The Active Compounds and Therapeutic Mechanisms of Pentaherbs Formula for Oral and Topical Treatment of Atopic Dermatitis Based on Network Pharmacology
Abstract
:1. Introduction
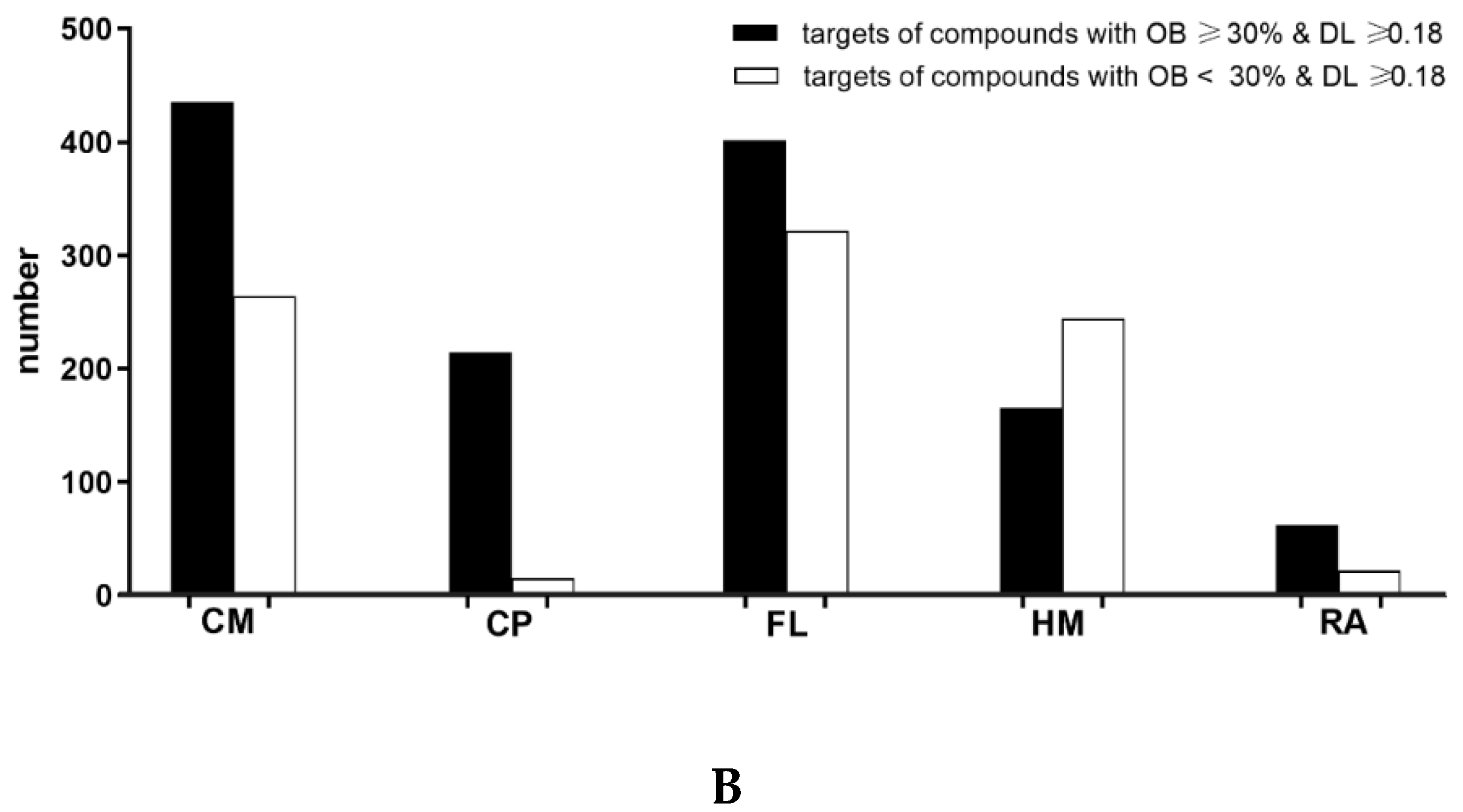
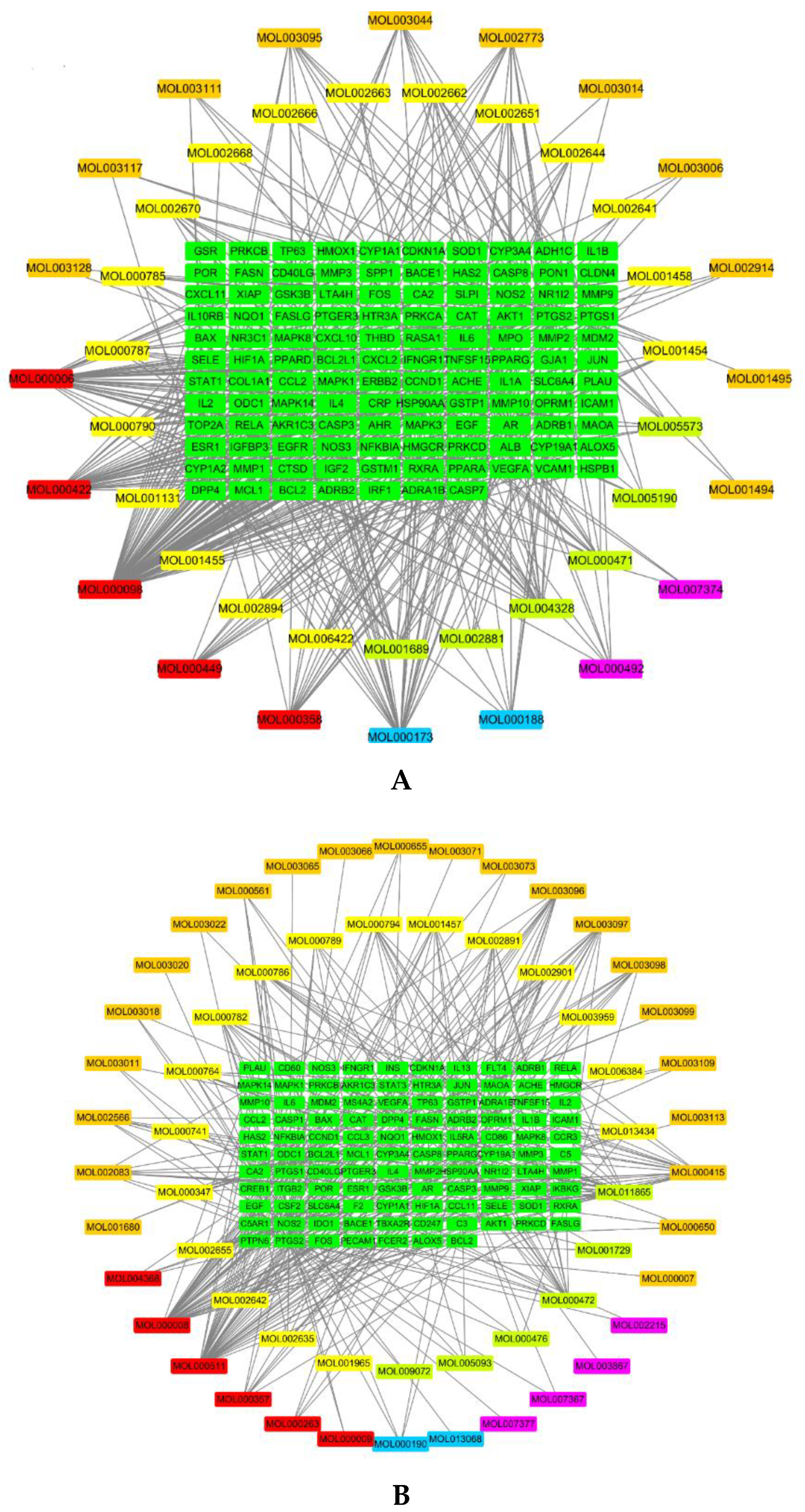
1.1. Active Compounds and Compound Targets in PHF
1.2. AD-Related Targets
1.3. Network Analysis
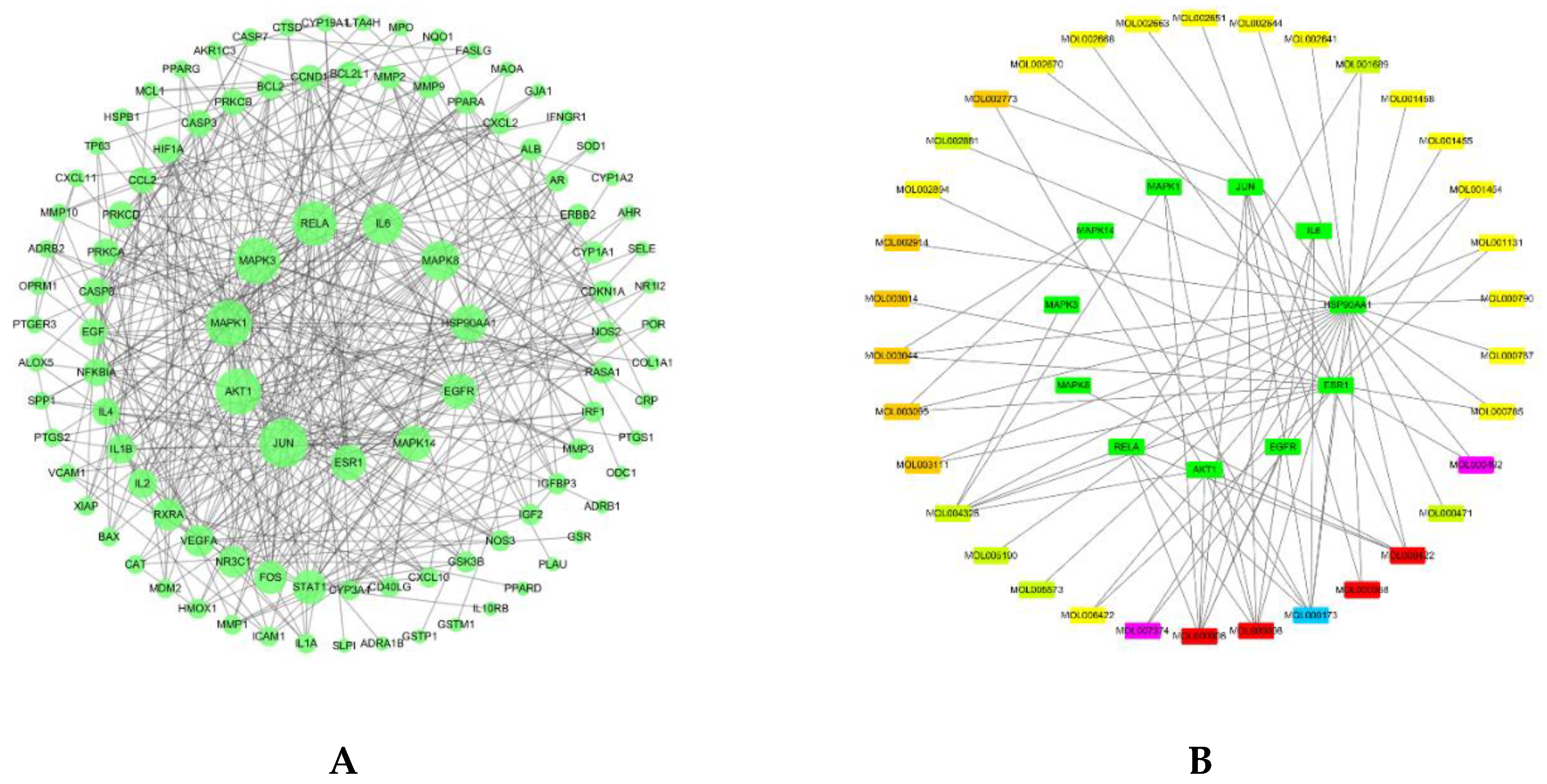
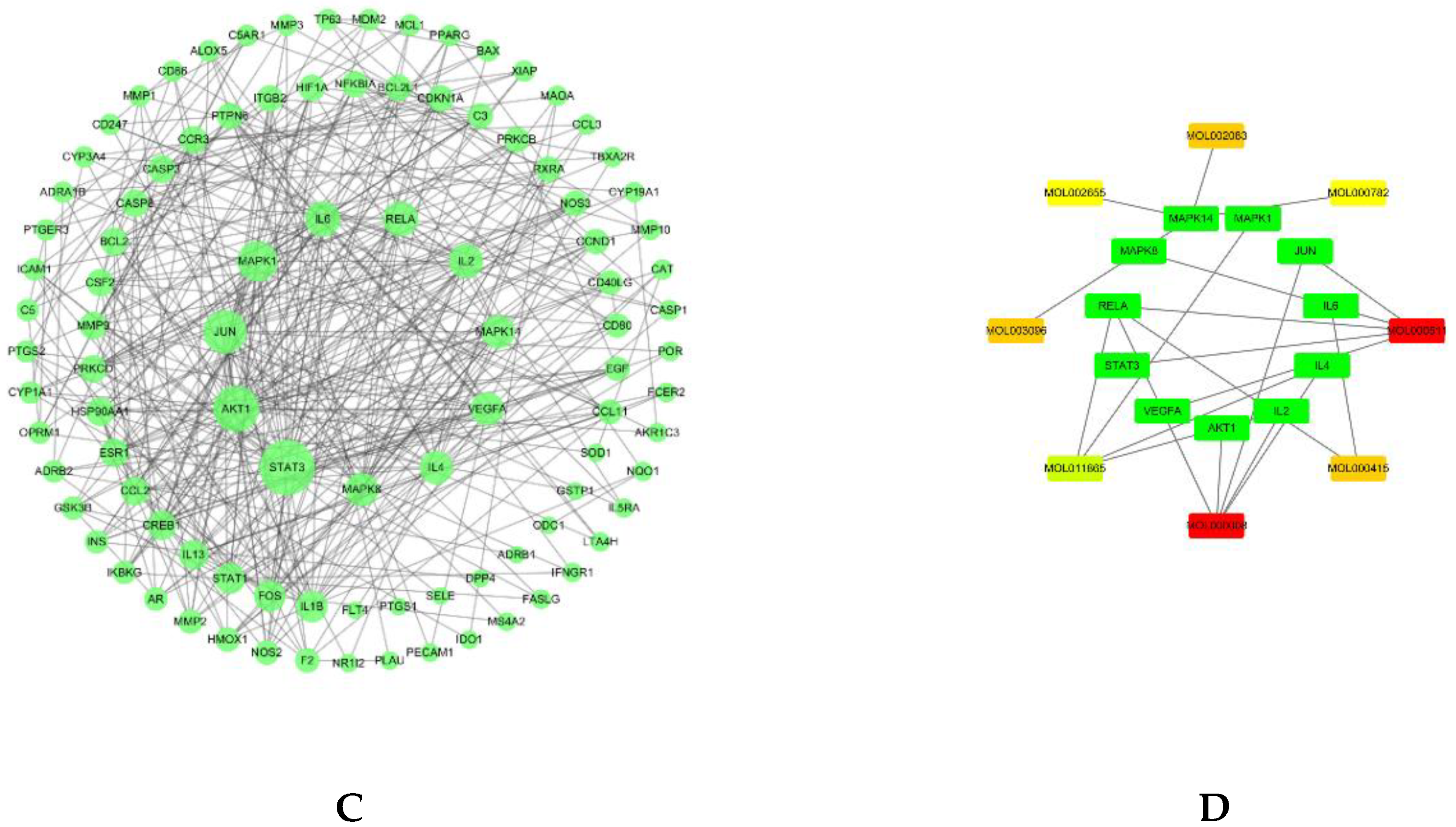
1.4. Protein–Protein Interaction and Network Analysis
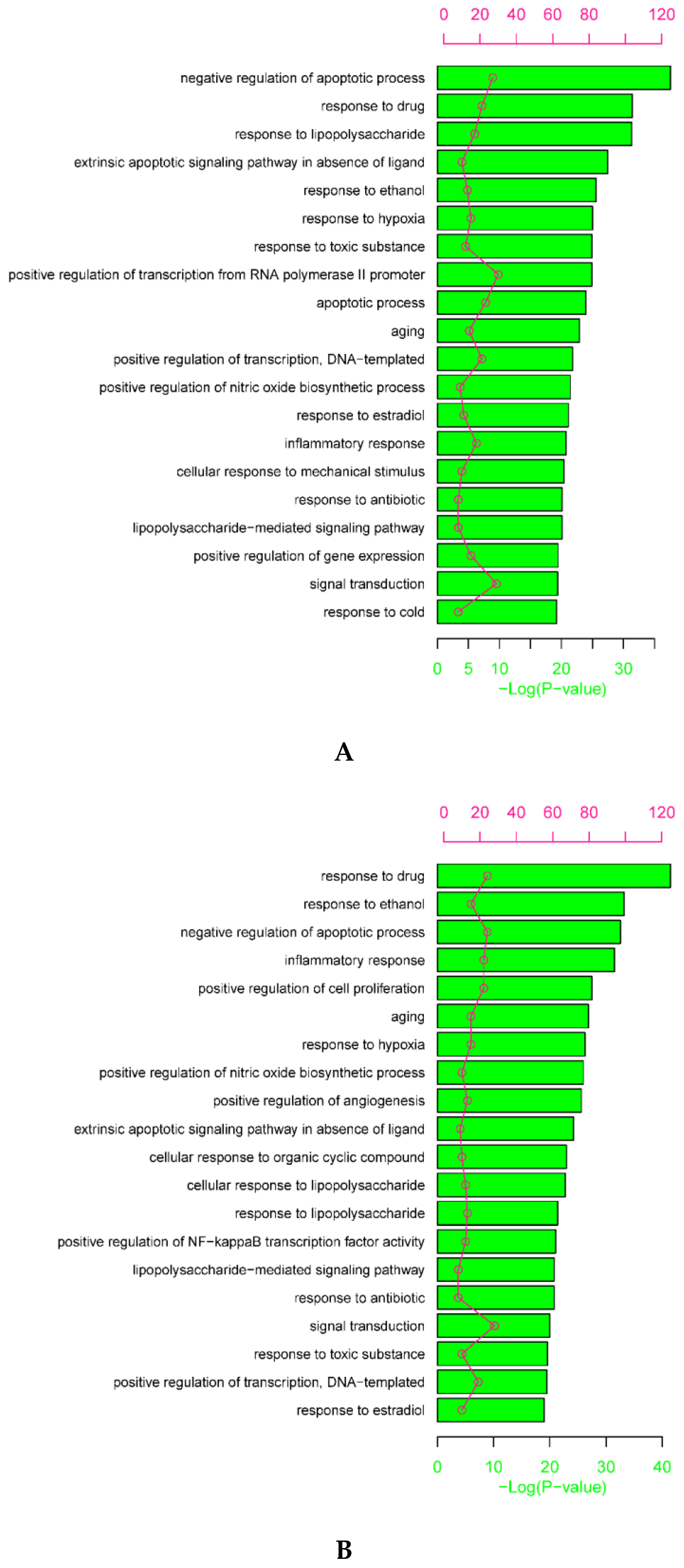
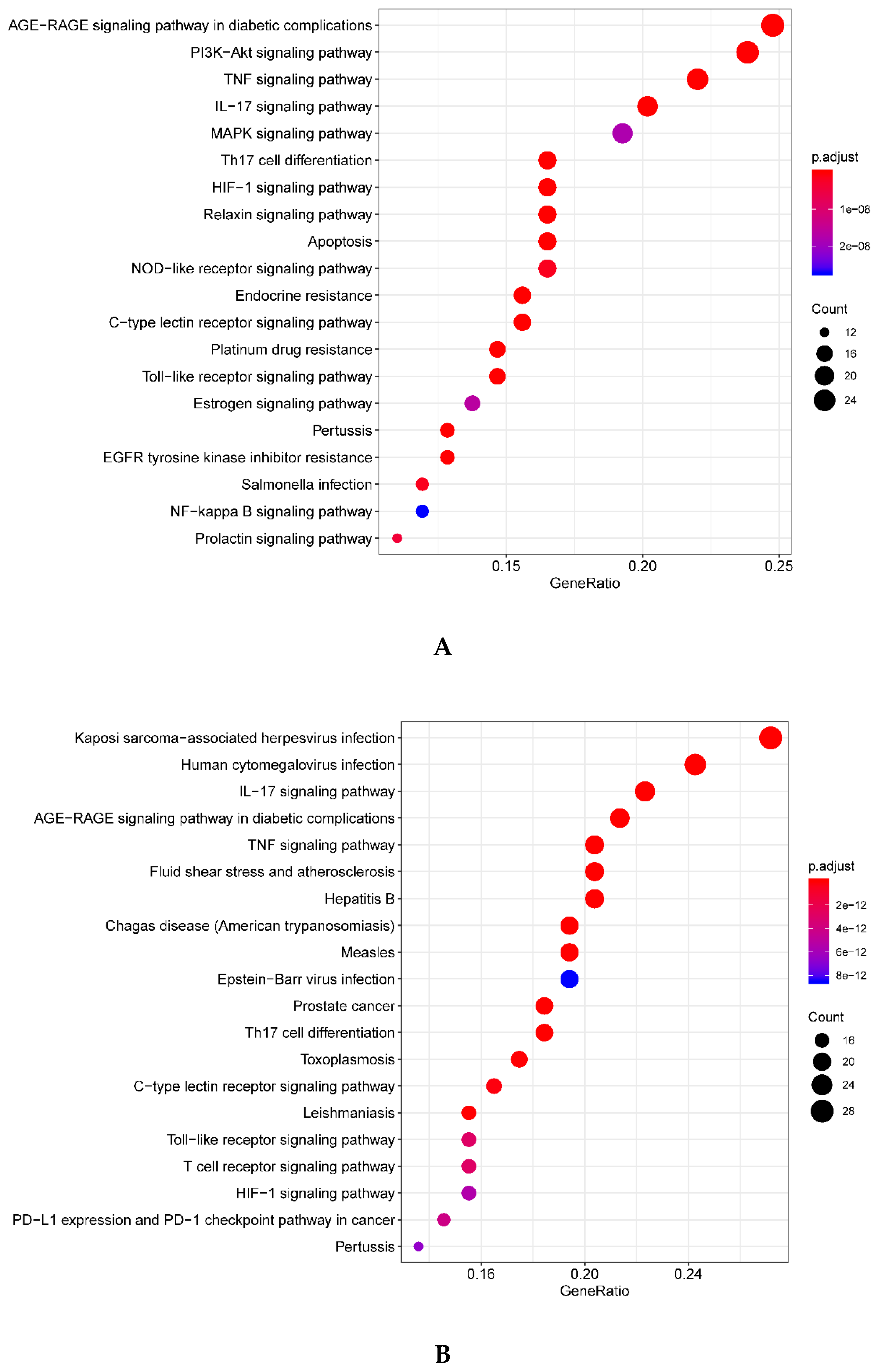
1.5. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Enrichment Analysis
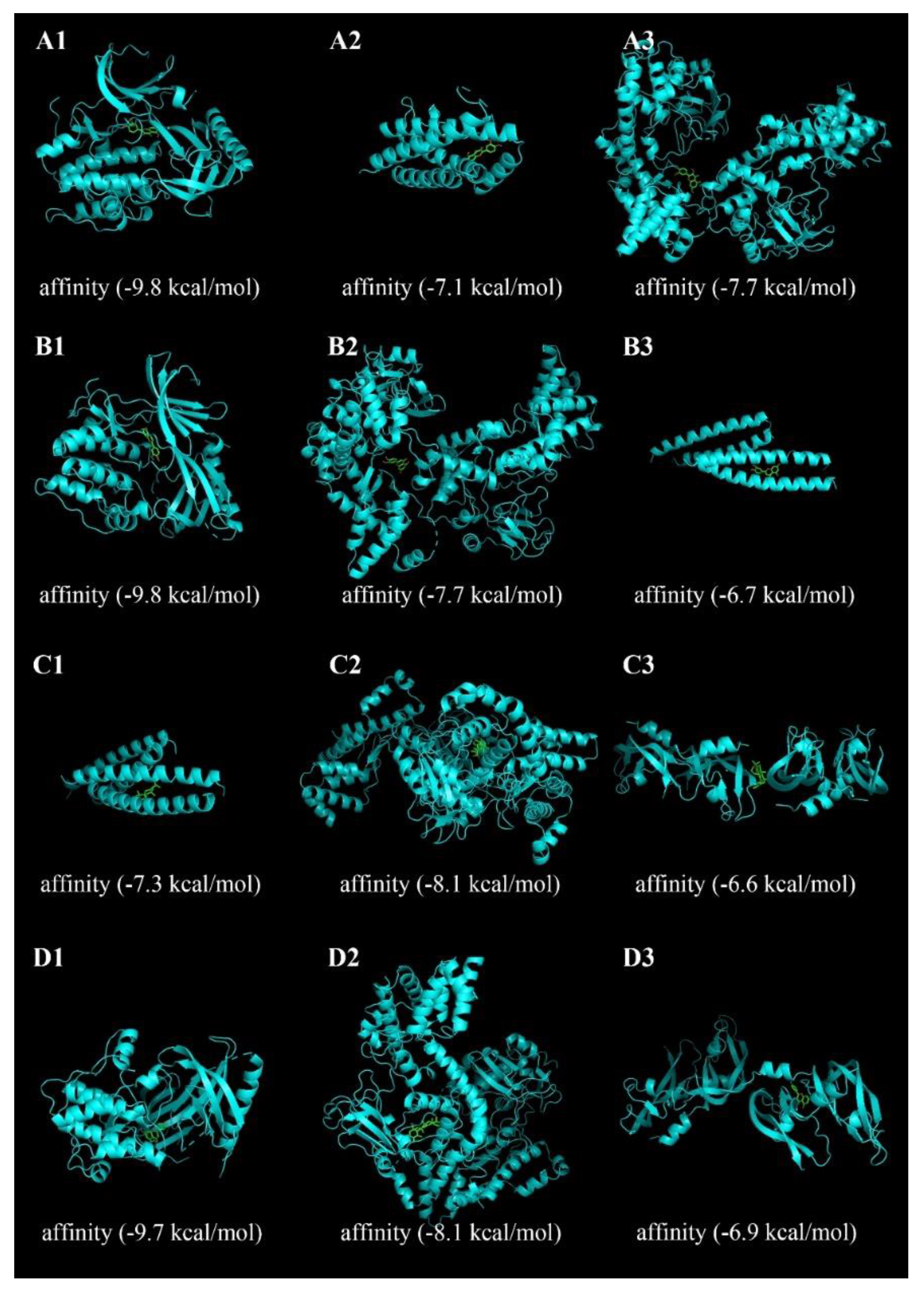
1.6. Molecular Docking Analysis
2. Discussion
3. Methods
3.1. Information of PHF Active Compounds, Compound Targets and AD-Related Targets
3.2. Network Construction
3.3. Protein–Protein Interaction Analysis
3.4. Gene Ontology and KEGG Enrichment Analysis
3.5. Docking Steps and Results Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AD | Atopic dermatitis |
| Th | T helper |
| TCM | Traditional Chinese Medicine |
| PHF | pentaherbs formula |
| CM | Cortex Moutan |
| CP | Cortex Phellodendri |
| FL | Flos Lonicerae |
| HM | Herba Menthae |
| RA | Rhizoma Atractylodis |
| TCMSP | Traditional Chinese Medicine Systems Pharmacology Database |
| ADME | The absorption, distribution, metabolism and excretion |
| OB | oral bioavailability |
| DL | drug-likeness |
| OMIM | Online Mendelian Inheritance in Man |
| PPI | protein–protein interaction |
| GO | gene ontology |
| BP | biological process |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| FDR | false discovery rate |
| AP-1 | activator protein 1 |
| AKT1 | RAC-alpha serine/threonine-protein kinase |
| MAPK1 | mitogen-activated protein kinase 1 |
| MAPK3 | mitogen-activated protein kinase 3 |
| RELA | transcription factor p65 |
| IL-6 | interleukin-6 |
| MAPK8 | mitogen-activated protein kinase 8 |
| HSP90AA1 | heat shock protein 90 alpha family class A member 1 |
| EGFR | epidermal growth factor receptor |
| MAPK14 | mitogen-activated protein kinase 14 |
| ESR1 | estrogen receptor |
| STAT3 | signal transducer and activator of transcription 3 |
| VEGFA | vascular endothelial growth factor A |
| HIF | hypoxia-inducible factor |
| IgE | immunoglobulin E |
| CUHK | The Chinese University of Hong Kong |
| DAVID | Database for Annotation, Visualization and Integrated Discovery |
| STRING | Search Tool for the Retrieval of Interacting Genes/Proteins |
| AGE-RAGE | advanced glycation end products-receptor for advanced glycation end products |
References
- Barbarot, S.; Auziere, S.; Gadkari, A.; Girolomoni, G.; Puig, L.; Simpson, E.L.; Margolis, D.J.; Bruin-Weller, M.; Eckert, L. Epidemiology of atopic dermatitis in adults: Results from an international survey. Allergy 2018, 73, 1284–1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sophie, N. Atopic Dermatitis: Global Epidemiology and Risk Factors. Ann. Nutr. Metab. 2015, 66, 8–16. [Google Scholar]
- Williams, H.C. Clinical practice. Atopic dermatitis. N. Engl. J. Med. 2005, 352, 2314–2324. [Google Scholar] [CrossRef] [PubMed]
- Williams, H.C. Is the prevalence of atopic dermatitis increasing? Clin. Exp. Dermatol. 2010, 17, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.W.; Hui, D.S.; Chan, H.; Fok, T.; Zhong, N.; Chen, Y.; Lai, C.K. Prevalence of respiratory and atopic disorders in Chinese schoolchildren. Chin. J. Tuberc. Respir. Dis. 2010, 31, 1225–1231. [Google Scholar] [CrossRef] [PubMed]
- Boguniewicz, M.; Leung, D.Y.M. Atopic dermatitis: A disease of altered skin barrier and immune dysregulation. Immunol. Rev. 2011, 242, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Elias, P.M.; Hatano, Y.; Williams, M.L. Basis for the barrier abnormality in atopic dermatitis: Outside-inside-outside pathogenic mechanisms. J. Allergy Clin. Immunol. 2008, 121, 1337–1343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, F.T.; Goodarzi, H.; Chen, H.Y. IgE, Mast Cells, and Eosinophils in Atopic Dermatitis. Clin. Rev. Allergy Immunol. 2011, 41, 298–310. [Google Scholar] [CrossRef]
- Jiao, D.; Wong, C.K.; Qiu, H.N.; Dong, J.; Cai, Z.; Chu, M.; Hon, K.L.; Tsang, M.S.M.; Lam, C.W.K. NOD2 and TLR2 ligands trigger the activation of basophils and eosinophils by interacting with dermal fibroblasts in atopic dermatitis-like skin inflammation. Cell. Mol. Immunol. 2016, 13, 535–550. [Google Scholar] [CrossRef]
- Alexander, T.; Maxim, E.; Cardwell, L.A.; Chawla, A.; Feldman, S.R. Prescriptions for atopic dermatitis: Oral corticosteroids remain commonplace. J. Dermatol. Treat. 2017, 29, 238–240. [Google Scholar] [CrossRef]
- Nghiem, P.; Pearson, G.; Langley, R.G. Tacrolimus and pimecrolimus: From clever prokaryotes to inhibiting calcineurin and treating atopic dermatitis. J. Am. Acad. Dermatol. 2002, 46, 228–241. [Google Scholar] [CrossRef] [Green Version]
- Ashcroft, D.M.; Dimmock, P.; Garside, R.; Stein, K.; Williams, H.C. Efficacy and tolerability of topical pimecrolimus and tacrolimus in the treatment of atopic dermatitis: Meta-analysis of randomised controlled trials. Br. Med. J. 2005, 330, 516–525. [Google Scholar] [CrossRef] [Green Version]
- Ormerod, A.D. Topical tacrolimus and pimecrolimus and the risk of cancer: How much cause for concern? Br. J. Dermatol. 2005, 153, 701–705. [Google Scholar] [CrossRef] [PubMed]
- Hon, K.L.E.; Leung, T.F.; Ng, P.C.; Lam, M.C.A.; Kam, W.Y.C.; Wong, K.Y.; Lee, K.C.K.; Sung, Y.T.; Cheng, K.F.; Fok, T.F.; et al. Efficacy and tolerability of a Chinese herbal medicine concoction for treatment of atopic dermatitis: A randomized, double-blind, placebo-controlled study. Br. J. Dermatol. 2007, 157, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Hon, K.L.E.; Leung, T.F.; Wong, Y.; Lam, W.K.C.; Guan, D.Q.B.; Ma, K.C.; Sung, Y.T.R.; Fok, T.F.; Leung, P.C. A Pentaherbs Capsule as a Treatment Option for Atopic Dermatitis in Children: An Open-Labeled Case Series. Am. J. Chin. Med. 2004, 32, 941–950. [Google Scholar] [CrossRef]
- Chan, B.C.L.; Hon, K.L.E.; Leung, P.C.; Sam, S.W.; Fung, K.P.; Lee, M.Y.H.; Lau, H.Y.A. Traditional Chinese medicine for atopic eczema: PentaHerbs formula suppresses inflammatory mediators release from mast cells. J. Ethnopharmacol. 2008, 120, 85–91. [Google Scholar] [CrossRef]
- Liu, K.Y.P.; Hu, S.Q.; Chan, B.C.L.; Wat, E.C.L.; Lau, C.B.S.; Hon, K.L.; Fung, K.P.; Leung, P.C.; Hui, P.C.L.; Lam, C.W.K. Anti-Inflammatory and Anti-Allergic Activities of Pentaherb Formula, Moutan Cortex (Danpi) and Gallic Acid. Molecules 2013, 18, 2483–2500. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.C.; Li, L.F.; Hu, S.Q.; Wat, E.; Wong, E.C.; Zhang, V.X.; Lau, C.B.; Wong, C.K.; Hon, K.L.; Hui, P.C.; et al. Gallic Acid Is the Major Active Component of Cortex Moutan in Inhibiting Immune Maturation of Human Monocyte-Derived Dendritic Cells. Molecules 2015, 20, 16388–16403. [Google Scholar] [CrossRef] [PubMed]
- Tsang, M.S.M.; Jiao, D.; Chan, B.C.L.; Hon, K.L.; Leung, P.C.; Lau, C.B.S.; Wong, E.C.W.; Cheng, L.; Chan, C.K.M.; Lam, C.W.K.; et al. Anti-Inflammatory Activities of Pentaherbs Formula, Berberine, Gallic Acid and Chlorogenic Acid in Atopic Dermatitis-Like Skin Inflammation. Molecules 2016, 21, 519–540. [Google Scholar] [CrossRef] [Green Version]
- Hopkins, A.L. Network pharmacology: The next paradigm in drug discovery. Nat. Chem. Biol. 2008, 4, 682–690. [Google Scholar] [CrossRef]
- Li, S.; Zhang, B. Traditional Chinese medicine network pharmacology: Theory, methodology and application. Chin. J. Nat. Med. 2013, 11, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhao, L.; Zhang, B.; Jiang, Y.; Wang, X.; Guo, Y.; Liu, H.; Li, S.; Tong, X. A network pharmacology approach to determine active compounds and action mechanisms of Ge-Gen-Qin-Lian decoction for treatment of type 2 diabetes. Evid.-Based Compl. Alt. Med. 2014, 2014, 495840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, M.; Tian, S.; Li, Y.; Li, Q.; Xu, X.; Wang, J.; Hou, T. Drug-likeness analysis of traditional Chinese medicines: 1. property distributions of drug-like compounds, non-drug-like compounds and natural compounds from traditional Chinese medicines. J. Cheminform. 2012, 4, 31–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, T. A Handbook of the Composition and Pharmacology of Common Chinese Drugs; China Medical Science and Technology: Beijing, China, 1994. [Google Scholar]
- Mehrbani, M.; Choopani, R.; Fekri, A.; Mehrabani, M.; Mosaddegh, M.; Mehrabani, M. The efficacy of whey associated with dodder seed extract on moderate-to-severe atopic dermatitis in adults: A randomized, double-blind, placebo-controlled clinical trial. J. Ethnopharmacol. 2015, 172, 325–332. [Google Scholar] [CrossRef]
- Weng, Z.; Zhang, B.; Asadi, S.; Sismanopoulos, N.; Butcher, A.; Fu, X.; Katsarou-Katsari, A.; Antoniou, C.; Theoharides, T.C. Quercetin is more effective than cromolyn in blocking human mast cell cytokine release and inhibits contact dermatitis and photosensitivity in humans. PLoS ONE 2012, 7, e33805. [Google Scholar] [CrossRef]
- Lee, E.J.; Ji, G.E.; Sung, M.K. Quercetin and kaempferol suppress immunoglobulin E-mediated allergic inflammation in RBL-2H3 and Caco-2 cells. Inflamm. Res. 2010, 59, 847–854. [Google Scholar] [CrossRef]
- Harwood, M.; Danielewska-Nikiel, B.; Borzelleca, J.F.; Flamm, G.W.; Williams, G.M.; Lines, T.C. A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food. Chem. Toxicol. 2007, 45, 2179–2205. [Google Scholar] [CrossRef]
- Hirano, T.; Higa, S.; Arimitsu, J.; Naka, T.; Ogata, A.; Shima, Y.; Fujimoto, M.; Yamadori, T.; Ohkawara, T.; Kuwabara, Y. Luteolin, a flavonoid, inhibits AP-1 activation by basophils. Biochem. Biophys. Res. Commun. 2006, 340, 1–7. [Google Scholar] [CrossRef]
- Seelinger, G.; Merfort, I.; Schempp, C. Anti-Oxidant, Anti-Inflammatory and Anti-Allergic Activities of Luteolin. Planta Med. 2008, 74, 1667–1677. [Google Scholar] [CrossRef]
- Liu, B.L.; Wu, W.W.; Tang, N. Topical application of luteolin inhibits scratching behavior associated with allergic cutaneous reaction in mice. Planta Med. 2005, 71, 424–428. [Google Scholar]
- Cortes, J.R.; Perez, G.M.; Rivas, M.D.; Zamorano, J. Kaempferol inhibits IL-4-induced STAT6 activation by specifically targeting JAK3. J. Immunol. 2007, 179, 3881–3887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Man, M.Q.; Hupe, M.; Sun, R.; Man, G.; Mauro, T.M.; Elias, P.M. Topical Apigenin Alleviates Cutaneous Inflammation in Murine Models. Evid.-Based Complement. Alternat. Med. 2012, 2012, 912028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yano, S.; Umeda, D.; Yamashita, S.; Yamada, K.; Tachibana, H. Dietary apigenin attenuates the development of atopic dermatitis-like skin lesions in NC/Nga mice. J. Nutr. Biochem. 2009, 20, 876–881. [Google Scholar] [CrossRef]
- Ross, J.A.; Kasum, C.M. Dietary flavonoids: Bioavailability, metabolic effects, and safety. Annu. Rev. Nutr. 2002, 22, 19–34. [Google Scholar] [CrossRef]
- Ikeda, Y.; Murakami, A.; Ohigashi, H. Ursolic acid: An anti- and pro-inflammatory triterpenoid. Mol. Nutr. Food Res. 2010, 52, 26–42. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Dai, X.; Kumar, A.P.; Tan, B.K.; Sethi, G.; Bishayee, A. Ursolic acid in cancer prevention and treatment: Molecular targets, pharmacokinetics and clinical studies. Biochem. Pharmacol. 2013, 85, 1579–1587. [Google Scholar] [CrossRef] [Green Version]
- Osakabe, N.; Yasuda, A.; Natsume, M.; Yoshikawa, T. Rosmarinic acid inhibits epidermal inflammatory responses: Anticarcinogenic effect of Perilla frutescens extract in the murine two-stage skin model. Carcinogenesis 2004, 25, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Trautmann, A.; Akdis, M.; Schmid-Grendelmeier, P.; Disch, R.; Bröcker, E.B.; Blaser, K.; Akdis, C.A. Targeting keratinocyte apoptosis in the treatment of atopic dermatitis and allergic contact dermatitis. J. Allergy Clin. Immunol. 2001, 108, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.J.; Richardson, T.; Seykora, J.T.; Cotsarelis, G.; Simon, M.C. Hypoxia-inducible factors regulate filaggrin expression and epidermal barrier function. J. Investig. Dermatol. 2015, 135, 454–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adler, B.L.; Friedman, A.J. Nitric oxide therapy for dermatologic disease. Future Sci. OA 2015, 1, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobi, A.; Antoni, C.; Manger, B.; Schuler, G.; Hertl, M. Infliximab in the treatment of moderate to severe atopic dermatitis. J. Am. Acad. Dermatol. 2005, 52, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Manresa, M.C.; Smith, L.; Casals-Diaz, L.; Fagundes, R.R.; Brown, E.; Radhakrishnan, P.; Murphy, S.J.; Crifo, B.; Strowitzki, M.J.; Halligan, D.N. Pharmacologic inhibition of hypoxia-inducible factor (HIF)-hydroxylases ameliorates allergic contact dermatitis. Allergy 2019, 74, 753–766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nograles, K.E.; Zaba, L.C.; Shemer, A.; Fuentes-Duculan, J.; Cardinale, I.; Kikuchi, T.; Ramon, M.; Bergman, R.; Krueger, J.G.; Guttman-Yassky, E. IL-22–producing “T22” T cells account for upregulated IL-22 in atopic dermatitis despite reduced IL-17–producing TH17 T cells. J. Allergy Clin. Immunol. 2009, 123, 1244–1252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, L.; Xue, H.B.; Guan, X.H.; Qi, R.Q.; An, R.Z.; Shu, C.M.; Zhang, Y.J.; Wei, Y.H.; Zhang, J.H. Possible role of Th17 cells and IL-17 in the pathogenesis of atopic dermatitis in northern China. J. Dermatol. Sci. 2012, 68, 66–68. [Google Scholar] [CrossRef]
- Quondamatteo, F. Skin and diabetes mellitus: What do we know? Cell Tissue Res. 2014, 355, 1–21. [Google Scholar] [CrossRef]
- Naeem, A.S.; Tommasi, C.; Cole, C. A mechanistic target of rapamycin complex 1/2 (mTORC1)/V-Akt murine thymoma viral oncogene homolog 1 (AKT1)/cathepsin H axis controls filaggrin expression and processing in skin, a novel mechanism for skin barrier disruption in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2017, 139, 1228–1241. [Google Scholar]
- Tamai, K.; Kaneda, Y.; Morishita, R. Development of NF-kappa B decoy ointment and clinical trial for atopic dermatitis. Dermatitis 2008, 19, 293–294. [Google Scholar]
- Melnik, B.C. The potential role of impaired Notch signalling in atopic dermatitis. Acta Derm.-Venereol. 2015, 95, 5–13. [Google Scholar] [CrossRef] [Green Version]
- Navarini, A.A.; French, L.E.; Hofbauer, G.F.L. Interrupting IL-6–receptor signaling improves atopic dermatitis but associates with bacterial superinfection. J. Allergy Clin. Immunol. 2011, 128, 1128–1130. [Google Scholar] [CrossRef]
- Esparza-Gordillo, J.; Schaarschmidt, H.; Liang, L. A functional IL-6 receptor (IL6R) variant is a risk factor for persistent atopic dermatitis. J. Allergy Clin. Immunol. 2013, 132, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Ru, J.; Li, P.; Wang, J.; Zhou, W.; Li, B.; Huang, C.; Li, P.; Guo, Z.; Tao, W.; Yang, Y. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 2014, 6, 13–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wishart, D.S.; Craig, K.; Guo, A.C.; Cheng, D.; Savita, S.; Dan, T.; Bijaya, G.; Murtaza, H. DrugBank: A knowledgebase for drugs, drug actions and drug targets. Nucleic Acids Res. 2007, 36, D901–D906. [Google Scholar] [CrossRef] [PubMed]
- Stelzer, G.; Rosen, N.; Plaschkes, I.; Zimmerman, S.; Twik, M.; Fishilevich, S.; Stein, T.I.; Nudel, R.; Lieder, I.; Mazor, Y. The GeneCards Suite: From Gene Data Mining to Disease Genome Sequence Analyses. Curr. Protoc. Bioinform. 2016, 54, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Hamosh, A.; Scott, A.F.; Amberger, J.S.; Bocchini, C.A.; McKusick, A.V. Online Mendelian Inheritance in Man (OMIM), a knowledgebase of human genes and genetic disorders. Nucleic Acids Res. 2005, 33, 514–517. [Google Scholar] [CrossRef] [PubMed]
- Lopes, C.T.; Franz, M.; Kazi, F.; Donaldson, S.L.; Bader, G.D. Cytoscape Web: An interactive web-based network browser. Bioinformatics 2010, 26, 2347–2348. [Google Scholar] [CrossRef] [Green Version]
- von Mering, C.; Huynen, M.; Jaeggi, D.; Schmidt, S.; Bork, P.; Snel, B. STRING: A database of predicted functional associations between proteins. Nucleic Acids Res. 2003, 31, 258–261. [Google Scholar] [CrossRef]
- Da, W.H.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2008, 4, 44–57. [Google Scholar]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [Green Version]

| MOL ID | MOL Name | OB (%) | DL | Herb Source | Targets (No.) | |
|---|---|---|---|---|---|---|
| 1 | MOL000098 | quercetin | 46.43 | 0.28 | CM, FL, CP | 84 |
| 2 | MOL000006 | luteolin | 36.16 | 0.25 | HM, FL | 37 |
| 3 | MOL000422 | kaempferol | 41.88 | 0.24 | CM, FL | 34 |
| 4 | MOL000173 | wogonin | 30.68 | 0.23 | RA | 28 |
| 5 | MOL004328 | naringenin | 59.29 | 0.21 | HM | 19 |
| 6 | MOL001689 | acacetin | 34.97 | 0.24 | HM | 17 |
| 7 | MOL002773 | beta-carotene | 37.18 | 0.58 | FL | 16 |
| 8 | MOL000358 | beta-sitosterol | 36.91 | 0.75 | CP, FL | 14 |
| 9 | MOL000471 | aloe-emodin | 83.38 | 0.24 | HM | 12 |
| 10 | MOL003095 | 5-hydroxy-7-methoxy-2-(3,4,5-trimethoxyphenyl)chromone | 51.96 | 0.41 | FL | 12 |
| 11 | MOL002662 | rutaecarpine | 40.3 | 0.6 | CP | 11 |
| 12 | MOL000449 | Stigmasterol | 43.83 | 0.76 | CP, FL | 10 |
| 13 | MOL002670 | Cavidine | 35.64 | 0.81 | CP | 10 |
| 14 | MOL000790 | Isocorypalmine | 35.77 | 0.59 | CP | 10 |
| 15 | MOL003044 | Chryseriol | 35.85 | 0.27 | FL | 10 |
| 16 | MOL001455 | (S)-Canadine | 53.83 | 0.77 | CP | 9 |
| 17 | MOL000188 | 3β-acetoxyatractylone | 40.57 | 0.22 | RA | 8 |
| 18 | MOL001454 | berberine | 36.86 | 0.78 | CP | 8 |
| 19 | MOL002651 | Dehydrotanshinone II A | 43.76 | 0.4 | CP | 8 |
| 20 | MOL000785 | palmatine | 64.6 | 0.65 | CP | 8 |
| 21 | MOL000787 | Fumarine | 59.26 | 0.83 | CP | 8 |
| 22 | MOL005573 | Genkwanin | 37.13 | 0.24 | HM | 7 |
| 23 | MOL000492 | (+)-catechin | 54.83 | 0.24 | CM | 7 |
| 24 | MOL006422 | thalifendine | 44.41 | 0.73 | CP | 7 |
| 25 | MOL002644 | Phellopterin | 40.19 | 0.28 | CP | 6 |
| 26 | MOL002894 | berberrubine | 35.74 | 0.73 | CP | 6 |
| 27 | MOL002881 | Diosmetin | 31.14 | 0.27 | HM | 5 |
| 28 | MOL005190 | eriodictyol | 71.79 | 0.24 | HM | 5 |
| 29 | MOL001458 | coptisine | 30.67 | 0.86 | CP | 5 |
| 30 | MOL003111 | Centauroside_qt | 55.79 | 0.5 | FL | 5 |
| 31 | MOL002668 | Worenine | 45.83 | 0.87 | CP | 4 |
| 32 | MOL001131 | phellamurin_qt | 56.6 | 0.39 | CP | 4 |
| 33 | MOL007374 | 5-[[5-(4-methoxyphenyl)-2-furyl]methylene]barbituric acid | 43.44 | 0.3 | CM | 3 |
| 34 | MOL002666 | Chelerythrine | 34.18 | 0.78 | CP | 3 |
| 35 | MOL002914 | Eriodyctiol (flavanone) | 41.35 | 0.24 | FL | 3 |
| 36 | MOL003006 | (-)-(3R,8S,9R,9aS,10aS)-9-ethenyl-8-(beta-D-glucopyranosyloxy)-2,3,9,9a,10,10a-hexahydro-5-oxo-5H,8H-pyrano[4,3-d]oxazolo[3,2-a]pyridine-3-carboxylic acid_qt | 87.47 | 0.23 | FL | 3 |
| 37 | MOL003117 | Ioniceracetalides B_qt | 61.19 | 0.19 | FL | 3 |
| 38 | MOL002641 | Phellavin_qt | 35.86 | 0.44 | CP | 2 |
| 39 | MOL002663 | Skimmianin | 40.14 | 0.2 | CP | 2 |
| 40 | MOL001494 | Mandenol | 42 | 0.19 | FL | 2 |
| 41 | MOL003014 | secologanic dibutylacetal_qt | 53.65 | 0.29 | FL | 2 |
| 42 | MOL003128 | dinethylsecologanoside | 48.46 | 0.48 | FL | 2 |
| 43 | MOL001495 | Ethyl linolenate | 46.1 | 0.2 | FL | 1 |
| MOL ID | MOL Name | OB (%) | DL | Herb Source | Targets (No.) | |
|---|---|---|---|---|---|---|
| 1 | MOL000008 | apigenin | 23.06 | 0.21 | HM, FL | 40 |
| 2 | MOL000511 | ursolic acid | 16.77 | 0.75 | HM, FL | 38 |
| 3 | MOL011865 | rosmarinic acid | 1.38 | 0.35 | HM | 24 |
| 4 | MOL000415 | rutin | 3.20 | 0.68 | FL | 18 |
| 5 | MOL000472 | emodin | 24.4 | 0.24 | HM | 16 |
| 6 | MOL000782 | Menisporphine | 24.33 | 0.52 | CP | 12 |
| 7 | MOL000786 | STOCK1N-14407 | 22.28 | 0.64 | CP | 11 |
| 8 | MOL002891 | magnoflorine | 0.48 | 0.55 | CP | 11 |
| 9 | MOL003096 | 2-(3,4-dimethoxyphenyl)-5-hydroxy-7-methoxy-chromone | 29.24 | 0.34 | FL | 11 |
| 10 | MOL003097 | Flavone der. | 27.12 | 0.27 | FL | 11 |
| 11 | MOL000794 | menisperine | 26.17 | 0.59 | CP | 10 |
| 12 | MOL002083 | tricin | 27.86 | 0.34 | FL | 10 |
| 13 | MOL000764 | magnoflorine | 26.69 | 0.55 | CP | 9 |
| 14 | MOL001457 | columbamine | 26.94 | 0.59 | CP | 9 |
| 15 | MOL002642 | phellodendrine | 2.61 | 0.58 | CP | 8 |
| 16 | MOL002655 | Amurensin_qt | 26.37 | 0.44 | CP | 8 |
| 17 | MOL003098 | 2-(2,4-dimethoxyphenyl)-3-hydroxy-7-methoxy-chromone | 12.94 | 0.33 | FL | 8 |
| 18 | MOL000357 | Sitogluside | 20.63 | 0.62 | CP, HM, FL | 7 |
| 19 | MOL000789 | jatrorrizine | 19.65 | 0.59 | CP | 7 |
| 20 | MOL002566 | 3-O-Methylquercetin | 10.1 | 0.3 | FL | 7 |
| 21 | MOL002635 | (±)-lyoniresinol | 4.87 | 0.54 | CP | 7 |
| 22 | MOL002901 | phellodendrine | 2.5 | 0.58 | CP | 7 |
| 23 | MOL000347 | Syrigin | 14.64 | 0.32 | CP | 6 |
| 24 | MOL013434 | Auraptene | 25.62 | 0.24 | CP | 6 |
| 25 | MOL000561 | Astragalin | 14.03 | 0.74 | FL | 5 |
| 26 | MOL000190 | 3,5-dimethoxy-4-glucosyloxyphenylallylalcohol | 29 | 0.32 | RA | 4 |
| 27 | MOL000263 | oleanolic acid | 29.02 | 0.76 | CM, HM, FL | 4 |
| 28 | MOL000476 | Physcion | 22.29 | 0.27 | HM | 4 |
| 29 | MOL004368 | Hyperin | 6.94 | 0.77 | CP, FL | 4 |
| 30 | MOL005093 | Diosmin | 12.70 | 0.66 | HM | 4 |
| 31 | MOL000650 | 1H,3H-Pyrano(3,4-c)pyran-1-one, 5-ethenyl-6-(beta-D-glucopyranosyloxy)-4,4a,5,6-tetrahydro-, (4aS-(4aalpha,5beta,6alpha))- | 4.96 | 0.38 | FL | 3 |
| 32 | MOL000655 | Loganic acid | 4.92 | 0.4 | FL | 3 |
| 33 | MOL000741 | (2S,3S)-3,5,7-trihydroxy-2-(4-hydroxyphenyl)chroman-4-one | 24.15 | 0.24 | CP | 3 |
| 34 | MOL001729 | Crysophanol | 18.64 | 0.21 | HM | 3 |
| 35 | MOL001965 | Dauricine (8CI) | 23.65 | 0.37 | CP | 3 |
| 36 | MOL003011 | Secologanate | 17.56 | 0.33 | FL | 3 |
| 37 | MOL003018 | SCG | 23.59 | 0.36 | FL | 3 |
| 38 | MOL003109 | Caeruloside C_qt | 5.4 | 0.37 | FL | 3 |
| 39 | MOL000009 | luteolin-7-o-glucoside | 7.29 | 0.78 | HM, FL | 2 |
| 40 | MOL001680 | Loganin | 5.9 | 0.44 | FL | 2 |
| 41 | MOL002215 | Oleanic acid | 8.41 | 0.77 | CM | 2 |
| 42 | MOL003022 | Secoxyloganin | 3.79 | 0.39 | FL | 2 |
| 43 | MOL003073 | 8-epiloganin | 11.68 | 0.44 | FL | 2 |
| 44 | MOL003099 | 7-epi-Loganin | 4.78 | 0.44 | FL | 2 |
| 45 | MOL007367 | paeonoside | 18.52 | 0.24 | CM | 2 |
| 46 | MOL000007 | Cosmetin | 9.68 | 0.74 | FL | 1 |
| 47 | MOL003020 | secologanoside 7-methylester | 3.88 | 0.45 | FL | 1 |
| 48 | MOL003065 | 4-caffeoylquinic acid | 10.48 | 0.33 | FL | 1 |
| 49 | MOL003066 | Neochlorogenic acid | 10.65 | 0.33 | FL | 1 |
| 50 | MOL003071 | secologanoside | 26.92 | 0.37 | FL | 1 |
| 51 | MOL003113 | Dehydroxymorroniside | 20.69 | 0.46 | FL | 1 |
| 52 | MOL003867 | Paeonolide | 6.3 | 0.64 | CM | 1 |
| 53 | MOL003959 | limonin | 21.3 | 0.57 | CP | 1 |
| 54 | MOL006384 | 4-[(1R,3aS,4R,6aS)-4-(4-hydroxy-3,5-dimethoxyphenyl)-1,3,3a,4,6,6a-hexahydrofuro[4,3-c]furan-1-yl]-2,6-dimethoxyphenol | 3.29 | 0.72 | CP | 1 |
| 55 | MOL007377 | mudanoside A | 13.39 | 0.29 | CM | 1 |
| 56 | MOL009072 | Prunin | 9.33 | 0.74 | HM | 1 |
| 57 | MOL013068 | Oroxindin | 7.07 | 0.77 | RA | 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, M.; Tsang, M.S.-M.; He, R.; Lam, C.W.-K.; Quan, Z.B.; Wong, C.K. The Active Compounds and Therapeutic Mechanisms of Pentaherbs Formula for Oral and Topical Treatment of Atopic Dermatitis Based on Network Pharmacology. Plants 2020, 9, 1166. https://doi.org/10.3390/plants9091166
Chu M, Tsang MS-M, He R, Lam CW-K, Quan ZB, Wong CK. The Active Compounds and Therapeutic Mechanisms of Pentaherbs Formula for Oral and Topical Treatment of Atopic Dermatitis Based on Network Pharmacology. Plants. 2020; 9(9):1166. https://doi.org/10.3390/plants9091166
Chicago/Turabian StyleChu, Man, Miranda Sin-Man Tsang, Ru He, Christopher Wai-Kei Lam, Zhi Bo Quan, and Chun Kwok Wong. 2020. "The Active Compounds and Therapeutic Mechanisms of Pentaherbs Formula for Oral and Topical Treatment of Atopic Dermatitis Based on Network Pharmacology" Plants 9, no. 9: 1166. https://doi.org/10.3390/plants9091166





