Room Temperature UV-Activated NO2 and NO Detection by ZnO/rGO Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Synthesis
2.2. Materials Characterization
2.3. Measurements of the Gas Sensor Properties
3. Results
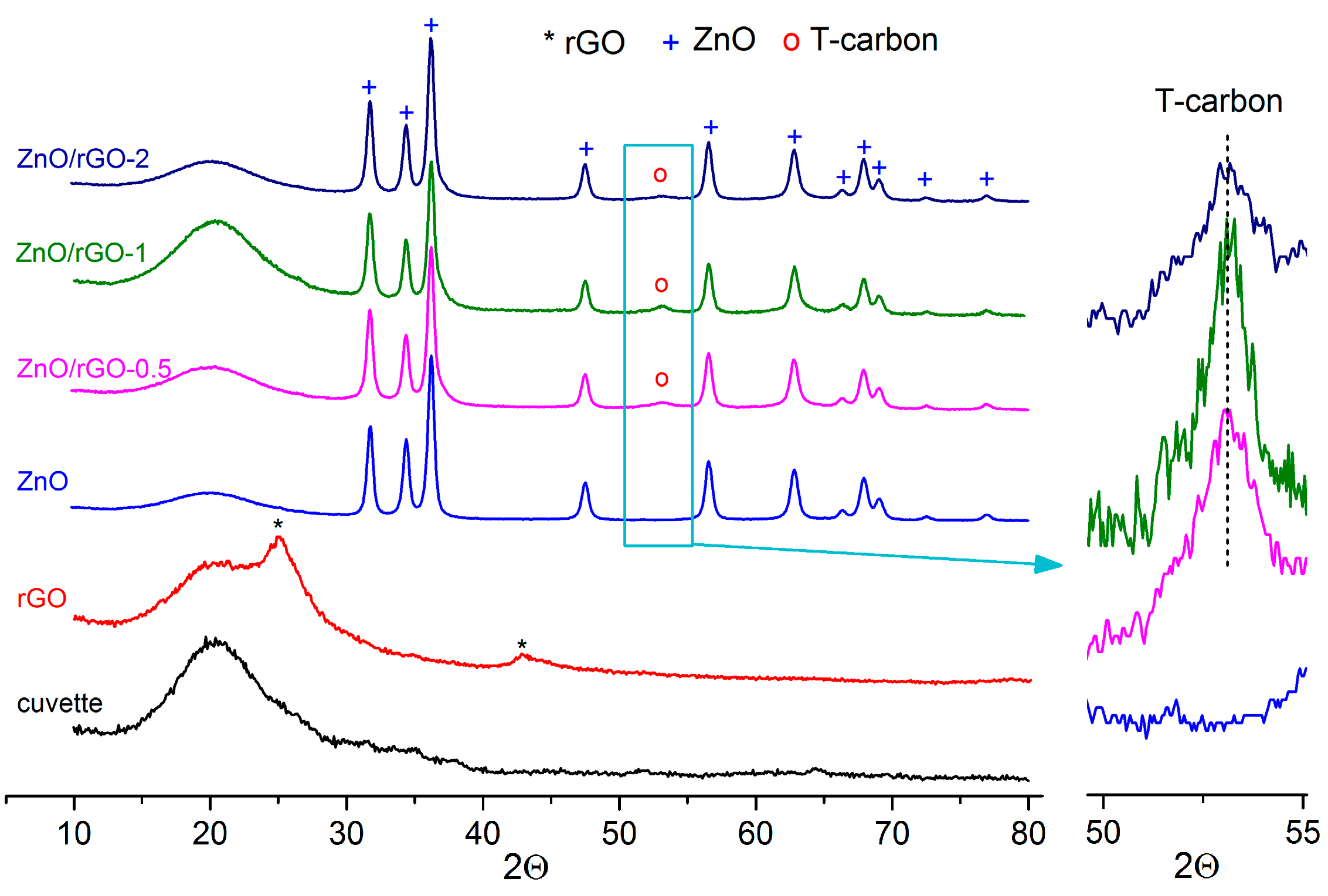
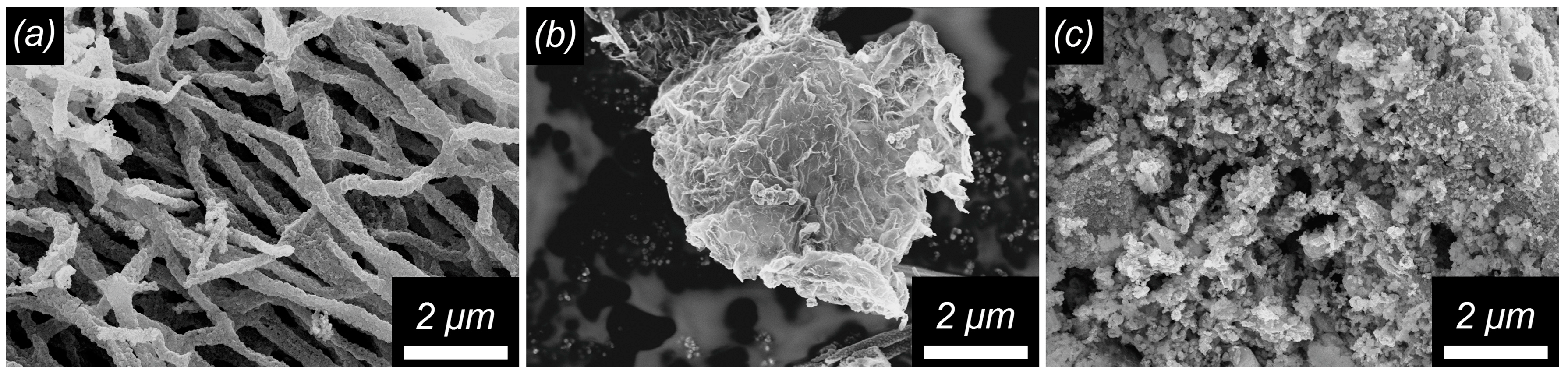
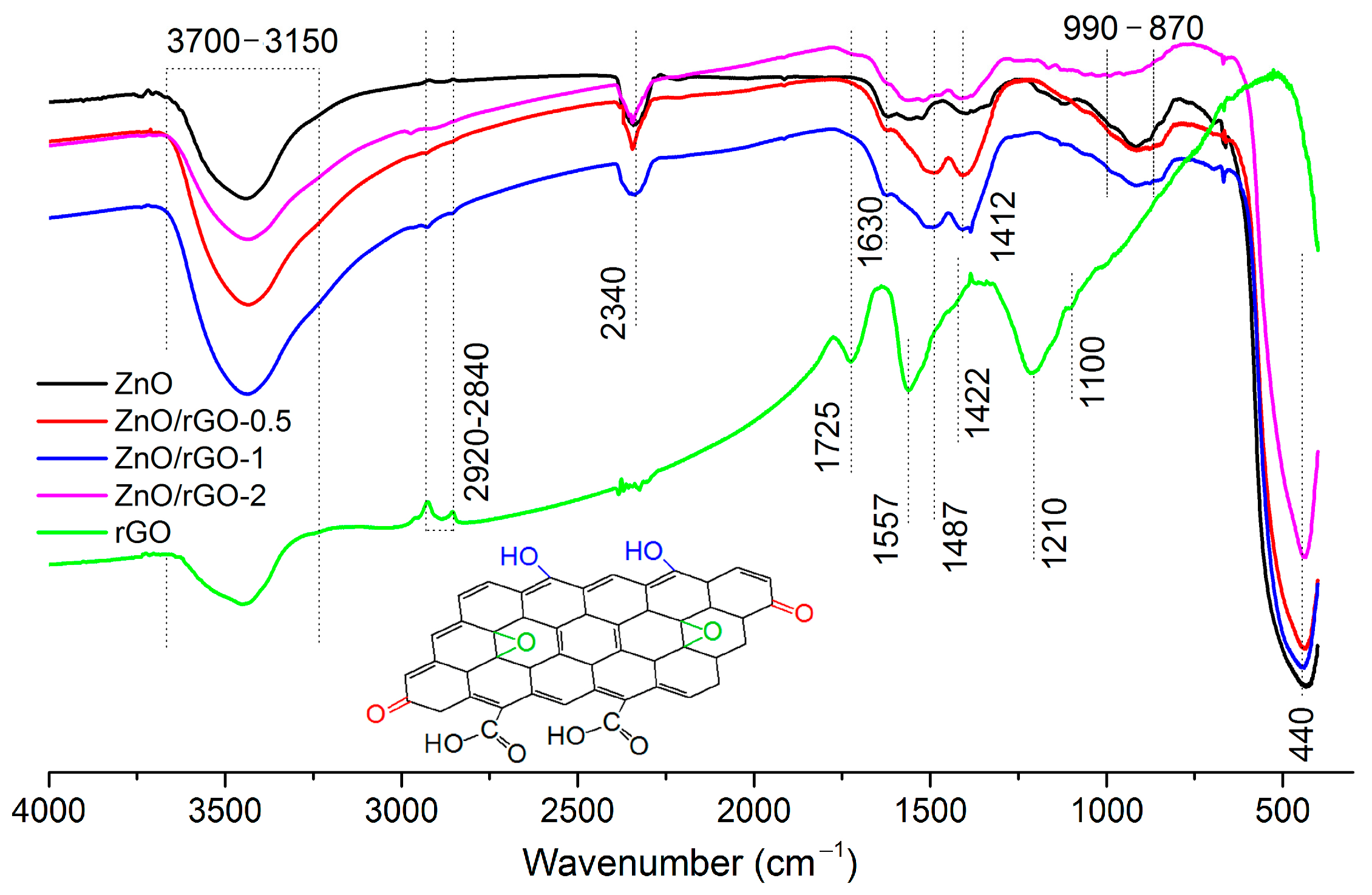
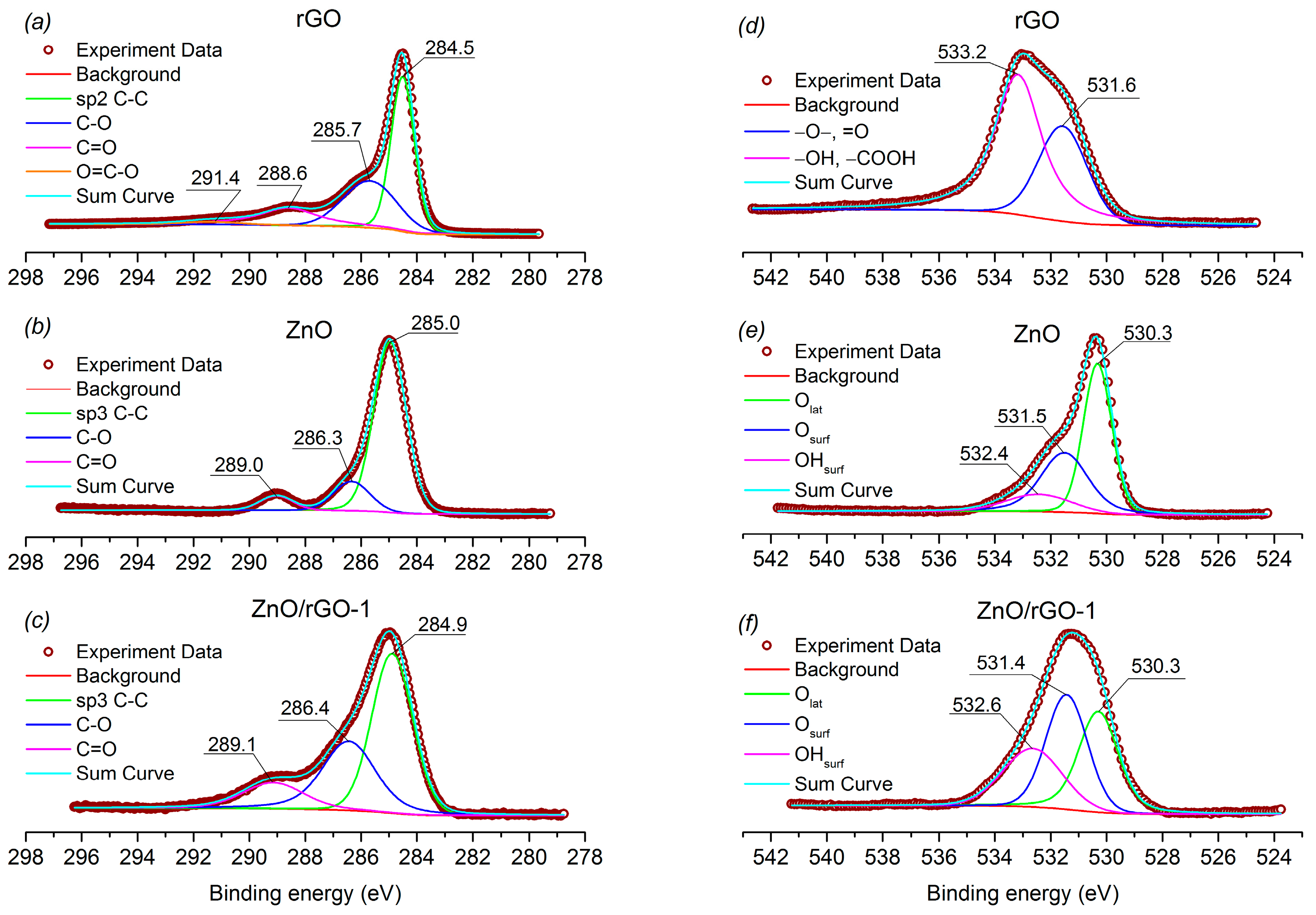
3.1. Structure, Morphology, and Surface Characterization
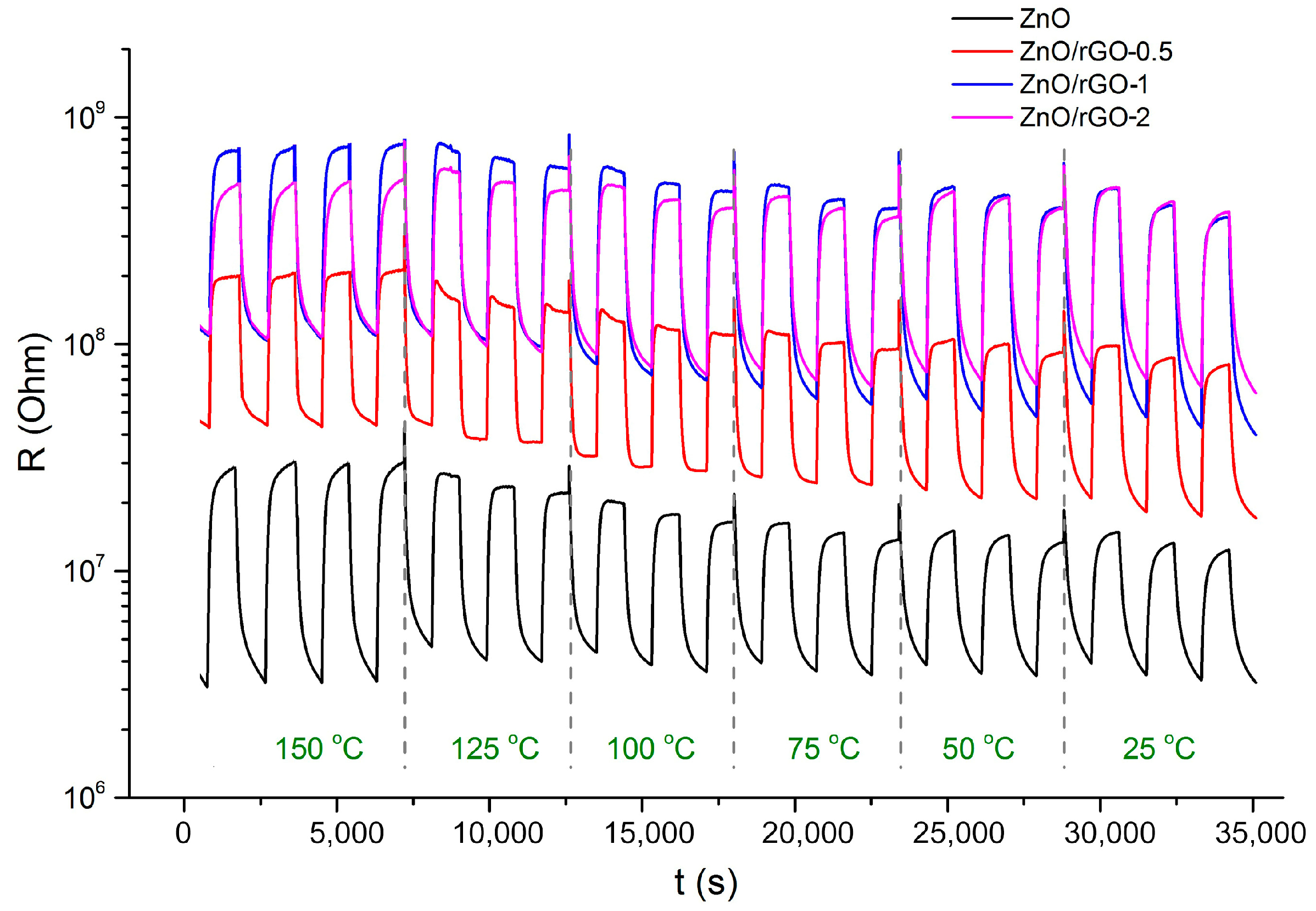
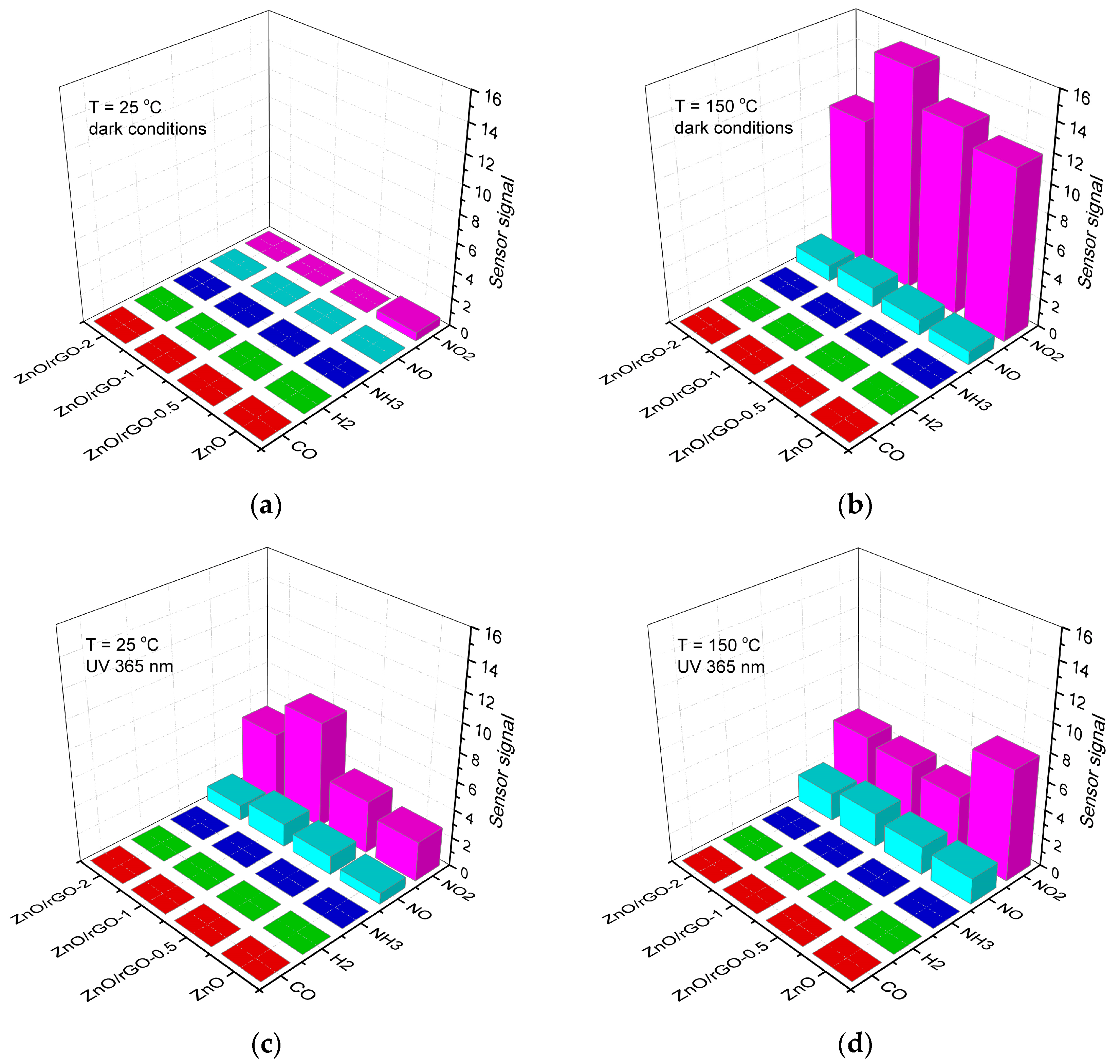
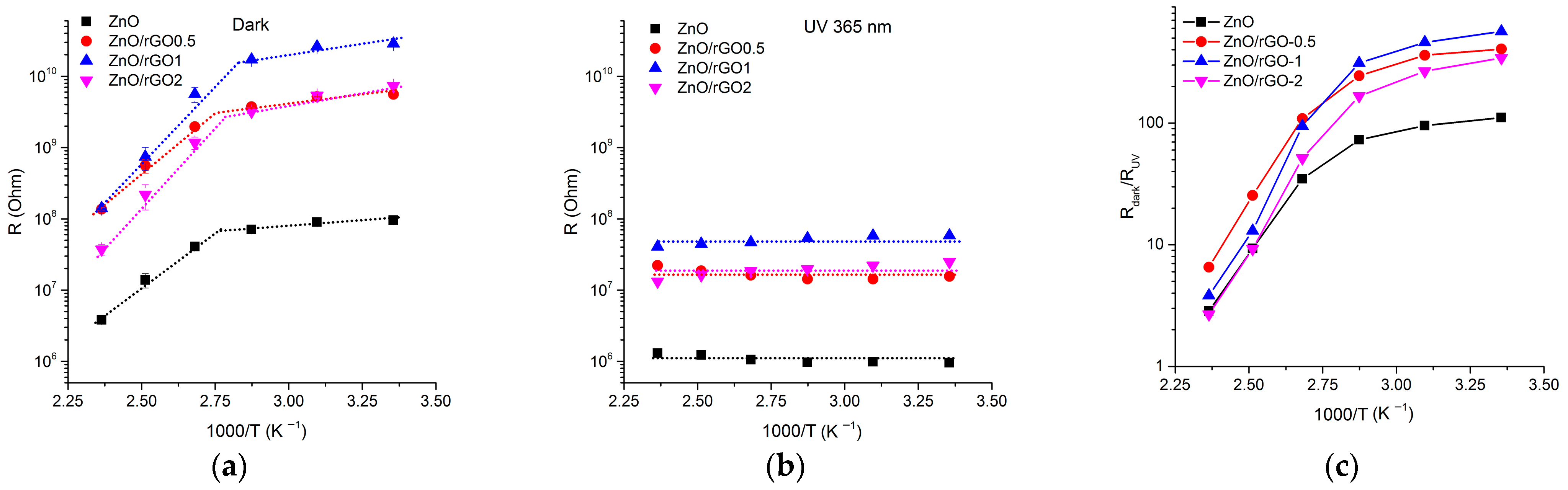
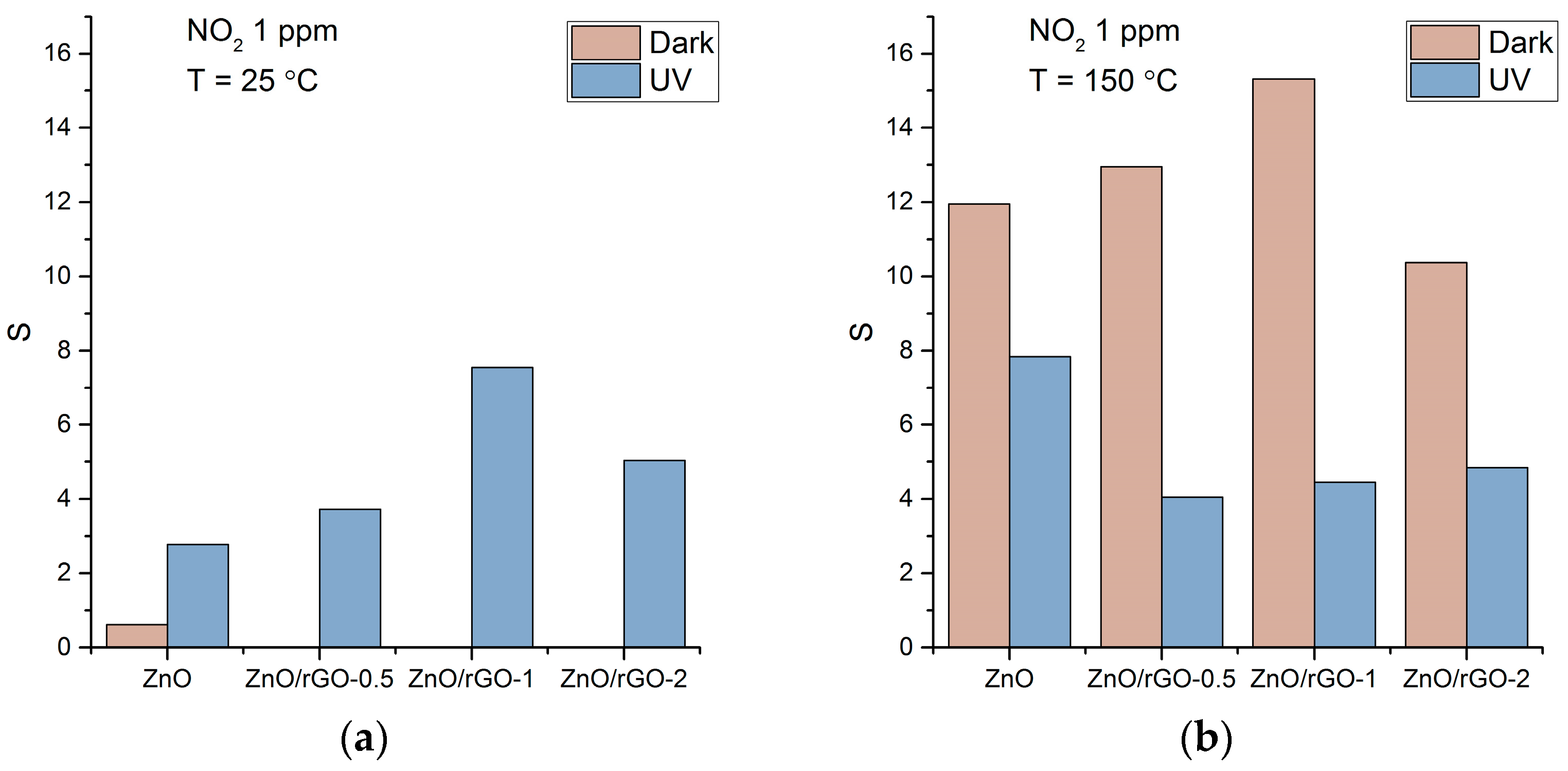
3.2. Gas Sensor Properties
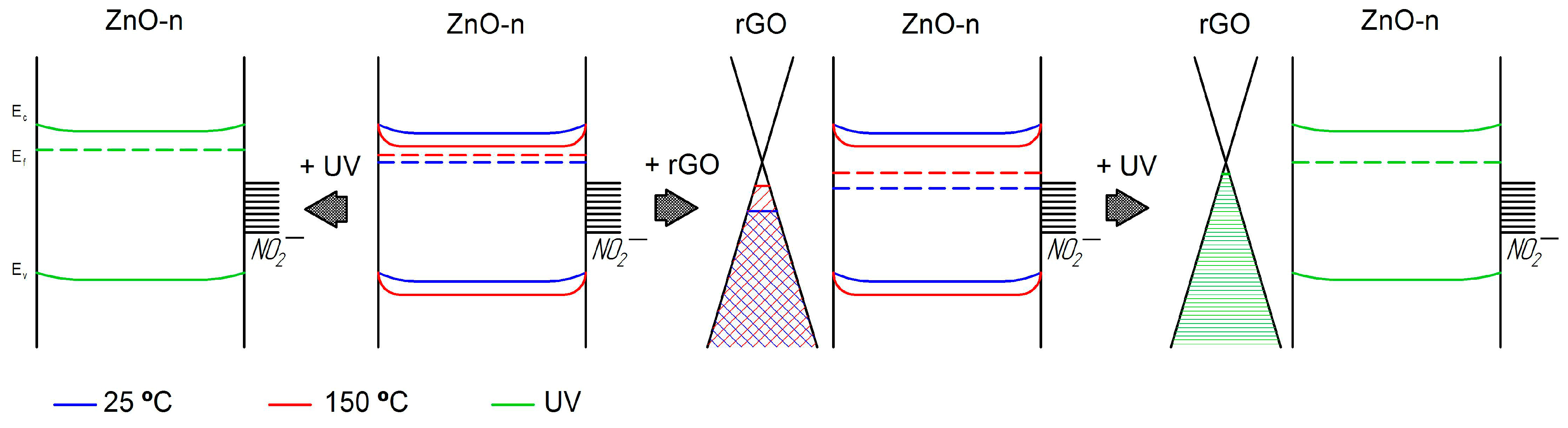
4. Discussion
4.1. Electrical Properties of ZnO/rGO Interface
4.2. NO2 Detection
4.3. NO Detection
4.4. Reducing Gases Detection
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geim, A.K. Graphene: Status and prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef]
- Butler, S.Z.; Hollen, S.M.; Cao, L.; Cui, Y.; Gupta, J.A.; Gutiérrez, H.R.; Heinz, T.F.; Hong, S.S.; Huang, J.; Ismach, A.F.; et al. Progress, Challenges, and Opportunities in Two-Dimensional Materials Beyond Graphene. ACS Nano 2013, 7, 2898–2926. [Google Scholar] [PubMed]
- Liu, X.; Ma, T.; Pinna, N.; Zhang, J. Two-Dimensional Nanostructured Materials for Gas Sensing. Adv. Funct. Mater. 2017, 27, 1702168. [Google Scholar]
- Wang, X.; Li, X.; Zhao, Y.; Chen, Y.; Yu, J.; Wang, J. The influence of oxygen functional groups on gas sensing properties of reduced graphene oxide. RSC Adv. 2016, 6, 52339–52346. [Google Scholar] [CrossRef]
- Leenaerts, O.; Pantoens, B.; Peeters, F. Adsorption of H2O, NH3, CO, and NO2 on graphene: A first-principles study. Phys. Rev. B 2008, 77, 125416. [Google Scholar] [CrossRef]
- Prezioso, S.; Perrozzi, F.; Giancaterini, L.; Cantalini, C.; Ottaviano, L. Graphene oxide as a practical solution to high sensitivity gas sensing. J. Phys. Chem. C 2013, 117, 10683. [Google Scholar] [CrossRef]
- Rumyantsev, S.; Liu, G.; Potyrailo, R.A.; Balandin, A.; Shur, M.S. Selective sensing of individual gases using graphene devices. IEEE Sens. 2013, 13, 2818–2822. [Google Scholar] [CrossRef]
- Yang, S.; Jiang, C.; Wei, S. Gas sensing in 2D materials. Appl. Phys. Rev. 2017, 4, 021304. [Google Scholar] [CrossRef]
- Joshi, N.; Hayasaka, T.; Liu, Y.; Liu, H.; Oliveira, O.N.; Lin, L. A review on chemiresistive room temperature gas sensors based on metal oxide nanostructures, graphene and 2D transition metal dichalcogenides. Microchim. Acta 2018, 185, 213. [Google Scholar] [CrossRef]
- Sun, D.; Luo, Y.; Debliquy, M.; Zhang, C. Graphene-enhanced metal oxide gas sensors at room temperature: A review. Beilstein J. Nanotechnol. 2018, 9, 2832–2844. [Google Scholar] [CrossRef]
- Rumjit, N.P.; Thmas, P.; Lai, C.W.; Wong, Y.H. Review—Recent Advancements of ZnO/rGO Nanocomposites (NCs) for Electrochemical Gas Sensor Applications. J. Electrochem. Soc. 2021, 168, 027506. [Google Scholar] [CrossRef]
- Yuan, S.; Zhang, S. Recent progress on gas sensors based on graphene-like 2D/2D nanocomposites. J. Semicond. 2019, 40, 11608. [Google Scholar] [CrossRef]
- Norizan, M.N.; Abdullah, N.; Halim, N.A.; Demon, S.Z.N.; Mohamad, I.S. Heterojunctions of rGO/Metal Oxide Nanocomposites as Promising Gas-Sensing Materials—A Review. Nanomaterials 2022, 12, 2278. [Google Scholar] [CrossRef]
- Drewniak, S.; Drewniak, Ł.; Pustelny, T. Mechanisms of NO2 Detection in Hybrid Structures Containing Reduced Graphene Oxide: A Review. Sensors 2022, 22, 5316. [Google Scholar]
- Majhi, S.M.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Reduced Graphene Oxide (rGO)-Loaded Metal-Oxide Nanofiber Gas Sensors: An Overview. Sensors 2021, 21, 1352. [Google Scholar] [CrossRef]
- Qi, L.; Yu, L.; Liu, Z.; Guo, F.; Gu, Y.; Fan, X. An enhanced optoelectronic NO2 gas sensors based on direct growth ZnO nanowalls in situ on porous rGO. J. Alloys Compd. 2018, 749, 244–249. [Google Scholar] [CrossRef]
- Nasriddinov, A.; Shatalova, T.; Maksimov, S.; Li, X.; Rumyantseva, M. Humidity Effect on Low-Temperature NH3 Sensing Behavior of In2O3/rGO Composites under UV Activation. Sensors 2023, 23, 1517. [Google Scholar] [CrossRef]
- Espid, E.; Taghipour, F. UV-LED Photo-activated Chemical Gas Sensors: A Review. Crit. Rev. Solid State Mater. Sci. 2017, 42, 416–432. [Google Scholar] [CrossRef]
- Chizhov, A.; Rumyantseva, M.; Gaskov, A. Light Activation of Nanocrystalline Metal Oxides for Gas Sensing: Principles, Achievements, Challenges. Nanomaterials 2021, 11, 892. [Google Scholar] [CrossRef]
- Wang, J.; Shen, H.; Xia, Y.; Komarneni, S. Light-activated room-temperature gas sensors based on metal oxide nanostructures: A review on recent advances. Ceram. Int. 2021, 47, 7353–7368. [Google Scholar]
- Suh, J.M.; Eom, T.H.; Cho, S.H.; Kim, T.; Jang, H.W. Light-activated gas sensing: A perspective of integration with micro-LEDs and plasmonic nanoparticles. Mater. Adv. 2021, 2, 827–844. [Google Scholar]
- Xu, F.; HO, H.P. Light-Activated Metal Oxide Gas Sensors: A Review. Micromachines 2017, 8, 333. [Google Scholar] [PubMed]
- Kumar, R.; Liu, X.; Zhang, J. Room-Temperature Gas Sensors under Photoactivation: From Metal Oxides to 2D Materials. Nano-Micro Lett. 2020, 12, 164. [Google Scholar]
- Wagner, T.; Kohl, C.D.; Malagù, C.; Donato, N.; Latino, M.; Neri, G.; Tiemann, M. UV Light-Enhanced NO2 Sensing by Mesoporous In2O3: Interpretation of Results by a New Sensing Model. Sens. Actuators B 2013, 187, 488–494. [Google Scholar]
- Ilin, A.; Martyshov, M.; Forsh, E.; Forsh, P.; Rumyantseva, M.; Abakumov, A.; Gaskov, A.; Kashkarov, P. UV Effect on NO2 Sensing Properties of Nanocrystalline In2O3. Sens. Actuators B 2016, 231, 491–496. [Google Scholar]
- Liu, B.; Luo, Y.; Li, K.; Wang, H.; Gao, L.; Duan, G. Room-Temperature NO2 Gas Sensing with Ultra-Sensitivity Activated by Ultraviolet Light Based on SnO2 Monolayer Array Film. Adv. Mater. Interfaces 2019, 6, 1900376. [Google Scholar]
- Saboor, F.H.; Ueda, T.; Kamada, T.; Hyodo, T.; Mortazavi, Y.; Khodadadi, A.A.; Shimizu, Y. Enhanced NO2 gas sensing performance of bare and Pd-loaded SnO2 thick film sensors under UV-light irradiation at room temperature. Sens. Actuators B 2016, 223, 429–439. [Google Scholar]
- Zhang, B.; Zhang, S.; Xia, Y.; Yu, P.; Xu, Y.; Dong, Y.; Wei, Q.; Wang, J. High-Performance Room- Temperature NO2 Gas Sensor Based on Au-Loaded SnO2 Nanowires under UV Light Activation. Nanomaterials 2022, 12, 4062. [Google Scholar]
- Li, W.; Guo, J.; Cai, L.; Qi, W.; Sun, Y.; Xu, J.-L.; Sun, M.; Zhu, H.; Xiang, L.; Xie, D.; et al. UV light irradiation enhanced gas sensor selectivity of NO2 and SO2 using rGO functionalized with hollow SnO2 nanofibers. Sens. Actuators B 2019, 290, 443–452. [Google Scholar]
- Espid, E.; Noce, A.S.; Taghipour, F. The Effect of Radiation Parameters on the Performance of Photo-Activated Gas Sensors. J. Photochem. Photobiol. A 2019, 374, 95–105. [Google Scholar]
- Wang, J.; Shen, Y.; Li, X.; Xia, Y.; Yang, C. Synergistic effects of UV activation and surface oxygen vacancies on the room-temperature NO2 gas sensing performance of ZnO nanowires. Sens. Actuators B 2019, 298, 126858. [Google Scholar] [CrossRef]
- Wang, J.; Yu, M.; Xia, Y.; Li, X.; Yang, C.; Komarneni, S. On-chip grown ZnO nanosheet-array with interconnected nanojunction interfaces for enhanced optoelectronic NO2 gas sensing at room temperature. J. Colloid Interface Sci. 2019, 554, 19–28. [Google Scholar] [CrossRef]
- Procek, M.; Stolarczyk, A.; Pustelny, T. Impact of temperature and UV irradiation on dynamics of NO2 sensors based on ZnO nanostructures. Nanomaterials 2017, 7, 312. [Google Scholar] [CrossRef]
- Benamara, M.; Soreto Teixeira, S.; Graça, M.P.F.; Valente, M.A.; Jakka, S.K.; Dahman, H.; Dhahri, E.; El Mir, L.; Debliquy, M.; Lahem, D. Study of ZnO room temperature NO2 sensor under illumination prepared by auto-combustion. Appl. Phys. A 2021, 127, 706. [Google Scholar] [CrossRef]
- Wang, J.; Yu, M.; Li, X.; Xia, Y. UV-enhanced NO2 gas sensing properties of polystyrene sulfonate functionalized ZnO nanowires at room temperature. Inorg. Chem. Front. 2019, 6, 176–183. [Google Scholar] [CrossRef]
- Jaballah, S.; Hjiri, M.; Zahmouli, N.; Albargi, H.B.; Dhahri, R.; Dahman, H.; El Mir, L.; Neri, G. Room temperature UV-Vis activated NO2 gas sensor based Mg-doped zinc oxide nanopowders. J. Mater. Sci. Mater. Electron. 2023, 34, 137. [Google Scholar] [CrossRef]
- Sayago, I.; Santos, J.P.; Sánchez-Vicente, C. The Effect of Rare Earths on the Response of Photo UV-Activate ZnO Gas Sensors. Sensors 2022, 22, 8150. [Google Scholar] [CrossRef]
- Espid, E.; Taghipour, F. Facile Synthesis and UV-Activated Gas Sensing Performance of Ag:ZnO Nano-Ellipsoids. ECS J. Solid State Sci. Technol. 2018, 7, Q3089. [Google Scholar] [CrossRef]
- Mun, Y.; Park, S.; An, S.; Lee, C.; Kim, H.W. NO2 Gas Sensing Properties of Au-Functionalized Porous ZnO Nanosheets Enhanced by UV Irradiation. Ceram. Int. 2013, 39, 8615–8622. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Li, G.; Liu, J.; Liang, Q.; Wang, H.; Zhu, Y.; Gao, J.; Lu, H. In2O3–ZnO Nanotubes for the Sensitive and Selective Detection of Ppb-Level NO2 under UV Irradiation at Room Temperature. Sens. Actuators B 2022, 355, 131322. [Google Scholar] [CrossRef]
- Lu, G.; Xu, J.; Sun, J.; Yu, Y.; Zhang, Y.; Liu, F. UV-enhanced room temperature NO2 sensor using ZnO nanorods modified with SnO2 nanoparticles. Sens. Actuators B 2012, 162, 82–88. [Google Scholar] [CrossRef]
- Wu, T.; Wang, Z.; Tian, M.; Miao, J.; Zhang, H.; Sun, J. UV excitation NO2 gas sensor sensitized by ZnO quantum dots at room temperature. Sens. Actuators B 2018, 259, 526–531. [Google Scholar] [CrossRef]
- Espid, E.; Taghipour, F. Development of Highly Sensitive ZnO/In2O3 Composite Gas Sensor Activated by UV-LED. Sens. Actuators B 2017, 241, 828–839. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, L.; Liu, Y.; Liu, F.; Liang, X.; Liu, F.; Gao, Y.; Yan, X.; Lu, G. UV-activated ultrasensitive and fast reversible ppb NO2 sensing based on ZnO nanorod modified by constructing interfacial electric field with In2O3 nanoparticles. Sens. Actuators B 2020, 305, 127498. [Google Scholar] [CrossRef]
- Espid, E.; Lo, A.-Y.; Taghipour, F. High performance UV-LED activated gas sensors based on ordered carbon mesoporous materials loaded with ZnO nanoparticles. Mater. Sci. Eng. B 2023, 288, 116203. [Google Scholar] [CrossRef]
- Zhou, Y.; Gao, C.; Guo, Y. UV assisted ultrasensitive trace NO2 gas sensing based on few-layer MoS2 nanosheet–ZnO nanowire heterojunctions at room temperature. J. Mater. Chem. A 2018, 6, 10286–10296. [Google Scholar] [CrossRef]
- Kumar, R.R.; Murugesan, T.; Dash, A.; Hsu, C.H.; Gupta, S.; Manikandan, A.; Anbalagan, A.k.; Lee, C.H.; Tai, N.H.; Chueh, Y.L.; et al. Ultrasensitive and Light-Activated NO2 Gas Sensor Based on Networked MoS2/ZnO Nanohybrid with Adsorption/Desorption Kinetics Study. Appl. Surf. Sci. 2021, 536, 147933. [Google Scholar] [CrossRef]
- Park, S.; Kim, S.; Ko, H.; Lee, C. Light-Enhanced Gas Sensing of ZnS-Core/ZnO-Shell Nanowires at Room Temperature. J. Electroceram. 2014, 33, 75–81. [Google Scholar] [CrossRef]
- Park, S.; Ko, H.; Lee, S.; Kim, H.; Lee, C. Light-Activated Gas Sensing of Bi2O3-Core/ZnO-Shell Nanobelt Gas Sensors. Thin Solid Films 2014, 570, 298–302. [Google Scholar] [CrossRef]
- Karaduman, I.; Yildiz, D.E.; Sincar, M.M.; Acar, S. UV Light Activated Gas Sensor for NO2 Detection. Mater. Sci. Semicond. Process. 2014, 28, 43–47. [Google Scholar] [CrossRef]
- Park, S.; Sun, G.J.; Kheel, H.; Ko, T.; Kim, H.W.; Lee, C. Light-Activated NO2 Gas Sensing of the Networked CuO-Decorated ZnS Nanowire Gas Sensor. Appl. Phys. A 2016, 122, 504. [Google Scholar] [CrossRef]
- Zhou, Y.; Zou, C.; Lin, X.; Guo, Y. UV Light Activated NO2 Gas Sensing Based on Au Nanoparticles Decorated Few-Layer MoS2 Thin Film at Room Temperature. Appl. Phys. Lett. 2018, 113, 2–7. [Google Scholar] [CrossRef]
- Yan, X.; Wu, Y.; Li, R.; Shi, C.; Moro, R.; Ma, Y.; Ma, L. High-Performance UV-Assisted NO2 Sensor Based on Chemical Vapor Deposition Graphene at Room Temperature. ASC Omega 2019, 4, 14179–14187. [Google Scholar] [CrossRef]
- Thakur, S.; Karak, N. Green reduction of graphene oxide by aqueous phytoextracts. Carbon 2012, 50, 5331–5339. [Google Scholar] [CrossRef]
- Cui, P.; Lee, J.; Hwang, E.; Lee, H. One-pot reduction of graphene oxide at subzero temperatures. Chem. Commun. 2011, 47, 12370–12372. [Google Scholar] [CrossRef] [PubMed]
- Low, F.W.; Lai, C.W.; Abd Hamid, S.B. Easy preparation of ultrathin reduced graphene oxide sheets at a high stirring speed. Ceram. Int. 2015, 41, 5798–5806. [Google Scholar] [CrossRef]
- Li, Z.Q.; Lu, C.J.; Xia, Z.P.; Zhou, Y.; Luo, Z. X-ray diffraction patterns of graphite and turbostratic carbon. Carbon 2007, 45, 1686–1695. [Google Scholar] [CrossRef]
- Tu, N.D.K.; Choi, J.; Park, C.R.; Kim, H. Remarkable Conversion Between n- and p-Type Reduced Graphene Oxide on Varying the Thermal Annealing Temperature. Chem. Mater. 2015, 27, 7362–7369. [Google Scholar] [CrossRef]
- Mizsei, J. How can sensitive and selective semiconductor gas sensor be made? Sens. Actuators B 1995, 23, 173–176. [Google Scholar] [CrossRef]
- Bârsan, N.; Weimar, U. Conduction model of metal oxide gas sensors. J. Electroceram. 2001, 7, 143–167. [Google Scholar] [CrossRef]
- Nasiri, N.; Bo, R.; Wang, F.; Fu, L.; Tricoli, A. Ultraporous Electron-Depleted ZnO Nanoparticle Networks for Highly Sensitive Portable Visible-Blinda UV Photodetectors. Adv. Mater. 2015, 27, 4336–4343. [Google Scholar] [CrossRef] [PubMed]
- Ginley, D.S.; Hosono, H.; Paine, D.C. Handbook on Transparent Conductors; Springer: New York, NY, USA, 2011. [Google Scholar]
- Kumar, P.V.; Bernardi, M.; Grossman, J.C. The impact of functionalization on the stability, work function, and photoluminescence of reduced graphene oxide. ACS Nano 2013, 7, 1638–1645. [Google Scholar] [CrossRef] [PubMed]
- Sygellou, L.; Paterakis, G.; Galiotis, C.; Tasis, D. Work function tuning of reduced graphene oxide thin films. J. Phys. Chem. C 2016, 120, 281–290. [Google Scholar] [CrossRef]
- Ji, S.; Min, B.K.; Kim, S.K.; Myung, S.; Kang, M.; Shin, H.S.; Song, W.; Heo, J.; Lim, J.; An, K.S.; et al. Work function engineering of graphene oxide via covalent functionalization for organic field-effect transistors. Appl. Surf. Sci. 2017, 419, 252–258. [Google Scholar] [CrossRef]
- Gutmann, S.; Conrad, M.; Wolak, M.A.; Beerbom, M.M.; Schlaf, R. Work function measurements on nano-crystalline zinc oxide surfaces. J. Appl. Phys. 2012, 111, 123710. [Google Scholar] [CrossRef]
- Li, J.; Qi, X.; Hao, G.; Ren, L.; Zhong, J. In-Situ investigation of graphene oxide under UV irradiation: Evolution of work function. AIP Adv. 2015, 5, 067154. [Google Scholar] [CrossRef]
- Chizhov, A.; Kutukov, P.; Gulin, A.; Astafiev, A.; Rumyantseva, M. UV-Activated NO2 Gas Sensing by Nanocrystalline ZnO: Mechanistic Insights from Mass Spectrometry Investigations. Chemosensors 2022, 10, 147. [Google Scholar] [CrossRef]
- Chizhov, A.; Kutukov, P.; Astafiev, A.; Rumyantseva, M. Photoactivated Processes on the Surface of Metal Oxides and Gas Sensitivity to Oxygen. Sensors 2023, 23, 1055. [Google Scholar] [CrossRef]
- Wang, C.Y.; Becker, R.W.; Passow, T.; Pletschen, W.; Köhler, K.; Cimalla, V.; Ambacher, O. Photon stimulated sensor based on indium oxide nanoparticles I: Wide-concentration-range ozone monitoring in air. Sens. Actuators B 2011, 152, 235–240. [Google Scholar] [CrossRef]
- da Silva, L.F.; M’Peko, J.C.; Catto, A.C.; Bernatdini, S.; Mastelaro, V.R.; Aguir, K.; Ribeiro, C.; Longo, E. UV-enhanced ozone gas sensing response of ZnO-SnO2 heterojunctions at room temperature. Sens. Actuators B 2017, 240, 573–579. [Google Scholar] [CrossRef]
- Yang, L.; Marikutsa, A.; Rumyantseva, M.; Konstantinova, E.; Khmelevsky, N.; Gaskov, A. Quasi similar routes of NO2 and NO sensing by nanocrystalline WO3: Evidence by in situ DRIFT spectroscopy. Sensors 2019, 19, 3405. [Google Scholar] [CrossRef] [PubMed]
- Nasriddinov, A.; Rumyantseva, M.; Shatalova, T.; Tokarev, S.; Yaltseva, P.; Fedorova, O.; Khmelevsky, N.; Gaskov, A. Organic-inorganic hybrid materials for room temperature light-activated sub-ppm NO detection. Nanomaterials 2020, 10, 70. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Gao, H.; Liu, R.; Du, B. Study of structure and property for the NO2 + NO2− electron transfer system. J. Mol. Struct. THEOCHEM 2001, 545, 179–186. [Google Scholar] [CrossRef]
- Šulka, M.; Pitoňák, M.; Neogrády, P.; Urban, M. Electron affinity of the O2 molecule: CCSD(T) calculations using the optimized virtual orbitals space approach. Int. J. Quantum Chem. 2008, 108, 2159–2171. [Google Scholar] [CrossRef]
- Sergent, N.; Epifani, M.; Comini, E.; Faglia, G.; Pagnier, T. Interactions of nanocrystalline tin oxide powder with NO2: A Raman spectroscopic study. Sens. Actuators B 2007, 126, 1–5. [Google Scholar] [CrossRef]
- Chizhov, A.; Vasiliev, R.; Rumyantseva, M.; Krylov, I.; Drozdov, K.; Batuk, M.; Hadermann, J.; Abakumov, A.; Gaskov, A. Light-Activated Sub-ppm NO2 Detection by Hybrid ZnO/QD Nanomaterials vs. Charge Localization in Core-Shell QD. Front. Mater. 2019, 6, 231. [Google Scholar] [CrossRef]
- Vasiliev, R.; Babynina, A.; Maslova, O.; Rumyantseva, M.; Ryabova, L.; Dobrovolsky, A.; Drozdov, K.; Khokhlov, D.; Abakumov, A.; Gaskov, A. Photoconductivity of nanocrystalline SnO2 sensitized with colloidal CdSe quantum dots. J. Mater. Chem. C 2013, 1, 1005–1010. [Google Scholar]
- Nasriddinov, A.; Rumyantseva, M.; Konstantinova, E.; Marikutsa, A.; Tokarev, S.; Yaltseva, P.; Fedorova, O.; Gaskov, A. Effect of humidity on light-activated NO and NO2 gas sensing by hybrid materials. Nanomaterials 2020, 10, 915. [Google Scholar] [CrossRef]
- Chen, E.S.; Wentworth, W.E.; Chen, E.C.M. The electron affinities of NO and O2. J. Mol. Struct. 2002, 606, 1–7. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, L.; He, C.; Ma, D.; Lu, Z. Adsorption and oxidation of NO on various SnO2 (110) surfaces: A density functional theory study. Sens. Actuators B 2015, 221, 717–722. [Google Scholar] [CrossRef]
- Drmosh, Q.A.; Hendi, A.H.; Hossain, M.K.; Yamani, Z.H.; Moqbel, R.A.; Hezam, A.; Gondal, M.A. UV-activated gold decorated rGO/ZnO heterostructured nanocomposite sensor for efficient room temperature H2 detection. Sens. Actuators B 2019, 290, 666–675. [Google Scholar] [CrossRef]


| Material | NO2 Concentration, ppm | Sensor Response, S = 100% × (Rgas − Rair)/Rair | Ref. |
|---|---|---|---|
| In2O3 | 5 | 900 | [24] |
| In2O3 | 8 | 17,900 | [25] |
| SnO2 | 10 | 1000 | [26] |
| Pd/SnO2 | 5 | 180,000 | [27] |
| Au/SnO2 | 5 | 6400 | [28] |
| SnO2/rGO | 10 | 120 | [29] |
| WO3 | 5 | 11,300 | [30] |
| ZnO | 1 | 708 | [31] |
| ZnO | 1 | 610 | [32] |
| ZnO | 1 | 304 | [33] |
| ZnO | 1 | 8820 | [34] |
| ZnO | 0.05 | 157 | [35] |
| ZnO(Mg) | 5 | 100 | [36] |
| ZnO(Dy) | 0.3 | 65 | [37] |
| Ag/ZnO | 5 | 98 | [38] |
| Au/ZnO | 5.0 | 455 | [39] |
| In2O3/ZnO | 0.5 | 3170 | [40] |
| ZnO/SnO2 | 0.5 | 126,600 | [41] |
| ZnO/SnO2 | 0.05 | 220 | [42] |
| ZnO/In2O3 | 5 | 221 | [43] |
| ZnO/In2O3 | 0.7 | 11,600 | [44] |
| ZnO/rGO | 50 | 3431 | [16] |
| ZnO/CMK-3 | 5 | 191 | [45] |
| ZnO/MoS2 | 0.2 | 188 | [46] |
| MoS2/ZnO | 0.5 | 2310 | [47] |
| ZnS/ZnO | 1.0 | 339 | [48] |
| Bi2O3/ZnO | 1.0 | 227 | [49] |
| Al/TiO2/Al2O3/p-Si | 20 | 11.5 | [50] |
| CuO/ZnS | 5.0 | 955 | [51] |
| Au/MoS2 | 2.5 | 30 | [52] |
| Graphene | 100 | 27.5 | [53] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Platonov, V.; Malinin, N.; Vasiliev, R.; Rumyantseva, M. Room Temperature UV-Activated NO2 and NO Detection by ZnO/rGO Composites. Chemosensors 2023, 11, 227. https://doi.org/10.3390/chemosensors11040227
Platonov V, Malinin N, Vasiliev R, Rumyantseva M. Room Temperature UV-Activated NO2 and NO Detection by ZnO/rGO Composites. Chemosensors. 2023; 11(4):227. https://doi.org/10.3390/chemosensors11040227
Chicago/Turabian StylePlatonov, Vadim, Nikolai Malinin, Roman Vasiliev, and Marina Rumyantseva. 2023. "Room Temperature UV-Activated NO2 and NO Detection by ZnO/rGO Composites" Chemosensors 11, no. 4: 227. https://doi.org/10.3390/chemosensors11040227
APA StylePlatonov, V., Malinin, N., Vasiliev, R., & Rumyantseva, M. (2023). Room Temperature UV-Activated NO2 and NO Detection by ZnO/rGO Composites. Chemosensors, 11(4), 227. https://doi.org/10.3390/chemosensors11040227









