Decoding Immuno-Competence: A Novel Analysis of Complete Blood Cell Count Data in COVID-19 Outcomes
Abstract
1. Introduction
1.1. Dynamic, Polychotomous and Personalized Methods That Prevent Confounding
1.2. Complex and Dynamic Interactions That Structure the Data and Show Patterns
1.3. COVID-19-Related Dynamic and Personalized Immuno-Competence
2. Materials and Methods
2.1. Clinical Data
2.2. Data Structure and Analysis
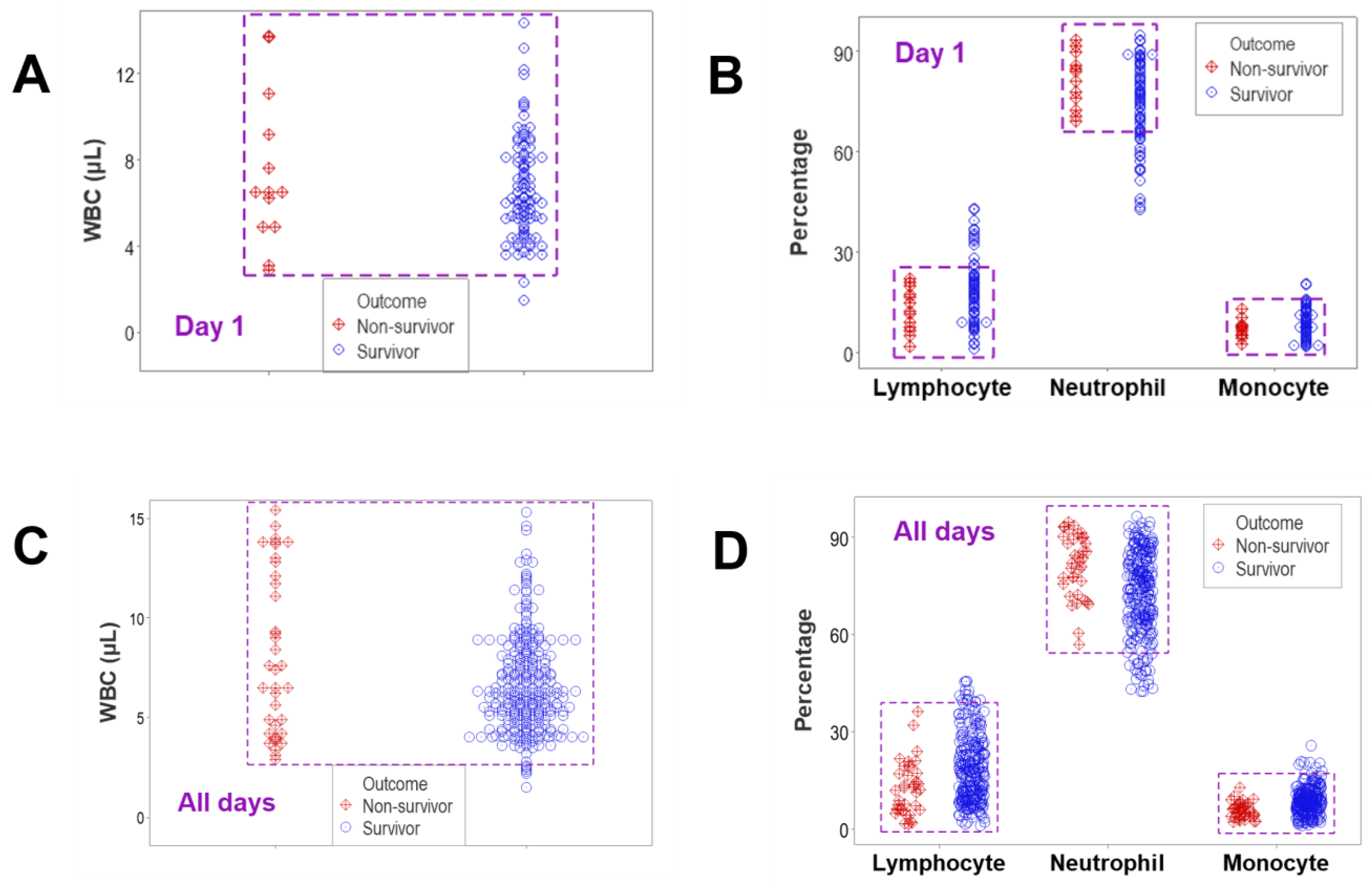
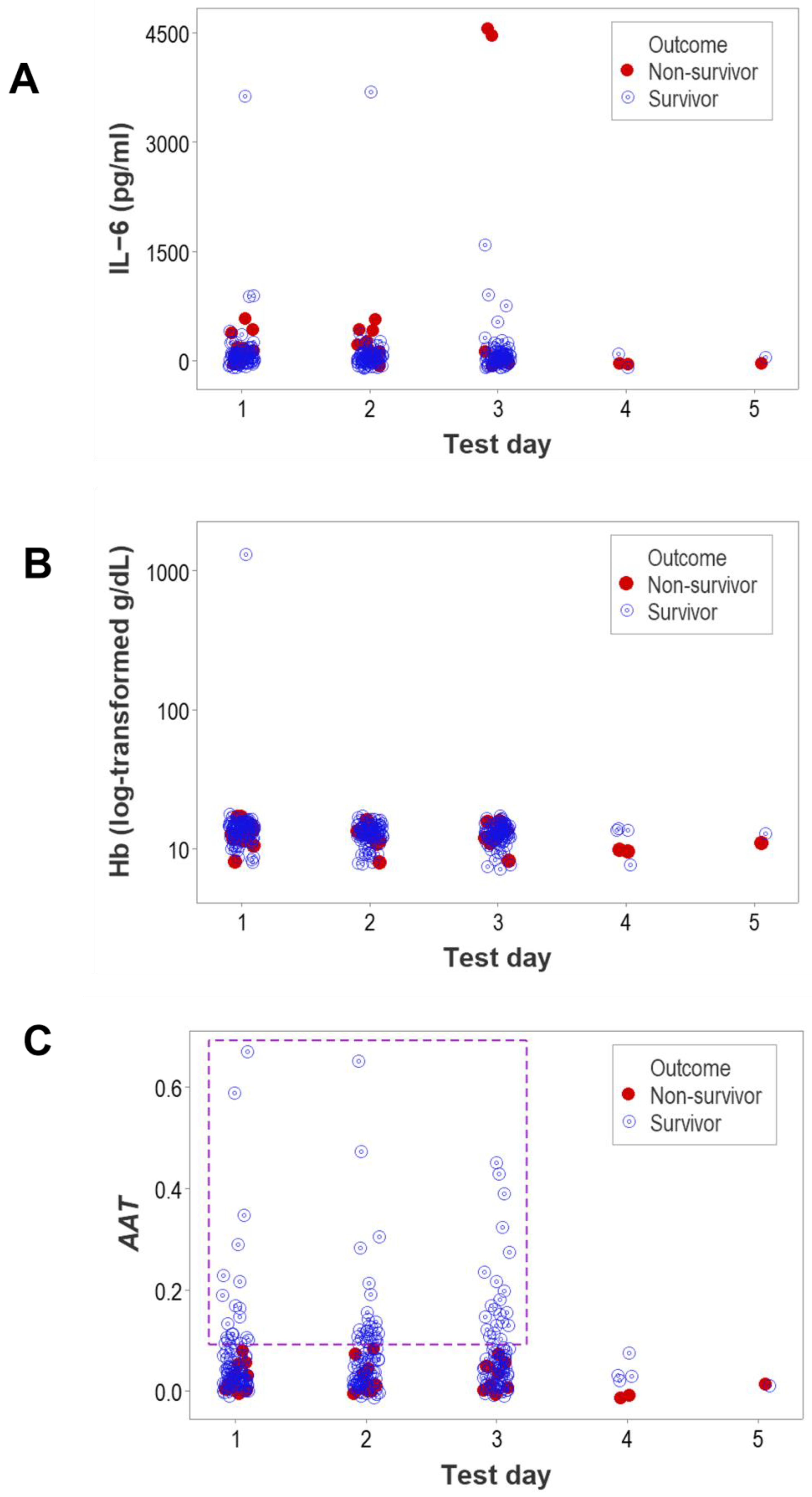
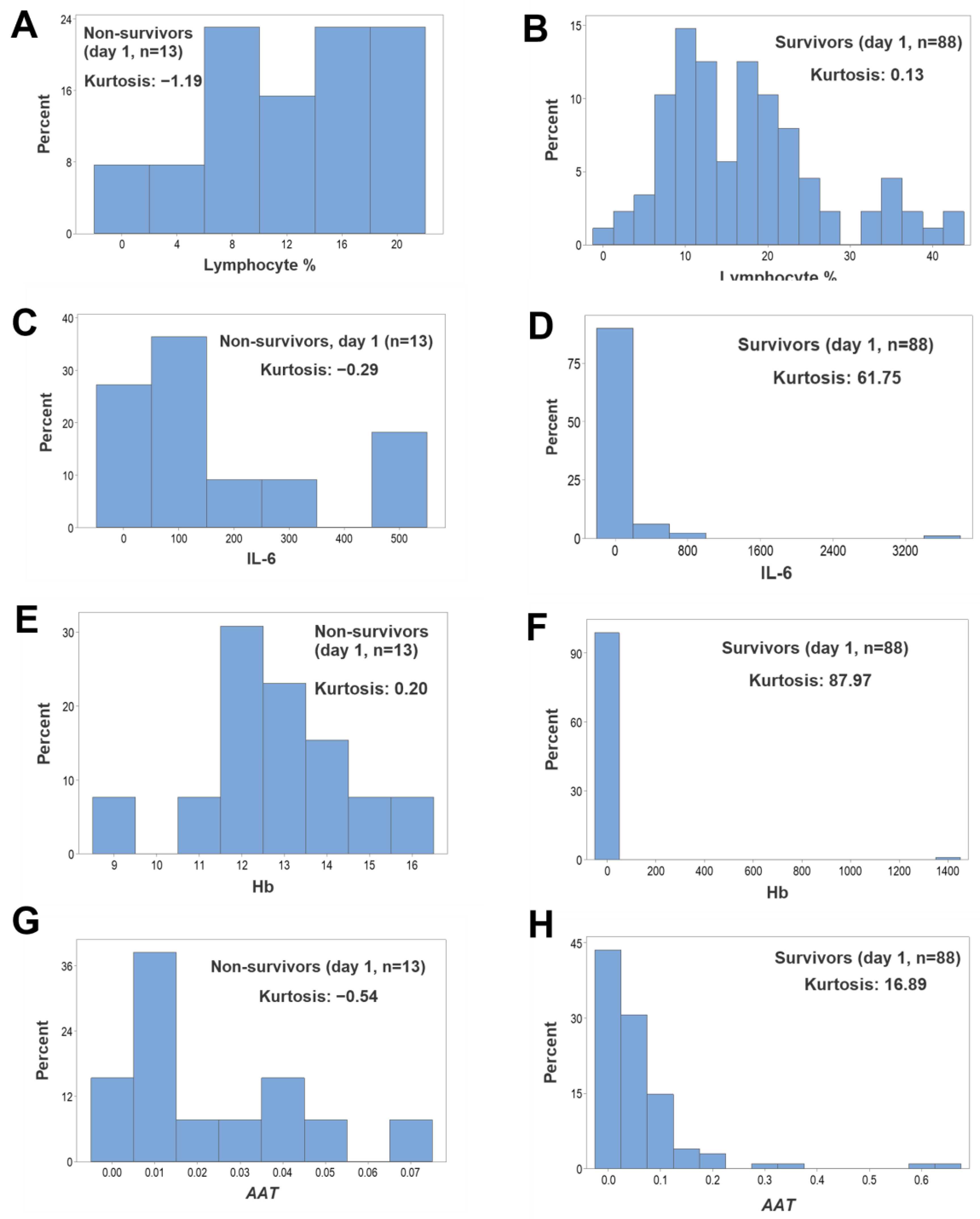
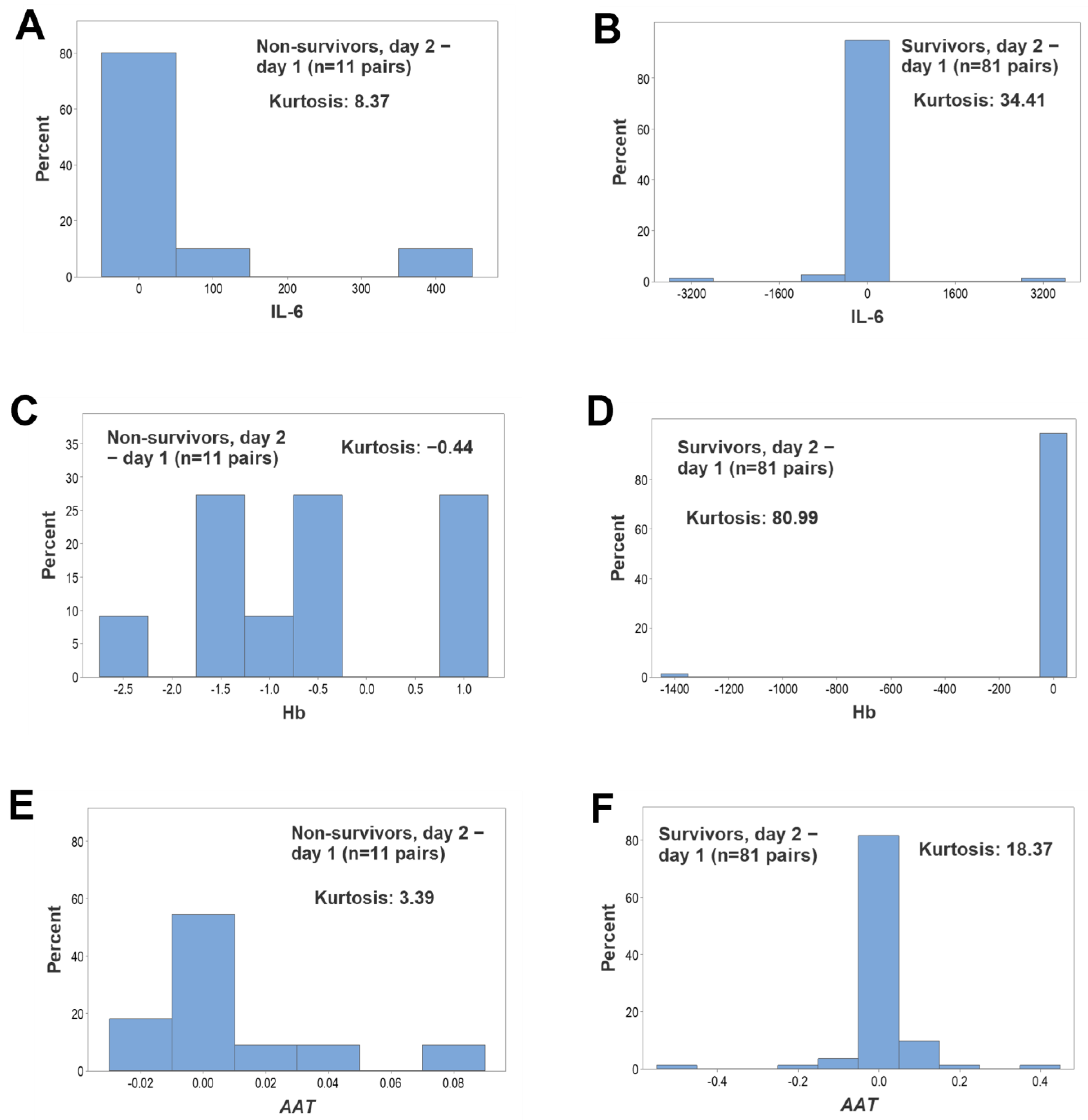
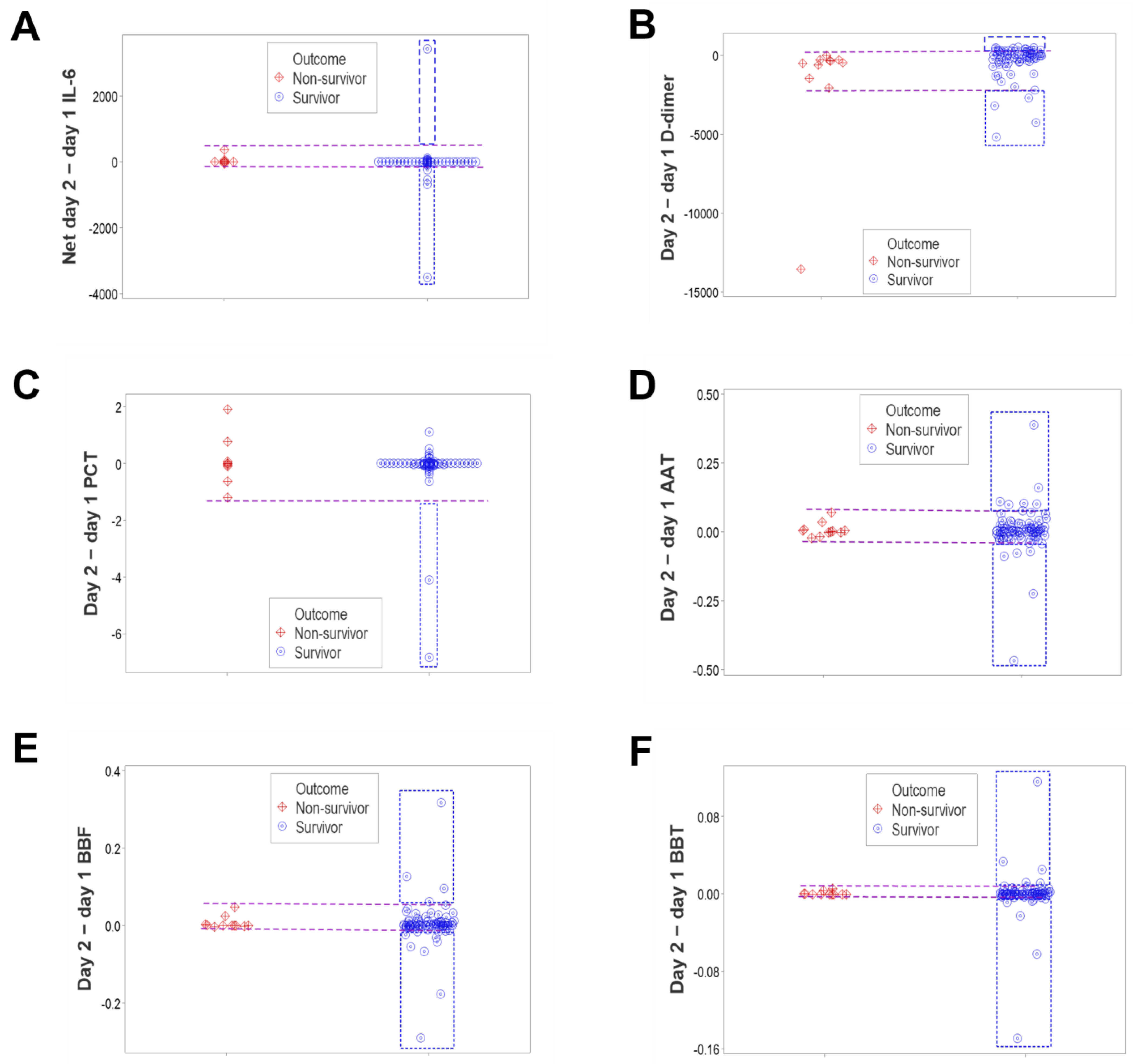
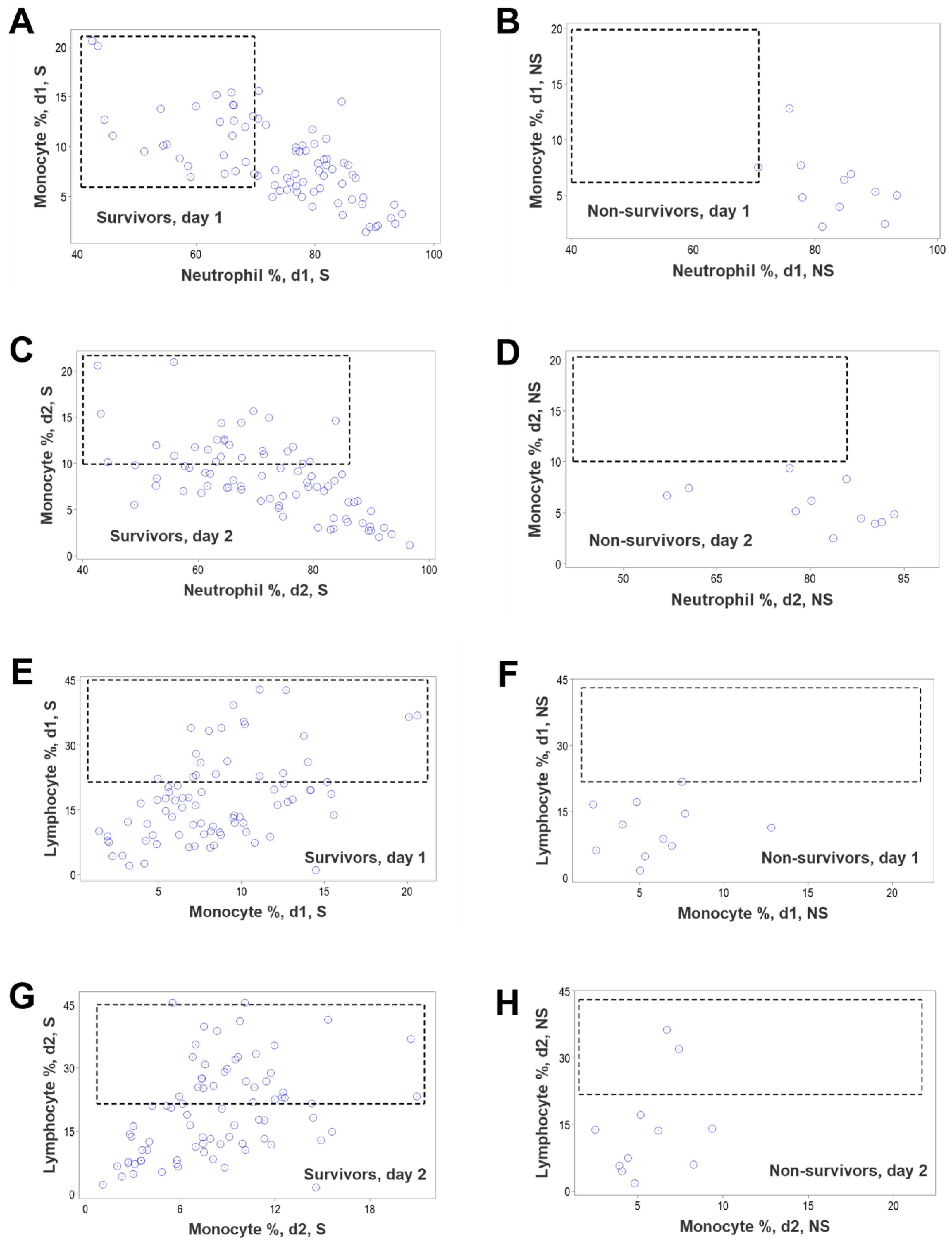
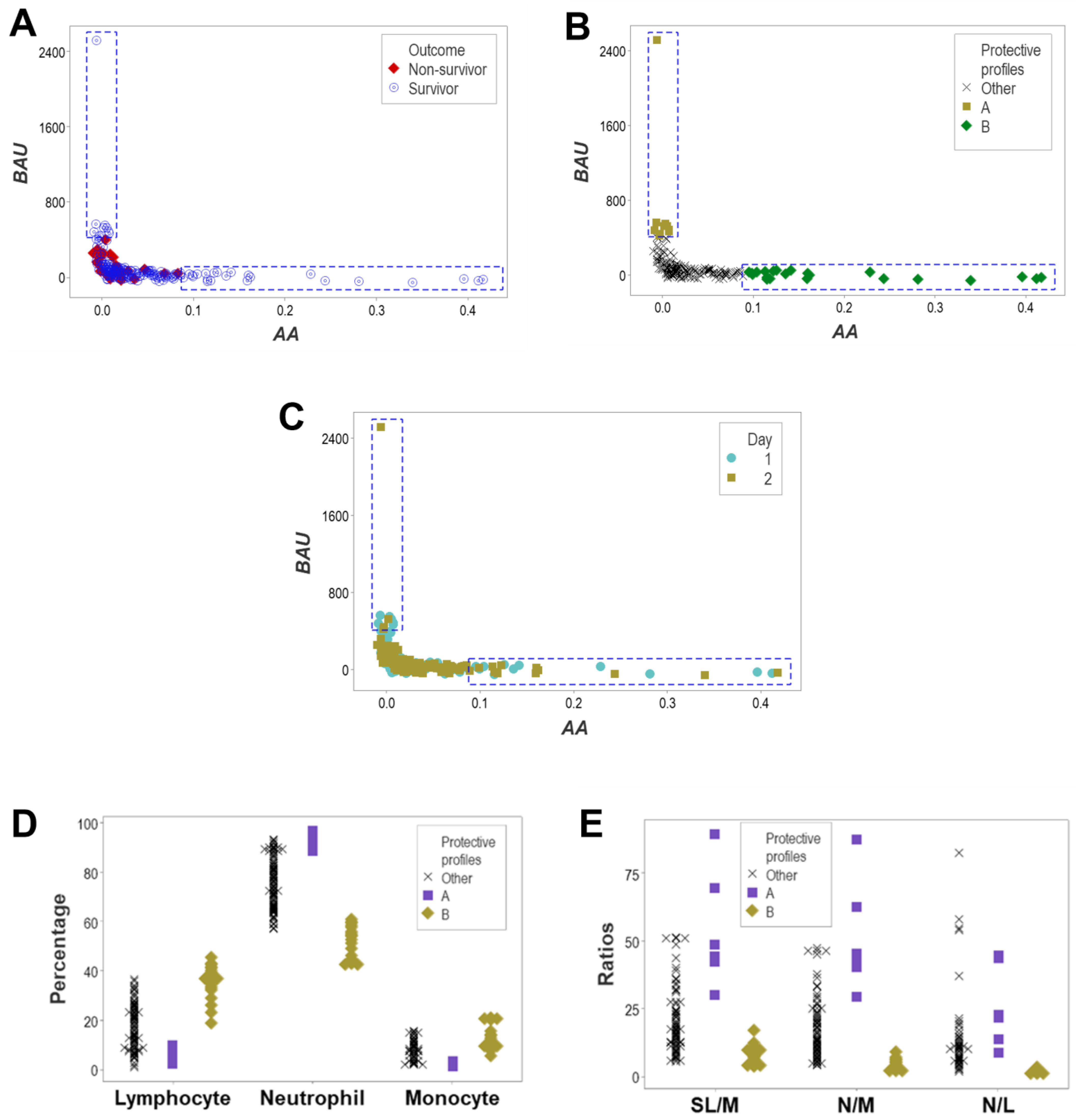
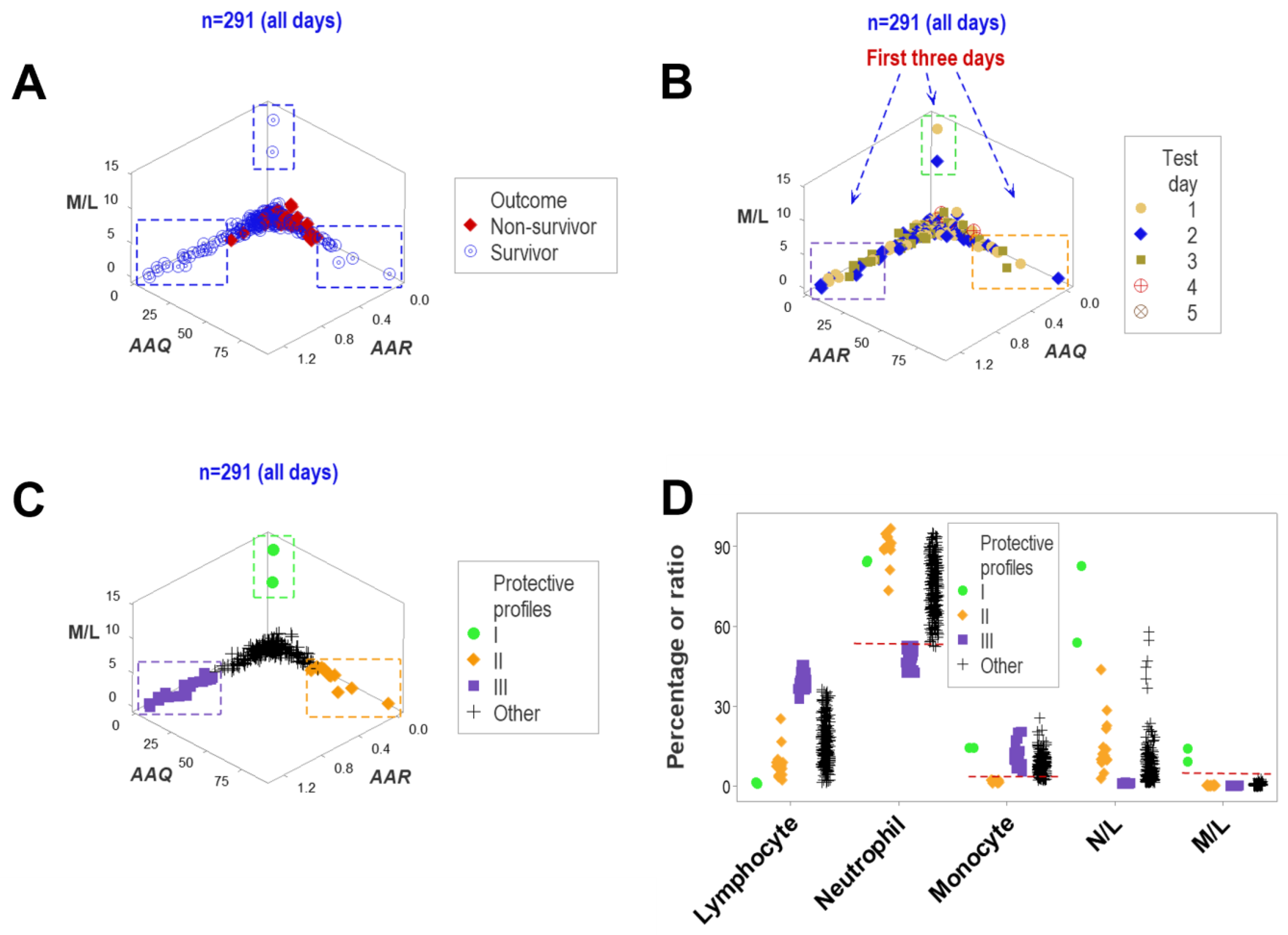
3. Results
4. Discussion
4.1. Caveats
4.2. Seven Major Findings
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bland, J.S. COVID-19 Risk: Clinical Tools for Assessing and Personalizing Immunity. Integr. Med. 2021, 20, 18–23. [Google Scholar]
- Chang, S.; Kohrt, H.; Maecker, H.T. Monitoring the immune competence of cancer patients to predict outcome. Cancer Immunol. Immunother. 2014, 63, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.C.; Restrepo, M.I.; Harper, N.; Manoharan, M.S.; Smith, A.M.; Meunier, J.A.; Sanchez-Reilly, S.; Ehsan, A.; Branum, A.P.; Winter, C.; et al. Immunologic resilience and COVID-19 survival advantage. J. Allergy Clin. Immunol. 2021, 148, 1176–1191. [Google Scholar] [CrossRef] [PubMed]
- Grimm, C.; Dickel, S.; Grundmann, J.; Payen, D.; Schanz, J.; Zautner, A.E.; Tampe, B.; Moerer, O.; Winkler, M.S. Case Report: Interferon-g Rescues Monocytic Human Leukocyte Antigen Receptor (mHLA-DR) Function in a COVID-19 Patient With ARDS and Superinfection With Multiple MDR 4MRGN Bacterial Strains. Front. Immunol. 2021, 12, 753849. [Google Scholar] [CrossRef]
- Li, Q.; Cao, Y.; Chen, L.; Wu, D.; Yu, J.; Wang, H.; He, W.; Chen, L.; Dongv, F.; Chen, W.; et al. Hematological features of persons with COVID-19. Leukemia 2020, 34, 2163–2172. [Google Scholar] [CrossRef] [PubMed]
- Grossman, Z.; Berke, G. Tumor escape from immune elimination. J. Theor. Biol. 1980, 83, 267–296. [Google Scholar] [CrossRef] [PubMed]
- Sontag, E.D. A Dynamic Model of Immune Responses to Antigen Presentation Predicts Different Regions of Tumor or Pathogen Elimination. Cell Syst. 2017, 22, 231–241.e11. [Google Scholar] [CrossRef] [PubMed]
- Assmus, H.E.; Herwig, R.; Cho, K.-H.; Wolkenhauer, O. Dynamics of biological systems: Role of systems biology in medical research. Expert Rev. Mol. Diagn. 2006, 6, 891–902. [Google Scholar] [CrossRef] [PubMed]
- Lesterhuis, W.J.; Bosco, A.; Millward, M.J.; Small, M.; Nowak, A.K.; Lake, R.A. Dynamic versus static biomarkers in cancer immune checkpoint blockade: Unravelling complexity. Nat. Rev. Drug Discov. 2017, 16, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Giannoula, A.; Gutierrez-Sacristán, A.; Bravo, Á.; Sanz, F.; Furlong, L.I. Identifying temporal patterns in patient disease trajectories using dynamic time warping: A population-based study. Sci. Rep. 2018, 8, 4216. [Google Scholar] [CrossRef] [PubMed]
- Taber, D.J.; Palanisamy, A.P.; Srinivas, T.R.; Titte, R.; Gebregziabher, M.; Odeghe, J.; Chavin, K.D.; Egede, L.E.; Baliga, P.K. Inclusion of dynamic clinical data improves the predictive performance of a 30-day readmission risk model in kidney transplantation. Transplantation 2015, 99, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Christaki, E.; Giamarellos-Bourboulis, E.J. The beginning of personalized medicine in sepsis: Small steps to a bright future. Clin. Genet. 2014, 86, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Tleyjeh, I.M.; Kashour, T.; Mandrekar, J.; Petitti, D.B. Overlooked Shortcomings of Observational Studies of Interventions in Coronavirus Disease 2019: An Illustrated Review for the Clinician. Open Forum Infect. Dis. 2021, 8, ofab317. [Google Scholar] [CrossRef] [PubMed]
- Millard, L.A.C.; Fernández-Sanlés, A.; Carter, A.R.; Hughes, R.A.; Tilling, K.; Morris, T.P.; Major-Smith, D.; Griffith, G.J.; Clayton, G.L.; Kawabata, E.; et al. Exploring the impact of selection bias in observational studies of COVID-19: A simulation study. Int. J. Epidemiol. 2023, 52, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Fung, K.; Jones, M.; Doshi, P. Sources of bias in observational studies of COVID-19 vaccine effectiveness. J. Eval. Clin. Pract. 2024, 30, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Accorsi, E.K.; Qiu, X.; Rumpler, E.; Kennedy-Shaffer, L.; Kahn, R.; Joshi, K.; Goldstein, E.; Stensrud, M.J.; Niehus, R.; Cevik, M.; et al. How to detect and reduce potential sources of biases in studies of SARS-CoV-2 and COVID-19. Eur. J. Epidemiol. 2021, 36, 179–196. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Tang, J.; Tam, W.W.; Mao, C.; Yuan, J.; Di, M.; Yang, Z. Comparing the Overall Result and Interaction in Aggregate Data Meta-Analysis and Individual Patient Data Meta-Analysis. Medicine 2016, 95, e3312. [Google Scholar] [CrossRef] [PubMed]
- Lyman, G.H.; Kuderer, N.M. The strengths and limitations of meta-analyses based on aggregate data. BMC Med. Res. Methodol. 2005, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Mueller, Y.M.; Schrama, T.J.; Ruijten, R.; Schreurs, M.W.; Grashof, D.G.; van de Werken, H.J.; Lasinio, G.J.; Álvarez-Sierra, D.; Kiernan, C.H.; Castro Eiro, M.D.; et al. Stratification of hospitalized COVID-19 patients into clinical severity progression groups by immuno-phenotyping and machine learning. Nat. Commun. 2022, 13, 915. [Google Scholar] [CrossRef] [PubMed]
- Altman, D.G.; Royston, P. What do we mean by validating a prognostic model? Statist. Med. 2000, 19, 453–473. [Google Scholar] [CrossRef]
- Leitner, G.; Blum, S.; Rivas, A.L. Visualizing the indefinable: Three-dimensional complexity of ‘infectious diseases. PLoS ONE 2015, 10, e0123674. [Google Scholar] [CrossRef] [PubMed]
- Lo, A.; Chernoff, H.; Zheng, T.; Lo, S.H. Why significant variables aren’t automatically good predictors. Proc. Natl. Acad. Sci. USA 2015, 112, 13892–13897. [Google Scholar] [CrossRef] [PubMed]
- Anjum, R.L.; Copeland, S.; Rocca, E. Rethinking Causality and Evidence for the Unique Patient; Springer: Berlin/Heidelberg, Germany, 2020; Available online: http://library.oapen.org/handle/20.500.12657/39574 (accessed on 3 March 2024).
- Rivas, A.L.; Leitner, G.; Jankowski, M.D.; Hoogesteijn, A.L.; Iandiorio, M.J.; Chatzipanagiotou, S.; Ioannidis, A.; Blum, S.E.; Piccinini, R.; Antoniades, A.; et al. Nature and consequences of biological reductionism for the immunological study of infectious diseases. Front. Immunol. 2017, 8, 612. [Google Scholar] [CrossRef]
- Agur, Z.; Elishmereni, M.; Foryś, U.; Kogan, Y. Accelerating the Development of Personalized Cancer Immunotherapy by Integrating Molecular Patients’ Profiles with Dynamic Mathematical Models. Clin. Pharmacol. Ther. 2020, 108, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Relecom, A.; Merhi, M.; Inchakalody, V.; Uddin, S.; Rinchai, D.; Bedognetti, D.; Dermime, S. Emerging dynamics pathways of response and resistance to PD-1 and CTLA-4 blockade: Tackling uncertainty by confronting complexity. J. Exp. Clin. Cancer Res. 2021, 40, 74. [Google Scholar] [CrossRef] [PubMed]
- Verma, J.S.; Libertin, C.R.; Gupta, Y.; Khanna, G.; Kumar, R.; Arora, B.S.; Krishna, L.; Fasina, F.O.; Hittner, J.B.; Antoniades, A.; et al. Multi-Cellular Immunological Interactions Associated With COVID-19 Infections. Front. Immunol. 2022, 13, 794006. [Google Scholar] [CrossRef]
- Rivas, A.L.; Jankowski, M.D.; Piccinini, R.; Leitner, G.; Schwarz, D.; Anderson, K.L.; Fair, J.M.; Hoogesteijn, A.L.; Wolter, W.; Chaffer, M.; et al. Feedback-based, system-level properties of vertebrate-microbial interactions. PLoS ONE 2013, 8, e53984. [Google Scholar] [CrossRef] [PubMed]
- Libertin, C.R.; Kempaiah, P.; Gupta, Y.; Fair, J.M.; van Regenmortel, M.H.V.; Antoniades, A.; Rivas, A.L.; Hoogesteijn, A.L. Data structuring may prevent ambiguity and improve personalized medical prognosis. Mol. Aspects Med. 2022, 15, 101142. [Google Scholar] [CrossRef] [PubMed]
- Greiff, V.; Bhat, P.; Cook, S.C.; Menzel, U.; Kang, W.; Reddy, S.T. A bioinformatic framework for immune repertoire diversity profiling enables detection of immunological status. Genome Med. 2015, 7, 49. [Google Scholar] [CrossRef]
- Miho, E.; Yermanos, A.; Weber, C.R.; Berger, C.T.; Reddy, S.T.; Greiff, V. Computational Strategies for Dissecting the High-Dimensional Complexity of Adaptive Immune Repertoires. Front. Immunol. 2018, 9, 224. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.V. The insidious L-shaped distribution. Bull. Psychon. Soc. 1982, 20, 85–88. [Google Scholar] [CrossRef]
- Raab, P.; Hattingen, E.; Franz, K.; Zanella, F.E.; Lanfermann, H. Cerebral gliomas: Diffusional kurtosis imaging analysis of microstructural differences. Radiology 2010, 254, 876–881. [Google Scholar] [CrossRef]
- Hittner, J.B.; Fasina, F.O.; Hoogesteijn, A.L.; Piccinini, R.; Maciorowski, D.; Kempaiah, P.; Smith, S.D.; Rivas, A.L. Testing-related and geo-demographic indicators strongly predict COVID-19 deaths in the United States during March of 2020. Biomed. Environ. Sci. 2021, 34, 734–738. [Google Scholar] [CrossRef]
- Van Cauter, S.; Veraart, J.; Sijbers, J.; Peeters, R.R.; Himmelreich, U.; De Keyzer, F.; Van Gool, S.W.; Van Calenbergh, F.; De Vleeschouwer, S.; Van Hecke, W.; et al. Gliomas: Diffusion kurtosis MR imaging in grading. Radiology 2012, 263, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Houwen, B. The Differential Cell Count. Lab. Hematol. 2001, 7, 89–100. [Google Scholar]
- Hamers, L.; Kox, M.; Pickkers, P. Sepsis-induced immunoparalysis: Mechanisms, markers, and treatment options. Minerva Anestesiol. 2015, 81, 426–439. [Google Scholar] [PubMed]
- Peters van Ton, A.M.; Kox, M.; Abdo, W.F.; Pickkers, P. Precision Immunotherapy for Sepsis. Front. Immunol. 2018, 9, 1926. [Google Scholar] [CrossRef] [PubMed]
- Rivas, A.L.; Hoogesteijn, A.L.; Antoniades, A.; Tomazou, M.; Buranda, T.; Perkins, D.J.; Fair, J.M.; Durvasula, R.; Fasina, F.O.; Tegos, G.P.; et al. Assessing the dynamics and complexity of disease pathogenicity using 4-dimensional immunological data. Front. Immunol. 2019, 10, 1258. [Google Scholar] [CrossRef] [PubMed]
- Chatzipanagiotou, S.; Ioannidis, A.; Trikka-Graphakos, E.; Charalampaki, N.; Sereti, C.; Piccinini, R.; Higgins, A.M.; Buranda, T.; Durvasula, R.; Hoogesteijn, A.L.; et al. Detecting the hidden properties of immunological data and predicting the mortality risks of infectious syndromes. Front. Immunol. 2016, 7, 217. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Venet, F.; Monneret, G. Advances in the understanding and treatment of sepsis-induced immunosuppression. Nat. Rev. Nephrol. 2018, 14, 121–137. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, K.; Yi, Z.; Geng, S.; Li, L. Development of exhausted memory monocytes and underlying mechanisms. Front. Immunol. 2021, 12, 778830. [Google Scholar] [CrossRef] [PubMed]
- Jerne, N.K. The generative grammar of the immune system. EMBO J. 1985, 4, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Satashia, P.H.; Franco, P.M.; Rivas, A.L.; Isha, S.; Hanson, A.; Narra, S.A.; Singh, K.; Jenkins, A.; Bhattacharyya, A.; Guru, P.; et al. From numbers to medical knowledge: Harnessing combinatorial data patterns to predict COVID-19 resource needs and distinguish patient subsets. Front. Med. 2023, 10, 240426. [Google Scholar] [CrossRef] [PubMed]
- Mazzoccoli, G.; Sothern, R.B.; Greco, A.; Pazienza, V.; Vinciguerra, M.; Liu, S.; Cai, Y. Time-related dynamics of variation in core clock gene expression levels in tissues relevant to the immune system. Int. J. Immunopathol. Pharmacol. 2011, 24, 869–879. [Google Scholar] [CrossRef] [PubMed]
- Mazzoccoli, G.; De Cata, A.; Greco, A.; Damato, M.; De Pinto, D.G.; Montrano, M.; Marzulli, N.; Dagostino, M.P.; Carughi, S. Opposing circadian rhythms of CD3+, CD4+ and CD3+, CD8+ lymphocyte subpopulations in healthy humans. Biol. Rhythm. Res. 2011, 42, 111–118. [Google Scholar] [CrossRef]
- Mazzoccoli, G.; Vendemiale, G.; De Cata, A.; Carughi, S.; Tarquini, R. Altered time structure of neuro-endocrine-immune system function in lung cancer patients. BMC Cancer 2010, 10, 314. [Google Scholar] [CrossRef]
- Fricke, G.M.; Letendre, K.A.; Moses, M.E.; Cannon, J.L. Persistence and Adaptation in Immunity: T Cells Balance the Extent and Thoroughness of Search. PLoS Comput. Biol. 2016, 12, e1004818. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, F.R.; Lorenzi, T.; Chaplain, M.A.J. Modelling the Immune Response to Cancer: An Individual-Based Approach Accounting for the Difference in Movement Between Inactive and Activated T Cells. Bull. Math. Biol. 2018, 80, 1539–1562. [Google Scholar] [CrossRef] [PubMed]
- Potthoff, R.F.; Tudor, G.E.; Pieper, K.S.; Hasselblad, V. Can one assess whether missing data are missing at random in medical studies? Stat. Methods Med. Res. 2006, 15, 213–234. [Google Scholar] [CrossRef]
- Lakshmikanth, T.; Muhammad, S.A.; Olin, A.; Chen, Y.; Mikes, J.; Fagerberg, L.; Gummesson, A.; Bergström, G.; Uhlen, M.; Brodin, P. Human Immune System Variation during 1 Year. Cell Rep. 2020, 32, 107923. [Google Scholar] [CrossRef] [PubMed]
- Cain, M.K.; Zhang, Z.; Yuan, K.H. Univariate and multivariate skewness and kurtosis for measuring nonnormality: Prevalence, influence and estimation. Behav. Res. Methods 2017, 49, 1716–1735. [Google Scholar] [CrossRef] [PubMed]
- Grossman, Z. Immunological Paradigms, Mechanisms, and Models: Conceptual Understanding Is a Prerequisite to Effective Modeling. Front. Immunol. 2019, 10, 2522. [Google Scholar] [CrossRef] [PubMed]
- Panda, A.; Chen, S.; Shaw, A.C.; Allore, H.G. Statistical approaches for analyzing immunologic data of repeated observations: A practical guide. J. Immunol. Methods 2013, 398–399, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Grossman, Z.; Greenblatt, C.L.; Cohen, I.R. Parasite immunology and lymphocyte population dynamics. J. Theor Biol. 1986, 121, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Jurema, M.W.; Bracero, N.J.; Garcia, J.E. Fine tuning cycle day 3 hormonal assessment of ovarian reserve improves in vitro fertilization outcome in gonadotropin-releasing hormone antagonist cycles. Fertil. Steril. 2003, 80, 1156–1561. [Google Scholar] [CrossRef] [PubMed]
- Corey, L.; Beyrer, C.; Cohen, M.S.; Michael, N.L.; Bedford, T.; Rolland, M. SARS-CoV-2 Variants in Patients with Immunosuppression. N. Engl. J. Med. 2021, 385, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Thng, Z.X.; De Smet, M.D.; Lee, C.S.; Gupta, V.; Smith, J.R.; McCluskey, P.J.; Thorne, J.E.; Kempen, J.H.; Zierhut, M.; Nguyen, Q.D.; et al. COVID-19 and immunosuppression: A review of current clinical experiences and implications for ophthalmology patients taking immunosuppressive drugs. Br. J. Ophthalmol. 2021, 105, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Goldman, J.D.; Robinson, P.C.; Uldrick, T.S.; Ljungman, P. COVID-19 in immunocompromised populations: Implications for prognosis and repurposing of immunotherapies. J. Immunother. Cancer 2021, 9, e002630. [Google Scholar] [CrossRef] [PubMed]
- Tassone, D.; Thompson, A.; Connell, W.; Lee, T.; Ungaro, R.; An, P.; Ding, Y.; Ding, N.S. Immunosuppression as a risk factor for COVID-19: A meta-analysis. Intern. Med. J. 2021, 51, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Baek, M.S.; Lee, M.-T.; Kim, W.-Y.; Choi, J.C.; Jung, S.-Y. COVID-19-related outcomes in immunocompromised patients: A nationwide study in Korea. PLoS ONE 2021, 16, e0257641. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, J. Researchers Tie Severe Immunosuppression to Chronic COVID-19 and Virus Variants. JAMA 2021, 325, 2033–2035. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.H.J.; Sparks, J.A. Immunosuppression and SARS-CoV-2 breakthrough infections. Lancet Rheumatol. 2022, 4, e379–e380. [Google Scholar] [CrossRef] [PubMed]
- Di Felice, G.; Visci, G.; Teglia, F.; Angelini, M.; Boffetta, P. Effect of cancer on outcome of COVID-19 patients: A systematic review and meta-analysis of studies of unvaccinated patients. eLife 2022, 11, e74634. [Google Scholar] [CrossRef]
- Bilich, T.; Roerden, M.; Maringer, Y.; Nelde, A.; Heitmann, J.S.; Dubbelaar, M.L.; Peter, A.; Hörber, S.; Bauer, J.; Rieth, J.; et al. Preexisting and Post-COVID-19 Immune Responses to SARS-CoV-2 in Patients with Cancer. Cancer Discov. 2021, 11, 1982–1995. [Google Scholar] [CrossRef] [PubMed]
- Primorac, D.; Vrdoljak, K.; Brlek, P.; Pavelič, E.; Molnar, V.; Matišić, V.; Erceg Ivkošić, I.; Parčina, M. Adaptive Immune Responses and Immunity to SARS-CoV-2. Front. Immunol. 2022, 13, 848582. [Google Scholar] [CrossRef] [PubMed]
- Bobcakova, A.; Petriskova, J.; Vysehradsky, R.; Kocan, I.; Kapustova, L.; Barnova, M.; Diamant, Z.; Jesenak, M. Immune Profile in Patients With COVID-19: Lymphocytes Exhaustion Markers in Relationship to Clinical Outcome. Front. Cell. Infect. Microbiol. 2021, 11, 646688. [Google Scholar] [CrossRef] [PubMed]
- Bange, E.M.; Han, N.A.; Wileyto, P.; Kim, J.; Gouma, S.; Robinson, J.; Greenplate, A.R.; Hwee, M.A.; Porterfield, F.; Owoyemi, O.; et al. CD8+ T cells contribute to survival in patients with COVID-19 and hematologic cancer. Nat. Med. 2021, 27, 1280–1289. [Google Scholar] [CrossRef] [PubMed]
- Başcı, S.; Ata, N.; Altuntaş, F.; Yiğenoğlu, T.N.; Dal, M.S.; Korkmaz, S.; Namdaroğlu, S.; Baştürk, A.; Hacıbekiroğlu, T.; Doğu, M.J.; et al. Patients with hematologic cancers are more vulnerable to COVID-19 compared to patients with solid cancers. Intern. Emerg. Med. 2022, 17, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Urbistondo, M.; Gutiérrez-Rojas, A.; Andrés, A.; Gutiérrez, I.; Escudero, G.; García, S.; Gutiérrez, A.; Sánchez, E.; Herráiz, J.; De La Fuente, S.; et al. Severe Lymphopenia as a Predictor of COVID-19 Mortality in Immunosuppressed Patients. J. Clin. Med. 2021, 10, 3595. [Google Scholar] [CrossRef] [PubMed]
- Garbo, R.; Valent, F.; Gigli, G.L.; Valente, M. Pre-Existing Lymphopenia Increases the Risk of Hospitalization and Death after SARS-CoV-2 Infection. Infect. Dis. Rep. 2022, 14, 20–25. [Google Scholar] [CrossRef]
- Giacaman, A.; Henriquez, W.; Tolosa, G.; Prado, A.; Jerez, R.; Reveco, Y.; Martínez, C.; Baumert, C.; Rodríguez, B.; Sanhueza, B.; et al. Hematological abnormalities in immunosuppressed patients with COVID-19: Evidence from a single center. A cross sectional study. Int. Immunopharmacol. 2022, 109, 108862. [Google Scholar] [CrossRef] [PubMed]
- Mara, C.A.; Peugh, J.L. Validity of Data Collected from Randomized Behavioral Clinical Trials During the COVID-19 Pandemic. J. Pediatr. Psychol. 2020, 45, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Rubina, K.; Shmakova, A.; Shabanov, A.; Andreev, Y.; Borovkova, N.; Kulabukhov, V.; Evseev, A.; Popugaev, K.; Petrikov, S.; Semina, E. Novel prognostic determinants of COVID-19-related mortality: A pilot study on severely-ill patients in Russia. PLoS ONE 2022, 17, e0264072. [Google Scholar] [CrossRef] [PubMed]
- Zein, J.G.; Strauss, R.; Attaway, A.H.; Hu, B.; Milinovich, A.; Jawhari, N.; Chamat, S.S.; Ortega, V.E. Eosinophilia Is Associated with Improved COVID-19 Outcomes in Inhaled Corticosteroid-Treated Patients. J. Allergy Clin. Immunol. Pract. 2022, 10, 742–750.e14. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Nie, H.X.; Hu, K.; Wu, X.-J.; Zhang, Y.-T.; Wang, M.-M.; Wang, T.; Zheng, Z.-S.; Li, X.-C.; Zeng, S.-L. Abnormal immunity of non-survivors with COVID-19: Predictors for mortality. Infect. Dis. Poverty 2020, 9, 108. [Google Scholar] [CrossRef] [PubMed]
- Qin, R.; He, L.; Yang, Z.; Jia, N.; Chen, R.; Xie, J.; Fu, W.; Chen, H.; Lin, X.; Huang, R.; et al. Identification of Parameters Representative of Immune Dysfunction in Patients with Severe and Fatal COVID-19 Infection: A Systematic Review and Meta-analysis. Clinic. Rev. Allerg. Immunol. 2022, 64, 33–65. Available online: https://doi-org.libproxy.unm.edu/10.1007/s12016-021-08908-8 (accessed on 3 March 2024). [CrossRef] [PubMed]
- Limon-de la Rosa, N.; Cervantes-Alvarez, E.; Méndez-Guerrero, O.; Gutierrez Gallardo, M.A.; Kershenobich, D.; Navarro-Alvarez, N. Time-Dependent Changes of Laboratory Parameters as Independent Predictors of All-Cause Mortality in COVID-19 Patients. Biology 2022, 11, 580. [Google Scholar] [CrossRef]
- Ingraham, N.E.; Lotfi-Emran, S.; Thielen, B.K.; Techar, K.; Morris, R.S.; Holtan, S.G.; Dudley, R.A.; Tignanelli, C.J. Immunomodulation in COVID-19. Lancet Respir. Med. 2020, 8, 544–546. [Google Scholar] [CrossRef] [PubMed]
- Van Regenmortel, M.H. Reductionism and complexity in molecular biology. Scientists now have the tools to unravel biological and overcome the limitations of reductionism. EMBO Rep. 2004, 5, 1016–1020. [Google Scholar] [CrossRef] [PubMed]
- Fair, J.M.; Rivas, A.L. Systems Biology and ratio-based, real-time disease surveillance. Transb. Emerg. Dis. 2015, 62, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Heudel, P.; Favier, B.; Solodky, M.L.; Assaad, S.; Chaumard, N.; Tredan, O.; Bachelot, T.; Ray-Coquard, I.; Russias, B.; Fournier, M.L.; et al. Survival and risk of COVID-19 after SARS-CoV-2 vaccination in a series of 2391 cancer patients. Eur. J. Cancer 2022, 165, 174–183. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kempaiah, P.; Libertin, C.R.; Chitale, R.A.; Naeyma, I.; Pleqi, V.; Sheele, J.M.; Iandiorio, M.J.; Hoogesteijn, A.L.; Caulfield, T.R.; Rivas, A.L. Decoding Immuno-Competence: A Novel Analysis of Complete Blood Cell Count Data in COVID-19 Outcomes. Biomedicines 2024, 12, 871. https://doi.org/10.3390/biomedicines12040871
Kempaiah P, Libertin CR, Chitale RA, Naeyma I, Pleqi V, Sheele JM, Iandiorio MJ, Hoogesteijn AL, Caulfield TR, Rivas AL. Decoding Immuno-Competence: A Novel Analysis of Complete Blood Cell Count Data in COVID-19 Outcomes. Biomedicines. 2024; 12(4):871. https://doi.org/10.3390/biomedicines12040871
Chicago/Turabian StyleKempaiah, Prakasha, Claudia R. Libertin, Rohit A. Chitale, Islam Naeyma, Vasili Pleqi, Johnathan M. Sheele, Michelle J. Iandiorio, Almira L. Hoogesteijn, Thomas R. Caulfield, and Ariel L. Rivas. 2024. "Decoding Immuno-Competence: A Novel Analysis of Complete Blood Cell Count Data in COVID-19 Outcomes" Biomedicines 12, no. 4: 871. https://doi.org/10.3390/biomedicines12040871
APA StyleKempaiah, P., Libertin, C. R., Chitale, R. A., Naeyma, I., Pleqi, V., Sheele, J. M., Iandiorio, M. J., Hoogesteijn, A. L., Caulfield, T. R., & Rivas, A. L. (2024). Decoding Immuno-Competence: A Novel Analysis of Complete Blood Cell Count Data in COVID-19 Outcomes. Biomedicines, 12(4), 871. https://doi.org/10.3390/biomedicines12040871







