Exploring the Dimensions of Pre-Clinical Research: 3D Cultures as an Investigative Model of Cardiac Fibrosis in Chagas Disease
Abstract
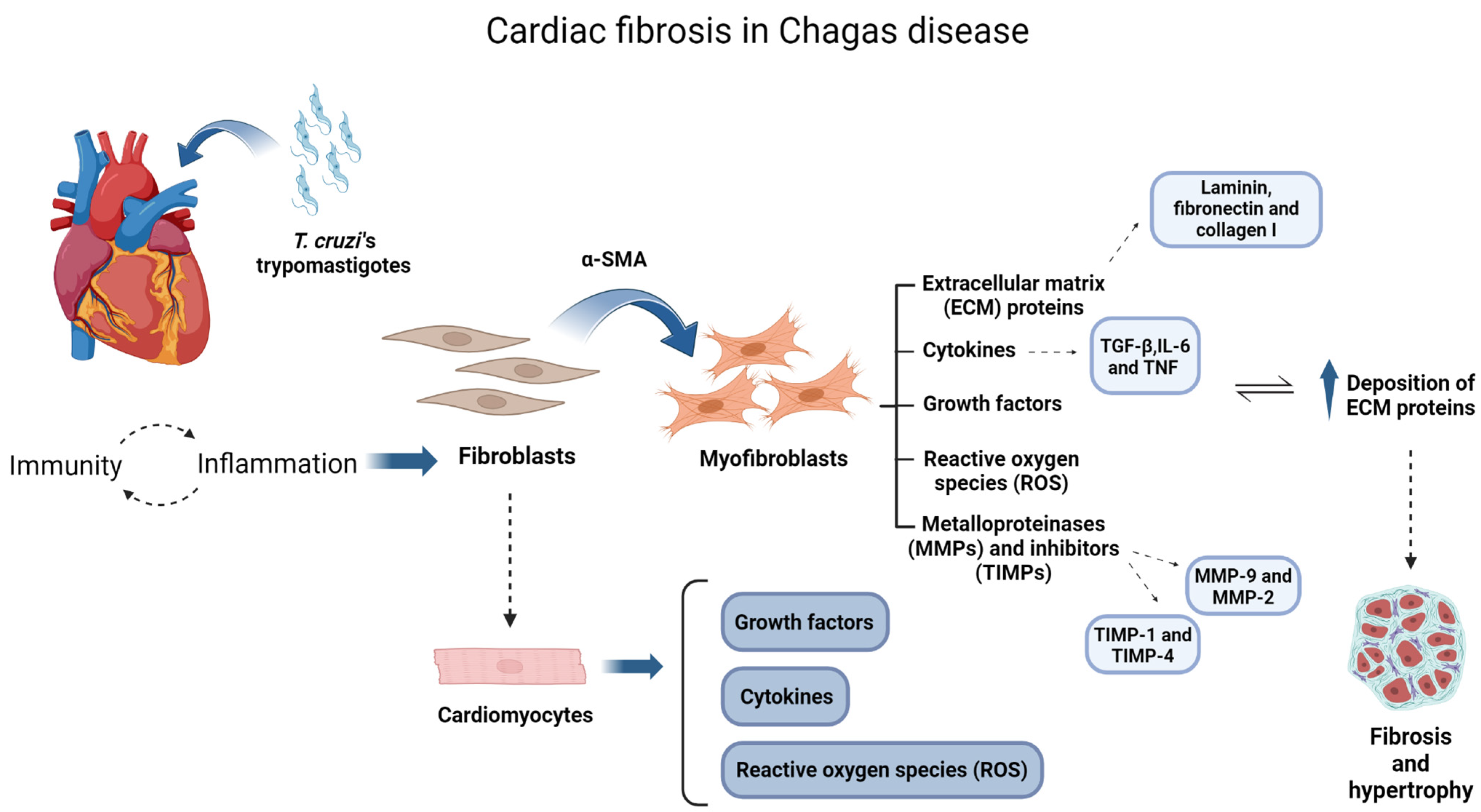
1. Introduction
2. Methods Used to Analyze Primary Cardiac Microtissues in CD
3. The Use of Spheroids in Chagas Disease
3.1. Fibrosis Characterization
3.2. Molecular Mechanisms
4. Three-Dimensional Cultures Applied for Drug Testing in Chagas Cardiomyopathy (CCC)
Disclosing New Treatments for Cardiac Fibrosis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Knight, E.; Przyborski, S. Advances in 3D cell culture technologies enabling tissue-like structures to be created in vitro. J. Anat. 2015, 227, 746–756. [Google Scholar] [CrossRef]
- Ong, C.S.; Zhou, X.; Han, J.; Huang, C.Y.; Nashed, A.; Khatri, S.; Mattson, G.; Fukunishi, T.; Zhang, H.; Hibino, N. In vivo therapeutic applications of cell spheroids. Biotechnol. Adv. 2018, 36, 494–505. [Google Scholar] [CrossRef]
- Ravi, M.; Paramesh, V.; Kaviya, S.R.; Anuradha, E.; Solomon, F.P. 3D cell culture systems: Advantages and applications. J. Cell. Physiol. 2015, 230, 16–26. [Google Scholar] [CrossRef]
- Jensen, C.; Teng, Y. Is it time to start transitioning from 2D to 3D cell culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar] [CrossRef]
- Zhao, L.; Xiu, J.; Liu, Y.; Zhang, T.; Pan, W.; Zheng, X.; Zhang, X. A 3D printed hanging drop dripper for tumor spheroids analysis without recovery. Sci. Rep. 2019, 9, 19717. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, M.; Zhang, Y.; Liu, H.; Han, L. Recent methods of droplet microfluidics and their applications in spheroids and organoids. Lab Chip 2023, 23, 1080–1096. [Google Scholar] [CrossRef]
- Metzger, W.; Sossong, D.; Bächle, A.; Pütz, N.; Wennemuth, G.; Pohlemann, T.; Oberringer, M. The liquid overlay technique is the key to formation of co-culture spheroids consisting of primary osteoblasts, fibroblasts and endothelial cells. Cytotherapy 2011, 13, 1000–1012. [Google Scholar] [CrossRef]
- Costa, E.C.; Gaspar, V.M.; Coutinho, P.; Correia, I.J. Optimization of liquid overlay technique to formulate heterogenic 3D co-cultures models. Biotechnol. Bioeng. 2014, 111, 1672–1685. [Google Scholar] [CrossRef] [PubMed]
- Carpenedo, R.L.; Sargent, C.Y.; McDevitt, T.C. Rotary suspension culture enhances the efficiency, yield, and homogeneity of embryoid body differentiation. Stem Cells 2007, 25, 2224–2234. [Google Scholar] [CrossRef] [PubMed]
- Maritan, S.M.; Lian, E.Y.; Mulligan, L.M. An efficient and flexible cell aggregation method for 3D spheroid production. J. Vis. Exp. 2017, 121, e55544. [Google Scholar] [CrossRef]
- Anil-Inevi, M.; Yaman, S.; Yildiz, A.A.; Mese, G.; Yalcin-Ozuysal, O.; Tekin, H.C.; Ozcivici, E. Biofabrication of in situ self assembled 3D cell cultures in a weightlessness environment generated using magnetic levitation. Sci. Rep. 2018, 8, 7239. [Google Scholar] [CrossRef]
- Ryu, N.E.; Lee, S.H.; Park, H. Spheroid culture system methods and applications for mesenchymal stem cells. Cells 2019, 8, 1620. [Google Scholar] [CrossRef]
- Shao, C.; Chi, J.; Zhang, H.; Fan, Q.; Zhao, Y.; Ye, F. Development of cell spheroids by advanced technologies. Adv. Mater. Technol. 2020, 5, 2000183. [Google Scholar] [CrossRef]
- Clevers, H. Modeling development and disease with organoids. Cell 2016, 165, 1586–1597. [Google Scholar] [CrossRef]
- Devanjali, D.; Clevers, H. Organoid culture systems to study host–pathogen interactions. Curr. Opin. Immunol. 2017, 48, 15–22. [Google Scholar]
- PAHO (Pan American Health Organization). Guidelines for the Diagnosis and Treatment of Chagas Disease; PAHO: Washington, DC, USA, 2019; Available online: http://iris.paho.org/xmlui/handle/123456789/49653 (accessed on 6 March 2024).
- Pérez-Molina, J.A.; Norman, F.; López-Vélez, R. Chagas disease in non-endemic countries: Epidemiology, clinical presentation and treatment. Curr. Infect. Dis. Rep. 2012, 14, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Echeverria, L.E.; Morillo, C.A. American trypanosomiasis (Chagas disease). Infect. Dis. Clin. 2019, 33, 119–134. [Google Scholar] [CrossRef] [PubMed]
- Rassi, A., Jr.; Rassi, A.; Little, W.C.; Xavier, S.S.; Rassi, S.G.; Rassi, A.G.; Rassi, G.G.; Hasslocher-Moreno, A.; Souza, A.S.; Scanavacca, M.I. Development and validation of a risk score for predicting death in Chagas’ heart disease. N. Engl. J. Med. 2006, 355, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Bestetti, R.B.; Cardinalli-Neto, A. Sudden cardiac death in Chagas’ heart disease in the contemporary era. Int. J. Cardiol. 2008, 131, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Prata, A. Clinical and epidemiological aspects of Chagas disease. Lancet Infect. Dis. 2001, 1, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Nunes, M.C.P.; Dones, W.; Morillo, C.A.; Encina, J.J.; Ribeiro, A.L.; Council on Chagas Disease of the Interamerican Society of Cardiology. Chagas disease: An overview of clinical and epidemiological aspects. J. Am. Coll. Cardiol. 2013, 62, 767–776. [Google Scholar] [CrossRef]
- Chagas, C. Nov a entidade mórbida no homem: Resumo gera l de estudos etiológicos e clínicos. Mem. Inst. Oswaldo Cruz. 1911, 3, 219–275. [Google Scholar] [CrossRef]
- Gascon, J.; Albajar, P.; Cañas, E.; Flores, M.; Herrera, R.N. Diagnosis, Management, and Treatment of Chronic Chagas’ Heart Disease in Areas Where Trypanosoma Cruzi Infection is Not Endemic. Rev. Esp. Cardiol. 2007, 60, 285–293. [Google Scholar] [CrossRef]
- Ferreira, R.R.; Waghabi, M.C.; Bailly, S.; Feige, J.J.; Hasslocher-Moreno, A.M.; Saraiva, R.M.; Araujo-Jorge, T.C. The search for biomarkers and treatments in Chagas disease: Insights from TGF-beta studies and immunogenetics. Front. Cell. Infect. Microbiol. 2022, 11, 767576. [Google Scholar] [CrossRef] [PubMed]
- Wesley, M.; Moraes, A.; Rosa, A.D.C.; Lott Carvalho, J.; Shiroma, T.; Vital, T.; Dias, N.; de Carvalho, B.; Rabello, D.A.; Borges, T.K.S.; et al. Correlation of parasite burden, kDNA integration, autoreactive antibodies, and cytokine pattern in the pathophysiology of chagas disease. Front. Microbiol. 2019, 10, 1856. [Google Scholar] [CrossRef]
- Pineda, M.A.; Cuervo, H.; Fresno, M.; Soto, M.; Bonay, P. Lack of galectin-3 prevents cardiac fibrosis and effective immune responses in a murine model of Trypanosoma cruzi infection. J. Infect. Dis. 2015, 7, 1160–1171. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, M.L.; Benvenuti, L.A.; Martins Reis, M.; Metzger, M. Pathophysiology of the heart in Chagas’ disease: Current status and new developments. Cardiovasc. Res. 2003, 60, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.A.; Tanowitz, H.B.; Malvestio, L.M.; Celes, M.R.; Campos, E.C.; Blefari, V.; Prado, C.M. Coronary microvascular disease in chronic Chagas cardiomyopathy including an overview on history, pathology, and other proposed pathogenic mechanisms. PLoS Negl. Trop. Dis. 2010, 4, e674. [Google Scholar] [CrossRef]
- Rassi, A., Jr.; Rassi, A.; Marcondes de Rezende, J. American trypanosomiasis (Chagas disease). Infect. Dis. Clin. N. Am. 2012, 26, 275–291. [Google Scholar] [CrossRef]
- Simões, M.V.; Oliveira, L.F.D.; Hiss, F.C.; Figueiredo, A.B.D.; Pintya, A.O.; Maciel, B.C.; Marin-Neto, J.A. Characterization of the apical aneurysm of chronic Chagas’ heart disease by scintigraphic image co-registration. Arq. Bras. Cardiol. 2007, 89, 131–134. [Google Scholar] [CrossRef]
- Rassi, A., Jr.; Rassi, S.G.; Rassi, A. Sudden death in Chagas’ disease. Arq. Bras. Cardiol. 2001, 76, 75–96. [Google Scholar] [CrossRef] [PubMed]
- Morillo, C.A.; Marin-Neto, J.A.; Avezum, A.; Sosa-Estani, S.; Rassi, A., Jr.; Rosas, F.; Villena, E.; Quiroz, R.; Bonilla, R.; Britto, C.; et al. Randomized trial of benznidazole for chronic chagas’ cardiomyopathy. N. Engl. J. Med. 2015, 373, 1295–1306. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, V.; Dias, N.; Paiva, T.; Hagström-Bex, L.; Nitz, N.; Pratesi, R.; Hecht, M. Current trends in the pharmacological management of Chagas disease. Int. J. Parasitol. Drugs Drug Resist. 2020, 12, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Garzoni, L.R.; Adesse, D.; Soares, M.J.; Rossi, M.I.D.; Borojevic, R.; de Nazareth Leal de Meirelles, M. Fibrosis and hypertrophy induced by Trypanosoma cruzi in a three-dimensional cardiomyocyte-culture system. J. Infect. Dis. 2008, 197, 906–915. [Google Scholar] [CrossRef] [PubMed]
- Ferrão, P.M.; Nisimura, L.M.; Moreira, O.C.; Land, M.G.; Pereira, M.C.; de Mendonça-Lima, L.; Araújo-Jorge, T.C.; Waghabi, M.C.; Garzoni, L.R. Inhibition of TGF-β pathway reverts extracellular matrix remodeling in T. cruzi-infected cardiac spheroids. Exp. Cell Res. 2018, 362, 260–267. [Google Scholar] [CrossRef]
- Nisimura, L.M.; Ferrão, P.M.; da Rocha Nogueira, A.; Waghabi, M.C.; Meuser-Batista, M.; Moreira, O.C.; Urbina, J.A.; Garzoni, L.R. Effect of Posaconazole in an in vitro model of cardiac fibrosis induced by Trypanosoma cruzi. Mol. Biochem. Parasitol. 2020, 238, 111283. [Google Scholar] [CrossRef]
- Garzoni, L.R.; Rossi, M.I.D.; de Barros, A.P.; Guarani, V.; Keramidas, M.; Balottin, L.B.; Adesse, D.; Takyia, C.M.; Manso, P.P.; Otazú, I.B.; et al. Dissecting coronary angiogenesis: 3D co-culture of cardiomyocytes with endothelial or mesenchymal cells. Exp. Cell Res. 2009, 315, 3406–3418. [Google Scholar] [CrossRef]
- de Almeida Fiuza, L.F.; Batista, D.D.G.J.; Nunes, D.F.; Moreira, O.C.; Cascabulho, C.; Soeiro, M.D.N.C. Benznidazole modulates release of inflammatory mediators by cardiac spheroids infected with Trypanosoma cruzi. Exp. Parasitol. 2021, 221, 108061. [Google Scholar] [CrossRef]
- Norman, F.F.; López-Vélez, R. Chagas Disease: Comments on the 2018 PAHO guidelines for diagnosis and management. J. Travel Med. 2019, 26, taz060. [Google Scholar] [CrossRef] [PubMed]
- Fares, R.C.G.; Gomes, J.D.A.S.; Garzoni, L.R.; Waghabi, M.C.; Saraiva, R.M.; Medeiros, N.I.; Oliveira-Prado, R.; Sangenis, L.H.C.; Chambela, M.C.; de Araújo, F.F.; et al. Matrix metalloproteinases 2 and 9 are differentially expressed in patients with indeterminate and cardiac clinical forms of Chagas disease. Infect. Immun. 2013, 81, 3600–3608. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.R.; de Souza, E.M.; Vilar-Pereira, G.; Degrave, W.; Abreu, R.D.S.; Meuser-Batista, M.; Ferreira, N.V.C.; Ledbeter, S.; Barker, R.H.; Bailly, S.; et al. In Chagas disease, transforming growth factor beta neutralization reduces Trypanosoma cruzi infection and improves cardiac performance. Front. Cell. Infect. Microbiol. 2022, 12, 1775. [Google Scholar] [CrossRef]
- Rassi, A.; Marin, J.A. Chronic Chagas cardiomyopathy: A review of the main pathogenic mechanisms and the efficacy of aetiological treatment following the BENznidazole evaluation for interrupting trypanosomiasis (BENEFIT) trial. Mem. Inst. Oswaldo Cruz. 2017, 112, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Rassi, A.; Marin-Neto, J.A. Chagas disease. Lancet 2010, 375, 1388–1402. [Google Scholar] [CrossRef] [PubMed]
- Coelho, L.L.; Pereira, I.R.; Pereira, M.C.D.S.; Mesquita, L.; Lannes-Vieira, J.; Adesse, D.; Garzoni, L.R. Trypanosoma cruzi activates mouse cardiac fibroblasts in vitro leading to fibroblast-myofibroblast transition and increase in expression of extracellular matrix proteins. Parasit. Vectors 2018, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.C.; Francisco, A.F.; Jayawardhana, S.; Calderano, S.G.; Lewis, M.D.; Olmo, F.; Taylor, M.C. Expanding the toolbox for Trypanosoma cruzi: A parasite line incorporating a bioluminescence-fluorescence dual reporter and streamlinedCRISPR/Cas9 functionality for rapid in vivo localization and phenotyping. PLoS Negl. Trop. Dis. 2018, 12, e0006388. [Google Scholar] [CrossRef] [PubMed]
- Gironès, N.; Cuervo, H.; Fresno, M. Trypanosoma cruzi-induced molecular mimicry and Chagas’ disease. In Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2005; pp. 89–123. [Google Scholar] [CrossRef]
- Teixeira, A.R.; Hecht, M.M.; Guimaro, M.C.; Sousa, A.O.; Nitz, N. Pathogenesis of chagas’ disease: Parasite persistence and autoimmunity. Clin. Microbiol. Rev. 2011, 24, 592–630. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.; Tanowitz, H.B.; Garg, N.J. Pathogenesis of chronic Chagas disease: Macrophages, mitochondria, and oxidative stress. Curr. Clin. Microbiol. Rep. 2018, 5, 45–54. [Google Scholar] [CrossRef]
- da Costa, A.W.F.; do Carmo Neto, J.R.; Braga, Y.L.L.; Silva, B.A.; Lamounier, A.B.; Silva, B.O.; dos Reis, M.A.; de Oliveira, F.A.; Celes, M.R.N.; Machado, J.R. Cardiac chagas disease: MMPs, TIMPs, galectins, and TGF-β as tissue remodelling players. Dis. Markers 2019. [Google Scholar] [CrossRef]
- Cruz, J.S.; Machado, F.S.; Ropert, C.; Roman-Campos, D. Molecular mechanisms of cardiac electromechanical remodeling during Chagas disease: Role of TNF and TGF-β. Trends Cardiovasc. Med. 2017, 27, 81–91. [Google Scholar] [CrossRef]
- Medina-Rincón, G.J.; Gallo-Bernal, S.; Jiménez, P.A.; Cruz-Saavedra, L.; Ramírez, J.D.; Rodríguez, M.J.; Medina-Mur, R.; Díaz-Nassif, G.; Valderrama-Achury, M.D.; Medina, H.M. Molecular and clinical aspects of chronic manifestations in Chagas disease: A state-of-the-art review. Pathogens 2021, 10, 1493. [Google Scholar] [CrossRef]
- Mehta, G.; Hsiao, A.Y.; Ingram, M.; Luker, G.D.; Takayama, S. Opportunities and challenges for use of tumor spheroids as models to test drug delivery and efficacy. J. Control. Release 2012, 164, 192–204. [Google Scholar] [CrossRef]
- Langhans, S.A. Three-Dimensional in vitro cell culture models in drug discovery and drug repositioning. Front. Pharmacol. 2018, 9, 334617. [Google Scholar] [CrossRef]
- Wang, H.; Brown, P.C.; Chow, E.C.; Ewart, L.; Ferguson, S.S.; Fitzpatrick, S.; Freedman, B.S.; Guo, G.L.; Hedrich, W.; Heyward, S.; et al. 3D cell culture models: Drug pharmacokinetics, safety assessment, and regulatory consideration. Clin. Transl. Sci. 2021, 14, 1659–1680. [Google Scholar] [CrossRef]
- Bern, C. Antitrypanosomal therapy for chronic Chagas’ disease. N. Engl. J. Med. 2011, 364, 2527–2534. [Google Scholar] [CrossRef]
- Malone, C.J.; Nevis, I.; Fernández, E.; Sanchez, A. A Rapid Review on the Efficacy and Safety of Pharmacological Treatments for Chagas Disease. Trop. Med. Infect. Dis. 2021, 12, 128. [Google Scholar] [CrossRef]
- Lamas, M.C.; Villaggi, L.; Nocito, I.; Bassani, G.; Leonardi, D.; Pascutti, F.; Serra, E.; Salomón, C.J. Development of parenteral formulations and evaluation of the biological activity of the trypanocide drug benznidazole. Int. J. Pharm. 2006, 307, 239–243. [Google Scholar] [CrossRef]
- Hoffman, K.A.; Reynolds, C.; Bottazzi, M.E.; Hotez, P.; Jones, K. Improved biomarker and imaging analysis for characterizing progressive cardiac fibrosis in a mouse model of chronic chagasic cardiomyopathy. J. Am. Heart Assoc. 2019, 8, e013365. [Google Scholar] [CrossRef]
- Bahia, M.T.; Diniz, L.D.F.; Mosqueira, V.C.F. Therapeutical Approaches under Investigation for Treatment of Chagas Disease. Expert Opin. Investig. Drugs 2014, 23, 1225–1237. [Google Scholar] [CrossRef] [PubMed]
- Bern, C. Chagas’ disease. N. Engl. J. Med. 2015, 373, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Kratz, J.M. Drug discovery for chagas disease: A viewpoint. Acta Trop. 2019, 198, 105107. [Google Scholar] [CrossRef] [PubMed]
- Prata, A.R.; Lopes, E.R.; Chapadeiro, E. Características da morte súbita tida como não esperada na doença de Chagas. Rev. Soc. Bras. Med. Trop. 1986, 19, 9–12. [Google Scholar] [CrossRef] [PubMed][Green Version]
| Title | Autor | Year | Main Findings | Focus |
|---|---|---|---|---|
| Fibrosis and Hypertrophy Induced by Trypanosoma cruzi in a Three-Dimensional Cardiomyocyte- Culture System | Garzoni [35] | 2008 | In this new three-dimensional system, cardiac spheroids showed spontaneous contractility, with typical cardiac morphology and production of extracellular matrix components. There were four- and six-fold increases, respectively, in the area and the volume of T. cruzi–infected cardiomyocytes and whole microtissues, together with a 50% reduction in the cell population. Immunofluorescence showed the increased expression of fibronectin, collagen IV and laminin in the microtissues 144 h after infection. T. cruzi infection induced an increase in both the cellular area and the extracellular matrix components in cardiac spheroids, which contributed to an increase in the total microtissue volume, making this a powerful three-dimensional in vitro model for the study of cardiac-tissue hypertrophy, fibrosis, and remodeling. |
|
| Matrix Metalloproteinases 2 and 9 Are Differentially Expressed in Patients with Indeterminate and Cardiac Clinical Forms of Chagas Disease | Fares [41] | 2013 | Using a new three-dimensional model of fibrosis, we observed that sera from patients with Chagas disease induced an increase in the extracellular matrix components in cardiac spheroids. Furthermore, MMP-2 and MMP-9 showed different correlations with matrix proteins and inflammatory cytokines in patients with Chagas disease. Our results suggest that MMP-2 and MMP-9 show distinct activities in Chagas disease pathogenesis. While MMP-9 seems to be involved in the inflammation and cardiac remodeling of Chagas disease, MMP-2 does not correlate with inflammatory molecules. |
|
| Inhibition of TGF-β pathway reverts extracellular matrix remodeling in T. cruzi-infected cardiac spheroids | Ferrão [36] | 2018 | Treatment with a selective inhibitor of TGF-β type I receptor, resulted in a reduction in the size of spheroids, and decreased parasite load and fibronectin expression as well as increased MMP-2 and a decrease TIMP-1 expression, which may be one of the mechanisms regulating extracellular matrix remodeling. The study discusses mechanisms by which the inhibition of TGF-β signaling reverses fibrosis and hypertrophy generated by T. cruzi. |
|
| Effect of Posaconazole in an in vitro model of cardiac fibrosis induced by Trypanosoma cruzi | Nisimura [37] | 2020 | Treatment with POS reduced parasite load by 50% according to real-time PCR and reduced fibrosis according to Western blot and immunofluorescence, which is associated with a reduction in fibronectin and laminin (45% and 54%, respectively). POS treatment also increased by 50% TGF-β and decreased by 58%; TIMP-4. |
|
| Benznidazole modulates release of inflammatory mediators by cardiac spheroids infected with Trypanosoma cruzi | Fiuza [39] | 2021 | BZ presented a low toxic profile on 3D matrices and a high potency in vitro according to a qPCR analysis of T. cruzi-infected cardiac spheroids. A flow cytometry appraisal of the inflammatory mediators released from the cellular supernatant showed increases in IL—6 and TNF in parasitized spheroids as compared to uninfected cultures. BZ at 10 μM suppressed the parasite load (92%) concomitantly decreasing in IL-6 (36%) and TNF (68%). Our findings corroborate the successful use of 3D cardiac matrices for in vitro identification of novel anti-parasitic agents and potential impact in host cell physiology. |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seydel, C.M.; Gonzaga, B.M.d.S.; Coelho, L.L.; Garzoni, L.R. Exploring the Dimensions of Pre-Clinical Research: 3D Cultures as an Investigative Model of Cardiac Fibrosis in Chagas Disease. Biomedicines 2024, 12, 1410. https://doi.org/10.3390/biomedicines12071410
Seydel CM, Gonzaga BMdS, Coelho LL, Garzoni LR. Exploring the Dimensions of Pre-Clinical Research: 3D Cultures as an Investigative Model of Cardiac Fibrosis in Chagas Disease. Biomedicines. 2024; 12(7):1410. https://doi.org/10.3390/biomedicines12071410
Chicago/Turabian StyleSeydel, Clara Monteiro, Beatriz Matheus de Souza Gonzaga, Laura Lacerda Coelho, and Luciana Ribeiro Garzoni. 2024. "Exploring the Dimensions of Pre-Clinical Research: 3D Cultures as an Investigative Model of Cardiac Fibrosis in Chagas Disease" Biomedicines 12, no. 7: 1410. https://doi.org/10.3390/biomedicines12071410
APA StyleSeydel, C. M., Gonzaga, B. M. d. S., Coelho, L. L., & Garzoni, L. R. (2024). Exploring the Dimensions of Pre-Clinical Research: 3D Cultures as an Investigative Model of Cardiac Fibrosis in Chagas Disease. Biomedicines, 12(7), 1410. https://doi.org/10.3390/biomedicines12071410






