RETRACTED: The cGAS-STING Pathway: A New Therapeutic Target for Ischemia–Reperfusion Injury in Acute Myocardial Infarction?
Abstract
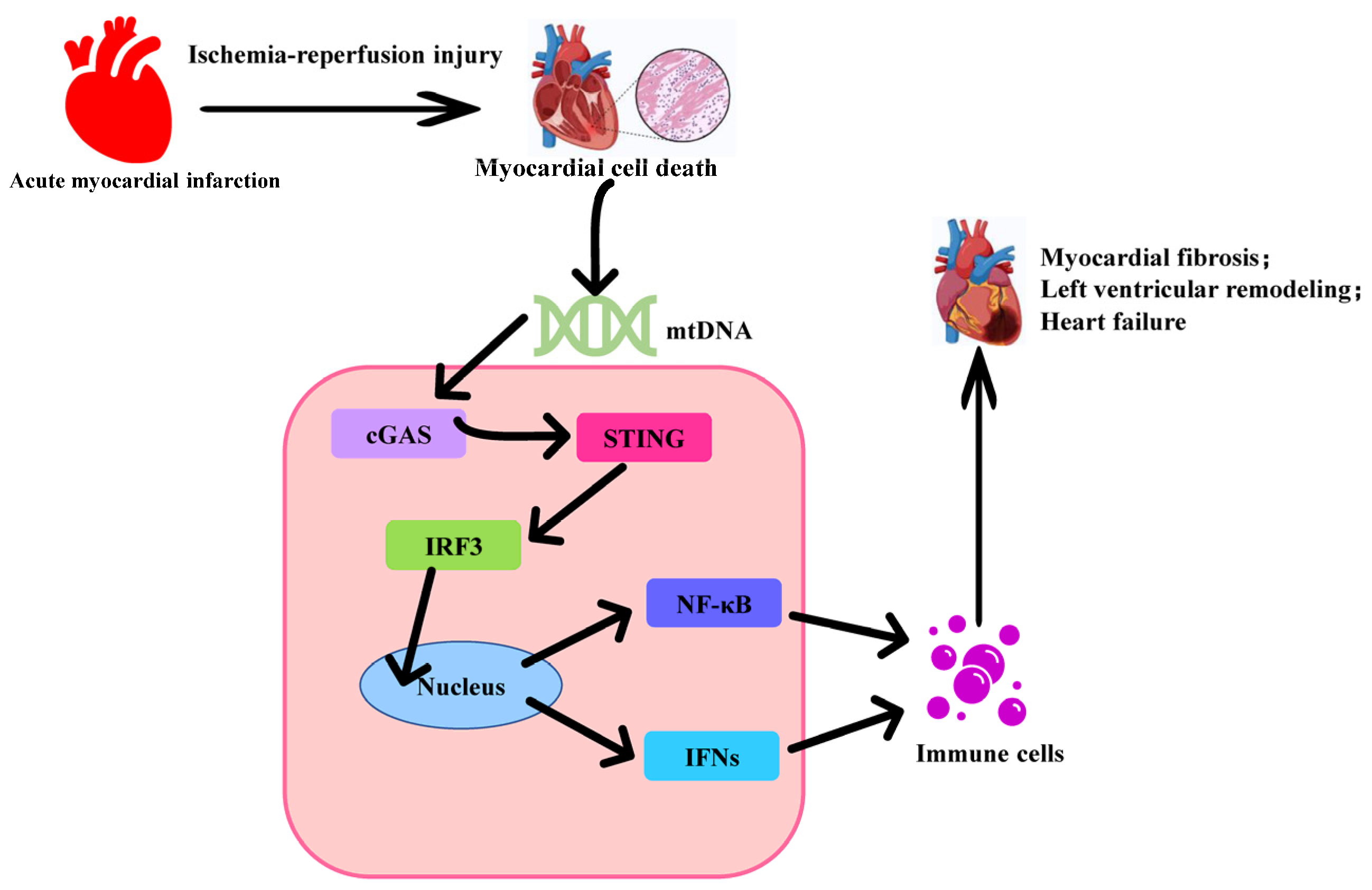
1. Backgrounds
2. The Activation of the cGAS-STING Signaling Pathway
3. The Role of cGAS-STING in Myocardial Infarction Injury
4. Inhibitors of the cGAS-STING Signaling Pathway
5. The Application of Nanomaterial Technology
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PRRs | pattern recognition receptors |
| cGAS | cyclic GMP–AMP synthase |
| STING | stimulator of interferon genes |
| AMI | acute myocardial infarction |
| IR | ischemia–reperfusion |
| dsDNA | double-stranded DNA |
| cGAMP | cyclic GMP-AMP |
| ER | endoplasmic reticulum |
| TBK1 | tank-binding kinase 1 |
| IKK | inhibitor of kappa B kinase |
| IRF3 | interferon regulatory factor 3 |
| IFN | interferon |
| NF-kB | nuclear factor kappa B |
| TAK1 | TGF-beta-activated kinase 1 |
References
- de Reuver, R.; Maelfait, J. Novel insights into double-stranded RNA-mediated immunopathology. Nat. Rev. Immunol. 2024, 24, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Cheng, J.; Ko, H.; Tang, Y. Cytosolic DNA sensors in neurodegenerative diseases: From physiological defenders to pathological culprits. EMBO Mol. Med. 2024, 16, 678–699. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liu, B.; Sinha, S.C.; Amin, S.; Gan, L. Mechanism and therapeutic potential of targeting cGAS-STING signaling in neurological disorders. Mol. Neurodegener. 2023, 18, 79. [Google Scholar] [CrossRef] [PubMed]
- Slavik, K.M.; Kranzusch, P.J. CBASS to cGAS-STING: The Origins and Mechanisms of Nucleotide Second Messenger Immune Signaling. Annu. Rev. Virol. 2023, 10, 423–453. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Gao, X.; Ge, S.; Li, H.; Wang, R.; Zhao, L. The Role of cGAS-STING Signalling in Metabolic Diseases: From Signalling Networks to Targeted Intervention. Int. J. Biol. Sci. 2024, 20, 152–174. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Mu, X.; Wu, X.; Liu, Y.; Deng, J.; Liu, Y.; Han, F.; Nie, X. The cGAS-STING pathway: A therapeutic target in diabetes and its complications. Burn. Trauma 2024, 12, tkad050. [Google Scholar] [CrossRef]
- Heusch, G.; Gersh, B.J. The pathophysiology of acute myocardial infarction and strategies of protection beyond reperfusion: A continual challenge. Eur. Heart J. 2017, 38, 774–784. [Google Scholar] [CrossRef] [PubMed]
- Welt, F.G.P.; Batchelor, W.; Spears, J.R.; Penna, C.; Pagliaro, P.; Ibanez, B.; Drakos, S.G.; Dangas, G.; Kapur, N.K. Reperfusion Injury in Patients With Acute Myocardial Infarction: JACC Scientific Statement. J. Am. Coll. Cardiol. 2024, 83, 2196–2213. [Google Scholar] [CrossRef]
- Barrère-Lemaire, S.; Vincent, A.; Jorgensen, C.; Piot, C.; Nargeot, J.; Djouad, F. Mesenchymal stromal cells for improvement of cardiac function following acute myocardial infarction: A matter of timing. Physiol. Rev. 2024, 104, 659–725. [Google Scholar] [CrossRef]
- Eckle, T.; Bertazzo, J.; Khatua, T.N.; Fatemi Tabatabaei, S.R.; Moori Bakhtiari, N.; Walker, L.A.; Martino, T.A. Circadian Influences on Myocardial Ischemia-Reperfusion Injury and Heart Failure. Circ. Res. 2024, 134, 675–694. [Google Scholar] [CrossRef]
- Algoet, M.; Janssens, S.; Himmelreich, U.; Gsell, W.; Pusovnik, M.; Van den Eynde, J.; Oosterlinck, W. Myocardial ischemia-reperfusion injury and the influence of inflammation. Trends Cardiovasc. Med. 2023, 33, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Cheng, J.; Kong, X.; Li, S.; Li, X.; Zhang, M.; Zhang, H.; Yang, T.; Dong, Y.; Li, J.; et al. HDAC3 inhibition ameliorates ischemia/reperfusion-induced brain injury by regulating the microglial cGAS-STING pathway. Theranostics 2020, 10, 9644–9662. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Zha, H.; Pan, Z.; Wang, J.; Xia, Y.; Li, H.; Huang, H.; Yue, R.; Song, Z.; Zhu, J. DUSP1 protects against ischemic acute kidney injury through stabilizing mtDNA via interaction with JNK. Cell Death Dis. 2023, 14, 724. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Yu, N.; Ye, Z.; Gu, Y.; Zhang, C.; Chen, M.; Wang, K. Inhibition of cGAS-STING pathway alleviates neuroinflammation-induced retinal ganglion cell death after ischemia/reperfusion injury. Cell Death Dis. 2023, 14, 615. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Xu, P. Cellular functions of cGAS-STING signaling. Trends Cell Biol. 2023, 33, 630–648. [Google Scholar] [CrossRef] [PubMed]
- Hopfner, K.P.; Hornung, V. Molecular mechanisms and cellular functions of cGAS-STING signalling. Nat. Rev. Mol. Cell Biol. 2020, 21, 501–521. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.J.; Yu, Y.; Xie, W. cGAMP-activated cGAS-STING signaling: Its bacterial origins and evolutionary adaptation by metazoans. Nat. Struct. Mol. Biol. 2023, 30, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Bai, X.C.; Chen, Z.J. Structures and Mechanisms in the cGAS-STING Innate Immunity Pathway. Immunity 2020, 53, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Li, X.D.; Wu, J.; Gao, D.; Wang, H.; Sun, L.; Chen, Z.J. Pivotal roles of cGAS-cGAMP signaling in antiviral defense and immune adjuvant effects. Science 2013, 341, 1390–1394. [Google Scholar] [CrossRef]
- Huiting, E.; Cao, X.; Ren, J.; Athukoralage, J.S.; Luo, Z.; Silas, S.; An, N.; Carion, H.; Zhou, Y.; Fraser, J.S.; et al. Bacteriophages inhibit and evade cGAS-like immune function in bacteria. Cell 2023, 186, 864–876.e21. [Google Scholar] [CrossRef]
- Slavik, K.M.; Morehouse, B.R.; Ragucci, A.E.; Zhou, W.; Ai, X.; Chen, Y.; Li, L.; Wei, Z.; Bähre, H.; König, M.; et al. cGAS-like receptors sense RNA and control 3’2’-cGAMP signalling in Drosophila. Nature 2021, 597, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Sun, L.; Chen, Z.J. Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing. Nat. Immunol. 2016, 17, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Skopelja-Gardner, S.; An, J.; Elkon, K.B. Role of the cGAS-STING pathway in systemic and organ-specific diseases. Nat. Rev. Nephrol. 2022, 18, 558–572. [Google Scholar] [CrossRef] [PubMed]
- Dunphy, G.; Flannery, S.M.; Almine, J.F.; Connolly, D.J.; Paulus, C.; Jønsson, K.L.; Jakobsen, M.R.; Nevels, M.M.; Bowie, A.G.; Unterholzner, L. Non-canonical Activation of the DNA Sensing Adaptor STING by ATM and IFI16 Mediates NF-κB Signaling after Nuclear DNA Damage. Mol. Cell 2018, 71, 745–760.e45. [Google Scholar] [CrossRef]
- Yum, S.; Li, M.; Fang, Y.; Chen, Z.J. TBK1 recruitment to STING activates both IRF3 and NF-κB that mediate immune defense against tumors and viral infections. Proc. Natl. Acad. Sci. USA 2021, 118, e2100225118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wei, X.; Wang, Z.; Liu, P.; Hou, Y.; Xu, Y.; Su, H.; Koci, M.D.; Yin, H.; Zhang, C. NF-κB activation enhances STING signaling by altering microtubule-mediated STING trafficking. Cell Rep. 2023, 42, 112185. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Chen, M.; Zhang, R.; Zhang, W.; Wang, C.; Zhang, Y.; Wei, X.; Guan, Y.; Liu, J.; Feng, K.; et al. Manganese is critical for antitumor immune responses via cGAS-STING and improves the efficacy of clinical immunotherapy. Cell Res. 2020, 30, 966–979. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Zhang, D. Research progress on the effects of novel hypoglycemic drugs in diabetes combined with myocardial ischemia/reperfusion injury. Ageing Res. Rev. 2023, 86, 101884. [Google Scholar] [CrossRef] [PubMed]
- Yanpiset, P.; Maneechote, C.; Sriwichaiin, S.; Siri-Angkul, N.; Chattipakorn, S.C.; Chattipakorn, N. Gasdermin D-mediated pyroptosis in myocardial ischemia and reperfusion injury: Cumulative evidence for future cardioprotective strategies. Acta Pharm. Sin. B 2023, 13, 29–53. [Google Scholar] [CrossRef]
- Hu, M.; Zhang, X.; Hu, C.; Ma, Z.G.; Wang, S.S.; Teng, T.; Zeng, X.F.; Tang, Q.Z. Isthmin-1 alleviates cardiac ischemia/reperfusion injury through cGMP-PKG signaling pathway. Cardiovasc. Res. 2024, 120, 1051–1064. [Google Scholar] [CrossRef]
- Xiong, Y.; Leng, Y.; Tian, H.; Deng, X.; Li, W.; Li, W.; Xia, Z. Decreased MFN2 activates the cGAS-STING pathway in diabetic myocardial ischaemia-reperfusion by triggering the release of mitochondrial DNA. Cell Commun. Signal. CCS 2023, 21, 192. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Hu, Q.; Ren, K.; Wu, P.; Wang, Y.; Lv, C. ALDH2 mitigates LPS-induced cardiac dysfunction, inflammation, and apoptosis through the cGAS/STING pathway. Mol. Med. 2023, 29, 171. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; You, Y.; Shang, F.F.; Wang, X.; Huang, B.; Zhao, B.; Lv, D.; Yang, S.; Xie, M.; Kong, L.; et al. iNOS aggravates pressure overload-induced cardiac dysfunction via activation of the cytosolic-mtDNA-mediated cGAS-STING pathway. Theranostics 2023, 13, 4229–4246. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.J.; Schiattarella, G.G.; Villalobos, E.; Jiang, N.; May, H.I.; Li, T.; Chen, Z.J.; Gillette, T.G.; Hill, J.A. Cytosolic DNA Sensing Promotes Macrophage Transformation and Governs Myocardial Ischemic Injury. Circulation 2018, 137, 2613–2634. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Chen, J.; Ruan, X.; Xu, X.; Li, X.; Bao, M.; Shao, Y.; Bian, X.; Li, R.; Jiang, Q.; et al. Cardiac injury activates STING signaling via upregulating SIRT6 in macrophages after myocardial infarction. Life Sci. 2024, 341, 122474. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Huang, S.; Du, Y.; Zhou, H.; Zhang, K.; He, J. Lats2 deficiency protects the heart against myocardial infarction by reducing inflammation and inhibiting mitochondrial fission and STING/p65 signaling. Int. J. Biol. Sci. 2023, 19, 3428–3440. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zhang, H.; Tang, B.; Luo, Y.; Yang, Y.; Zhong, X.; Chen, S.; Xu, X.; Huang, S.; Liu, C. Macrophages in cardiovascular diseases: Molecular mechanisms and therapeutic targets. Signal Transduct. Target. Ther. 2024, 9, 130. [Google Scholar] [CrossRef] [PubMed]
- King, K.R.; Aguirre, A.D.; Ye, Y.X.; Sun, Y.; Roh, J.D.; Ng, R.P., Jr.; Kohler, R.H.; Arlauckas, S.P.; Iwamoto, Y.; Savol, A.; et al. IRF3 and type I interferons fuel a fatal response to myocardial infarction. Nat. Med. 2017, 23, 1481–1487. [Google Scholar] [CrossRef] [PubMed]
- Bird, L. Cytokines: The inflamed heart. Nat. Rev. Immunol. 2017, 17, 732. [Google Scholar] [CrossRef]
- Lavine, K.J.; Mann, D.L. Recognition of self-DNA drives cardiac inflammation: Why broken hearts fail. Nat. Med. 2017, 23, 1400–1401. [Google Scholar] [CrossRef]
- Rech, L.; Abdellatif, M.; Pöttler, M.; Stangl, V.; Mabotuwana, N.; Hardy, S.; Rainer, P.P. Small molecule STING inhibition improves myocardial infarction remodeling. Life Sci. 2022, 291, 120263. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Gao, Y.; Gao, R.; Wang, Y.; Qu, Y.; Yang, J.; Wei, X.; Zhang, F.; Ge, J. The selective STING inhibitor H-151 preserves myocardial function and ameliorates cardiac fibrosis in murine myocardial infarction. Int. Immunopharmacol. 2022, 107, 108658. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.; Ke, Q.; Nie, Q.; Qi, R.; Zhu, X.; Liu, W.; Hu, X.; Sun, Q.; Fu, J.L.; Tang, X.; et al. Inhibition of cGAS-STING by JQ1 alleviates oxidative stress-induced retina inflammation and degeneration. Cell Death Differ. 2022, 29, 1816–1833. [Google Scholar] [CrossRef] [PubMed]
- Wiser, C.; Kim, B.; Vincent, J.; Ascano, M. Small molecule inhibition of human cGAS reduces total cGAMP output and cytokine expression in cells. Sci. Rep. 2020, 10, 7604. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.; Adura, C.; Gao, P.; Luz, A.; Lama, L.; Asano, Y.; Okamoto, R.; Imaeda, T.; Aida, J.; Rothamel, K.; et al. Small molecule inhibition of cGAS reduces interferon expression in primary macrophages from autoimmune mice. Nat. Commun. 2017, 8, 750. [Google Scholar] [CrossRef]
- Lama, L.; Adura, C.; Xie, W.; Tomita, D.; Kamei, T.; Kuryavyi, V.; Gogakos, T.; Steinberg, J.I.; Miller, M.; Ramos-Espiritu, L.; et al. Development of human cGAS-specific small-molecule inhibitors for repression of dsDNA-triggered interferon expression. Nat. Commun. 2019, 10, 2261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Du, J.; Liu, D.; Zhuo, J.; Chu, L.; Li, Y.; Gao, L.; Xu, M.; Chen, W.; Huang, W.; et al. Polystyrene microplastics induce pulmonary fibrosis by promoting alveolar epithelial cell ferroptosis through cGAS/STING signaling. Ecotoxicol. Environ. Saf. 2024, 277, 116357. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Li, G.; Tao, J.; Wu, N.N.; Kandadi, M.R.; Bi, Y.; Wang, S.; Pei, Z.; Ren, J. Double knockout of Akt2 and AMPK accentuates high fat diet-induced cardiac anomalies through a cGAS-STING-mediated mechanism. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165855. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.; Brault, A.; Vincent, F.; Weng, S.; Wang, H.; Dumlao, D.; Aulabaugh, A.; Aivazian, D.; Castro, D.; Chen, M.; et al. Discovery of PF-06928215 as a high affinity inhibitor of cGAS enabled by a novel fluorescence polarization assay. PLoS ONE 2017, 12, e0184843. [Google Scholar] [CrossRef]
- Wang, X.; Lu, W.; Xia, X.; Zhu, Y.; Ge, C.; Guo, X.; Zhang, N.; Chen, H.; Xu, S. Selenomethionine mitigate PM2.5-induced cellular senescence in the lung via attenuating inflammatory response mediated by cGAS/STING/NF-κB pathway. Ecotoxicol. Environ. Saf. 2022, 247, 114266. [Google Scholar] [CrossRef]
- Zhao, W.; Xiong, M.; Yuan, X.; Li, M.; Sun, H.; Xu, Y. In Silico Screening-Based Discovery of Novel Inhibitors of Human Cyclic GMP-AMP Synthase: A Cross-Validation Study of Molecular Docking and Experimental Testing. J. Chem. Inf. Model. 2020, 60, 3265–3276. [Google Scholar] [CrossRef] [PubMed]
- Eissa, N.; Hussein, H.; Kermarrec, L.; Elgazzar, O.; Metz-Boutigue, M.H.; Bernstein, C.N.; Ghia, J.E. Chromofungin (CHR: CHGA(47-66)) is downregulated in persons with active ulcerative colitis and suppresses pro-inflammatory macrophage function through the inhibition of NF-κB signaling. Biochem. Pharmacol. 2017, 145, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Kwak, S.C.; Lee, C.; Kim, J.Y.; Oh, H.M.; So, H.S.; Lee, M.S.; Rho, M.C.; Oh, J. Chlorogenic acid inhibits osteoclast differentiation and bone resorption by down-regulation of receptor activator of nuclear factor kappa-B ligand-induced nuclear factor of activated T cells c1 expression. Biol. Pharm. Bull. 2013, 36, 1779–1786. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Baek, S.I.; Yun, J.; Lee, S.; Yoon, D.Y.; Jung, J.K.; Jung, S.H.; Hwang, B.Y.; Hong, J.T.; Han, S.B.; et al. IRAK4 as a molecular target in the amelioration of innate immunity-related endotoxic shock and acute liver injury by chlorogenic acid. J. Immunol. 2015, 194, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Song, Z.; Shen, A.; Chen, T.; Zhang, A. Small molecules targeting the innate immune cGAS–STING–TBK1 signaling pathway. Acta Pharm. Sin. B 2020, 10, 2272–2298. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; You, Y.; Sun, L.; Sui, Q.; Liu, L.; Yuan, H.; Chen, C.; Liu, J.; Wen, X.; Dai, L.; et al. The STING antagonist H-151 ameliorates psoriasis via suppression of STING/NF-κB-mediated inflammation. Br. J. Pharmacol. 2021, 178, 4907–4922. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Xu, M.M.; Fan, C.; Feng, C.L.; Lu, Q.K.; Lu, H.M.; Xiang, C.G.; Bai, F.; Wang, H.Y.; Wu, Y.W.; et al. STING inhibitor ameliorates LPS-induced ALI by preventing vascular endothelial cells-mediated immune cells chemotaxis and adhesion. Acta Pharmacol. Sin. 2022, 43, 2055–2066. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Wang, M.; Liu, S.; Liu, H.; Jiang, A.; Zou, Y.; Deng, Y.; Qin, Q.; Song, Y.; Zheng, Y. A sequential scheme including PTT and 2’3’-cGAMP/CQ-LP reveals the antitumor immune function of PTT through the type I interferon pathway. Pharmacol. Res. 2023, 196, 106939. [Google Scholar] [CrossRef]
- Hong, Z.; Mei, J.; Li, C.; Bai, G.; Maimaiti, M.; Hu, H.; Yu, W.; Sun, L.; Zhang, L.; Cheng, D.; et al. STING inhibitors target the cyclic dinucleotide binding pocket. Proc. Natl. Acad. Sci. USA 2021, 118, e2105465118. [Google Scholar] [CrossRef]
- Montesinos, P.; Al-Ali, H.; Alonso-Dominguez, J.M.; Jentzsch, M.; Jongen-Lavrencic, M.; Martelli, M.P.; Röllig, C.; Sica, S.; Iadevaia, R.; Yablonski, K.; et al. Abstract CT124: A first-in-clinic phase 1 study of GSK3745417 STING agonist in relapsed/refractory acute myeloid leukemia and high-risk myelodysplastic syndrome. Cancer Res. 2023, 83, CT124. [Google Scholar] [CrossRef]
- Li, S.; Hong, Z.; Wang, Z.; Li, F.; Mei, J.; Huang, L.; Lou, X.; Zhao, S.; Song, L.; Chen, W.; et al. The Cyclopeptide Astin C Specifically Inhibits the Innate Immune CDN Sensor STING. Cell Rep. 2018, 25, 3405–3421.e7. [Google Scholar] [CrossRef]
- Ullah, T.R.; Balka, K.R.; Ambrose, R.L.; Pépin, G.; Wilce, M.C.J.; Wilce, J.A.; Thomas, B.J.; De Nardo, D.; Williams, B.R.G.; Gantier, M.P. Genistein Targets STING-Driven Antiviral Responses. mBio 2022, 13, e0206422. [Google Scholar] [CrossRef]
- Wen, J.; Qin, S.; Li, Y.; Zhang, P.; Zhan, X.; Fang, M.; Shi, C.; Mu, W.; Kan, W.; Zhao, J.; et al. Flavonoids derived from licorice suppress LPS-induced acute lung injury in mice by inhibiting the cGAS-STING signaling pathway. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2023, 175, 113732. [Google Scholar] [CrossRef]
- Gao, X.; Wang, J.; Wang, Y.; Liu, S.; Dong, K.; Wu, J.; Wu, X.; Shi, D.; Wang, F.; Guo, C. Fucoidan-ferulic acid nanoparticles alleviate cisplatin-induced acute kidney injury by inhibiting the cGAS-STING pathway. Int. J. Biol. Macromol. 2022, 223, 1083–1093. [Google Scholar] [CrossRef]
- Gu, J.; Liu, X.; Ji, Z.; Shen, M.; Zhu, M.; Ren, Y.; Guo, L.; Yang, K.; Liu, T.; Yi, X. Tumor Vascular Destruction and cGAS-STING Activation Induced by Single Drug-Loaded Nano-Micelles for Multiple Synergistic Therapies of Cancer. Small 2023, 19, e2303517. [Google Scholar] [CrossRef]
- Ji, X.; Meng, Y.; Wang, Q.; Tong, T.; Liu, Z.; Lin, J.; Li, B.; Wei, Y.; You, X.; Lei, Y.; et al. Cysteine-Based Redox-Responsive Nanoparticles for Fibroblast-Targeted Drug Delivery in the Treatment of Myocardial Infarction. ACS Nano 2023, 17, 5421–5434. [Google Scholar] [CrossRef]
- Li, Y.; Chen, X.; Jin, R.; Chen, L.; Dang, M.; Cao, H.; Dong, Y.; Cai, B.; Bai, G.; Gooding, J.J.; et al. Injectable hydrogel with MSNs/microRNA-21-5p delivery enables both immunomodification and enhanced angiogenesis for myocardial infarction therapy in pigs. Sci. Adv. 2021, 7, eabd6740. [Google Scholar] [CrossRef]
- Muñoz, M.; Eren Cimenci, C.; Goel, K.; Comtois-Bona, M.; Hossain, M.; McTiernan, C.; Zuñiga-Bustos, M.; Ross, A.; Truong, B.; Davis, D.R.; et al. Nanoengineered Sprayable Therapy for Treating Myocardial Infarction. ACS Nano 2022, 16, 3522–3537. [Google Scholar] [CrossRef]
- Wang, K.; Zhu, K.; Zhu, Z.; Shao, F.; Qian, R.; Wang, C.; Dong, H.; Li, Y.; Gao, Z.; Zhao, J. Triptolide with hepatotoxicity and nephrotoxicity used in local delivery treatment of myocardial infarction by thermosensitive hydrogel. J. Nanobiotechnol. 2023, 21, 227. [Google Scholar] [CrossRef]
- Zheng, Z.; Sun, J.; Wang, J.; He, S.; Liu, Z.; Xie, J.; Yu, C.Y.; Wei, H. Enhancing myocardial infarction treatment through bionic hydrogel-mediated spatial combination therapy via mtDNA-STING crosstalk modulation. J. Control. Release 2024, 371, 570–587. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, Y.; Bai, Y.; Wei, Y. Application of biomedical materials in the diagnosis and treatment of myocardial infarction. J. Nanobiotechnol. 2023, 21, 298. [Google Scholar] [CrossRef] [PubMed]

| Classification | Name | Features | Limitations |
|---|---|---|---|
| Direct inhibitors | RU.521 | Highly selective, capable of effectively inhibiting cGAS activity and reducing DNA-induced interferon production. | Clinical application data are limited, and there may be issues with specificity and pharmacokinetics. |
| G150 | A novel small molecule that directly binds to cGAS and inhibits its activity. It has shown good efficacy both in vitro and in vivo. | It is in the preclinical stage and requires further research to confirm its long-term safety and efficacy. | |
| Competitive inhibitors | PF-06928215 | Shows potential for various autoimmune and inflammatory diseases. | It is in the preclinical research stage and has not yet entered clinical trials. |
| Antisense oligonucleotides | IONIS-cGAS | Targets cGAS mRNA, reduces cGAS protein levels, and has shown significant anti-inflammatory effects in animal models. | Delivery and stability of antisense oligonucleotides are challenging, requiring solutions for cellular uptake and degradation issues. |
| Classification | Name | Features | Limitations |
|---|---|---|---|
| Small-molecule STING inhibitors | H-151 | High specificity, good drug tunability, and easy manufacturing and modification. | Potential off-target effects, poor metabolic stability, and high clinical trial risks. |
| C-176 | |||
| C-178 | |||
| SN-011 | |||
| GSK3745417 | |||
| Natural-product STING inhibitors | Astin C | Multifunctionality, lower toxicity, and good biocompatibility. | Difficulty in large-scale production through synthetic means; lower stability and bioavailability. |
| Flavonoids |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, M.; Li, F.; Pei, H. RETRACTED: The cGAS-STING Pathway: A New Therapeutic Target for Ischemia–Reperfusion Injury in Acute Myocardial Infarction? Biomedicines 2024, 12, 1728. https://doi.org/10.3390/biomedicines12081728
Tian M, Li F, Pei H. RETRACTED: The cGAS-STING Pathway: A New Therapeutic Target for Ischemia–Reperfusion Injury in Acute Myocardial Infarction? Biomedicines. 2024; 12(8):1728. https://doi.org/10.3390/biomedicines12081728
Chicago/Turabian StyleTian, Mengxiang, Fengyuan Li, and Haiping Pei. 2024. "RETRACTED: The cGAS-STING Pathway: A New Therapeutic Target for Ischemia–Reperfusion Injury in Acute Myocardial Infarction?" Biomedicines 12, no. 8: 1728. https://doi.org/10.3390/biomedicines12081728
APA StyleTian, M., Li, F., & Pei, H. (2024). RETRACTED: The cGAS-STING Pathway: A New Therapeutic Target for Ischemia–Reperfusion Injury in Acute Myocardial Infarction? Biomedicines, 12(8), 1728. https://doi.org/10.3390/biomedicines12081728








