External Support of Autologous Internal Jugular Vein Grafts with FRAME Mesh in a Porcine Carotid Artery Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Surgery
2.1.1. Implantation
2.1.2. Flowmetry
2.1.3. Angiography
2.1.4. Explantation
2.2. Macroscopic Examinations
2.3. Microscopical Examinations
2.3.1. Histology and Immunohistochemistry
2.3.2. Macro Photography
2.3.3. Confocal Microscopy
2.3.4. Scanning Electron Microscopy (SEM)
2.4. Statistical Analysis
3. Results
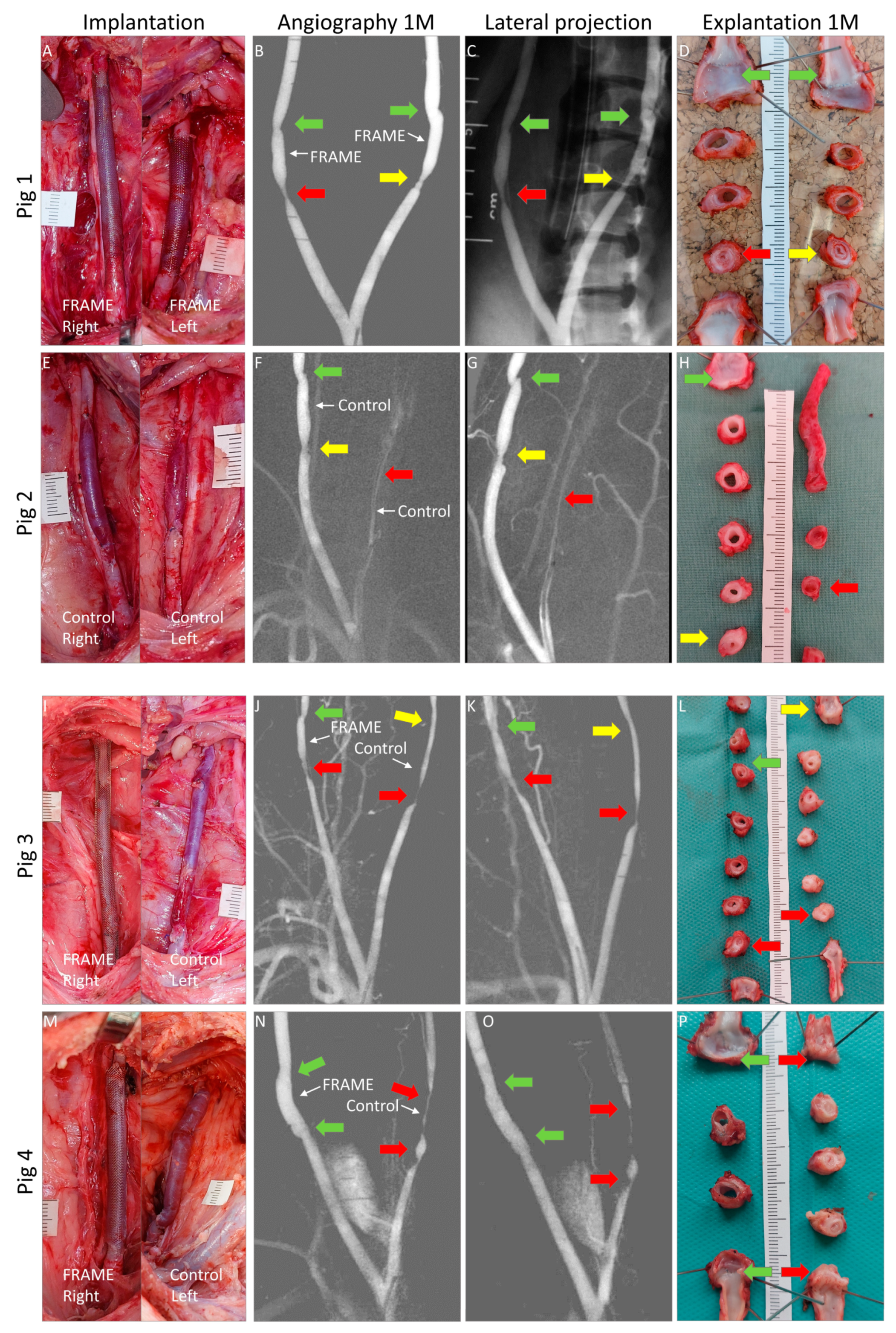
3.1. Surgery
3.1.1. Implantation
3.1.2. Flowmetry
3.1.3. Angiography
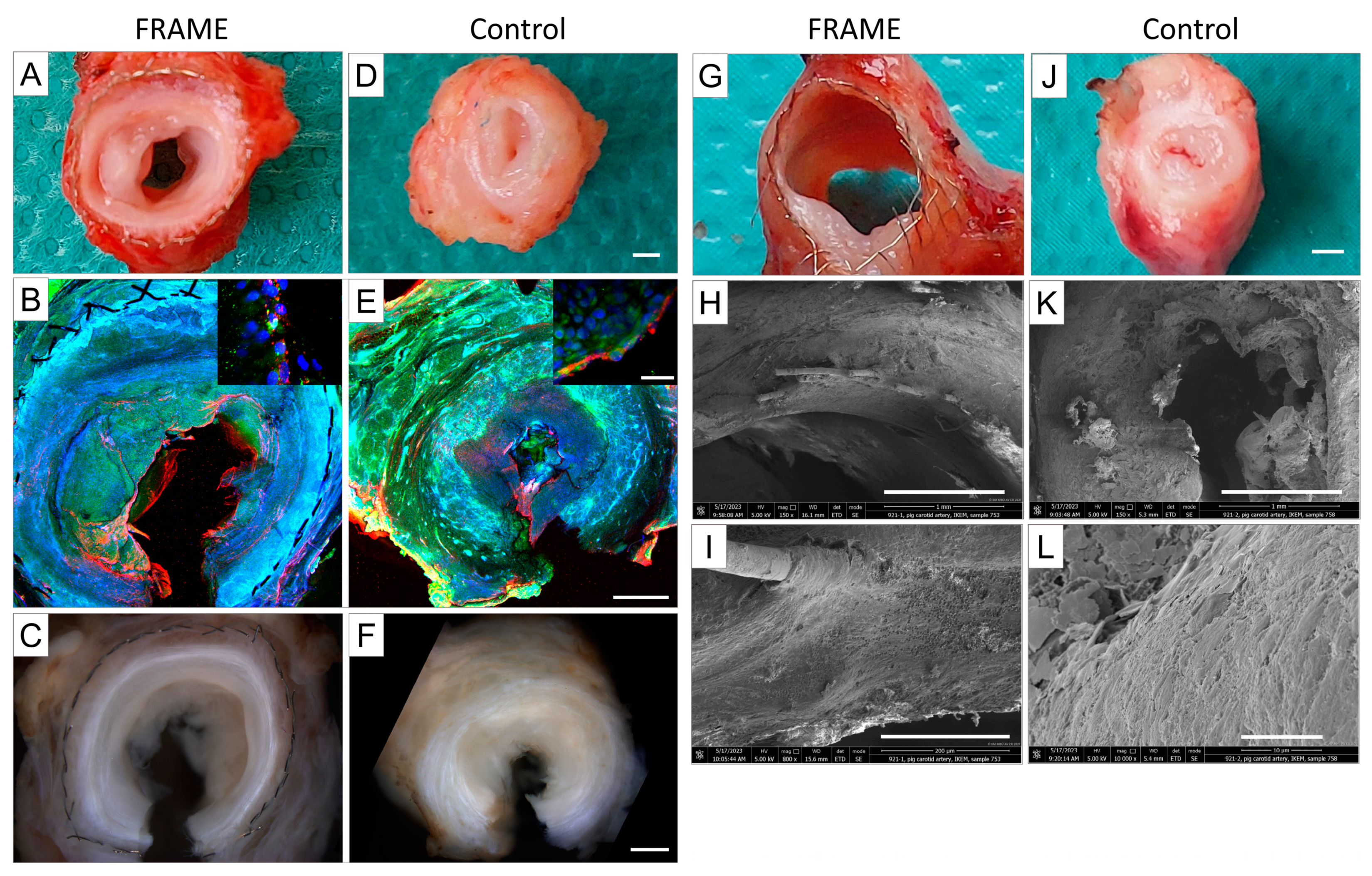
3.1.4. Explantation and Macroscopical Examinations
3.2. Microscopical Examinations
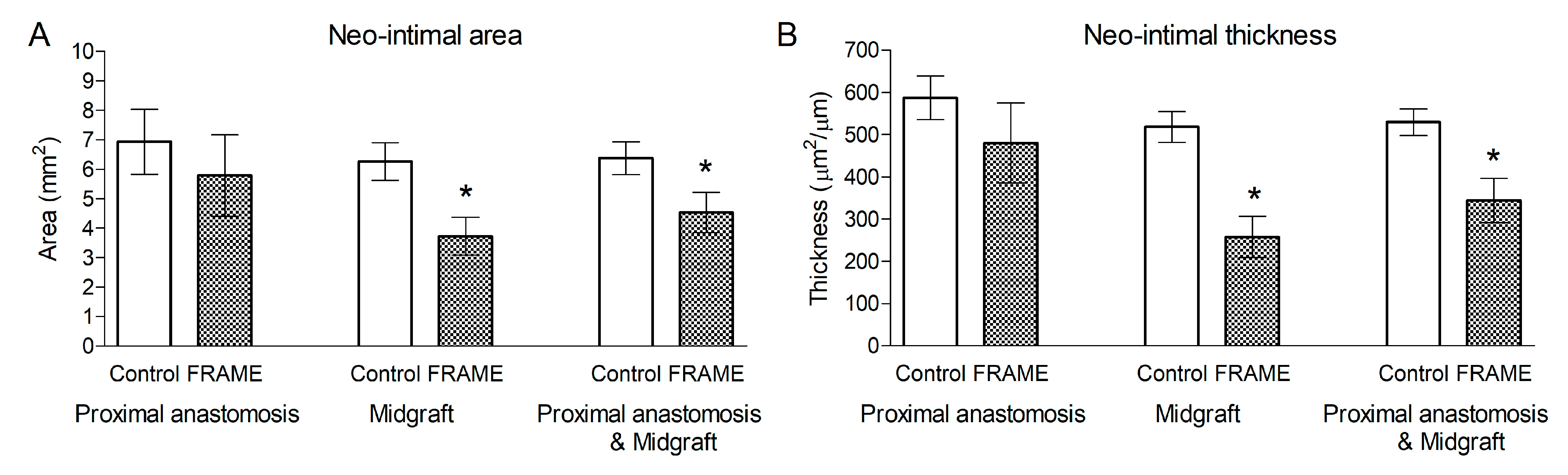
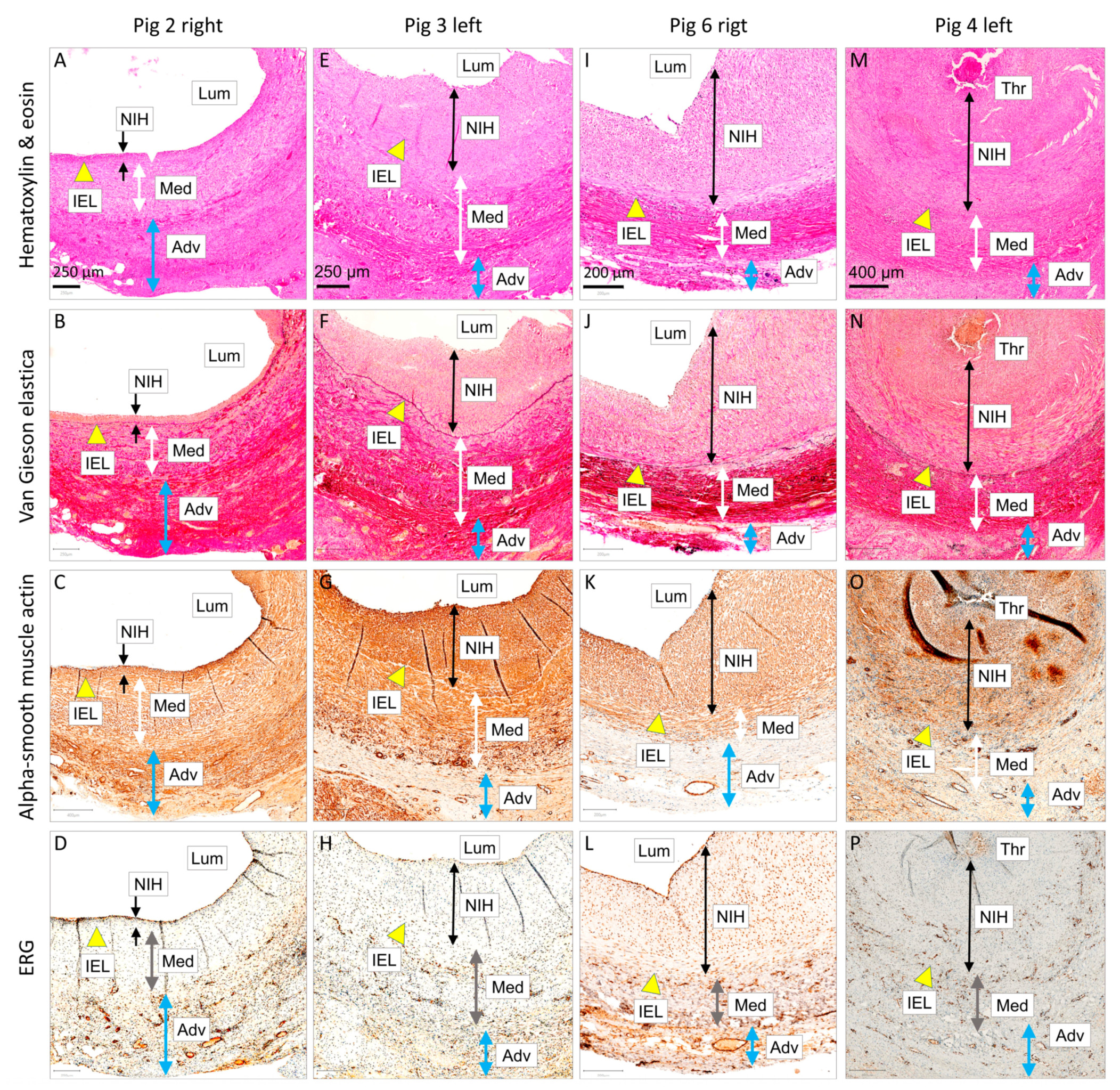
3.2.1. Histology and Immunohistochemistry
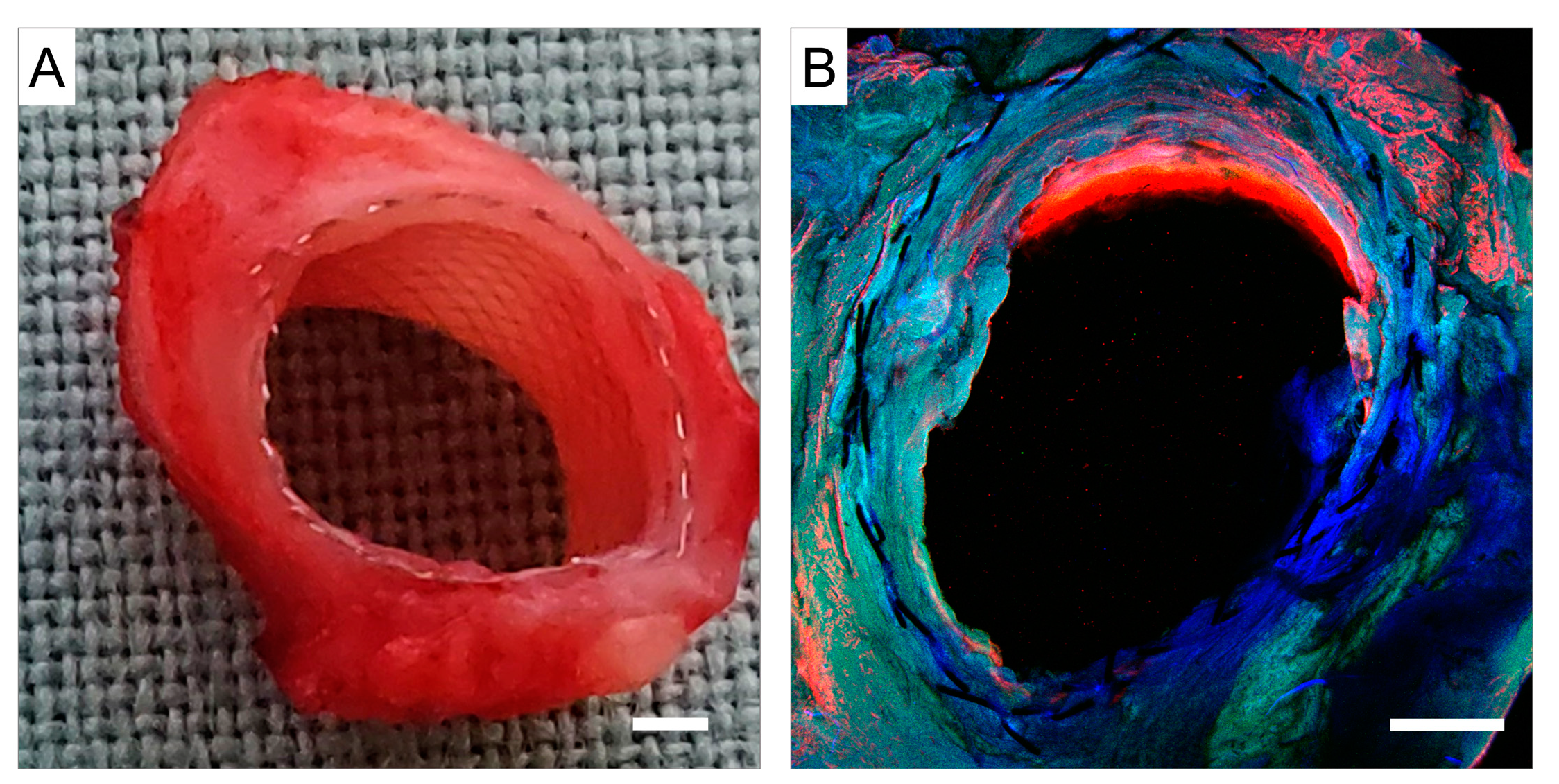
3.2.2. Confocal Microscopy and Scanning Electron Microscopy (SEM)
4. Discussion
4.1. Animal Model
4.2. Anastomosis Considerations
4.3. Existing Preclinical Data
4.4. Existing Clinical Data
4.4.1. Coronary Artery Bypass Grafting
4.4.2. Peripheral Vascular Surgery
4.4.3. Arterio-Venous Fistulas (AVFs) for Hemodialysis Access
4.5. Limitations
4.6. Clinical Implications and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Vries, M.R.; Simons, K.H.; Jukema, J.W.; Braun, J.; Quax, P.H. Vein graft failure: From pathophysiology to clinical outcomes. Nat. Rev. Cardiol. 2016, 13, 451–470. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, B.; Reyes-Hernández, C.G.; Quiroga-Garza, A.; Rodríguez-Rodríguez, V.E.; Esparza-Hernández, C.N.; Elizondo-Omaña, R.E.; Guzmán-López, S. Conduits Used in Coronary Artery Bypass Grafting: A Review of Morphological Studies. Ann. Thorac. Cardiovasc. Surg. 2017, 23, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.C. Animal models for studying vein graft failure and therapeutic interventions. Curr. Opin. Pharmacol. 2012, 12, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Guida, G.; Ward, A.O.; Bruno, V.D.; George, S.J.; Caputo, M.; Angelini, G.D.; Zakkar, M. Saphenous vein graft disease, pathophysiology, prevention, and treatment. A review of the literature. J. Card. Surg. 2020, 35, 1314–1321. [Google Scholar] [CrossRef] [PubMed]
- Samano, N.; Geijer, H.; Liden, M.; Fremes, S.; Bodin, L.; Souza, D. The no-touch saphenous vein for coronary artery bypass grafting maintains a patency, after 16 years, comparable to the left internal thoracic artery: A randomized trial. J. Thorac. Cardiovasc. Surg. 2015, 150, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Weltert, L.P.; Wolf, L.G.; Garufi, L.; Scaffa, R.; Salica, A.; Ricci, A.; Irace, F.G.; Fusca, S.; D’Aleo, S.; Chirichilli, I.; et al. External Stents for Vein Grafts in Coronary Artery Bypass Grafting: Targeting Intimal Hyperplasia. Surg. Technol. Int. 2020, 35, 197–201. [Google Scholar] [PubMed]
- Nitecki, S.; Yosef, L.; Tozzi, M.; Shofti, R. Inhibition of vein graft remodeling and neo-intimal formation using a cobalt chrome external support. Arch. Clin. Exp. Surg. 2018, 7, 108–115. [Google Scholar] [CrossRef]
- Fashina, O.; Abbasciano, R.G.; McQueen, L.W.; Ladak, S.; George, S.J.; Suleiman, S.; Punjabi, P.P.; Angelini, G.D.; Zakkar, M. Large animal model of vein grafts intimal hyperplasia: A systematic review. Perfusion 2023, 38, 894–930. [Google Scholar] [CrossRef] [PubMed]
- Gemelli, M.; Gallo, M.; Addonizio, M.; Pahwa, S.; Van den Eynde, J.; Trivedi, J.; Slaughter, M.S.; Gerosa, G. Venous External Support in Coronary Artery Bypass Surgery: A Systematic Review and Meta-Analysis. Curr. Probl. Cardiol. 2023, 48, 101687. [Google Scholar] [CrossRef]
- Emmert, M.Y.; Bonatti, J.; Caliskan, E.; Gaudino, M.; Grabenwöger, M.; Grapow, M.T.; Heinisch, P.P.; Kieser-Prieur, T.; Kim, K.-B.; Kiss, A.; et al. Consensus statement—Graft treatment in cardiovascular bypass graft surgery. Front. Cardiovasc. Med. 2024, 11, 1285685. [Google Scholar] [CrossRef]
- Hu, J.; Wan, S. External support in preventing vein graft failure. Asian Cardiovasc. Thorac. Ann. 2012, 20, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.J. Device profile of the VEST for external support of SVG Coronary artery bypass grafting: Historical development, current status, and future directions. Expert Rev. Med. Devices 2021, 18, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Soletti, G.J.; Dell’Aquila, M.; Harik, L.; Cancelli, G.; Alzghari, T.; Perezgrovas-Olaria, R.; Dimagli, A.; An, K.R.; Leith, J.; Rossi, C.S.; et al. The VEST External Support for Saphenous Vein Grafts in Coronary Surgery: A Review of Randomized Clinical Trials. J. Cardiovasc. Dev. Dis. 2023, 10, 453. [Google Scholar] [CrossRef] [PubMed]
- Vigliotti, R.C.; Montelione, N.; Franceschi, F.; Franceschini, E.; Zardi, E.; Spinelli, F.; Stilo, F. Externally Supported Extra-anatomical Venous Bypass to Treat Upper Limb Ischemia with Shoulder Prosthetic Infection. Ann. Vasc. Surg. 2020, 69, 453.e5–453.e10. [Google Scholar] [CrossRef] [PubMed]
- Ciftci, Ü.; Marti, R.; Fahrni, J.; Gähwiler, R.; Thalhammer, C.; Gürke, L.; Isaak, A. External stenting and disease progression in vein grafts 1 year after open surgical repair of popliteal artery aneurysm. J. Vasc. Surg. 2021, 74, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Chlupac, J.; Matejka, R.; Konarik, M.; Novotny, R.; Simunkova, Z.; Mrazova, I.; Fabian, O.; Zapletal, M.; Pulda, Z.; Lipensky, J.F.; et al. Vascular Remodeling of Clinically Used Patches and Decellularized Pericardial Matrices Recellularized with Autologous or Allogeneic Cells in a Porcine Carotid Artery Model. Int. J. Mol. Sci. 2022, 23, 3310. [Google Scholar] [CrossRef] [PubMed]
- Góes, A.M.O.; Chaves, R.H.F.; Furlaneto, I.P.; Rodrigues, E.M.; de Albuquerque, F.B.A.; Smit, J.H.A.; de Oliveira, C.P.; Abib, S.C.V. Comparative angiotomographic study of swine vascular anatomy: Contributions to research and training models in vascular and endovascular surgery. J. Vasc. Bras. 2021, 20, e20200086. [Google Scholar] [CrossRef] [PubMed]
- FRAME TM. External Support for Peripheral Vascular Reconstructions. Available online: https://www.cardion.cz/file/1466/lb461-rev02-frame-product-page.pdf (accessed on 27 July 2023).
- Mrowczynski, W.; Mugnai, D.; de Valence, S.; Tille, J.C.; Khabiri, E.; Cikirikcioglu, M.; Moller, M.; Walpoth, B.H. Porcine carotid artery replacement with biodegradable electrospun poly-e-caprolactone vascular prosthesis. J. Vasc. Surg. 2014, 59, 210–219. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef]
- Angelini, G.D.; Lloyd, C.; Bush, R.; Johnson, J.; Newby, A.C. An external, oversized, porous polyester stent reduces vein graft neointima formation, cholesterol concentration, and vascular cell adhesion molecule 1 expression in cholesterol-fed pigs. J. Thorac. Cardiovasc. Surg. 2002, 124, 950–956. [Google Scholar] [CrossRef]
- Moodley, L.; Franz, T.; Human, P.; Wolf, M.F.; Bezuidenhout, D.; Scherman, J.; Zilla, P. Protective constriction of coronary vein grafts with knitted nitinol. Eur. J. Cardio Thorac. Surg. 2013, 44, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Rippstein, P.; Black, M.K.; Boivin, M.; Veinot, J.P.; Ma, X.; Chen, Y.X.; Human, P.; Zilla, P.; O’Brien, E.R. Comparison of processing and sectioning methodologies for arteries containing metallic stents. J. Histochem. Cytochem. 2006, 54, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Honetschlägerová, Z.; Husková, Z.; Kikerlová, S.; Sadowski, J.; Kompanowska-Jezierska, E.; Táborský, M.; Vaňourková, Z.; Kujal, P.; Červenka, L. Renal sympathetic denervation improves pressure-natriuresis relationship in cardiorenal syndrome: Insight from studies with Ren-2 transgenic hypertensive rats with volume overload induced using aorto-caval fistula. Hypertens. Res. 2024, 47, 998–1016. [Google Scholar] [CrossRef] [PubMed]
- Kolesová, H.; Bartoš, M.; Hsieh, W.C.; Olejníčková, V.; Sedmera, D. Novel approaches to study coronary vasculature development in mice. Dev. Dyn. 2018, 247, 1018–1027. [Google Scholar] [CrossRef] [PubMed]
- Kolesová, H.; Čapek, M.; Radochová, B.; Janáček, J.; Sedmera, D. Comparison of different tissue clearing methods and 3D imaging techniques for visualization of GFP-expressing mouse embryos and embryonic hearts. Histochem. Cell Biol. 2016, 146, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Sochman, J.; Peregrin, J.H.; Pavcnik, D.; Uchida, B.T.; Timmermans, H.A.; Sedmera, D.; Benada, O.; Kofronova, O.; Keller, F.S.; Rosch, J. Reverse endoventricular artificial obturator in tricuspid valve position. Experimental feasibility research study. Physiol. Res. 2014, 63, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Zilla, P.; Moodley, L.; Scherman, J.; Krynauw, H.; Kortsmit, J.; Human, P.; Wolf, M.F.; Franz, T. Remodeling leads to distinctly more intimal hyperplasia in coronary than in infrainguinal vein grafts. J. Vasc. Surg. 2012, 55, 1734–1741. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.J.; Wilson, J.M.; Muller, D.W. Adenovirus-mediated gene transfer of soluble vascular cell adhesion molecule to porcine interposition vein grafts. Circulation 1994, 89, 1922–1928. [Google Scholar] [CrossRef] [PubMed]
- Bartels, C.; Erasmi, A.; Sayk, F.; Eggers, R.; Dendorfer, A.; Feyerabend, T.; Eichler, W.; Sievers, H.H. Prophylactic gamma radiation of unaffected vein grafts failed to prevent vein graft disease in a chronic hypercholesterolemic porcine model. Eur. J. Cardiothorac. Surg. 2003, 24, 92–97. [Google Scholar] [CrossRef]
- Jevon, M.; Ansari, T.I.; Finch, J.; Zakkar, M.; Evans, P.C.; Shurey, S.; Sibbons, P.D.; Hornick, P.; Haskard, D.O.; Dorling, A. Smooth muscle cells in porcine vein graft intimal hyperplasia are derived from the local vessel wall. Cardiovasc. Pathol. 2011, 20, e91–e94. [Google Scholar] [CrossRef]
- Quint, C.; Kondo, Y.; Manson, R.J.; Lawson, J.H.; Dardik, A.; Niklason, L.E. Decellularized tissue-engineered blood vessel as an arterial conduit. Proc. Natl. Acad. Sci. USA 2011, 108, 9214–9219. [Google Scholar] [CrossRef] [PubMed]
- Thim, T.; Hagensen, M.K.; Hørlyck, A.; Drouet, L.; Paaske, W.P.; Bøtker, H.E.; Falk, E. Oversized vein grafts develop advanced atherosclerosis in hypercholesterolemic minipigs. BMC Cardiovasc. Disord. 2012, 12, 24. [Google Scholar] [CrossRef]
- Kibbe, M.R.; Tzeng, E.; Gleixner, S.L.; Watkins, S.C.; Kovesdi, I.; Lizonova, A.; Makaroun, M.S.; Billiar, T.R.; Rhee, R.Y. Adenovirus-mediated gene transfer of human inducible nitric oxide synthase in porcine vein grafts inhibits intimal hyperplasia. J. Vasc. Surg. 2001, 34, 156–165. [Google Scholar] [CrossRef] [PubMed]
- El-Kurdi, M.S.; Hong, Y.; Stankus, J.J.; Soletti, L.; Wagner, W.R.; Vorp, D.A. Transient elastic support for vein grafts using a constricting microfibrillar polymer wrap. Biomaterials 2008, 29, 3213–3220. [Google Scholar] [CrossRef]
- Angelini, G.D.; Bryan, A.J.; Williams, H.M.; Soyombo, A.A.; Williams, A.; Tovey, J.; Newby, A.C. Time-course of medial and intimal thickening in pig venous arterial grafts: Relationship to endothelial injury and cholesterol accumulation. J. Thorac. Cardiovasc. Surg. 1992, 103, 1093–1103. [Google Scholar] [CrossRef]
- Angelini, G.D.; Bryan, A.J.; Williams, H.M.; Morgan, R.; Newby, A.C. Distention promotes platelet and leukocyte adhesion and reduces short-term patency in pig arteriovenous bypass grafts. J. Thorac. Cardiovasc. Surg. 1990, 99, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Angelini, G.D.; Izzat, M.B.; Bryan, A.J.; Newby, A.C. External stenting reduces early medial and neointimal thickening in a pig model of arteriovenous bypass grafting. J. Thorac. Cardiovasc. Surg. 1996, 112, 79–84. [Google Scholar] [CrossRef]
- Isaji, T.; Hashimoto, T.; Yamamoto, K.; Santana, J.M.; Yatsula, B.; Hu, H.; Bai, H.; Jianming, G.; Kudze, T.; Nishibe, T.; et al. Improving the Outcome of Vein Grafts: Should Vascular Surgeons Turn Veins into Arteries? Ann. Vasc. Dis. 2017, 10, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Conte, M.S.; Owens, C.D.; Belkin, M.; Creager, M.A.; Edwards, K.L.; Gasper, W.J.; Kenagy, R.D.; LeBoeuf, R.C.; Sobel, M.; Clowes, A. A single nucleotide polymorphism in the p27(Kip1) gene is associated with primary patency of lower extremity vein bypass grafts. J. Vasc. Surg. 2013, 57, 1179–1185.E2. [Google Scholar] [CrossRef]
- Ramachandra, A.B.; Wang, H.; Wnorowski, A.; Schwarz, E.L.; Pickering, J.; Heiler, J.C.; Lucian, H.J.; Hironaka, C.E.; Tran, N.A.; Liu, Y.; et al. Biodegradable external wrapping promotes favorable adaptation in an ovine vein graft model. Acta Biomater. 2022, 151, 414–425. [Google Scholar] [CrossRef]
- Zilla, P.; Human, P.; Wolf, M.; Lichtenberg, W.; Rafiee, N.; Bezuidenhout, D.; Samodien, N.; Schmidt, C.; Franz, T. Constrictive external nitinol meshes inhibit vein graft intimal hyperplasia in nonhuman primates. J. Thorac. Cardiovasc. Surg. 2008, 136, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Zilla, P.; Moodley, L.; Wolf, M.F.; Bezuidenhout, D.; Sirry, M.S.; Rafiee, N.; Lichtenberg, W.; Black, M.; Franz, T. Knitted nitinol represents a new generation of constrictive external vein graft meshes. J. Vasc. Surg. 2011, 54, 1439–1450. [Google Scholar] [CrossRef] [PubMed]
- Parsonnet, V.; Lari, A.A.; Shah, I.H. New Stent For Support of Veins in Arterial GRAFTS. Arch. Surg. 1963, 87, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Batellier, J.; Wassef, M.; Merval, R.; Duriez, M.; Tedgui, A. Protection from atherosclerosis in vein grafts by a rigid external support. Arterioscler. Thromb. 1993, 13, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Izzat, M.B.; Mehta, D.; Bryan, A.J.; Reeves, B.; Newby, A.C.; Angelini, G.D. Influence of external stent size on early medial and neointimal thickening in a pig model of saphenous vein bypass grafting. Circulation 1996, 94, 1741–1745. [Google Scholar] [CrossRef]
- Jeremy, J.Y.; Dashwood, M.R.; Mehta, D.; Izzat, M.B.; Shukla, N.; Angelini, G.D. Nitric oxide, prostacyclin and cyclic nucleotide formation in externally stented porcine vein grafts. Atherosclerosis 1998, 141, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Mehta, D.; George, S.J.; Jeremy, J.Y.; Izzat, M.B.; Southgate, K.M.; Bryan, A.J.; Newby, A.C.; Angelini, G.D. External stenting reduces long-term medial and neointimal thickening and platelet derived growth factor expression in a pig model of arteriovenous bypass grafting. Nat. Med. 1998, 4, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Caro, C.; Jeremy, J.; Watkins, N.; Bulbulia, R.; Angelini, G.; Smith, F.; Wan, S.; Yim, A.; Sherwin, S.; Peiró, J.; et al. The geometry of unstented and stented pig common carotid artery bypass grafts. Biorheology 2002, 39, 507–512. [Google Scholar] [PubMed]
- Jeremy, J.Y.; Bulbulia, R.; Johnson, J.L.; Gadsdon, P.; Vijayan, V.; Shukla, N.; Smith, F.C.; Angelini, G.D. A bioabsorbable (polyglactin), nonrestrictive, external sheath inhibits porcine saphenous vein graft thickening. J. Thorac. Cardiovasc. Surg. 2004, 127, 1766–1772. [Google Scholar] [CrossRef]
- Vijayan, V.; Shukla, N.; Johnson, J.L.; Gadsdon, P.; Angelini, G.D.; Smith, F.C.; Baird, R.; Jeremy, J.Y. Long-term reduction of medial and intimal thickening in porcine saphenous vein grafts with a polyglactin biodegradable external sheath. J. Vasc. Surg. 2004, 40, 1011–1019. [Google Scholar] [CrossRef]
- Human, P.; Franz, T.; Scherman, J.; Moodley, L.; Zilla, P. Dimensional analysis of human saphenous vein grafts: Implications for external mesh support. J. Thorac. Cardiovasc. Surg. 2009, 137, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Zilla, P.; Wolf, M.; Rafiee, N.; Moodley, L.; Bezuidenhout, D.; Black, M.; Human, P.; Franz, T. Utilization of shape memory in external vein-graft meshes allows extreme diameter constriction for suppressing intimal hyperplasia: A non-human primate study. J. Vasc. Surg. 2009, 49, 1532–1542. [Google Scholar] [CrossRef] [PubMed]
- Franz, T.; Human, P.; Dobner, S.; Reddy, B.D.; Black, M.; Ilsley, H.; Wolf, M.F.; Bezuidenhout, D.; Moodley, L.; Zilla, P. Tailored sizes of constrictive external vein meshes for coronary artery bypass surgery. Biomaterials 2010, 31, 9301–9309. [Google Scholar] [CrossRef] [PubMed]
- Zilla, P.; Bezuidenhout, D.; Human, P. Prosthetic vascular grafts: Wrong models, wrong questions and no healing. Biomaterials 2007, 28, 5009–5027. [Google Scholar] [CrossRef] [PubMed]
- Ben-Gal, Y.; Taggart, D.P.; Williams, M.R.; Orion, E.; Uretzky, G.; Shofti, R.; Banai, S.; Yosef, L.; Bolotin, G. Expandable external support device to improve Saphenous Vein Graft Patency after CABG. J. Cardiothorac. Surg. 2013, 8, 122. [Google Scholar] [CrossRef] [PubMed]
- Murphy, G.J.; Newby, A.C.; Jeremy, J.Y.; Baumbach, A.; Angelini, G.D. A randomized trial of an external Dacron sheath for the prevention of vein graft disease: The Extent study. J. Thorac. Cardiovasc. Surg. 2007, 134, 504–505. [Google Scholar] [CrossRef] [PubMed]
- Emery, R.W.; Solien, E.; Klima, U. Clinical Evaluation of the eSVS Mesh: First-In-Man Trial Outcomes. ASAIO J. 2015, 61, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Inderbitzin, D.T.; Bremerich, J.; Matt, P.; Grapow, M.T.; Eckstein, F.S.; Reuthebuch, O. One-year patency control and risk analysis of eSVS®-mesh-supported coronary saphenous vein grafts. J. Cardiothorac. Surg. 2015, 10, 108. [Google Scholar] [CrossRef]
- Taggart, D.P.; Ben Gal, Y.; Lees, B.; Patel, N.; Webb, C.; Rehman, S.M.; Desouza, A.; Yadav, R.; De Robertis, F.; Dalby, M.; et al. A Randomized Trial of External Stenting for Saphenous Vein Grafts in Coronary Artery Bypass Grafting. Ann. Thorac. Surg. 2015, 99, 2039–2045. [Google Scholar] [CrossRef]
- Taggart, D.P.; Gavrilov, Y.; Krasopoulos, G.; Rajakaruna, C.; Zacharias, J.; De Silva, R.; Channon, K.M.; Gehrig, T.; Donovan, T.J.; Friedrich, I.; et al. External stenting and disease progression in saphenous vein grafts two years after coronary artery bypass grafting: A multicenter randomized trial. J. Thorac. Cardiovasc. Surg. 2022, 164, 1532–1541.e2. [Google Scholar] [CrossRef]
- Goldstein, D.J.; Puskas, J.D.; Alexander, J.H.; Chang, H.L.; Gammie, J.S.; Marks, M.E.; Iribarne, A.; Vengrenyuk, Y.; Raymond, S.; Taylor, B.S.; et al. External Support for Saphenous Vein Grafts in Coronary Artery Bypass Surgery: A Randomized Clinical Trial. JAMA Cardiol. 2022, 7, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Taggart, D.P.; Webb, C.M.; Desouza, A.; Yadav, R.; Channon, K.M.; De Robertis, F.; Di Mario, C. Long-term performance of an external stent for saphenous vein grafts: The VEST IV trial. J. Cardiothorac. Surg. 2018, 13, 117. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, Z.; Si, K.; Wu, X.; Ni, H.; Tang, Y.; Liu, W.; Wang, Z. External stenting for saphenous vein grafts in coronary artery bypass grafting: A meta-analysis. Eur. J. Clin. Investig. 2023, 53, e14046. [Google Scholar] [CrossRef]
- Soletti, G.J.; Dimagli, A.; Harik, L.; Cancelli, G.; Perezgrovas-Olaria, R.; Alzghari, T.; Dell’Aquila, M.; Leith, J.; Castagnini, S.; Lau, C.; et al. External Stenting for Saphenous Vein Grafts in Coronary Surgery: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 7395. [Google Scholar] [CrossRef] [PubMed]
- Arvela, E.; Kauhanen, P.; Albäck, A.; Lepäntalo, M.; Neufang, A.; Adili, F.; Schmitz-Rixen, T. Initial Experience with a New Method of External Polyester Scaffolding for Infrainguinal Vein Grafts. Eur. J. Vasc. Endovasc. Surg. 2009, 38, 456–462. [Google Scholar] [CrossRef]
- Carella, G.S.; Stilo, F.; Benedetto, F.; David, A.; Risitano, D.C.; Buemi, M.; Spinelli, F. Femoro-Distal Bypass with Varicose Veins Covered by Prosthetic Mesh. J. Surg. Res. 2011, 168, e189–e194. [Google Scholar] [CrossRef]
- Berard, X.; Brizzi, V.; Mayeux, S.; Sassoust, G.; Biscay, D.; Ducasse, E.; Bordenave, L.; Corpataux, J.M.; Midy, D. Salvage Treatment for Venous Aneurysm Complicating Vascular Access Arteriovenous Fistula: Use of an Exoprosthesis to Reinforce the Vein after Aneurysmorrhaphy. Eur. J. Vasc. Endovasc. Surg. 2010, 40, 100–106. [Google Scholar] [CrossRef]
- Rokošný, S.; Baláž, P.; Wohlfahrt, P.; Palouš, D.; Janoušek, L. Reinforced Aneurysmorrhaphy for True Aneurysmal Haemodialysis Vascular Access. Eur. J. Vasc. Endovasc. Surg. 2014, 47, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Chemla, E.; Velazquez, C.C.; D’Abate, F.; Ramachandran, V.; Maytham, G. Arteriovenous fistula construction with the VasQ™ external support device: A pilot study. J. Vasc. Access 2016, 17, 243–248. [Google Scholar] [CrossRef]
- Matoussevitch, V.; Kalmykov, E.; Shahverdyan, R. Novel external stenting for reconstruction of high flow arteriovenous fistula. J. Vasc. Access 2022, 23, 864–870. [Google Scholar] [CrossRef]
- Vaes, R.H.; Wouda, R.; van Loon, M.; van Hoek, F.; Tordoir, J.H.; Scheltinga, M.R. Effectiveness of surgical banding for high flow in brachial artery-based hemodialysis vascular access. J. Vasc. Surg. 2015, 61, 762–766. [Google Scholar] [CrossRef] [PubMed]
- Kuemmerli, C.; Habrina, D.; Puchner, S.; Laminger, F.; Werzowa, J.; Roka, S. Primary External Stenting of an Autogenous Brachial-Basilic Upper Arm Transposition. Ann. Vasc. Surg. 2020, 65, 288.e1–288.e4. [Google Scholar] [CrossRef] [PubMed]
- Dashwood, M.R.; Loesch, A. The saphenous vein as a bypass conduit: The potential role of vascular nerves in graft performance. Curr. Vasc. Pharmacol. 2009, 7, 47–57. [Google Scholar] [CrossRef]
- Cooley, B.C. Murine Model of Neointimal Formation and Stenosis in Vein Grafts. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1180–1185. [Google Scholar] [CrossRef]
- Zwolak, R.M.; Adams, M.C.; Clowes, A.W. Kinetics of vein graft hyperplasia: Association with tangential stress. J. Vasc. Surg. 1987, 5, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.P.; Nili, N.; Jackson, Z.S.; Qiang, B.; Leong-Poi, H.; Jaffe, R.; Raanani, E.; Connelly, P.W.; Sparkes, J.D.; Strauss, B.H. Expansive remodeling in venous bypass grafts: Novel implications for vein graft disease. Atherosclerosis 2008, 196, 580–589. [Google Scholar] [CrossRef]
- Tian, X.D.; Zhou, N.K.; Li, B.J.; Xiao, C.S.; Liu, X.; Liang, C.Y.; Zhang, T.; Gao, C.Q. Effects and mechanisms of non-restrictive external stent for prevention of vein graft restenosis in a rabbit model. Chin. Med. J. 2010, 123, 2400–2404. [Google Scholar] [PubMed]
- You, Q.; Duan, L.; Wang, F.; Du, X.; Xiao, M. Characterization of the inhibition of vein graft intimal hyperplasia by a biodegradable vascular stent. Cell Biochem. Biophys. 2011, 59, 99–107. [Google Scholar] [CrossRef]
- Karayannacos, P.E.; Hostetler, J.R.; Bond, M.G.; Kakos, G.S.; Williams, R.A.; Kilman, J.W.; Vasko, J.S. Late failure in vein grafts: Mediating factors in subendothelial fibromuscular hyperplasia. Ann. Surg. 1978, 187, 183–188. [Google Scholar] [CrossRef]
- Barra, J.A.; Volant, A.; Leroy, J.P.; Braesco, J.; Airiau, J.; Boschat, J.; Blanc, J.J.; Penther, P. Constrictive perivenous mesh prosthesis for preservation of vein integrity. Experimental results and application for coronary bypass grafting. J. Thorac. Cardiovasc. Surg. 1986, 92, 330–336. [Google Scholar] [CrossRef]
- Goldstein, R.L.; McCormack, M.C.; Mallidi, S.; Runyan, G.; Randolph, M.A.; Austen, W.G., Jr.; Redmond, R.W. Photochemical Tissue Passivation of Arteriovenous Grafts Prevents Long-Term Development of Intimal Hyperplasia in a Swine Model. J. Surg. Res. 2020, 253, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Nantsios, A.; Vo, T.X.; Ruel, M. Commentary: External stenting of saphenous vein grafts-reinVESTing to achieve best returns in coronary artery bypass grafting. J. Thorac. Cardiovasc. Surg. 2022, 164, 1542–1543. [Google Scholar] [CrossRef] [PubMed]
- Zwischenberger, B.A.; Gaudino, M. Commentary: A device solution for the saphenous vein graft’s infamous foible? J. Thorac. Cardiovasc. Surg. 2022, 164, 1543–1545. [Google Scholar] [CrossRef] [PubMed]
- Conte, M.S.; Bandyk, D.F.; Clowes, A.W.; Moneta, G.L.; Seely, L.; Lorenz, T.J.; Namini, H.; Hamdan, A.D.; Roddy, S.P.; Belkin, M.; et al. Results of PREVENT III: A multicenter, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery. J. Vasc. Surg. 2006, 43, 742–751; discussion 751. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.H.; Hafley, G.; Harrington, R.A.; Peterson, E.D.; Ferguson, T.B., Jr.; Lorenz, T.J.; Goyal, A.; Gibson, M.; Mack, M.J.; Gennevois, D.; et al. Efficacy and safety of edifoligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: A randomized controlled trial. JAMA 2005, 294, 2446–2454. [Google Scholar] [CrossRef]






| Right Carotid Artery | Left Carotid Artery | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protocol # | Group | Graft | Beveling | Length (cm) | Group | Graft | Beveling | Length (cm) | |||||||||||
| Pig 1 | 50 | FRAME | IJV right | No | 5.0 | FRAME | IJV right | No | 3.5 | ||||||||||
| Pig 2 | 56 | Control | IJV right | Yes | 4.0 | Control | IJV right | Yes | 2.5 | ||||||||||
| Pig 3 | 60 | FRAME | IJV right | Yes | 5.0 | Control | IJV left | Yes | 5.0 | ||||||||||
| Pig 4 | 61 | FRAME | IJV right | Yes | 5.0 | Control | IJV left | Yes | 5.0 | ||||||||||
| Pig 5 | 20 | Control | IJV right | No | 2.5 | N/A | |||||||||||||
| Pig 6 | 29 | Control | IJV right | No | 4.0 | N/A | |||||||||||||
| Pig 7 | 48 | Control | IJV left | No | 4.0 | N/A | |||||||||||||
| FRAME | Control | ||||||||||||||||||
| Mean ± SD | 4.6 ± 0.8 cm (n = 4) | 3.8 ± 1.1 cm (n = 7) | n.s. p = 0.227 | ||||||||||||||||
| FRAME | Pre-Implantation | Post-Implantation | Explantation 1 M | |||
|---|---|---|---|---|---|---|
| Blood Flow (mL/min) | MAP (mmHg) | Blood Flow (mL/min) | MAP (mmHg) | Blood Flow (mL/min) | MAP (mmHg) | |
| Pig 1 right | 85 | 67 | 100 | 72 | 85 | 67 |
| Pig 1 left | 185 | 77 | 220 | 84 | 185 | 77 |
| Pig 3 right | 120 | 76 | 95 | 76 | 120 | 76 |
| Pig 4 right | 190 | 69 | 250 | 67 | 190 | 69 |
| Mean ± SD | 228 ± 51 | 73 ± 8 | 166 ± 80 | 75 ± 7 | 145 ± 51 | 72 ± 5 |
| ANOVA p = 0.208 | n.s. | n.s. | n.s. | |||
| ANOVA p = 0.845 | n.s. | n.s. | n.s. | |||
| Control | ||||||
| Pig 2 right | 160 | 65 | 95 | 56 | 230 | 67 |
| Pig 2 left | 450 | 65 | 60 | 58 | 0 | 70 |
| Pig 3 left | 310 | 88 | 190 | 71 | 22 | 80 |
| Pig 4 left | 320 | 77 | 130 | 68 | 0 | 73 |
| Pig 5 right | 400 | 66 | 20 | 60 | 0 | 82 |
| Pig 6 right | 160 | 56 | 75 | 51 | 70 | 74 |
| Pig 7 right | 270 | 68 | 90 | 89 | 0 | 80 |
| Mean ± SD | 296 ± 110 | 69 ± 10 | 94 ± 54 | 65 ± 13 | 46 ± 85 | 75 ± 6 |
| ANOVA p = 0.0001 | * vs. Pre-impl. | * vs. Pre-impl. | ||||
| ANOVA p = 0.177 | n.s. | n.s. | n.s. | |||
| FRAME vs. Control | p = 0.280 | p = 0.601 | p = 0.107 | p = 0.186 | p = 0.066 | p = 0.419 |
| FRAME | Proximal Anastomosis | Graft Body | Distal Anastomosis |
|---|---|---|---|
| Pig 1 right | Severe stenosis 68% | No stenosis * | Mild stenosis 36% |
| Pig 1 left | Moderate stenosis 60% | No stenosis | Mild stenosis 14% |
| Pig 3 right | Severe stenosis 66% | No stenosis * | Mild stenosis 40% |
| Pig 4 right | Mild stenosis 37% | No stenosis * | Mild stenosis 11% |
| Control | |||
| Pig 2 right | Moderate stenosis 56% | No stenosis | Mild stenosis 29% |
| Pig 2 left | Occlusion | Occlusion | Occlusion |
| Pig 3 left | Severe stenosis 76% | Mild long stenosis 25% | Medium stenosis 41% |
| Pig 4 left | Severe stenosis 73% | Long near occlusion 84% | Near occlusion 83% |
| Pig 5 right | Occlusion | Occlusion | Occlusion |
| Pig 6 right | Moderate stenosis 47% | Moderate long stenosis 40% | Moderate stenosis 52% |
| Pig 7 right | Occlusion | Occlusion | Occlusion |
| Neointimal Area (mm2) | Proximal Anastomosis | Midgraft | Prox. Anastomosis and Midgraft |
|---|---|---|---|
| Control | 6.94 ± 1.10 | 6.27 ± 0.64 | 6.38 ± 0.56 |
| FRAME | 5.79 ± 1.34 | 3.73 ± 0.64 | 4.53 ± 0.68 |
| Reduction (%) | 16.6% | 40.5% | 29% |
| t-test | n.s. p = 0.558 | * p = 0.022 | * p = 0.044 |
| Neointimal Thickness (μm2/μm) | |||
| Control | 587 ± 52 | 518 ± 36 | 530 ± 32 |
| FRAME | 480 ± 95 | 258 ± 49 | 344 ± 53 |
| Reduction (%) | 18.2% | 50.2% | 35.1% |
| t-test | n.s. p = 0.401 | * p = 0.0002 | * p = 0.002 |
| Study | Objective | Animal Model | Graft Harvest | Configuration | Heparin | Antiplatelet | Period | Patency |
|---|---|---|---|---|---|---|---|---|
| Chen et al. 1994 [29] | Vein graft gene transfer (iNOS) | Farm pig | N/R, prob. conventional | Interposition end-to-end | 300 IU/kg | Aspirin 150 mg (3 ds bef.) | 3 ds | 100% (8/8) |
| Kibbe et al., 2001 [34] | Vein graft gene transfer (VCAM) | Domestic pig | N/R, prob. conventional | Interposition end-to-end | 100 IU/kg | N/R | 21 ds | 100% (8/8) |
| Bartels et al., 2003 [30] | Vein graft brachytherapy, control group | Hyperchol. Landrace pig | N/R, prob. conventional | Bypass end-to-side | N/R | Aspirin 100 mg (post-op.) | 4 wks | 87.5% (14/16) |
| Jevon et al., 2011 [31] | Vein graft disease study | Inbred Landrace pig | N/R, prob. conventional | Interposition end-to-end | 1000 IU/kg | N/R | 4 wks | 100% (4/4) |
| Quint et al., 2011 [32] | Tissue engineering, control group | Yorkshire pig | N/R, prob. conventional | Bypass end-to-side | 100 IU/kg | Aspirin 5 mg/kg + clopidogrel 1 mg/kg, (1 d bef.) | 30 ds | 37.5% (3/8) |
| Thim et al., 2012 [33] | Vein graft disease study | Hyperchol. minipig | Conventional, no distension | Interposition end-to-end not beveled | Yes, dose N/R | Aspirin 150 mg (post-op) | 12–14 wks | 88.9% (8/9) |
| Our study | External stenting, control group | Domestic pig | Conventional, gentle distension | Interposition end-to-end | 200 IU/kg | Aspirin 100 mg (1 d bef.) | 4 wks | 42.9% (3/7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chlupac, J.; Frank, J.; Sedmera, D.; Fabian, O.; Simunkova, Z.; Mrazova, I.; Novak, T.; Vanourková, Z.; Benada, O.; Pulda, Z.; et al. External Support of Autologous Internal Jugular Vein Grafts with FRAME Mesh in a Porcine Carotid Artery Model. Biomedicines 2024, 12, 1335. https://doi.org/10.3390/biomedicines12061335
Chlupac J, Frank J, Sedmera D, Fabian O, Simunkova Z, Mrazova I, Novak T, Vanourková Z, Benada O, Pulda Z, et al. External Support of Autologous Internal Jugular Vein Grafts with FRAME Mesh in a Porcine Carotid Artery Model. Biomedicines. 2024; 12(6):1335. https://doi.org/10.3390/biomedicines12061335
Chicago/Turabian StyleChlupac, Jaroslav, Jan Frank, David Sedmera, Ondrej Fabian, Zuzana Simunkova, Iveta Mrazova, Tomas Novak, Zdenka Vanourková, Oldrich Benada, Zdenek Pulda, and et al. 2024. "External Support of Autologous Internal Jugular Vein Grafts with FRAME Mesh in a Porcine Carotid Artery Model" Biomedicines 12, no. 6: 1335. https://doi.org/10.3390/biomedicines12061335
APA StyleChlupac, J., Frank, J., Sedmera, D., Fabian, O., Simunkova, Z., Mrazova, I., Novak, T., Vanourková, Z., Benada, O., Pulda, Z., Adla, T., Kveton, M., Lodererova, A., Voska, L., Pirk, J., & Fronek, J. (2024). External Support of Autologous Internal Jugular Vein Grafts with FRAME Mesh in a Porcine Carotid Artery Model. Biomedicines, 12(6), 1335. https://doi.org/10.3390/biomedicines12061335







