Coefficient of Friction and Height Loss: Two Criteria Used to Determine the Mechanical Property and Stability of Regenerated Versus Natural Articular Cartilage
Abstract
1. Introduction
1.1. Coefficient of Friction of Articular Cartilage
1.2. Height Loss of the Cartilage Layer after Tribological Exposure
1.3. Articular Cartilage Defect Model in the Göttingen Minipig for Regenerative Treatments
1.4. The Effect Size
1.5. Focus of the Study
2. Materials and Methods
2.1. Animals and Treatment
2.2. Specimens
2.3. Examinations in the Tribometer
2.4. Calculation of the CoF
2.5. Calculation of the HL (Height Loss)
2.6. Exclusion Criteria of Data
- artificial damage to the cartilage at the pin or within the friction distance on the plate was macroscopically identified
- a pair specimen was stressed (loaded) before the experiment had started, so it moved through some cycles with the wrong test parameters
- the pin or plate wobbled during the friction experiment due to insufficient fixation
- the data records of the experiment showed undefined values because the position and movement direction data did not match
- if we suspected that the sample holder of the pin might have touched the cartilage surface of the plate before or during the experiment
- the testing device did not work correctly
2.7. Statistics
3. Results
3.1. Complications and Welfare of the Animals
3.2. The Specimens
3.3. Quality of the Cartilage Pin and Plate; Slope Angles of the Uneven Plate
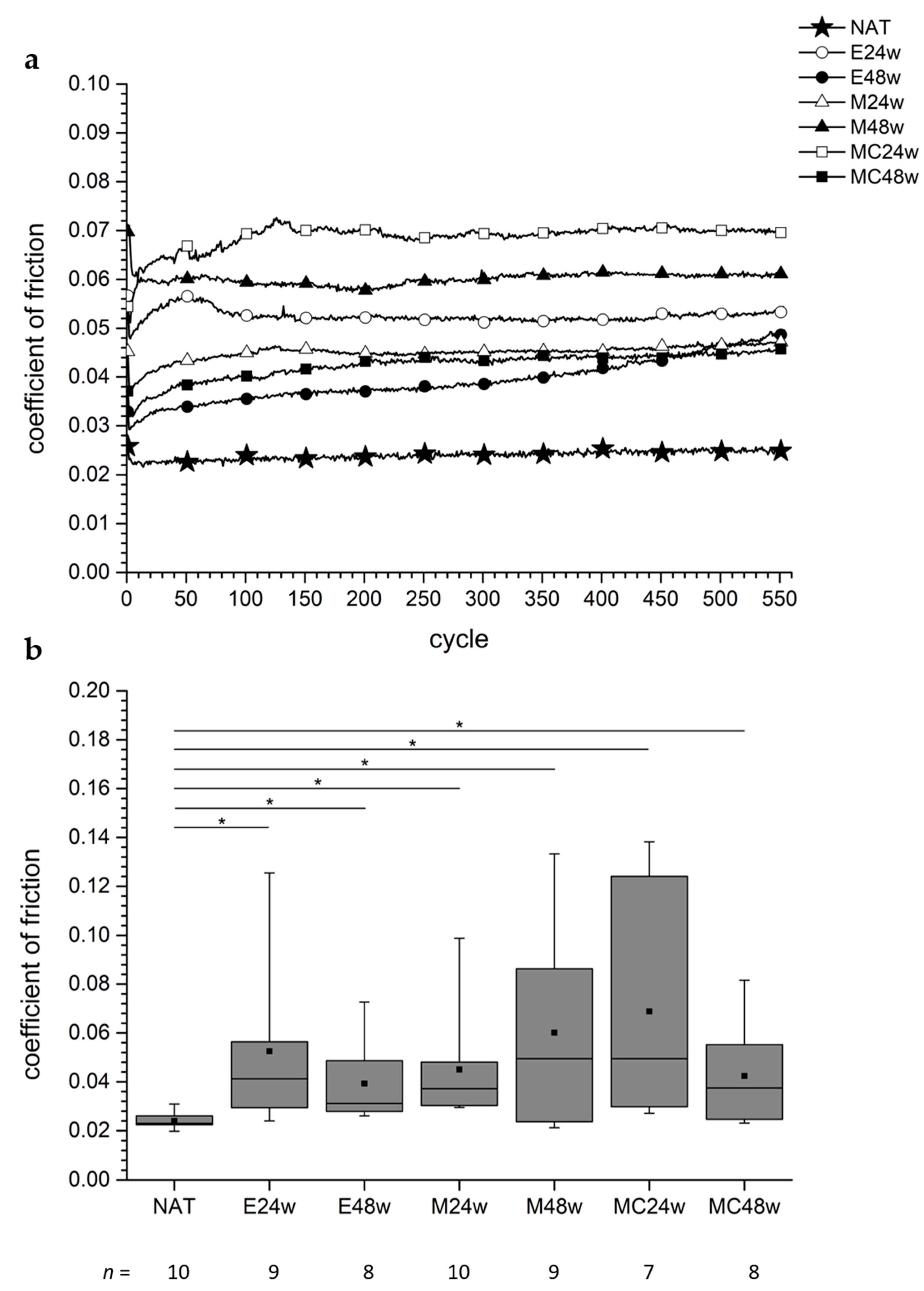
3.4. Coefficient of Friction
3.4.1. Defect areas
3.4.2. Non-Defect Areas
3.4.3. Comparison between Defect and Non-Defect Areas in the Groups
3.5. Height Loss
3.5.1. Defect Areas
3.5.2. Non-Defect Areas
3.5.3. Comparison between Defect and Non-Defect Areas in the Groups
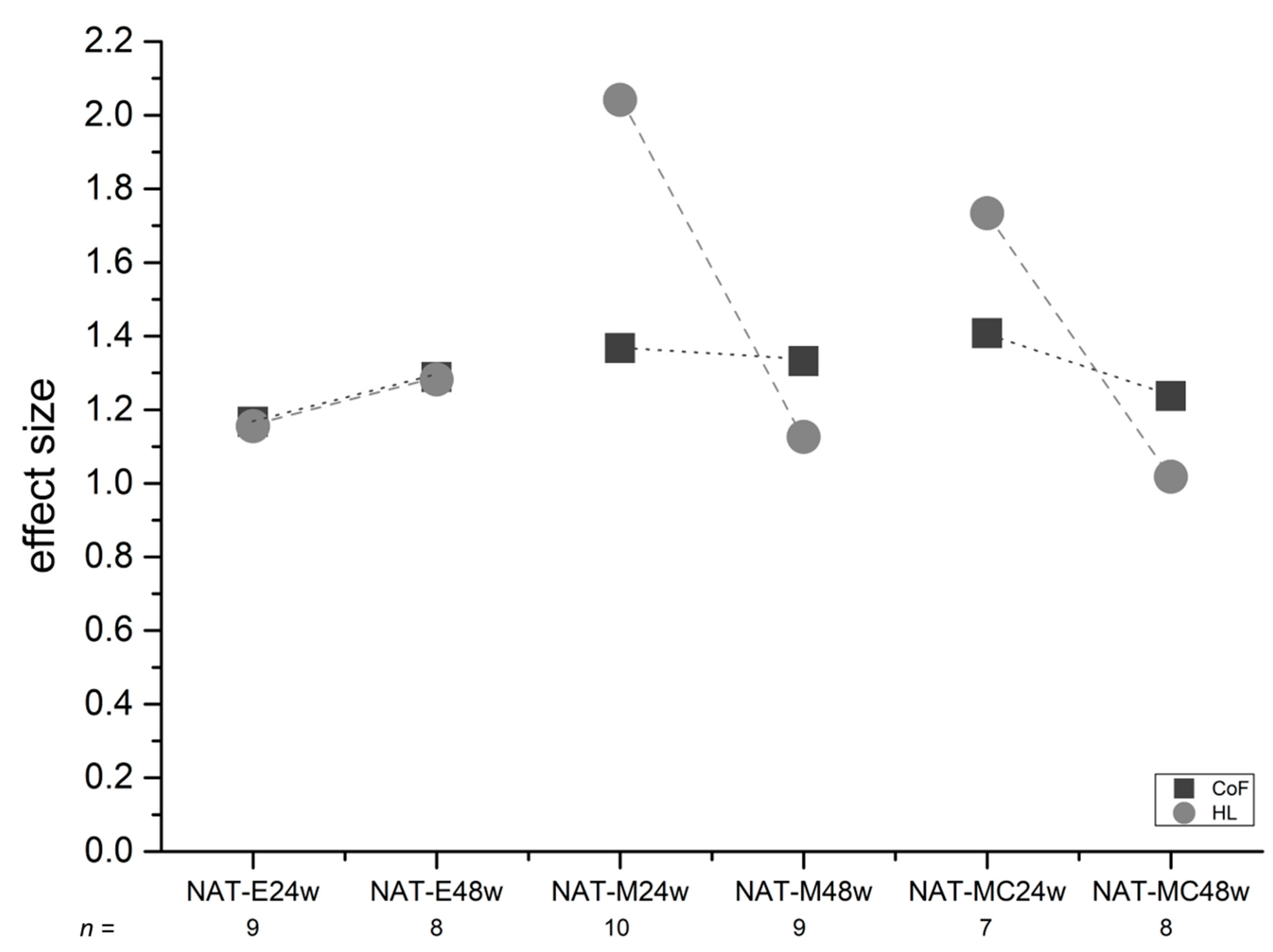
3.6. Effect Sizes (ES)
4. Discussion
4.1. CoF, HL, and Controls
4.2. Animal Model
4.3. Lost Values
4.4. Sample Size
4.5. Lubricant PBS
4.6. CoF
4.6.1. CoF in Pin-on-Plate Tribometers
4.6.2. CoF in Literature
4.6.3. CoF Regarding Internal and External Controls
4.6.4. Validity of the CoF Calculation
4.7. HL
4.7.1. HL after Applied Shear Stress
4.7.2. HL in Literature
4.7.3. HL Useful for Differentiation
4.7.4. HL Regarding Internal and External Controls
4.8. Effect Sizes (ES)
4.9. Limitations and Advantages of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Arakaki, K.; Kitamura, N.; Fujiki, H.; Kurokawa, T.; Iwamoto, M.; Ueno, M.; Kanaya, F.; Osada, Y.; Gong, J.P.; Yasuda, K. Artificial cartilage made from a novel double-network hydrogel: In vivo effects on the normal cartilage and ex vivo evaluation of the friction property. J. Biomed. Mater. Res. A 2010, 93, 1160–1168. [Google Scholar] [PubMed]
- Schütte, A.; Mack, M.; Behler, H.; Ruland, M.; Weiß, C.; Schwarz, M.L.R. Tribometer for measuring coefficients of friction of uneven surfaces like articular cartilage. Rev. Sci. Instrum. 2020, 91, 034102. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, M.L.; Schneider-Wald, B.; Krase, A.; Richter, W.; Reisig, G.; Kreinest, M.; Heute, S.; Pott, P.P.; Brade, J.; Schutte, A. Tribological assessment of articular cartilage. A system for the analysis of the friction coefficient of cartilage, regenerates and tissue engineering constructs; initial results. Orthopade 2012, 41, 827–836. [Google Scholar] [CrossRef] [PubMed]
- Bell, C.J.; Ingham, E.; Fisher, J. Influence of hyaluronic acid on the time-dependent friction response of articular cartilage under different conditions. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2006, 220, 23–31. [Google Scholar] [CrossRef]
- Bonnevie, E.D.; Galesso, D.; Secchieri, C.; Cohen, I.; Bonassar, L.J. Elastoviscous Transitions of Articular Cartilage Reveal a Mechanism of Synergy between Lubricin and Hyaluronic Acid. PLoS ONE 2015, 10, e0143415. [Google Scholar] [CrossRef]
- Caligaris, M.; Ateshian, G. Effects of sustained interstitial fluid pressurization under migrating contact area, and boundary lubrication by synovial fluid, on cartilage friction. Osteoarthr. Cartil. 2008, 16, 1220–1227. [Google Scholar] [CrossRef]
- Caligaris, M.; Canal, C.E.; Ahmad, C.S.; Gardner, T.R.; Ateshian, G.A. Investigation of the frictional response of osteoarthritic human tibiofemoral joints and the potential beneficial tribological effect of healthy synovial fluid. Osteoarthr. Cartil. 2009, 17, 1327–1332. [Google Scholar] [CrossRef]
- Farnham, M.S.; Larson, R.E.; Burris, D.L.; Price, C. Effects of mechanical injury on the tribological rehydration and lubrication of articular cartilage. J. Mech. Behav. Biomed. Mater. 2019, 101, 103422. [Google Scholar] [CrossRef]
- Forster, H.; Fisher, J. The Influence of Loading Time and Lubricant on the Friction of Articular Cartilage. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 1996, 210, 109–119. [Google Scholar] [CrossRef]
- Gleghorn, J.P.; Bonassar, L.J. Lubrication mode analysis of articular cartilage using Stribeck surfaces. J. Biomech. 2008, 41, 1910–1918. [Google Scholar] [CrossRef]
- Griffin, D.J.; Bonnevie, E.D.; Lachowsky, D.J.; Hart, J.C.; Sparks, H.D.; Moran, N.; Matthews, G.; Nixon, A.J.; Cohen, I.; Bonassar, L.J. Mechanical characterization of matrix-induced autologous chondrocyte implantation (MACI(R)) grafts in an equine model at 53 weeks. J. Biomech. 2015, 48, 1944–1949. [Google Scholar] [CrossRef] [PubMed]
- Katta, J.; Jin, Z.; Ingham, E.; Fisher, J. Effect of nominal stress on the long term friction, deformation and wear of native and glycosaminoglycan deficient articular cartilage. Osteoarthr. Cartil. 2009, 17, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, R.; Mariner, E.N.; Ateshian, G.A. Effect of dynamic loading on the frictional response of bovine articular cartilage. J. Biomech. 2005, 38, 1665–1673. [Google Scholar] [CrossRef] [PubMed]
- Link, J.M.; Salinas, E.Y.; Hu, J.C.; Athanasiou, K.A. The tribology of cartilage: Mechanisms, experimental techniques, and relevance to translational tissue engineering. Clin. Biomech. 2019, 79, 104880. [Google Scholar] [CrossRef]
- Middendorf, J.M.; Griffin, D.J.; Shortkroff, S.; Dugopolski, C.; Kennedy, S.; Siemiatkoski, J.; Cohen, I.; Bonassar, L.J. Mechanical properties and structure-function relationships of human chondrocyte-seeded cartilage constructs after in vitro culture. J. Orthop. Res. 2017, 35, 2298–2306. [Google Scholar] [CrossRef] [PubMed]
- Morita, Y.; Tomita, N.; Aoki, H.; Sonobe, M.; Wakitani, S.; Tamada, Y.; Suguro, T.; Ikeuchi, K. Frictional properties of regenerated cartilage in vitro. J. Biomech. 2006, 39, 103–109. [Google Scholar] [CrossRef]
- Northwood, E.; Fisher, J. A multi-directional in vitro investigation into friction, damage and wear of innovative chondroplasty materials against articular cartilage. Clin. Biomech. 2007, 22, 834–842. [Google Scholar] [CrossRef]
- Wong, B.L.; Bae, W.C.; Gratz, K.R.; Sah, R.L. Shear deformation kinematics during cartilage articulation: Effect of lubrication, degeneration, and stress relaxation. Mol. Cell. Biomech. 2008, 5, 197–206. [Google Scholar]
- Engelhardt, J.P.; Schütte, A.; Hetjens, S.; Reisig, G.; Schwarz, M.L. Resilience to height loss of articular cartilage of osteoarthritic stifle joints of old pigs, compared with healthy cartilage from young pigs in a tribological pin—On—Plate exposure, revealing similar friction forces. PLoS ONE 2021, 16, e0250244. [Google Scholar] [CrossRef]
- Niemeyer, P.; Albrecht, D.; Andereya, S.; Angele, P.; Ateschrang, A.; Aurich, M.; Baumann, M.; Bosch, U.; Erggelet, C.; Fickert, S.; et al. Autologous chondrocyte implantation (ACI) for cartilage defects of the knee: A guideline by the working group “Clinical Tissue Regeneration” of the German Society of Orthopaedics and Trauma (DGOU). Knee 2016, 23, 426–435. [Google Scholar] [CrossRef]
- Roessler, P.P.; Pfister, B.; Gesslein, M.; Figiel, J.; Heyse, T.J.; Colcuc, C.; Lorbach, O.; Efe, T.; Schüttler, K.F. Short-term follow up after implantation of a cell-free collagen type I matrix for the treatment of large cartilage defects of the knee. Int. Orthop. 2015, 39, 2473–2479. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, M.L.; Reisig, G.; Schutte, A.; Becker, K.; Serba, S.; Forsch, E.; Thier, S.; Fickert, S.; Lenz, T.; Weiss, C.; et al. Report on a large animal study with Gottingen Minipigs where regenerates and controls for articular cartilage were created in a large number. Focus on the conditions of the operated stifle joints and suggestions for standardized procedures. PLoS ONE 2019, 14, e0224996. [Google Scholar] [CrossRef] [PubMed]
- Gravius, S.; Schneider, U.; Mumme, T.; Bauer, D.; Maus, U.; Muller-Rath, R.; Berdel, P.; Siebert, C.; Andereya, S. Osteochondral marker proteins in the quantitative evaluation of matrix-based autologous chondrocyte transplantation CaRes. Z. Orthop. Unfall. 2007, 145, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Schneider, U.; Rackwitz, L.; Andereya, S.; Siebenlist, S.; Fensky, F.; Reichert, J.C.; Löer, I.; Barthel, T.; Rudert, M.; Nöth, U. A Prospective Multicenter Study on the Outcome of Type I Collagen Hydrogel–Based Autologous Chondrocyte Implantation (CaReS) for the Repair of Articular Cartilage Defects in the Knee. Am. J. Sports Med. 2011, 39, 2558–2565. [Google Scholar] [CrossRef]
- Strauss, E.; Goodrich, L.R.; Chen, C.-T.; Hidaka, C.; Nixon, A.J. Biochemical and Biomechanical Properties of Lesion and Adjacent Articular Cartilage after Chondral Defect Repair in an Equine Model. Am. J. Sports Med. 2005, 33, 1647–1653. [Google Scholar] [CrossRef]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef]
- Schwarz, M.L.; Schneider-Wald, B.; Brade, J.; Schleich, D.; Schütte, A.; Reisig, G. Instruments for reproducible setting of defects in cartilage and harvesting of osteochondral plugs for standardisation of preclinical tests for articular cartilage regeneration. J. Orthop. Surg. Res. 2015, 10, 117. [Google Scholar] [CrossRef]
- Shi, L.; Brunski, D.B.; Sikavitsas, V.I.; Johnson, M.B.; Striolo, A. Friction coefficients for mechanically damaged bovine articular cartilage. Biotechnol. Bioeng. 2012, 109, 1769–1778. [Google Scholar] [CrossRef]
- Szarko, M.; Muldrew, K.; Bertram, J.E. Freeze-thaw treatment effects on the dynamic mechanical properties of articular cartilage. BMC Musculoskelet. Disord. 2010, 11, 231. [Google Scholar] [CrossRef]
- Bronstein, I.N.; Semendjajew, K.A.; Musiol, G.; Mühlig, H. Taschenbuch der Mathematik; 19. Auflage; Verlag Harri Deutsch: Frankfurt/Main, Germany, 1993. [Google Scholar]
- Coulomb, C.A. Théorie des Machines Simples, en Ayant Egard au Frottement de Leurs Parties, et à la Roideur des Cordages; Bachelier: Paris, France, 1821; Available online: https://books.google.de/books?id=A5k5AAAAcAAJ (accessed on 13 May 2020).
- Czichos, H.; Habig, K.; Celis, J.-P.; Cowan, R.S.; Fischer, A.; Gerschwiler, K.; Gradt, T.; Kleinlein, E.; Klocke, F.; Knoll, G.; et al. Tribologie-Handbuch; 3. Auflage; Czichos, H., Habig, K., Eds.; Vieweg+Teubner Verlag/Springer Fachmedien Wiesbaden GmbH: Wiesbaden, Germany, 2010. [Google Scholar]
- Boettcher, K.; Kienle, S.; Nachtsheim, J.; Burgkart, R.; Hugel, T.; Lieleg, O. The structure and mechanical properties of articular cartilage are highly resilient towards transient dehydration. Acta Biomater. 2016, 29, 180–187. [Google Scholar] [CrossRef]
- Bonnevie, E.D.; Baro, V.J.; Wang, L.; Burris, D.L. In Situ Studies of Cartilage Microtribology: Roles of Speed and Contact Area. Tribol. Lett. 2010, 41, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.; Burris, D. Tribological rehydration of cartilage and its potential role in preserving joint health. Osteoarthr. Cartil. 2016, 25, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Neu, C.P.; Komvopoulos, K.; Reddi, A.H. The Interface of Functional Biotribology and Regenerative Medicine in Synovial Joints. Tissue Eng. Part B Rev. 2008, 14, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Ateshian, G.A. The role of interstitial fluid pressurization in articular cartilage lubrication. J. Biomech. 2009, 42, 1163–1176. [Google Scholar] [CrossRef]
- Kanca, Y.; Milner, P.; Dini, D.; Amis, A.A. Tribological evaluation of biomedical polycarbonate urethanes against articular cartilage. J. Mech. Behav. Biomed. Mater. 2018, 82, 394–402. [Google Scholar] [CrossRef]
- Setton, L.A.; Zhu, W.; Mow, V.C. The biphasic poroviscoelastic behavior of articular cartilage: Role of the surface zone in governing the compressive behavior. J. Biomech. 1993, 26, 581–592. [Google Scholar] [CrossRef]
- Mostakhdemin, M.; Nand, A.; Ramezani, M. Tribological Evaluation of Silica Nanoparticle Enhanced Bilayer Hydrogels as A Candidate for Cartilage Replacement. Polymers 2022, 14, 3593. [Google Scholar] [CrossRef]
- Schinhan, M.; Gruber, M.; Vavken, P.; Dorotka, R.; Samouh, L.; Chiari, C.; Gruebl-Barabas, R.; Nehrer, S. Critical-size defect induces unicompartmental osteoarthritis in a stable ovine knee. J. Orthop. Res. 2011, 30, 214–220. [Google Scholar] [CrossRef]
- Shon, O.J.; Park, S.J.; Shim, B.J.; Lee, D.Y. Comparative Study of Clinical and Radiographic Outcomes of High Tibial Osteotomy in Patients with Kissing Lesions and Non-Kissing Lesions. Knee Surg. Relat. Res. 2017, 29, 288–294. [Google Scholar] [CrossRef]
- Cone, S.; Warren, P.B.; Fisher, M.B. Rise of the Pigs: Utilization of the Porcine Model to Study Musculoskeletal Biomechanics and Tissue Engineering During Skeletal Growth. Tissue Eng. Part C Methods 2017, 23, 763–780. [Google Scholar] [CrossRef]
- Gotterbarm, T.; Breusch, S.J.; Schneider, U.; Jung, M. The minipig model for experimental chondral and osteochondral defect repair in tissue engineering: Retrospective analysis of 180 defects. Lab. Anim. 2008, 42, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Hennerbichler, A.; Rosenberger, R.; Arora, R.; Hennerbichler, D. Biochemical, biomechanical and histological properties of osteoarthritic porcine knee cartilage: Implications for osteochondral transplantation. Arch. Orthop. Trauma. Surg. 2007, 128, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Kääb, M.J.; Gwynn, I.A.; Notzli, H.P. Collagen fibre arrangement in the tibial plateau articular cartilage of man and other mammalian species. J. Anat. 1998, 193, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Kreinest, M.; Reisig, G.; Ströbel, P.; Fickert, S.; Brade, J.; Wennemuth, G.; Lipp, P.; Schwarz, M.L. Analysis of Gene Expression and Ultrastructure of Stifle Menisci from Juvenile and Adult Pigs. Comp. Med. 2016, 66, 30–40. [Google Scholar]
- Reisig, G.; Kreinest, M.; Richter, W.; Wagner-Ecker, M.; Dinter, D.; Attenberger, U.; Schneider-Wald, B.; Fickert, S.; Schwarz, M.L. Osteoarthritis in the Knee Joints of Gottingen Minipigs after Resection of the Anterior Cruciate Ligament? Missing Correlation of MRI, Gene and Protein Expression with Histological Scoring. PLoS ONE 2016, 11, e0165897. [Google Scholar] [CrossRef][Green Version]
- Li, Y.; Yuan, Z.; Yang, H.; Zhong, H.; Peng, W.; Xie, R. Recent Advances in Understanding the Role of Cartilage Lubrication in Osteoarthritis. Molecules 2021, 26, 6122. [Google Scholar] [CrossRef]
- Schmidt, T.; Sah, R. Effect of synovial fluid on boundary lubrication of articular cartilage. Osteoarthr. Cartil. 2007, 15, 35–47. [Google Scholar] [CrossRef]
- Wong, B.L.; Bae, W.C.; Chun, J.; Gratz, K.R.; Lotz, M.; Sah, R.L. Biomechanics of cartilage articulation: Effects of lubrication and degeneration on shear deformation. Arthritis Care Res. 2008, 58, 2065–2074. [Google Scholar] [CrossRef]
- Kosinska, M.K.; Ludwig, T.E.; Liebisch, G.; Zhang, R.; Siebert, H.-C.; Wilhelm, J.; Kaesser, U.; Dettmeyer, R.B.; Klein, H.; Ishaque, B.; et al. Articular Joint Lubricants during Osteoarthritis and Rheumatoid Arthritis Display Altered Levels and Molecular Species. PLoS ONE 2015, 10, e0125192. [Google Scholar] [CrossRef]
- Katta, J.; Stapleton, T.; Ingham, E.; Jin, Z.M.; Fisher, J. The effect of glycosaminoglycan depletion on the friction and deformation of articular cartilage. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2008, 222, 1–11. [Google Scholar] [CrossRef]
- Sivan, S.; Schroeder, A.; Verberne, G.; Merkher, Y.; Diminsky, D.; Priev, A.; Maroudas, A.; Halperin, G.; Nitzan, D.; Etsion, I.; et al. Liposomes Act as Effective Biolubricants for Friction Reduction in Human Synovial Joints. Langmuir 2010, 26, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Basalo, I.M.; Chen, F.H.; Hung, C.T.; Ateshian, G.A. Frictional Response of Bovine Articular Cartilage Under Creep Loading Following Proteoglycan Digestion With Chondroitinase ABC. J. Biomech. Eng. 2006, 128, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Warnecke, D.; Schild, N.B.; Klose, S.; Joos, H.; Brenner, R.E.; Kessler, O.; Skaer, N.; Walker, R.; Freutel, M.; Ignatius, A.; et al. Friction properties of a new silk fibroin scaffold for meniscal replacement. Tribol. Int. 2017, 109, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Jahn, S.; Seror, J.; Klein, J. Lubrication of Articular Cartilage. Annu. Rev. Biomed. Eng. 2016, 18, 235–258. [Google Scholar] [CrossRef]
- O’Driscoll, S.W.; Keeley, F.W.; Salter, R.B. The chondrogenic potential of free autogenous periosteal grafts for biological resurfacing of major full-thickness defects in joint surfaces under the influence of continuous passive motion. An experimental investigation in the rabbit. J. Bone Jt. Surg. Am. 1986, 68, 1017–1035. [Google Scholar] [CrossRef]
- Moore, A.; Burris, D. An analytical model to predict interstitial lubrication of cartilage in migrating contact areas. J. Biomech. 2013, 47, 148–153. [Google Scholar] [CrossRef]
- Moore, A.C.; Schrader, J.L.; Ulvila, J.J.; Burris, D.L. A review of methods to study hydration effects on cartilage friction. Tribol. -Mater. Surf. Interfaces 2017, 11, 202–214. [Google Scholar] [CrossRef]
- Gao, L.L.; Zhang, C.Q.; Gao, H.; Liu, Z.D.; Xiao, P.P. Depth and rate dependent mechanical behaviors for articular cartilage: Experiments and theoretical predictions. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 38, 244–251. [Google Scholar] [CrossRef]
- Diermeier, T.; Venjakob, A.; Byrne, K.; Burgkart, R.; Foehr, P.; Milz, S.; Imhoff, A.B.; Vogt, S. Effects of focal metallic implants on opposing cartilage–an in-vitro study with an abrasion test machine. BMC Musculoskelet. Disord. 2020, 21, 261. [Google Scholar] [CrossRef]
- Fermor, H.L.; McLure, S.W.; Taylor, S.D.; Russell, S.L.; Williams, S.; Fisher, J.; Ingham, E. Biological, biochemical and biomechanical characterisation of articular cartilage from the porcine, bovine and ovine hip and knee. Bio-Medical Mater. Eng. 2015, 25, 381–395. [Google Scholar] [CrossRef]
- Moore, A.C.; Burris, D.L. Tribological and material properties for cartilage of and throughout the bovine stifle: Support for the altered joint kinematics hypothesis of osteoarthritis. Osteoarthr. Cartil. 2015, 23, 161–169. [Google Scholar] [CrossRef] [PubMed]


| Group | Filling of Defects | Observation Period | Number of Animals (n) |
|---|---|---|---|
| NAT | no defects set; external control | 24 weeks | 10 |
| E24w | no filling, empty defects | 24 weeks | 10 |
| E48w | no filling, empty defects | 48 weeks | 10 |
| M24w | matrices without cells | 24 weeks | 10 |
| M48w | matrices without cells | 48 weeks | 10 |
| MC24w | matrices laden with autologous cells | 24 weeks | 10 |
| MC48w | matrices laden with autologous cells | 48 weeks | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwarz, M.L.; Reisig, G.; Schneider-Wald, B.; Weiß, C.; Hauk, L.; Schütte, A. Coefficient of Friction and Height Loss: Two Criteria Used to Determine the Mechanical Property and Stability of Regenerated Versus Natural Articular Cartilage. Biomedicines 2022, 10, 2685. https://doi.org/10.3390/biomedicines10112685
Schwarz ML, Reisig G, Schneider-Wald B, Weiß C, Hauk L, Schütte A. Coefficient of Friction and Height Loss: Two Criteria Used to Determine the Mechanical Property and Stability of Regenerated Versus Natural Articular Cartilage. Biomedicines. 2022; 10(11):2685. https://doi.org/10.3390/biomedicines10112685
Chicago/Turabian StyleSchwarz, Markus L., Gregor Reisig, Barbara Schneider-Wald, Christel Weiß, Luisa Hauk, and Andy Schütte. 2022. "Coefficient of Friction and Height Loss: Two Criteria Used to Determine the Mechanical Property and Stability of Regenerated Versus Natural Articular Cartilage" Biomedicines 10, no. 11: 2685. https://doi.org/10.3390/biomedicines10112685
APA StyleSchwarz, M. L., Reisig, G., Schneider-Wald, B., Weiß, C., Hauk, L., & Schütte, A. (2022). Coefficient of Friction and Height Loss: Two Criteria Used to Determine the Mechanical Property and Stability of Regenerated Versus Natural Articular Cartilage. Biomedicines, 10(11), 2685. https://doi.org/10.3390/biomedicines10112685





