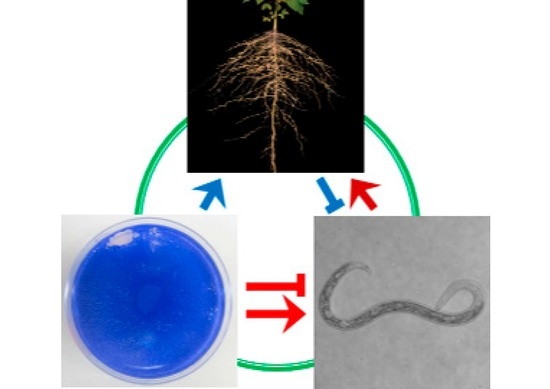
Current Utility of Plant Growth-Promoting Rhizobacteria as Biological Control Agents towards Plant-Parasitic Nematodes
Abstract
:1. Introduction
2. Recent Increases in Agronomic Burden by PPN
Current Management Method against PPN
3. Current utility of PGPR as BCAs of PPN; Mode of actions of PGPR
3.1. Direct Antagonism of PGPR against PPN
3.2. Indirect Antagonism, ISR of PGPR against PPN
3.3. Potential Roles of SA in the Indirect Antagonism, SAR of PGPR against PPN
3.4. Crosstalk between SA and JA Signaling
4. Commercialization of PGPR
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nicol, J.M.; Turner, S.J.; Coyne, D.L.; den Nijs, L.; Hockland, S.; Maafi, Z.T. Current nematode threats to world agriculture. In Genomics and Molecular Genetics of Plant-Nematode Interaction; Jones, J., Gheyse, G., Ffenoll, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 21–43. [Google Scholar]
- Aktar, M.W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timper, P. Conserving and enhancing biological control of nematodes. J. Nematol. 2014, 46, 75–89. [Google Scholar] [PubMed]
- Xiang, N.; Lawrence, K.; Donald, P. Biological control potential of plant growth-promoting rhizobacteria suppression of Meloidogyne incognita on cotton and Heterodera glycines on soybean: A review. J. Phytopathol. 2018, 166, 449–458. [Google Scholar] [CrossRef] [Green Version]
- Kirkpatrick, T.L.; Sasser, J.N. Crop rotation and races of Meloidogyne incognita in cotton root-knot management. J. Nematol. 1984, 16, 323–328. [Google Scholar] [PubMed]
- Pieterse, C.M.J.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; van Wees, S.C.M.; Bakker, P.A.H.M. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vlot, A.C.; Klessig, D.F.; Park, S.W. Systemic acquired resistance: The elusive signal(s). Curr. Opin. Plant Biol. 2008, 11, 436–442. [Google Scholar] [CrossRef] [Green Version]
- De Vrieze, J. The littlest farmhands. Science 2015, 349, 680–683. [Google Scholar] [CrossRef]
- Oka, Y.; Chet, I.; Spiegel, Y. Control of the rootknot nematode Meloidogyne javanica by Bacillus cereus. Biocontrol Sci. Technol. 1993, 3, 115–126. [Google Scholar] [CrossRef]
- Gao, H.; Qi, G.; Yin, R.; Zhang, H.; Li, C.; Zhao, X. Bacillus cereus strain S2 shows high nematicidal activity against Meloidogyne incognita by producing sphingosine. Sci. Rep. 2016, 6, 28756. [Google Scholar] [CrossRef]
- Ahmed, S. Bacillus cereus a potential strain infested cereal cyst nematode (Heterodera avenae). Pak. J. Nematol. 2019, 37, 53–61. [Google Scholar] [CrossRef]
- Ambo, P.B.N.; Ethiopia, E.A.; Serfoji, P.; Rajeshkumar, S.; Selvaraj, T. Management of root-knot nematode, Meloidogyne incognita on tomato cv Pusa Ruby by using vermicompost, AM fungus, Glomus aggregatum and mycorrhiza helper bacterium, Bacillus coagulans. J. Agric. Sci. Technol. 2010, 6, 37–45. [Google Scholar]
- Serfoji, P.; Smithra, P.; Saravavanan, K.; Durai Raj, K. Plant growth promotion and management of root-knot nematode Meloidogyne incognita through Glomus aggregatum and Bacillus coagulans and vermicomposting in tomato. Int. J. Pharm. Biol. Arch. 2013, 4, 532–536. [Google Scholar]
- Giannakou, I.O.; Karpouzas, D.G.; Prophetou-Athanasiadou, D. A novel non-chemical nematicide for the control of root-knot nematodes. Appl. Soil Ecol. 2004, 26, 69–79. [Google Scholar] [CrossRef]
- Mendoza, A.R.; Kiewnick, S.; Sikora, R.A. In vitro activity of Bacillus firmus against the burrowing nematode Radopholus similis, the root-knot nematode Meloidogyne incognita and the stem nematode Ditylenchus dipsaci. Biocontrol Sci. Technol. 2008, 18, 377–389. [Google Scholar] [CrossRef]
- Terefe, M.; Tefera, T.; Sakhuja, P.K. Effect of a formulation of Bacillus firmus on root-knot nematode Meloidogyne incognita infestation and the growth of tomato plants in the greenhouse and nursery. J. Invertebr. Pathol. 2009, 100, 94–99. [Google Scholar] [CrossRef]
- Terefe, M.; Tefera, T.; Sakhuja, P.K. Biocontrol (formulation of Bacillus firmus (BioNem)) of root-knot nematode, Meloidogyne incognita on tomato plants in the field. Ethiop. J. Agric. Sci. 2012, 22, 102–116. [Google Scholar]
- Xiong, J.; Zhou, Q.; Luo, H.; Xia, L.; Li, L.; Sun, M.; Yu, Z. Systemic nematicidal activity and biocontrol efficacy of Bacillus firmus against the root-knot nematode Meloidogyne incognita. World J. Microbiol. Biotechnol. 2015, 31, 661–667. [Google Scholar] [CrossRef]
- Geng, C.; Nie, X.; Tang, Z.; Zhang, Y.; Lin, J.; Sun, M.; Peng, D. A novel serine protease, Sep1, from Bacillus firmus DS-1 has nematicidal activity and degrades multiple intestinal-associated nematode proteins. Sci Rep. 2016, 6, 25012. [Google Scholar] [CrossRef] [Green Version]
- Bayer Crop Science. Available online: www.cropscience.bayer.us/products/seedgrowth/ponchovotivo/ (accessed on 18 August 2020).
- Siddiqui, Z.A.; Husain, S.I. Studies on the biological control of root-knot nematode. Curr. Nematol. 1991, 2, 5–6. [Google Scholar]
- Siddiqui, Z.A.; Mahmood, I. Biological control of root-rot disease complex of chickpea caused by Meloidogyne incognita Race 3 and Macrophomina phaseolina. Nematol. Medit. 1992, 20, 199–202. [Google Scholar]
- Jeong, M.-H.; Yang, S.-Y.; Lee, Y.-S.; Ahn, Y.-S.; Park, Y.-S.; Han, H.; Kim, K.-Y. Selection and characterization of Bacillus licheniformis MH48 for the biocontrol of pine wood nematode (Bursaphelenchus xylophilus). J. Korean For. Soc. 2015, 104, 512–518. [Google Scholar] [CrossRef] [Green Version]
- El-Nagdi, W.M.A.; Abd-El-Khair, H.; Soliman, G.M.; Ameen, H.H.; El-Sayed, G.M. Application of protoplast fusants of Bacillus licheniformis and Pseudomonas aeruginosa on Meloidogyne incognita in tomato and eggplant. Middle East J. Appl. Sci. 2019, 9, 622–629. [Google Scholar]
- Kloepper, J.W.; Beauchamp, C.J. A review of issues related to measuring of plant roots by bacteria. Can. J. Microbiol. 1992, 38, 1219–1232. [Google Scholar] [CrossRef]
- Padgham, J.L.; Sikora, R.A. Biological control potential and modes of action of Bacillus megaterium against Meloidogyne graminicola on rice. Crop Prot. 2007, 26, 971–977. [Google Scholar] [CrossRef]
- Mostafa, F.A.M.; Khalil, A.E.; Nour El-Deen, A.H.; Ibrahim, D.S. The role of Bacillus megaterium and other bio-agents in controlling root-knot nematodes infecting sugar beet under field conditions. Egypt. J. Biol. Pest Control 2018, 28, 66. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kim, K.Y. Antagonistic potential of Bacillus pumilus L1 against root-knot nematode, Meloidogyne arenaria. J. Phytopathol. 2016, 164, 29–39. [Google Scholar] [CrossRef]
- Forghani, F.; Hajihassani, A. Recent advances in the development of environmentally benign treatments to control root-knot nematodes. Front. Plant Sci. 2020, 11, 1125. [Google Scholar] [CrossRef]
- Prakob, W.; Nguen-Hom, J.; Jaimasit, P.; Thanunchai, J.; Chaisuk, P. Biological control of lettuce root-knot disease by the used of Pseudomonas aeruginosa, Bacillus subtilis and Paecilomyces lilacinus. J. Agric. Technol. 2009, 13, 179–191. [Google Scholar]
- Kavitha, P.G.; Jonathan, E.L.; Nakkeeran, S. Effects of crude antibiotic of Bacillus subtilis on hatching of eggs and mortality of juveniles of Meloidogyne incognita. Nematol. Mediterr. 2012, 40, 211–215. [Google Scholar]
- Basyony, A.G.; Abo-Zaid, G.A. Biocontrol of the root-knot nematode, Meloidogyne incognita, using an eco-friendly formulation from Bacillus subtilis, lab and greenhouse studies. Egypt. J. Biol. Pest Control 2018, 28, 87. [Google Scholar] [CrossRef]
- de Mazzuchelli, R.C.L.; Mazzuchelli, E.H.L.; de Araujo, F.F. Efficiency of Bacillus subtilis for root-knot and lesion nematodes management in sugarcane. Biol. Control 2020, 143, 104185. [Google Scholar] [CrossRef]
- Gautam, A.; Siddiqui, Z.; Mahmood, I. Integrated management of Meloidogyne incognita on tomato. Nematol. Mediterr. 1995, 23, 245–248. [Google Scholar]
- Noel, G.R. Evaluation of thuringiensin for control of Heterodera glycines on soybean. J. Nematol. 1990, 22, 763–766. [Google Scholar] [PubMed]
- Wei, J.-Z.; Hale, K.; Carta, L.; Platzer, E.; Wong, C.; Fang, S.-C.; Aroian, R.V. Bacillus thuringiensis crystal proteins that target nematodes. Proc. Natl. Acad. Sci. USA 2003, 100, 2760–2765. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, S.; Saedy, M.; Enan, M.; Ibrahim, N.E.; Ghareeb, A.; Moustafa, S. Biocontrol efficiency of Bacillus thuringiensis toxins against root-knot nematode, Meloidogyne incognita. J. Cell Mol. Biol. 2008, 7, 57–66. [Google Scholar]
- Mena, J.; Pimentel, E. Mechanism of action of Corynebacterium pauronetabolum strain C-924 on nematodes. Nematology 2002, 4, 287. [Google Scholar]
- Mankau, R.; Imbriani, J.L.; Bell, A.H. SEM observations on nematode cuticle penetration by Bacillus penetrans. J. Nematol. 1976, 8, 179–181. [Google Scholar]
- Mankau, R.; Prasad, N. Possibilities and problems in use of a sporozoan endoparasite for biological control of plant parasitic nematodes. Nematropica 1977, 2, 7–8. [Google Scholar]
- Dube, B.; Smart, G.C. Biological control of Meloidogyne incognita by Paecilomyces lilacinus and Pasteuria penetrans. J. Nematol. 1987, 19, 222–227. [Google Scholar]
- Sayre, R.M.; Starr, M.P. Pasteuria penetrans a mycelial and endospore-forming bacterium parasitic in plant-parasitic nematodes. Proc. Helminthol. Soc. Wash 1985, 52, 149–165. [Google Scholar]
- Bhuiyan, S.A.; Garlick, K.; Anderson, J.M.; Wickramasinghe, P.; Stirling, G.R. Biological control of root-knot nematode on sugarcane in soil naturally or artificially infested with Pasteuria penetrans. Australas. Plant Pathol. 2018, 47, 45–52. [Google Scholar] [CrossRef]
- Atibalentja, N.; Noel, G.R.; Domier, L.L. Phylogenetic position of the North American isolate of Pasteuria that parasitizes the soybean cyst nematode, Heterodera glycines, as inferred from 16S RDNA sequence analy-sis. Int. J. Syst. Evol. Microbiol. 2000, 50, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Sayre, R.M.; Wergin, W.P.; Schmldt, J.M.; Starr, M.P. Pasteuria nishizawae sp. nov., a mycelial and endospore-forming bacterium parasitic on cyst nematodes of genera Heterodera and Globodera. Res. Microbiol. 1991, 142, 551–564. [Google Scholar] [CrossRef] [Green Version]
- Siddiqui, I.A.; Ehteshamul-Haque, S. Suppression of the root rot–root knot disease complex by Pseudomo-nas aeruginosa in tomato: The influence of inoculum density, nematode populations, moisture and other plant-associated bacteria. Plant Soil 2001, 237, 81–89. [Google Scholar] [CrossRef]
- Gallagher, L.A.; Manoil, C. Pseudomonas aeruginosa PAO1 kills Caenorhabditis elegans by cyanide poisoning. J. Bacteriol. 2001, 183, 6207–6214. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.; Siddiqui, Z.A. Biocontrol of root-knot nematode Meloidogyne incognita by the isolates of Pseudomonas on tomato. Arch. Phytopathol. Plant Protect. 2010, 43, 1423–1434. [Google Scholar] [CrossRef]
- Cronin, D.; Moenne-Loccoz, Y.; Fenton, A.; Dunne, C.; Dowling, D.N.; O’gara, F. Role of 2,4-diacetyl-phloroglucinol in the interactions of the biocontrol Pseudomonad strain F113 with the potato cyst nematode Globodera rostochiensis. Appl. Environ. Microbiol. 1997, 63, 1357–1361. [Google Scholar] [CrossRef] [Green Version]
- Siddiqui, I.; Shaukat, S. Suppression of root-knot disease by Pseudomonas fluorescens CHA0 in tomato: Importance of bacterial secondary metabolite, 2,4-diacetylpholoroglucinol. Soil Biol. Biochem. 2003, 35, 1615–1623. [Google Scholar] [CrossRef]
- Hamid, M.; Siddiqui, I.A.; Shahid Shaukat, S. Improvement of Pseudomonas fluorescens CHA0 biocontrol activity against root-knot nematode by the addition of ammonium molybdate. Lett. Appl. Microbiol. 2003, 36, 239–244. [Google Scholar] [CrossRef]
- Siddiqui, I.A.; Haas, D.; Heeb, S. Extracellular protease of Pseudomonas fluorescens CHA0, a biocontrol factor with activity against the root-knot nematode Meloidogyne incognita. Appl. Environ. Microbiol. 2005, 71, 5646–5649. [Google Scholar] [CrossRef] [Green Version]
- Timper, P.; Koné, D.; Yin, J.; Ji, P.; McSpadden Gardener, B.B. Evaluation of an antibiotic-producing strain of Pseudomonas fluorescens for suppression of plant-parasitic nematodes. J. Nematol. 2009, 41, 234–240. [Google Scholar]
- Khan, M.R.; Mohidin, F.A.; Khan, U.; Ahamad, F. Native Pseudomonas spp. suppressed the root-knot nematode in in vitro and in vivo, and promoted the nodulation and grain yield in the field grown mungbean. Biol. Control 2016, 101, 159–168. [Google Scholar] [CrossRef]
- Zabaketa-Mejia, E. The effect of soil bacteria on Meloidogyne incognita (Kofoid & White) Chitwood infection. Diss. Abstr. Interact. 1985, 46, 1018. [Google Scholar]
- Rahul, S.; Chandrashekhar, P.; Hemant, B.; Chandrakant, N.; Laxmikant, S.; Satish, P. Nematicidal activity of microbial pigment from Serratia marcescens. Nat. Prod. Res. 2014, 28, 1399–1404. [Google Scholar] [CrossRef] [PubMed]
- Hasky-Guenther, K.; Hoffmann-Hergarten, S.; Sikora, R.A. Resistance against the potato cyst nematode Globodera pallida systemically induced by the rhizobacteria Agrobacterium radiobacter (G12) and Bacillus sphaericus (B43). Fundam. Appl. Nematol. 1998, 21, 511–517. [Google Scholar]
- Hackenberg, C.; Sikora, R.A. Influence of cultivar, temperature, and soil moisture on the antagonistic potential of Agrobacterium radiobacter against Globodera pallida. J. Nematol. 1992, 24, 594. [Google Scholar]
- Racke, J.; Sikora, R.A. Influence of the plant health-promoting rhizobacteria Agrobacterium radiobacter and Bacillus sphaericus on Globodera pallida root infection of potato and subsequent plant growth. J. Phytopathol. 1992, 134, 198–208. [Google Scholar] [CrossRef]
- Hackenberg, C.; Vrain, T.C.; Sikora, R.A. Rhizosphere colonization pattern of Agrobacterium Radiobacter strain G12A, an antagonistic rhizobacterium to the potato cyst nematode Globodera pallida. Microbiol. Res. 1999, 154, 57–61. [Google Scholar] [CrossRef]
- Ryu, C.-M.; Farag, M.A.; Hu, C.-H.; Reddy, M.S.; Kloepper, J.W.; Paré, P.W. Bacterial volatiles induce systemic resistance in Arabidopsis. Plant Physiol. 2004, 134, 1017–1026. [Google Scholar] [CrossRef] [Green Version]
- Beris, D.; Theologidis, I.; Skandalis, N.; Vassilakos, N. Bacillus amyloliquefaciens strain MBI600 induces Sali-cylic acid dependent resistance in tomato plants against tomato spotted wilt virus and potato virus Y. Sci. Rep. 2018, 8, 10320. [Google Scholar] [CrossRef] [Green Version]
- Burkett-Cadena, M.; Kokalis-Burelle, N.; Lawrence, K.S.; van Santen, E.; Kloepper, J.W. Suppressiveness of root-knot nematodes mediated by rhizobacteria. Biol. Control. 2008, 47, 55–59. [Google Scholar] [CrossRef]
- Choudhary, D.K.; Johri, B.N. Interactions of Bacillus spp. and plants—with special reference to induced systemic resistance (ISR). Microbiol. Res. 2009, 164, 493–513. [Google Scholar] [CrossRef]
- Li, J.; Zou, C.; Xu, J.; Ji, X.; Niu, X.; Yang, J.; Huang, X.; Zhang, K.-Q. Molecular mechanisms of nematode-nematophagous microbe interactions: Basis for biological control of plant-parasitic nematodes. Annu. Rev. Phytopathol. 2015, 53, 67–95. [Google Scholar] [CrossRef]
- Xiang, N.; Lawrence, K.S.; Kloepper, J.W.; Donald, P.A.; McInroy, J.A.; Lawrence, G.W. Biological control of Meloidogyne incognita by spore-forming plant growth-promoting rhizobacteria on cotton. Plant Dis. 2016, 101, 774–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, S.; Jiang, H.; Ding, T.; Xu, Q.; Chai, W.; Cheng, B. Bacillus amyloliquefaciens FZB42 represses plant MiR846 to induce systemic resistance via a jasmonic acid-dependent signalling pathway. Mol. Plant Pathol. 2018, 19, 1612–1623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Halfeld-Vieira, B.A.; Vieira Júnior, J.R.; da Romeiro, R.S.; Silva, H.S.A.; Baracat-Pereira, M.C. Induction of systemic resistance in tomato by the autochthonous phylloplane resident Bacillus cereus. Pesqui. Agropecu. Bras. 2006, 41, 1247–1252. [Google Scholar] [CrossRef] [Green Version]
- Niu, D.-D.; Liu, H.-X.; Jiang, C.-H.; Wang, Y.-P.; Wang, Q.-Y.; Jin, H.-L.; Guo, J.-H. The plant growth-promoting rhizobacterium Bacillus cereus AR156 induces systemic resistance in Arabidopsis thaliana by simultaneously activating salicylate- and jasmonate/ethylene-dependent signaling pathways. Mol. Plant Microbe Interact. 2011, 24, 533–542. [Google Scholar] [CrossRef] [Green Version]
- Jiang, C.; Fan, Z.; Li, Z.; Niu, D.; Li, Y.; Zheng, M.; Wang, Q.; Jin, H.; Guo, J. Bacillus cereus AR156 triggers induced systemic resistance against Pseudomonas syringae pv. tomato DC3000 by suppressing MiR472 and activating CNLs-mediated basal immunity in Arabidopsis. Mol. Plant Pathol. 2020, 21, 854–870. [Google Scholar] [CrossRef]
- Liu, K.; Garrett, C.; Fadamiro, H.; Kloepper, J.W. Induction of systemic resistance in chinese cabbage against black rot by plant growth-promoting rhizobacteria. Biol. Control. 2016, 99, 8–13. [Google Scholar] [CrossRef] [Green Version]
- Bargabus, R.L.; Zidack, N.; Sherwood, J.E.; Jacobsen, B.J. Characterisation of systemic resistance in sugar beet elicited by a non-pathogenic, phyllosphere-colonizing Bacillus mycoides biological control agent. Physiol. Mol. Plant Pathol. 2002, 61, 289–298. [Google Scholar] [CrossRef]
- Ryu, C.-M.; Farag, M.A.; Hu, C.-H.; Reddy, M.S.; Wei, H.-X.; Paré, P.W.; Kloepper, J.W. Bacterial volatiles promote growth in Arabidopsis. Proc. Natl. Acad. Sci. USA 2003, 100, 4927–4932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Moyne, A.-L.; Reddy, M.S.; Kloepper, J.W. The role of salicylic acid in induced systemic resistance elicited by plant growth-promoting rhizobacteria against blue mold of tobacco. Biol. Control. 2002, 25, 288–296. [Google Scholar] [CrossRef]
- Bargabus, R.; Zidack, N.; Sherwood, J.; Jacobsen, B. Screening for the identification of potential biological control agents that induce systemic acquired resistance in sugar beet. Biol. Control Biol. Control 2004, 30, 342–350. [Google Scholar] [CrossRef]
- Kavitha, J.; Jonathan, E.I.; Umamaheswari, R. Field application of Pseudomonas fluorescens, Bacillus subtilis and Trichoderma viride for the control of Meloidogyne incognita (Kofoid and White) chitwood on sugarbeet. J. Biol. Control 2007, 21, 211–215. [Google Scholar]
- Choudhary, D.K.; Prakash, A.; Johri, B.N. Induced systemic resistance (ISR) in plants: Mechanism of action. Indian J. Microbiol. 2007, 47, 289–297. [Google Scholar] [CrossRef] [Green Version]
- Lastochkina, O.; Pusenkova, L.; Yuldashev, R.; Babaev, M.; Garipova, S.; Blagova, D.; Khairullin, R.; Aliniaeifard, S. Effects of Bacillus subtilis on some physiological and biochemical parameters of Triticum aestivum L. (wheat) under salinity. Plant Physiol. Biochem. 2017, 121, 80–88. [Google Scholar] [CrossRef]
- Akram, W.; Mahboob, A.; Javed, A.A. Bacillus thuringiensis strain 199 can induce systemic resistance in tomato against Fusarium wilt. Eur. J. Microbiol. Immunol. 2013, 3, 275–280. [Google Scholar] [CrossRef] [Green Version]
- Zuckerman, B.M.; Dicklow, M.B.; Acosta, N. A strain of Bacillus thuringiensis for the control of plant-parasitic nematodes. Biocontrol Sci. Technol. 1993, 3, 41–46. [Google Scholar] [CrossRef]
- Audenaert, K.; Pattery, T.; Cornelis, P.; Höfte, M. Induction of systemic resistance to Botrytis cinerea in tomato by Pseudomonas aeruginosa 7NSK2: Role of salicylic acid, pyochelin, and pyocyanin. Mol. Plant Microbe Interact. 2002, 15, 1147–1156. [Google Scholar] [CrossRef] [Green Version]
- Fatima, S.; Anjum, T. Identification of a potential ISR determinant from Pseudomonas aeruginosa PM12 against Fusarium wilt in tomato. Front. Plant Sci. 2017, 8, 848. [Google Scholar] [CrossRef]
- Krechel, A.; Faupel, A.; Hallmann, J.; Ulrich, A.; Berg, G. Potato-associated bacteria and their antagonistic potential towards plant-pathogenic fungi and the plant-parasitic nematode Meloidogyne incognita (Kofoid & White) Chitwood. Can. J. Microbiol. 2002, 48, 772–786. [Google Scholar] [PubMed]
- Saikia, S.K.; Tiwari, S.; Pandey, R. Rhizospheric biological weapons for growth enhancement and Meloidogyne incognita management in Withania somnifera cv. Poshita. Biol. Control. 2013, 65, 225–234. [Google Scholar] [CrossRef]
- De Vleesschauwer, D.; Djavaheri, M.; Bakker, P.A.H.M.; Höfte, M. Pseudomonas fluorescens WCS374r-induced systemic resistance in rice against Magnaporthe oryzae is based on pseudobactin-mediated priming for a salicylic acid-repressible multifaceted defense response. Plant Physiol. 2008, 148, 1996–2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leeman, M.; van Pelt, J.A.; den Ouden, F.M.; Heinsbroek, M.; Bakker, P.A.H.M.; Schippers, B. Induction of systemic resistance by Pseudomonas fluorescens in radish cultivars differing in susceptibility to Fusarium wilt, using a novel bioassay. Eur. J. Plant Pathol. 1995, 101, 655–664. [Google Scholar] [CrossRef]
- Almaghrabi, O.A.; Massoud, S.I.; Abdelmoneim, T.S. Influence of inoculation with plant growth promoting rhizobacteria (PGPR) on tomato plant growth and nematode reproduction under greenhouse conditions. Saudi J. Biol. Sci. 2013, 20, 57–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reitz, M.; Rudolph, K.; Schröder, I.; Hoffmann-Hergarten, S.; Hallmann, J.; Sikora, R.A. lipopolysaccharides of Rhizobium etli strain G12 act in potato roots as an inducing agent of systemic resistance to infection by the cyst nematode Globodera pallida. Appl. Environ. Microbiol. 2000, 66, 3515–3518. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Medina, A.; Fernandez, I.; Lok, G.B.; Pozo, M.J.; Pieterse, C.M.J.; Wees, S.C.M.V. Shifting from priming of salicylic acid- to jasmonic acid-regulated defences by Trichoderma protects tomato against the root knot nematode Meloidogyne incognita. New Phytol. 2017, 213, 1363–1377. [Google Scholar] [CrossRef] [Green Version]
- BioNemaGonTM. Available online: http://www.agrilife.in/biopesti_microrigin_nemagon.htm (accessed on 18 August 2020).
- Clariva® pn. Available online: https://www.syngenta-us.com/seed-treatment/clariva-pn (accessed on 18 August 2020).
- Nematode: Alternative Controls. Available online: www.attra.ncat.org/attra-pub/PDF/nematode.pdf (accessed on 18 August 2020).
- MeloCon®WG. Available online: https://www.certisusa.com/products/bionematicides/melocon-wg (accessed on 18 August 2020).
- Mistures Comprising a Bacillus Strain and a Pesticide. Available online: https://patentswarm.com/patents/US10251400B2 (accessed on 18 August 2020).
- US EPA, Pesticide Product Label, NewPro. Available online: https://www3.epa.gov/pesticides/chem_search/ppls/085004-00011-20130422.pdf (accessed on 18 August 2020).
- Nortica 10 WP. Available online: http://www.tomirwin.com/pdf/labels/Nortica%2010WP.pdf (accessed on 18 August 2020).
- VoTIVo FS. Available online: https://agrobaseapp.com/united-states/pesticide/votivo-fs (accessed on 18 August 2020).
- Bansal, R.K.; Verma, V. kumar. Antagonistic efficacy of Azotobacter chroococcum against Meloidogyne javanica infecting brinjal. Indian J. Nematol. 2002, 32, 132–134. [Google Scholar]
- Crow, W.T. Effects of a commercial formulation of Bacillus firmus I-1582 on golf course bermudagrass infested with Belonolaimus longicaudatus. J. Nematol. 2014, 46, 331–335. [Google Scholar]
- Tiwari, S.; Pandey, S.; Chauhan, P.; Pandey, R. Biocontrol agents in co-inoculation manages root knot nematode [Meloidogyne incognita (Kofoid & White) Chitwood] and enhances essential oil content in Ocimum basilicum L. Ind. Crops Prod. 2017, 97, 292–301. [Google Scholar]
- Siddiqui, Z.A.; Shakeel, U. Screening of Bacillus isolates for potential biocontrol of the wilt disease complex of pigeon pea (Cajanus cajan) under greenhouse and small-scale field conditions. J. Plant Pathol. 2007, 89, 179–183. [Google Scholar]
- Zhou, L.; Yuen, G.; Wang, Y.; Wei, L.; Ji, G. Evaluation of bacterial biological control agents for control of rootknot nematode disease on tomato. Crop Prot. 2016, 84, 8–13. [Google Scholar] [CrossRef]
- Khan, M.R.; Akram, M. Effect of certain antagonistic fungi and rhizobacteria on wilt disease complex caused by Meloidogyne incognita and Fusarium oxysporium f. sp. lycopersici on tomato. Nematol. Mediterr. 2000, 28, 139–144. [Google Scholar]
- Meyer, S.L.F.; Massood, S.I.; Chitwood, D.J.; Roberts, D.P. Evaluation of Trichoderma virens and Burkholderia cepacia for antagonistic activity against root-knot nematode, Meloidogyne incognita. Nematology 2000, 2, 871–879. [Google Scholar]
- Colagiero, M.; Rosso, L.C.; Ciancio, A. Diversity and biocontrol potential of bacterial consortia associated to root-knot nematodes. Biol. Control. 2018, 120, 11–16. [Google Scholar] [CrossRef]
- Son, S.H.; Khan, Z.; Kim, S.G.; Kim, Y.H. Plant growth-promoting rhizobacteria, Paenibacillus polymyxa and Paenibacillus lentimorbus suppress disease complex caused by root-knot nematode and Fusarium wilt fungus. J. Appl. Microbiol. 2009, 107, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, D.F.; Campos, V.P.; Amaral, D.R.; Nunes, A.S.; Pantaleão, J.A.; Costa, D.A. Selection of rhizobacteria able to produce metabolites active against Meloidogyne exigua. Eur. J. Plant Pathol. 2007, 119, 477–479. [Google Scholar] [CrossRef]
- Kermarrec, A.; Jacqua, G.; Anais, J. Effect of Fusarium solani and Pseudomonas solanacearum on the infestation of aubergine with the plant-parasitic nematode Rotylenchulus reniformis. Nematologica 1994, 40, 152–154. [Google Scholar]
- Siddiqui, Z.A.; Singh, L.P. Effects of fly ash, Pseudomonas striata and Rhizobium on the reproduction of nematode Meloidogyne incognita and on the growth and transpiration of pea. J. Environ. Biol. 2005, 26, 117–122. [Google Scholar]
- Seenivasan, N.; Parameswaran, S.; Sridar, P.; Gopalakrishnan, C.; Gnanamurthy, P. Application of Bioagents and Neem Cake as Soil Application for the Management of Root-knot Nematode in Turmeric. National Congress on Centenary of Nematology in India Appraisal and Future Plans; Indian Agricultural Research Institute: New Delhi, India, 2001; p. 164. [Google Scholar]
- Insunza, V.; Alström, S.; Eriksson, K.B. Root bacteria from nematicidal plants and their biocontrol potential against trichodorid nematodes in potato. Plant Soil. 2002, 241, 271–278. [Google Scholar] [CrossRef]
- Rashad, F.; Fathy, H.; Samir, A.; Elghonaimy, A. Isolation and characterization of multifunctional Streptomyces species with antimicrobial, nematicidal and phytohormone activities from marine environments in Egypt. Microbiol. Res. 2015, 175, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Hussey, R.S.; Grundler, F.M. Nematode parasitism of plants. In Physiology and Biochemistry of Free-Living and Plant Parasitic Nematodes; Perry, R.N., Wright, J., Eds.; 11 CAB International Press: Oxford, UK, 1988; pp. 213–243. [Google Scholar]
- Singh, S.; Singh, B.; Singh, A.P. Nematodes: A threat to sustainability of agriculture. Procedia Environ. Sci. 2015, 29, 215–216. [Google Scholar] [CrossRef] [Green Version]
- Sikkens, R.B.; Weaver, D.B.; Lawrence, K.S.; Moore, S.R.; van Santen, E. LONREN upland cotton germ-plasm response to Rotylenchulus reniformis inoculum level. Nematropica 2011, 6, 68–74. [Google Scholar]
- Roshi, R.A.; King, R.L.; Lawrence, G.W. Classification of Rothylenhulus reniformis numbers in cotton using remotely sensed hyperspectral data on self-organizing maps. J. Nematol. 2010, 42, 179–193. [Google Scholar]
- Weaver, D.B. Cotton nematodes. In Cotton, 2nd ed.; Fang, D.D., Rercy, R.G., Eds.; ASA, CSSA, and SSSA: Madison, WI, USA, 2015; pp. 547–570. [Google Scholar]
- Elhady, A.; Giné, A.; Topalovic, O.; Jacquiod, S.; Sørensen, S.J.; Sorribas, F.J.; Heuer, H.; Castagnone-Sereno, P. Microbiomes associated with infective stages of root-knot and lesion nematodes in soil. PLoS ONE 2017, 12, e0177145. [Google Scholar] [CrossRef] [Green Version]
- Bernard, G.C.; Egnin, M.; Bonsi, C. The impact of plant-parasitic nematodes on agriculture and methods of control. In Nematology—Concepts, Diagnosis and Control; Shah, M.M., Mohammod, M., Eds.; IntechOpen: London, UK, 2017; pp. 121–151. [Google Scholar]
- Pimentel, D. Environmental and economic costs of the application of pesticides primarily in the United States. Environ. Dev. Sustain. 2005, 7, 229–252. [Google Scholar] [CrossRef]
- Mitiku, M. Plant-parasitic nematodes and their management: A review. Agric. Res. Technol. Open Access J. 2016, 16, 555980. [Google Scholar] [CrossRef]
- Shepherd, R.L.; Huck, M.G. Progression of root-knot nematode symptoms and infection on resistant and susceptible cottons. J. Nematol. 1989, 2, 235–241. [Google Scholar]
- Rhoads, M.L. Cholinesterase in the parasitic nematode, Stephanurus denatus. J. Biochem. 1981, 17, 9316–9323. [Google Scholar]
- Chitwood, B.G. “Root-knot Nematodes”—Part I. A revision of the genus Meloidogyne goeldi, 1887. Proc. Helminthol. Soc. Wah. 1949, 16, 90–104. [Google Scholar]
- Johnson, A.; Dowler, C.; Handoo, Z. Population dynamics of Meloidogyne incognita, M. arenaria and other nematodes and crop yields in rotations of cotton, peanut, and wheat under minimum tillage. J. Nematol. 2000, 32, 52–61. [Google Scholar] [PubMed]
- Vyska, M.; Cunniffe, N.; Gilligan, C. Trade-off between disease resistance and crop yield: A landscape-scale mathematical modeling perspective. J. R. Soc. Interface 2016, 13, 20160451. [Google Scholar] [CrossRef] [Green Version]
- Trudgill, D.L. Resistance to and tolerance of plant parasitic nematodes in plants. Ann. Rev. Phytopathol. 1991, 29, 167–192. [Google Scholar] [CrossRef]
- Siddiqui, Z.A.; Mahmood, I. Role of bacteria in the management of plant parasitic nematodes: A Review. Bioresour. Technol. 1999, 69, 167–179. [Google Scholar] [CrossRef]
- Wani, A.H. Plant growth-promoting rhizobacteria as biocontrol agents of phytonematodes. In Biocontrol Agents of Phytonematodes; Askary, T.H., Martinelli, P.R.P., Eds.; CAB International: Wallingford, UK, 2015; pp. 339–362. [Google Scholar]
- Lugtenberg, B.; Kamilova, F. Plant-growth-promoting rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Loon, L.C.; Bakker, P.A.H.M.; Pieterse, C.M.J. Systemic resistance induced by rhizosphere bacteria. Annu. Rev. Phytopathol. 1998, 36, 453–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bishop, A. Pasteuria penetrans and its parasitic interaction with plant parasitic nematodes. In Endospore-Forming Soil Bacteria; Logan, N.A., de Vos, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 181–201. [Google Scholar]
- Starr, M.P.; Sayre, R.M. Pasteuria thornei sp. nov. and Pasteuria penetrans sensu stricto emend., mycelial and endospore-forming bacteria parasitic, respectively, on plant-parasitic nematodes of the genera Pratylenchus and Meloidogyne. Ann. Inst. Pasteur. Microbiol. 1988, 139, 11–31. [Google Scholar] [CrossRef]
- Migula, W. Über ein neues System der Bakterien. Arab Bacteriol. Inst. Karlsr. 1984, 1, 235–328. [Google Scholar]
- Migula, W. System der Bakteriem; Gustav Fischer: Jena, Germany, 1900; Volume 2. [Google Scholar]
- Maksimov, I.V.; Abizgil’dina, R.R.; Pusenkova, L.I. Plant growth promoting rhizobacteria as alternative to chemical crop protectors from pathogens (Review). Appl. Biochem. Microbiol. 2011, 47, 333–345. [Google Scholar] [CrossRef]
- Frankland, S.G.; Frankland, R.F. Studies on some new microorganisms obtained from air. Royal Soc. Lon-don Phil. Trans. Ser. B Biol. Sci. 1887, 178, 257–287. [Google Scholar]
- Bredemann, G.; Werner, W. Botanische beschreibung haufinger am buttersaureabbau beteiligter sporenbildender bakterienspezies. Zentralbl. Bakteriol. Parasitenkd. Infektionskr. Hyg. Abt. II 1933, 87, 446–475. [Google Scholar]
- Chester, F.D. A Manual of Determinative Bacteriology; The Macmillan Co.: New York, NY, USA, 1901; pp. 1–401. [Google Scholar]
- De Bary, A. Vergleichende Morphologie und Biologie der Pilze, Mycetozoen und Bacterien; Wilhelm Engelmann: Leipzig, Germany, 1884. [Google Scholar]
- Cohn, F. Untersuchungen uber Bakterien. Beitr. Biol. Pflanz. 1872, 1, 127–224. [Google Scholar]
- Collins, M.D.; Smida, J.; Dorsch, M.; Stackebrandt, E. Tsukamurella gen. nov. harboring Corynebacterium paurometabolum and Rhodococcus aurantiacus. Int. J. Syst. Bacteriol. 1988, 38, 385–391. [Google Scholar] [CrossRef]
- Ramamoorthy, V.; Viswanathan, R.; Raguchander, T.; Prakasam, V.; Samiyappan, R. Induction of systemic resistance by plant growth promoting rhizobacteria in crop plants against pests and diseases. Crop Prot. 2001, 20, 1–11. [Google Scholar] [CrossRef]
- Babalola, O.O. Beneficial bacteria of agricultural importance. Biotechnol. Lett. 2010, 32, 1559–1570. [Google Scholar] [CrossRef]
- Ichinohe, M. On the soybean nematode, Heterodera glycines n. sp., from Japan (trans.). Oyo-Dobutsugaku-Zasshi 1952, 17, 1–4. [Google Scholar]
- Koshy, P.K. A new species of Heterodera from India. Indian Phytopathol. 1967, 20, 272–274. [Google Scholar]
- Stone, A.R. Heterodera pallida n. sp. (Nematoda: Heteroderidae), a second species of potato cyst nematode. Nematologica 1973, 18, 591–606. [Google Scholar] [CrossRef]
- Priest, F.G.; Goodfellow, M.; Shute, L.A.; Berkeley, R.C.W. Bacillus amyloliquefaciens sp. nov., nom. rev. Int. J. Syst. Bacteriol. 1987, 37, 69–71. [Google Scholar] [CrossRef]
- Golden, A.M.; Birchfield, W. Rice root-knot nematode (Meloidogyne graminicola) as a new pest of rice. Plant Dis. Rep. 1968, 52, 423. [Google Scholar]
- Krall, E.L.; Krall, H.A. Revision of the plant nematodes of the family Heteroderidae on the basis of the trophic specialization of these parasites and their co-evolution with their host plants. In Fitogel’Mintologi-Cheskie Issledovaniya. Moscow; Nauka: Moscow, USSR, 1978; pp. 39–56. [Google Scholar]
- Schmidt, A. Über den Rüben-Nematoden (Heterodera schachtii A.S.). Z. Ver. Die Rüben-Zucker-Ind. Zollverein 1871, 21, 1–19. [Google Scholar]
- Filipjev, I.N.; Schuurmans Stekhoven, J.H., Jr. A Manual of Agricultural Helminthology; Brill: Leiden, The Netherland, 1941; p. 878. [Google Scholar]
- Nahar, K.; Kyndt, T.; De Vleesschauwer, D.; Höfte, M.; Gheysen, G. The jasmonate pathway is a key player in systemically induced defense against root knot nematodes in rice. Plant Physiol. 2011, 157, 305–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujimoto, T.; Tomitaka, Y.; Abe, H.; Tsuda, S.; Futai, K.; Mizukubo, T. Expression profile of jasmonic acid-induced genes and the induced resistance against the root-knot nematode (Meloidogyne incognita) in tomato plants (Solanum lycopersicum ) after foliar treatment with methyl jasmonate. J. Plant Physiol. 2011, 168, 1084–1097. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, K.E.; Nishimura, N.; Hitomi, K.; Getzoff, E.D.; Schroeder, J.I. Early abscisic acid signal transduction mechanisms: Newly discovered components and newly emerging questions. Genes Dev. 2010, 24, 1695–1708. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.C.; Luan, S. ABA signal transduction at the crossroad of biotic and abiotic stress responses. Plant Cell Environ. 2012, 35, 53–60. [Google Scholar] [CrossRef]
- Daszkowska-Golec, A.; Szarejko, I. The molecular basis of ABA-mediated plant response to drought. In Abiotic Stress—Plant Responses and Applications in Agriculture; Vahdati, K., Leslie, C., Eds.; IntechOpen: Rijeka, Croatia, 2013; Available online: https://www.intechopen.com/books/abiotic-stress-plant-responses-and-applications-in-agriculture/the-molecular-basis-of-aba-mediated-plant-response-to-drought (accessed on 3 September 2020).
- Der Ent, S.V.; Hulten, M.V.; Pozo, M.J.; Czechowski, T.; Udvardi, M.K.; Pieterse, C.M.J.; Ton, J. Priming of plant innate immunity by rhizobacteria and β-aminobutyric acid: Differences and similarities in regulation. New Phytol. 2009, 183, 419–431. [Google Scholar] [CrossRef] [Green Version]
- de Toni, J.B.; Trevisan, V. Schizomycetaceae Naeg. In Sylloge Fungorum Omnium Hujusque Cognitorum; Saccardo, P.A., Ed.; Sumptibus Auctoris: Berlin, Germany, 1889; Volume 8, pp. 923–1087. [Google Scholar]
- Pieterse, C.M.; van Wees, S.C.; van Pelt, J.A.; Knoester, M.; Laan, R.; Gerrits, H.; Weisbeek, P.J.; van Loon, L.C. A novel signaling pathway controlling induced systemic resistance in Arabidopsis. Plant Cell 1998, 10, 1571–1580. [Google Scholar] [CrossRef] [Green Version]
- Ramírez, V.; Van der Ent, S.; García-Andrade, J.; Coego, A.; Pieterse, C.M.; Vera, P. OCP3 is an important modulator of NPR1-mediated jasmonic acid-dependent induced defenses in Arabidopsis. BMC Plant Biol. 2010, 10, 199. [Google Scholar] [CrossRef] [Green Version]
- Cominelli, E.; Galbiati, M.; Vavasseur, A.; Conti, L.; Sala, T.; Vuylsteke, M.; Leonhardt, N.; Dellaporta, S.L.; Tonelli, C. A guard-cell-specific MYB transcription factor regulates stomatal movements and plant drought tolerance. Curr. Biol. 2005, 15, 1196–1200. [Google Scholar]
- Lim, C.W.; Baek, W.; Jung, J.; Kim, J.-H.; Lee, S.C. Function of ABA in stomatal defense against biotic and drought stresses. Int. J. Mol. Sci. 2015, 16, 15251–15270. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Gao, H.; Liu, B.; Qi, T.; Tong, J.; Xiao, L.; Xie, D.; Song, S. Arabidopsis MYB24 regulates jasmonate-mediated stamen development. Front. Plant Sci. 2017, 8, 1525. [Google Scholar] [CrossRef]
- Feys, B.; Benedetti, C.E.; Penfold, C.N.; Turner, J.G. Arabidopsis mutants selected for resistance to the phytotoxin coronatine are male sterile, insensitive to methyl jasmonate, and resistant to a bacterial pathogen. Plant Cell 1994, 6, 751–759. [Google Scholar] [CrossRef] [Green Version]
- Mosblech, A.; Thurow, C.; Gatz, C.; Feussner, I.; Heilmann, I. Jasmonic acid perception by COI1 involves inositol polyphosphates in Arabidopsis thaliana. Plant J. 2011, 65, 949–957. [Google Scholar] [CrossRef]
- Liu, Y.; Lai, Q.; Shao, Z. Genome analysis-based reclassification of Bacillus weihenstephanensis as a later heterotypic synonym of Bacillus mycoides. Int. J. Syst. Evol. Microbiol. 2018, 68, 106–112. [Google Scholar] [CrossRef]
- Meyer, A.; Gottheil, O. Botanische beschreibung einiger bodenbakterien. Zentralbl. Bakteriol. Parasitenkd. Infektionskr. Hyg. Abt. II 1901, 7, 680–691. [Google Scholar]
- Chaverri, P.; Samuels, G.J. Hypocrea lixii, the teleomorph of Trichoderma harzianum. Mycol. Prog. 2002, 1, 283–286. [Google Scholar] [CrossRef]
- Martinez- Medina, A.; Appels, F.V.W.; van Wees, S.C.M. Impact of salicylic acid- and jasmonic acid-defences on root colonization of Trichoderma harzianum T-78. Plant Signal. Behav. 2017, 12, 1345414. [Google Scholar] [CrossRef] [Green Version]
- Glazebrook, J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Ann. Rev. Phytopathol. 2005, 43, 205–227. [Google Scholar] [CrossRef]
- Gimenez-Ibanez, S.; Solano, R. Nuclear jasmonate and salicylate signaling and crosstalk in defense against pathogens. Front. Plant Sci. 2013, 4, 72. [Google Scholar] [CrossRef] [Green Version]
- Pena-Cortés, H.; Albrecht, T.; Prat, S.; Weiler, E.W.; Willmitzer, L. Aspirin prevents wound-induced gene expression in tomato leaves by blocking jasmonic acid biosynthesis. Planta 1993, 191, 123–128. [Google Scholar] [CrossRef]
- Uppalapati, S.R.; Ishiga, Y.; Wangdi, T.; Kunkel, B.N.; Anand, A.; Mysore, K.S.; Bender, C.L. The phytotoxin coronatine contributes to pathogen fitness and is required for suppression of salicylic acid accumulation in tomato inoculated with Pseudomonas syringae pv. tomato DC3000. Mol. Plant Microbe Interact. 2007, 20, 955–965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamaoki, D.; Seo, S.; Yamada, S.; Kano, A.; Miyamoto, A.; Shishido, H.; Miyoshi, S.; Taniguchi, S.; Akimitsu, K.; Gomi, K. Jasmonic acid and salicylic acid activate a common defense system in rice. Plant Signal. Behav. 2013, 8, 24260. [Google Scholar] [CrossRef] [PubMed]
- Van der Does, D.; Leon-Reyes, A.; Koornneef, A.; Van Verk, M.C.; Rodenburg, N.; Pauwels, L.; Goossens, A.; Körbes, A.P.; Memelink, J.; Ritsema, T.; et al. Salicylic acid suppresses jasmonic acid signaling downstream of SCFCOI1-JAZ by targeting GCC promoter motifs via transcription factor ORA59. Plant Cell. 2013, 25, 744–761. [Google Scholar] [CrossRef] [Green Version]
- Whetzel, H.H. A synopsis of the genera and species of the Sclerotiniaceae, a family of stromatic inoperculate discomycetes. Mycologia 1945, 37, 648–714. [Google Scholar] [CrossRef]
- Mitter, N.; Kazan, K.; Way, H.M.; Broekaert, W.F.; Manners, J.M. Systemic induction of an Arabidopsis plant defensin gene promoter by tobacco mosaic virus and jasmonic acid in transgenic tobacco. Plant Sci. 1998, 136, 169–180. [Google Scholar] [CrossRef]
- Clarke, J.D.; Volko, S.M.; Ledford, H.; Ausubel, F.M.; Dong, X. Roles of salicylic acid, jasmonic acid, and ethylene in cpr-induced resistance in Arabidopsis. Plant Cell 2000, 12, 2175–2190. [Google Scholar] [CrossRef] [Green Version]
- Mur, L.A.J.; Kenton, P.; Atzorn, R.; Miersch, O.; Wasternack, C. The outcomes of concentration-specific interactions between salicylate and jasmonate signaling include synergy, antagonism, and oxidative stress leading to cell death. Plant Physiol. 2006, 140, 249–262. [Google Scholar] [CrossRef] [Green Version]
- Shafi, J.; Tian, H.; Ji, M. Bacillus species as versatile weapons for plant pathogens: A review. Biotechnol. Biotechnol. Equip. 2017, 31, 446–459. [Google Scholar] [CrossRef] [Green Version]
- Kloepper, J.W.; Lifshitz, R.; Zablotowicz, R. Free-living bacterial inocula for enhancing crop productivity. Trends Biotechnol. 1989, 7, 39–44. [Google Scholar] [CrossRef]
- Vacheron, J.; Desbrosses, G.; Bouffaud, M.-L.; Touraine, B.; Moënne-Loccoz, Y.; Muller, D.; Legendre, L.; Wisniewski-Dyé, F.; Prigent-Combaret, C. Plant growth-promoting rhizobacteria and root system functioning. Front. Plant Sci. 2013, 4, 356. [Google Scholar] [CrossRef] [Green Version]
- Kloepper, J.W.; Ryu, C.-M.; Zhang, S. Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology 2004, 94, 1259–1266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| PGPR | Target PPN | Molecules or Modes | References |
|---|---|---|---|
| Bacillus cereus | Heterodera avenae, Meloidogyne incognita, Meloidogyne javanica | Sphingosine, Protease, Chitinase, Antibiotic production, Secondary metabolites | Oka et al., 2008 [9]; Gao et al., 2016 [10]; Ahmed, 2019 [11] |
| Bacillus coagulans | Meloidogyne incognita | Hydrolytic enzymes | Ambo et al., 2010 [12]; Serfoji et al., 2013 [13]; Xiang et al., 2018 [4] |
| Bacillus firmus | Ditylenchus dipasi, Heterodera spp., Meloidogyne incognita, Pratylenchus spp., Radopholus similis | Sep 1 protease, Secondary metabolites | Giannakou et al., 2004 [14]; Mendoza et al., 2008 [15]; Terefe et al., 2009 [16]; Terefe et al., 2012 [17]; Xiong et al., 2015 [18]; Geng et al., 2016 [19]; Bayer Crop Science [20] |
| Bacillus licheniformis | Bursaphelenchulus xylophi lus, Meloidogyne incognita | Protease, Chitinase | Siddiqui and Husain 1991 [21]; Siddiqui and Mahmood 1992 [22]; Jeong et al., 2015 [23]; El-Nagdi et al., 2019 [24] |
| Bacillus megaterium | Heterodera glycines,Meloidogyne incognita, Meloidogyne graminicola | Protease, Secondary metabolites | Kloepper et al., 1992 [25]; Padgham et al., 2007 [26]; Mostafa et al., 2018 [27] |
| Bacillus pumilus L1 | Heterodera glycines, Meloidogyne arenaria | Protease, Chitinase | Lee and Kim 2015 [28]; Forghani and Hajihassani et al., 2020 [29] |
| Bacillus subtilis | Helicotylenchus multicinctus, Meloidogyne graminicola, Meloidogyne incognita, Meloidogyne javanica, Rotylenchulus reniformis | Lipopeptide antibiotics, Hydrolytic enzymes, Secondary metabolites | Prakob et al., 2009 [30]; Kavitha et al., 2012 [31]; Basyony and Abo-Zaid, 2018 [32]; Mazzuchelli et al., 2020 [33]; Gautam et al., 1995 [34] |
| Bacillus thuringiensis | Heterodera glycines, Meloidogyne incognita | Bt crystal protein (toxin protein), Thuringiensin (β-exotoxin) | Noel, 1990 [35]; Wei et al., 2003 [36]; Mohammed et al., 2008 [37] |
| Corynebacterium paurometabolum | Meloidogyne incognita | Hydrogen sulfide, Chitinase | Mena and Pimentel, 2002 [38] |
| Pasteuria penetrans1 | Meloidogyne spp. | Predation | Mankau et al., 1976 [39]; Mankau and Prasad, 1977 [40]; Dube and Smart, [41]; Sayre and Starr 1975 [42]; Bhuiyan et al., 2018 [43] |
| Pasteuria thornei1 | Pratylenchus spp. | Predation | Mankau et al., 1976 [41]; Atibalentja et al., 2000 [44] |
| Pasteuria nishizawae1 | Globodera spp., Heterodera spp. | Predation | Sayre and Wergin, 1991 [45] |
| Pseudomonas aeruginosa | Caenorhabditis elegans, Meloidogyne incognita, Meloidogyne javanica | Hydrogen cyanide (HCN) | Siddiqui and Ehteshamul-Haque, 2001 [46]; Gallagher and Manoil, 2001 [47]; Singh and Siddiqui, 2010 [48] |
| Pseudomonas. fluorescens F113 | Globodera rostochinensis | 2,4-diacetylphloroglucinol (DAPG) | Cronin et al., 1997 [49] |
| Pseudomonas fluorescens CHA0 | Meloidogyne incognita, Meloidogynejavanica | HCN, DAPG, Pyoluteorin, Extracellular protease | Siddiqui and Shaukat, 2003 [50]; Hamid et al., 2003 [51]; Siddiqui et al., 2005 [52] |
| Pseudomonas fluorescens Wood1R | Meloidogyne incognita | DAPG | Timper et al., 2009 [53] |
| Pseudomonas stutzeri | Meloidogyne incognita | HCN | Khan et al., 2016 [54] |
| Serratia marcescens | Meloidogyne incognita, Meloidogyne javanica, Radopholus similis | Volatile metabolites, Prodigiosin | Zabaketa-Mejia, 1985 [55]; Rahul et al., 2014 [56] |
| PGPR | Target PPN | Modes | References |
|---|---|---|---|
| Agrobacterium radiobacter (G12) | Globodera spp. | ISR | Hasky-Guenther et al., 1998 [57]; Hackenberg and Sikora, 1992 [58]; Racke and Sikora, 1992 [59]; Hackenberg et al., 1999 [60] |
| Bacillus amyloliquefaciens (syn. Bacillus velezensis) | Heterodera glycine, Meloidogyne incognita | ISR and SAR | Ryu et al., 2004 [61]; Beris et al., 2018 [62]; Burkett- Cadena et al., 2008 [63]; Choudhary et al., 2009 [64]; Li et al., 2015 [65]; Xiang et al., 2016 [66]; Xie et al., 2018 [67] |
| Bacillus cereus | Meloidogyne javanica, Meloidogyne incognita | ISR | Xiang et al., 2016 [66]; Halfeld-Vieira et al., 2006 [68]; Niu et al., 2011 [69]; Jiang et al., 2020 [70] |
| Bacillus mojavensis | Meloidogyne incognita | ISR | Xiang et al., 2016 [66]; Liu et al., 2016 [71] |
| Bacillus mycoides | Meloidogyne incognita | ISR and SAR | Xiang et al., 2016 [66]; Barbagus et al., 2004 [72] |
| Bacillus pasteurii | Meloidogyne incognita | ISR | Xiang et al., 2016 [66]; Ryu et al., 2003 [73] |
| Bacillus pumilus | Heterodera glycine, Meloidogyne incognita | ISR and SAR | Xiang et al., 2016 [66]; Zhang et al., 2002 [74]; Barbagus et al., 2004 [75]; Kavitha et al., 2007 [76]; Choudhary et al., 2007 [77]; Lastochkina et al., 2017 [78] |
| Bacillus sphaericus | Globodera pallida, Meloidogyne incognita | ISR | Hasky-Guenther et al., 1998 [57]; Racke and Sikora 1992 [59]; Xiang et al., 2016 [66] |
| Bacillus subtilis | Heterodera cajani, Meloidogyne arenaria, Meloidogyne incognita, Meloidogyne javanica | ISR and SAR | Ryu et al., 2004 [61]; Xiang et al., 2016 [66]; Kavitha et al., 2007 [76]; Choudhary et al., 2007 [77]; Lastochkina et al., 2017 [78] |
| Bacillus thuringiensis | Aphelenchus avenae, Meloidogyne incognita | ISR | Zhang et al., 2002 [74] Akram et al., 2013 [79]; Zuckerman et al.; 1993 [80] |
| Pseudomonas aeruginosa | Meloidogyne javanica | ISR and SAR | Audenaert et al.,2013 [81]; Fatima et al., 2017 [82] |
| Pseudomonas fluorescens | Meloidogyne incognita, Meloidogyne javanica | ISR and SAR | Siddiqui and Shaukat 2003 [50]; Choudhary et al., 2007 [77]; Krechel et al., 2002 [83]; Saikia et al., 2013 [84]; de Vleesschauwer et al., 2012 [85]; Leeman et al., 1995 [86] |
| Pseudomonas putida | Meloidogyne incognita | ISR | Krechel et al., 2002 [83]; Almaghrabi et al., 2013 [87] |
| Rhizobium etli | Meloidogyne spp. | ISR | Reitz et al., 2000 [88] |
| Serratia marcescens | Meloidogyne incognita | ISR | Zhang et al., 2002 [74]; Almaghrabi et al., 2013 [87] |
| Trichoderma harzianum1 | Meloidogyne incognita | ISR and SAR | Martínez-Medina et al., 2017 [89] |
| Commercial Products | PGPR | Applications | References |
|---|---|---|---|
| BioNemaGonTM | Bacillus firmus | Reduce nematode population and root infestation by nematodes in vegetables and herbs | [90] |
| BioYieldTM | Bacillus subtilis GB03, Bacillus amyloliquefaciens | Nematodes in tomato, strawberry, and bell pepper | [65] |
| Clariva® pn | Pasteuria nishizawae Pn1 | Seed treatment; Target Heterodera glycines to reduce feeding and reproduction, and increase yields under heavy PPN pressure. | [91] |
| Deny, Blue Circle | Burlkholderia cepacia | Inhibit egg hatching and mobility of nematode juveniles | [92] |
| MeloCon®, BioAct and NemOut | Purpureocillium lilacinus 251 | Inhibit root knot, burring, cyst, reniform, spiral, sting, and root lesion nematodes. | [93] |
| Naviva ST | Pasteuria sp. Ph3 | Seed treatment; Inhibit Rotylenchulus reniformis in cotton, soy, vegetables, cucurbits, and floriculture. | [94] |
| NewPro | Pasteuria usgae Bl1 + Pasteuria sp. Ph3 | Inhibit lance and sting nematodes in turf (Bermudagrass and St. Augustine grass) | [95] |
| Nortica 10 WP | Bacillus firmus I-1582 | Inhibit cyst, lance, lesion, ring, root knot sheath, spiral, sting, and stunt nematodes in turf. | [96] |
| VOTiVO FS | Bacillus firmus I-1582 | Seed treatment; inhibit a broad range of nematodes. Available also as premix with insecticide | [97] |
| PGPR | Target PPN | Target Crops | References |
|---|---|---|---|
| Alcaligenes faecalis | Meloidogyne incognita | Chickpea | Siddiqui and Mahmood, 1992 [22] |
| Azotobacter chroococcum | Meloidogyne incognita, Meloidogyne javanica | Eggplant, Tomato | Bansal et al., 2002 [98] |
| Bacillus altitudinis, Bacillus aerophilus, Bacillus aryabhattai, Bacillus galliciensis, Bacillus psychrosaccharolyticus, Bacillus safensis, Bacillus siamensis, Bacillus simplex, Bacillus toyonensis, Bacillus weihenstephanensis | Meloidogyne incognita | Cotton | Xiang et al., 2016 [66] |
| Bacillus firmus | Belonolaimus longicaudatus | Bermudagrass | Crow, 2014 [99] |
| Bacillus flexus | Meloidogyne incognita | Basil | Tiwari et al., 2017 [100] |
| Bacillus isolates | Heterodera cajani, Meloidogyne incognita | Pigeon pea | Siddiqui and Shakeel, 2007 [101] |
| Bacillus methylotrophicus | Meloidogyne incognita | Tomato | Zhou et al., 2016 [102] |
| Bacillus polymyxa | Meloidogyne incognita | Tomato | Khan and Akram, 2000 [103] |
| Bacillus tequilensis | Meloidogyne incognita | Basil | Tiwari et al., 2017 [100] |
| Burkholderia cepacia | Meloidogyne incognita | Tomato | Meyer et al., 2000 [104] |
| Lysinibacillus sphaericus | Meloidogyne incognita | Tomato | Colagiero et al., 2018 [105] |
| Lysobacter spp. | Meloidogyne incognita | Tomato | Zhou et al., 2016 [102] |
| Paenibacillus lentimorbus, Paenibacillus polymyxa | Meloidogyne incognita | Tomato | Son et al., 2009 [106] |
| Paenibacillus macerans | Meloidogyne exigua | Coffee | Oliveira et al., 2007 [107] |
| Pseudomonas solanacearum | Rotylenchulus reniformis | Eggplant | Kermarrec et al.,1994 [108] |
| Pseudomonas striata | Meloidogyne incognita | Pea | Siddiqui and Singh, 2005 [109] |
| Pseudomonas stutzeri | Meloidogyne incognita | Chickpea | Seenivasan et al., 2001 [110] |
| Stenotrophomonas maltophilia | Paratrichodorus pachydermus, Trichodorus primitivus | Potato | Insunza et al., 2002 [111] |
| Streptomyces spp. | Meloidogyne incognita | Eggplant, Tomato | Rashad et al., 2015 [112] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subedi, P.; Gattoni, K.; Liu, W.; Lawrence, K.S.; Park, S.-W. Current Utility of Plant Growth-Promoting Rhizobacteria as Biological Control Agents towards Plant-Parasitic Nematodes. Plants 2020, 9, 1167. https://doi.org/10.3390/plants9091167
Subedi P, Gattoni K, Liu W, Lawrence KS, Park S-W. Current Utility of Plant Growth-Promoting Rhizobacteria as Biological Control Agents towards Plant-Parasitic Nematodes. Plants. 2020; 9(9):1167. https://doi.org/10.3390/plants9091167
Chicago/Turabian StyleSubedi, Pratima, Kaitlin Gattoni, Wenshan Liu, Kathy S. Lawrence, and Sang-Wook Park. 2020. "Current Utility of Plant Growth-Promoting Rhizobacteria as Biological Control Agents towards Plant-Parasitic Nematodes" Plants 9, no. 9: 1167. https://doi.org/10.3390/plants9091167
APA StyleSubedi, P., Gattoni, K., Liu, W., Lawrence, K. S., & Park, S.-W. (2020). Current Utility of Plant Growth-Promoting Rhizobacteria as Biological Control Agents towards Plant-Parasitic Nematodes. Plants, 9(9), 1167. https://doi.org/10.3390/plants9091167