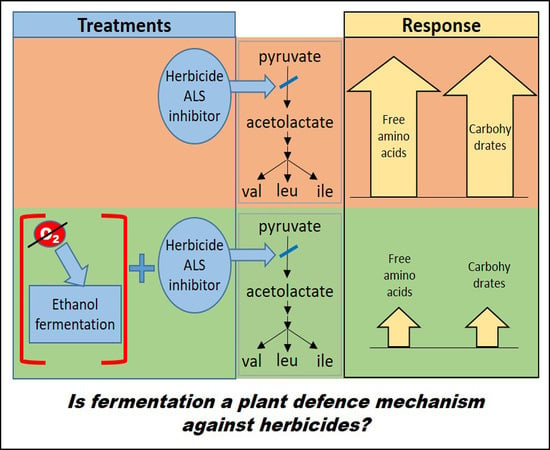
Hypoxic Treatment Decreases the Physiological Action of the Herbicide Imazamox on Pisum sativum Roots
Abstract
:1. Introduction
2. Results
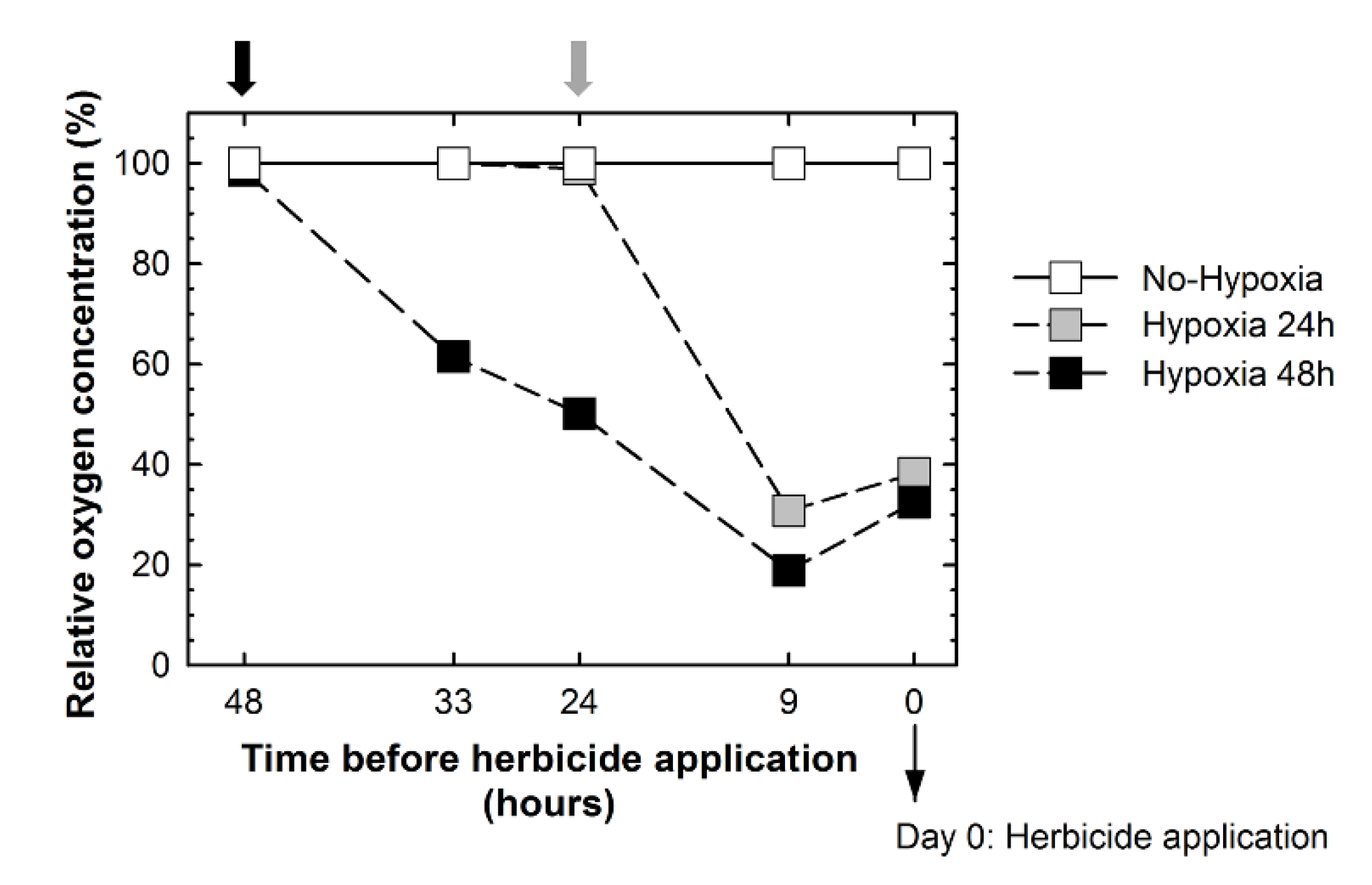
2.1. Validation of the Experiment
2.2. Growth Parameters
2.3. Carbohydrate Content
2.4. Free Amino Acid Profile
3. Discussion
4. Materials and Methods
4.1. Plant Material and Treatment Application
4.2. PDC and ADH Enzymatic Activities
4.3. PDC and ADH Immunoblotting
4.4. Metabolites Determination
4.4.1. Amino Acids
4.4.2. Total Soluble Sugars and Starch
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Singh, B.K. Biosynthesis of valine, leucine and isoleucine. In Plant Amino Acids: Biochemistry and Biotechnology; Singh, B.K., Ed.; Marcel Dekker: New York, NY, USA, 1999; pp. 227–247. [Google Scholar]
- Peters, B.; Strek, H.J. Herbicide discovery in light of rapidly spreading resistance and ever-increasing regulatory hurdles. Pest Manag. Sci. 2018, 74, 2211–2215. [Google Scholar] [CrossRef]
- Duggleby, R.G.; McCourt, J.A.; Guddat, L.W. Structure and mechanism of inhibition of plant acetohydroxyacid synthase. Plant Physiol. Biochem. 2008, 46, 309–324. [Google Scholar] [CrossRef]
- Wittenbach, V.; Abell, L.M. Inhibition of valine, leucine and isoleucine biosynthesis. In Plant Amino Acids: Biochemistry and Biotechnology; Singh, B.K., Ed.; Marcel Dekker: New York, NY, USA, 1999; pp. 385–416. [Google Scholar]
- Cobb, A.; Reade, J. The inhibition of amino acid biosynthesis. In Herbicides and Plant Physiology; Wiley-Blackwell, Ed.; Wiley-Blackwell: Oxford, UK, 2010; pp. 176–199. ISBN 978-1-4051-2935-0. [Google Scholar]
- Shaner, D.L.; Reider, M.L. Physiological responses of corn (Zea mays) to AC 243,997 in combination with valine, leucine, and isoleucine. Pestic. Biochem. Physiol. 1986, 25, 248–257. [Google Scholar] [CrossRef]
- Zulet, A.; Gil-Monreal, M.; Villamor, J.G.; Zabalza, A.; van der Hoorn, R.A.L.; Royuela, M. Proteolytic pathways induced by herbicides that inhibit amino acid biosynthesis. PLoS ONE 2013, 8, e73847. [Google Scholar] [CrossRef] [Green Version]
- Zabalza, A.; Orcaray, L.; Gaston, S.; Royuela, M. Carbohydrate accumulation in leaves of plants treated with the herbicide chlorsulfuron or imazethapyr is due to a decrease in sink strength. J. Agric. Food Chem. 2004, 52, 7601–7606. [Google Scholar] [CrossRef]
- Armendáriz, O.; Gil-Monreal, M.; Zulet, A.; Zabalza, A.; Royuela, M. Both foliar and residual applications of herbicides that inhibit amino acid biosynthesis induce alternative respiration and aerobic fermentation in pea roots. Plant Biol. 2016, 18, 382–390. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Escalada, M.; Zulet-González, A.; Gil-Monreal, M.; Royuela, M.; Zabalza, A. Physiological performance of glyphosate and imazamox mixtures on Amaranthus palmeri sensitive and resistant to glyphosate. Sci. Rep. 2019, 9, 18225. [Google Scholar] [CrossRef] [Green Version]
- Gaston, S.; Zabalza, A.; Gonzalez, E.M.; Arrese-Igor, C.; Aparicio-Tejo, P.M.; Royuela, M. Imazethapyr, an inhibitor of the branched-chain amino acid biosynthesis, induces aerobic fermentation in pea plants. Physiol. Plant. 2002, 114, 524–532. [Google Scholar] [CrossRef]
- Gaston, S.; Ribas-Carbo, M.; Busquets, S.; Berry, J.A.; Zabalza, A.; Royuela, M. Changes in mitochondrial electron partitioning in response to herbicides inhibiting branched-chain amino acid biosynthesis in soybean. Plant Physiol. 2003, 133, 1351–1359. [Google Scholar] [CrossRef] [Green Version]
- Zabalza, A.; González, E.M.; Arrese-Igor, C.; Royuela, M. Fermentative metabolism is induced by inhibiting different enzymes of the branched-chain amino acid biosynthesis pathway in pea plants. J. Agric. Food Chem. 2005, 53, 7486–7493. [Google Scholar] [CrossRef]
- Zulet, A.; Gil-Monreal, M.; Zabalza, A.; van Dongen, J.T.; Royuela, M. Fermentation and alternative oxidase contribute to the action of amino acid biosynthesis-inhibiting herbicides. J. Plant Physiol. 2015, 175, 102–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gil-Monreal, M.; Fernandez-Escalada, M.; Royuela, M.; Zabalza, A. An aerated axenic hydroponic system for the application of root treatments: Exogenous pyruvate as a practical case. Plant Methods 2018, 14, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loreti, E.; van Veen, H.; Perata, P. Plant responses to flooding stress. Curr. Opin. Plant Biol. 2016, 33, 64–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Dongen, J.T.; Licausi, F. Oxygen sensing and signaling. Annu. Rev. Plant Biol. 2015, 66, 345–367. [Google Scholar] [CrossRef]
- Perata, P.; Alpi, A. Plant responses to anaerobiosis. Plant Sci. 1993, 93, 1–17. [Google Scholar] [CrossRef]
- Christie, P.J.; Hahn, M.; Walbot, V. Low-temperature accumulation of alcohol dehydrogenase-1 mRNA and protein activity in maize and rice seedlings. Plant Physiol. 1991, 95, 699–706. [Google Scholar] [CrossRef] [Green Version]
- Kato-Noguchi, H. Osmotic stress increases alcohol dehydrogenase activity in maize seedlings. Biol. Plant. 2000, 43, 621–623. [Google Scholar] [CrossRef]
- Dolferus, R.; Jacobs, M.; Peacock, W.J.; Dennis, E.S. Differential interactions of promoter elements in stress responses of the Arabidopsis Adh gene. Plant Physiol. 1994, 105, 1075–1087. [Google Scholar] [CrossRef] [Green Version]
- Minhas, D.; Grover, A. Transcript levels of genes encoding various glycolytic and fermentation enzymes change in response to abiotic stresses. Plant Sci. 1999, 146, 41–51. [Google Scholar] [CrossRef]
- Kürsteiner, O.; Dupuis, I.; Kuhlemeier, C. The Pyruvate decarboxylase1 gene of Arabidopsis is required during anoxia but not other environmental stresses. Plant Physiol. 2003, 132, 968–978. [Google Scholar] [CrossRef] [Green Version]
- Peters, J.S.; Frenkel, C. Relationship between alcohol dehydrogenase activity and low-temperature in two maize genotypes, Silverado F1 and Adh1-Adh2 - doubly null. Plant Physiol. Biochem. 2004, 42, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Tadege, M.; Dupuis, I.; Kuhlemeier, C. Ethanolic fermentation: New functions for an old pathway. Trends Plant Sci. 1999, 4, 320–325. [Google Scholar] [CrossRef]
- Schwartz, D.; Endo, T. Alcohol dehydrogenase polymorphism in maize-simple and compound loci. Genetics 1966, 53, 709–715. [Google Scholar] [PubMed]
- Orcaray, L.; Igal, M.; Marino, D.; Zabalza, A.; Royuela, M. The possible role of quinate in the mode of action of glyphosate and acetolactate synthase inhibitors. Pest Manag. Sci. 2010, 66, 262–269. [Google Scholar] [CrossRef]
- Fernández-Escalada, M.; Gil-Monreal, M.; Zabalza, A.; Royuela, M. Characterization of the Amaranthus palmeri physiological response to glyphosate in susceptible and resistant populations. J. Agric. Food Chem. 2016, 64, 95–106. [Google Scholar] [CrossRef] [Green Version]
- Millar, A.A.; Olive, M.R.; Dennis, E.S. The expression and anaerobic induction of alcohol-dehydrogenase in cotton. Biochem. Genet. 1994, 32, 279–300. [Google Scholar] [CrossRef]
- Zabalza, A.; Gaston, S.; Sandalio, L.M.; del Río, L.A.; Royuela, M. Oxidative stress is not related to the mode of action of herbicides that inhibit acetolactate synthase. Environ. Exp. Bot. 2007, 59, 150–159. [Google Scholar] [CrossRef]
- Qian, H.; Hu, H.; Mao, Y.; Ma, J.; Zhang, A.; Liu, W.; Fu, Z. Enantioselective phytotoxicity of the herbicide imazethapyr in rice. Chemosphere 2009, 76, 885–892. [Google Scholar] [CrossRef]
- Wang, Q.; Ge, L.; Zhao, N.; Zhang, L.; You, L.; Wang, D.; Liu, W.; Wang, J. A Trp-574-Leu mutation in the acetolactate synthase (ALS) gene of Lithospermum arvense L. confers broad-spectrum resistance to ALS inhibitors. Pestic. Biochem. Physiol. 2019, 158, 12–17. [Google Scholar] [CrossRef]
- Qian, H.; Wang, R.; Hu, H.; Lu, T.; Chen, X.; Ye, H.; Liu, W.; Fu, Z. Enantioselective phytotoxicity of the herbicide imazethapyr and its effect on rice physiology and gene transcription. Environ. Sci. Technol. 2011, 45, 7036–7043. [Google Scholar] [CrossRef]
- Zabalza, A.; Orcaray, L.; Igal, M.; Schauer, N.; Fernie, A.R.; Geigenberger, P.; van Dongen, J.T.; Royuela, M. Unraveling the role of fermentation in the mode of action of acetolactate synthase inhibitors by metabolic profiling. J. Plant Physiol. 2011, 168, 1568–1575. [Google Scholar] [CrossRef] [PubMed]
- Zabalza, A.; Gaston, S.; Ribas-Carbo, M.; Orcaray, L.; Igal, M.; Royuela, M. Nitrogen assimilation studies using 15N in soybean plants treated with imazethapyr, an inhibitor of branched-chain amino acid biosynthesis. J. Agric. Food Chem. 2006, 54, 8818–8823. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, D.; Hogan, A.L.; Deal, L.; Jamieson, G.C.; Haworth, P. Amino acid metabolism of Lemna minor L.: II. Responses to chlorsulfuron. Plant Physiol. 1987, 84, 775–780. [Google Scholar] [CrossRef] [Green Version]
- Trenkamp, S.; Eckes, P.; Busch, M.; Fernie, A.R. Temporally resolved GC-MS-based metabolic profiling of herbicide treated plants treated reveals that changes in polar primary metabolites alone can distinguish herbicides of differing mode of action. Metabolomics 2009, 5, 277–291. [Google Scholar] [CrossRef] [Green Version]
- Less, H.; Angelovici, R.; Tzin, V.; Galili, G. Principal transcriptional regulation and genome-wide system interactions of the Asp-family and aromatic amino acid networks of amino acid metabolism in plants. Amino Acids 2010, 39, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Parry, M.; Noctor, G. Markers and signals associated with nitrogen assimilation in higher plants. J. Exp. Bot. 2003, 54, 585–593. [Google Scholar] [CrossRef]
- Rocha, M.; Licausi, F.; Araújo, W.L.; Nunes-Nesi, A.; Sodek, L.; Fernie, A.R.; van Dongen, J.T. Glycolysis and the tricarboxylic acid cycle are linked by alanine aminotransferase during hypoxia induced by waterlogging of Lotus japonicus. Plant Physiol. 2010, 152, 1501–1513. [Google Scholar] [CrossRef] [Green Version]
- Shelp, B.J.; Bown, A.W.; Mclean, M.D. Metabolism and functions of gamma-aminobutyric acid. Trends Plant Sci. 1999, 4, 446–452. [Google Scholar] [CrossRef]
- Narsai, R.; Rocha, M.; Geigenberger, P.; Whelan, J.; van Dongen, J.T. Comparative analysis between plant species of transcriptional and metabolic responses to hypoxia. New Phytol. 2011, 190, 472–487. [Google Scholar] [CrossRef]
- Zabalza, A.; Zulet, A.; Gil-Monreal, M.; Igal, M.; Royuela, M. Branched-chain amino acid biosynthesis inhibitors: Herbicide efficacy is associated with an induced carbon-nitrogen imbalance. J. Plant Physiol. 2013, 170, 814–821. [Google Scholar] [CrossRef] [Green Version]
- Gil-Monreal, M.; Giuntoli, B.; Zabalza, A.; Licausi, F.; Royuela, M. ERF-VII transcription factors induce ethanol fermentation in response to amino acid biosynthesis-inhibiting herbicides. J. Exp. Bot. 2019, 70, 5839–5851. [Google Scholar] [CrossRef] [PubMed]
- Gil-Monreal, M.; Zabalza, A.; Missihoun, T.D.; Dörmann, P.; Bartels, D.; Royuela, M. Induction of the PDH bypass and upregulation of the ALDH7B4 in plants treated with herbicides inhibiting amino acid biosynthesis. Plant Sci. 2017, 264, 16–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zabalza, A.; van Dongen, J.T.; Froehlich, A.; Oliver, S.N.; Faix, B.; Gupta, K.J.; Schmälzlin, E.; Igal, M.; Orcaray, L.; Royuela, M.; et al. Regulation of respiration and fermentation to control the plant internal oxygen concentration. Plant Physiol. 2009, 149, 1087–1098. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Group | Abbreviation | Treatment Description |
|---|---|---|
| No-Hypoxia | 0-C | No treatment was applied. |
| 0-IMX | Application of 5 mg L−1 of imazamox at day 0. | |
| Hypoxia-24h | 24h-C | The aeration was removed for 24 h before the day 0. Aeration was again placed at day 0 until the end of the experiment. No herbicide was applied. |
| 24h-IMX | The aeration was removed for 24 h before the day 0. The aeration was again placed at day 0 until the end of the experiment. At day 0 imazamox was applied at a final concentration of 5 mg L−1. | |
| Hypoxia-48h | 48h-C | The aeration was removed for 48 h before the day 0. Aeration was again placed at day 0 until the end of the experiment. No herbicide was applied. |
| 48h-IMX | The aeration was removed for 48 h before the day 0. Aeration was again placed at day 0 until the end of the experiment. The day 0 imazamox was applied at a final concentration of 5 mg L−1. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gil-Monreal, M.; Royuela, M.; Zabalza, A. Hypoxic Treatment Decreases the Physiological Action of the Herbicide Imazamox on Pisum sativum Roots. Plants 2020, 9, 981. https://doi.org/10.3390/plants9080981
Gil-Monreal M, Royuela M, Zabalza A. Hypoxic Treatment Decreases the Physiological Action of the Herbicide Imazamox on Pisum sativum Roots. Plants. 2020; 9(8):981. https://doi.org/10.3390/plants9080981
Chicago/Turabian StyleGil-Monreal, Miriam, Mercedes Royuela, and Ana Zabalza. 2020. "Hypoxic Treatment Decreases the Physiological Action of the Herbicide Imazamox on Pisum sativum Roots" Plants 9, no. 8: 981. https://doi.org/10.3390/plants9080981