MicroRNAs in Sepsis
Abstract
:1. Introduction
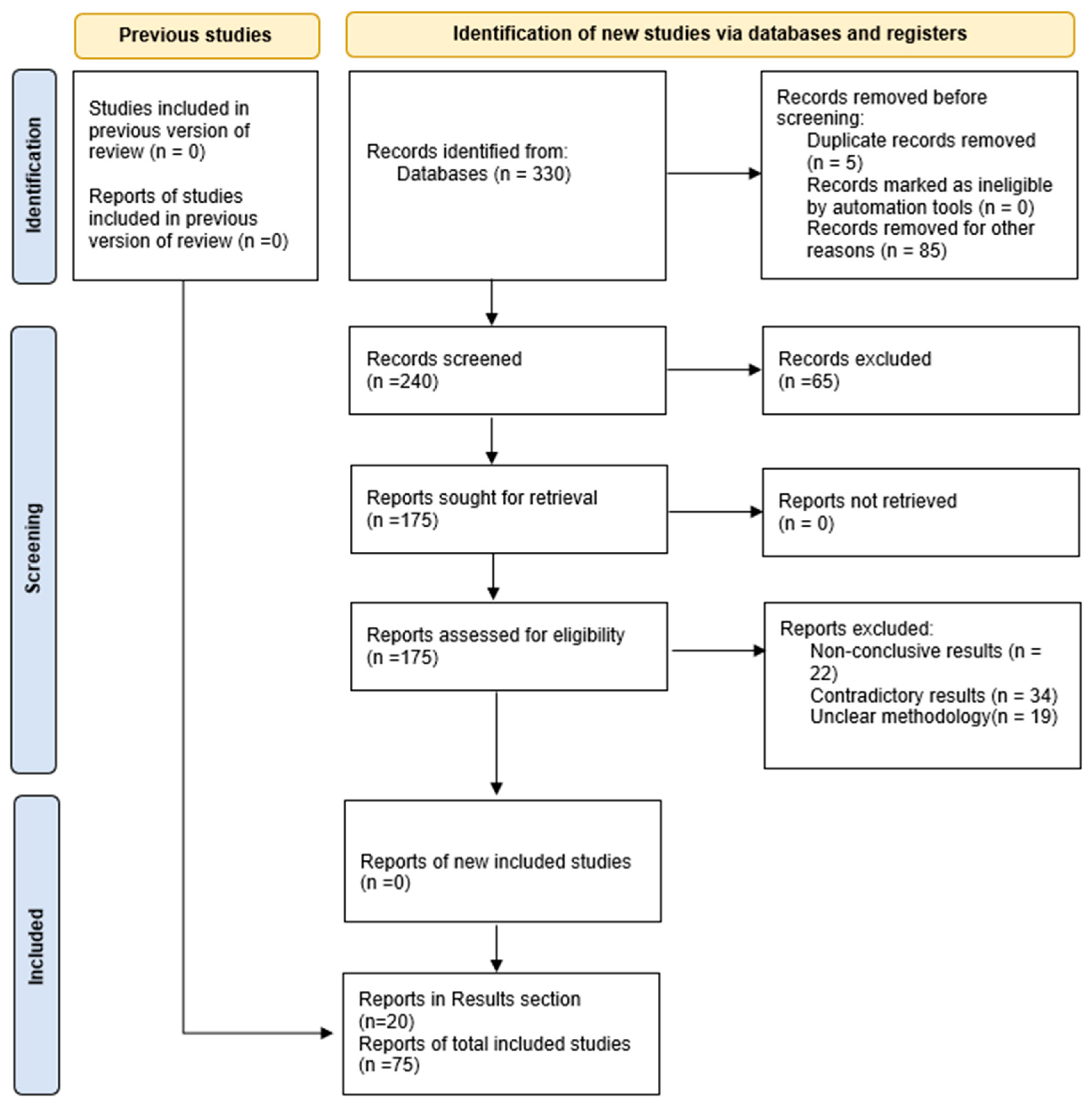
2. Materials and Methods
- PHASE I. Utilization of the keywords sepsis, non-coding RNAs, microRNAs, inflammation, immune response, biomarkers, and diagnosis using various combinations.
- PHASE II. Further investigation is based on specific miRs of interest by their coded term, in combination with sepsis effects.
- PHASE III. Evaluation of information obtained from thorough and recent review articles and cross-referencing of their sources.
3. Results
3.1. Correlation of miR with Specific Sepsis-Related Cellular Functions
3.2. miR Diagnostic Value Evaluation Based on MIR Function
- Inhibition of the slit2Robo4 pathway that regulates sepsis-induced endothelial activation is correlated with MIR-218 down-regulation. MIR-218 has been postulated to modulate endothelial inflammation [62];
- ADAMS15 disintegrin–metalloproteinase modulates endothelial permeability, and its mRNA expression has been shown to be inhibited by MIR-147b [45];
- MIRs-146a/b block components of pro-inflammation pathways such as NF-kB, AP-1 and MAPK [63];
- Angiopoietin-1 (AP-1) seems to be blocked also by MIR-150 [64];
- Inhibition of MIR-146a aggravates endothelial VCAM-1 expression [46].
- The inflammation mechanism involved in sepsis. Whether anti-inflammatory pathways are suppressed (MIR-181b, MIR-150 and MIR-223-5p) or pro-inflammatory pathways are augmented (MIR-155).
- Identifying which organs are affected by sepsis since different miRs may be activated or silenced in different tissues and/or cell types, as discussed earlier with MIR-181b in different brain regions.
- The age of the septic patient since, during differentiation and maturation, different miRs may be silenced or activated. For example, MIR-223 is shown to be up-regulated in pediatric sepsis, while MIR-146a is down-regulated.
- The condition of the septic patient, e.g., MIR-141 is down-regulated in sepsis during pregnancy, and MIR-23b pinpoints disease severity [48].
3.3. Positive Aspects for the Utilization of miRs as Biomarkers for Sepsis
- miRs have proven to be very stable and easily detectable in small samples of blood and blood fractions of serum, plasma, WBCs and any other cell component to be analyzed, as well as tissue and organ biopsies and aspirations.
- Since each miR has been mapped to its specific mRNA target, the relevance with sepsis is associated with specific gene products known to regulate inflammation processes during the disease, thus validating the observed miR levels compared to healthy controls.
- Since miR expression levels or silencing depend on cell and tissue differentiation, they can be used selectively as markers for age-dependent screening. For example, Fatmi et al. (2022) proposed a large set of 20 miRs as reliable biomarkers for neonatal sepsis based on increased or decreased levels of expression in sepsis compared to controls, all analyzed with the same RT-PCR technique [47].
- Up or down-regulation of each suspect miR can easily correspond with inverse levels of target mRNAs and final protein products, confirming the validity of the observations correlating with specific cellular dysfunctions in the disease state.
- miRs have been shown to be valuable tools in identifying or confirming the infection causative agent. For example, it has been shown that detectable consistent alterations of certain miRs are directly correlated with the source of infection, such as MIR-155 for DNA virus infections, MIR-96 for down-regulation in Gram (+) bacterial infections, and MIR-101 also down-regulated in Gram (−) bacterial infections [48].
- miR expression levels can be used to monitor sepsis progression or attenuation; e.g., MIR-23b can be proposed as a biomarker for disease severity.
4. Discussion
- The proposed panel for neonatal sepsis;
- Panel for confirming or even identifying sepsis causative infection;
- Panel for pro-inflammatory miRs and another for anti-inflammatory miRs;
- Panel for both miRs and corresponding competitive LNCs;
- Panel for detecting progression rate or disease attenuation;
- Panel for organ-specific miR functional dysregulation.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Bone, R.C.; Balk, R.A.; Cerra, F.B.; Dellinger, R.P.; Fein, A.M.; Knaus, W.A.; Schein, R.M.H.; Sibbald, W.J. Definitions for Sepsis and Organ Failure and Guidelines for the Use of Innovative Therapies in Sepsis. Chest 1992, 101, 1644–1655. [Google Scholar] [CrossRef] [PubMed]
- Erlanger, D.; Assous, M.V.; Wiener-Well, Y.; Yinnon, A.M.; Ben-Chetrit, E. Clinical manifestations, risk factors and prognosis of patients with Morganella morganii sepsis. J. Microbiol. Immunol. Infect. 2019, 52, 443–448. [Google Scholar] [CrossRef]
- Antonakos, N.; Gilbert, C.; Théroude, C.; Schrijver, I.T.; Roger, T. Modes of action and diagnostic value of miRNAs in sepsis. Front. Immunol. 2022, 13, 951798. [Google Scholar] [CrossRef] [PubMed]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Cecconi, M.; Evans, L.; Levy, M.; Rhodes, A. Sepsis and septic shock. Lancet 2018, 392, 75–87. [Google Scholar] [CrossRef]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensiv. Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef]
- Dellinger, R.P.; Levy, M.M.; Rhodes, A.; Annane, D.; Gerlach, H.; Opal, S.M.; Sevransky, J.E.; Sprung, C.L.; Douglas, I.S.; Jaeschke, R.; et al. Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup. Sur-viving sepsis campaign: International guidelines for management of severe sepsis and septic shock: 2012. Crit. Care Med. 2013, 41, 580–637. [Google Scholar] [CrossRef]
- Walayat, A.; Yang, M.; Xiao, D. Therapeutic Implication of miRNA in Human Disease. In Antisense Therapy; Sharad, S., Kapur, S., Eds.; IntechOpen: London, UK, 2019; ISBN 978-1-78984-532-7. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef]
- Dexheimer, P.J.; Cochella, L. MicroRNAs: From Mechanism to Organism. Front. Cell Dev. Biol. 2020, 8, 409. [Google Scholar] [CrossRef]
- Reichholf, B.; Herzog, V.A.; Fasching, N.; Manzenreither, R.A.; Sowemimo, I.; Ameres, S.L. Time-Resolved Small RNA Sequencing Unravels the Molecular Principles of MicroRNA Homeostasis. Mol. Cell 2019, 75, 756–768.e7. [Google Scholar] [CrossRef] [PubMed]
- Backes, Y.; Van Der Sluijs, K.F.; Mackie, D.P.; Tacke, F.; Koch, A.; Tenhunen, J.J.; Schultz, M.J. Usefulness of suPAR as a biological marker in patients with systemic inflammation or infection: A systematic review. Intensiv. Care Med. 2012, 38, 1418–1428. [Google Scholar] [CrossRef] [PubMed]
- Wacker, C.; Prkno, A.; Brunkhorst, F.M.; Schlattmann, P. Procalcitonin as a diagnostic marker for sepsis: A systematic review and meta-analysis. Lancet Infect. Dis. 2013, 13, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Kyriazopoulou, E.; Leventogiannis, K.; Tavoulareas, G.; Mainas, E.; Toutouzas, K.; Mathas, C.; Prekates, A.; Sakka, V.; Panagopoulos, P.; Syrigos, K.; et al. Presepsin as a diagnostic and prognostic biomarker of severe bacterial infections and COVID-19. Sci. Rep. 2023, 13, 3814. [Google Scholar] [CrossRef]
- Dépret, F.; Hollinger, A.; Cariou, A.; Deye, N.; Vieillard-Baron, A.; Fournier, M.-C.; Jaber, S.; Damoisel, C.; Lu, Q.; Monnet, X.; et al. Incidence and Outcome of Subclinical Acute Kidney Injury Using penKid in Critically Ill Patients. Am. J. Respir. Crit. Care Med. 2020, 202, 822–829. [Google Scholar] [CrossRef]
- Samaras, C.; Kyriazopoulou, E.; Poulakou, G.; Reiner, E.; Kosmidou, M.; Karanika, I.; Petrakis, V.; Adamis, G.; Gatselis, N.K.; Fragkou, A.; et al. Interferon gamma-induced protein 10 (IP-10) for the early prognosis of the risk for severe respiratory failure and death in COVID-19 pneumonia. Cytokine 2022, 162, 156111. [Google Scholar] [CrossRef]
- Pool, R.; Gomez, H.; Kellum, J.A. Mechanisms of Organ Dysfunction in Sepsis. Crit. Care Clin. 2017, 34, 63–80. [Google Scholar] [CrossRef]
- Harbarth, S.; Holeckova, K.; Froidevaux, C.; Pittet, D.; Ricou, B.; Grau, G.E.; Vadas, L.; Pugin, J.; The Geneva Sepsis Network. Diagnostic Value of Procalcitonin, Interleukin-6, and Interleukin-8 in Critically Ill Patients Admitted with Suspected Sepsis. Am. J. Respir. Crit. Care Med. 2001, 164, 396–402. [Google Scholar] [CrossRef]
- Hou, T.; Huang, D.; Zeng, R.; Ye, Z.; Zhang, Y. Accuracy of serum interleukin (IL)-6 in sepsis diagnosis: A systematic review and meta-analysis. Int. J. Clin. Exp. Med. 2015, 8, 15238–15245. [Google Scholar] [PubMed]
- Holub, M.; Džupová, O.; Růžková, M.; Stráníková, A.; Bartáková, E.; Máca, J.; Beneš, J.; Herwald, H.; Beran, O. Selected Biomarkers Correlate with the Origin and Severity of Sepsis. Mediat. Inflamm. 2018, 2018, 7028267. [Google Scholar] [CrossRef]
- Huang, M.; Cai, S.; Su, J. The Pathogenesis of Sepsis and Potential Therapeutic Targets. Int. J. Mol. Sci. 2019, 20, 5376. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Meng, K.; Chen, W.J.; Feng, D.; Jia, Y.; Xie, L. Serum miR-574-5p: A prognostic predictor of sepsis patients. Shock 2012, 37, 263–267. [Google Scholar] [CrossRef]
- Lill, M.; Kõks, S.; Soomets, U.; Schalkwyk, L.C.; Fernandes, C.; Lutsar, I.; Taba, P. Peripheral blood RNA gene expression profiling in patients with bacterial meningitis. Front. Neurosci. 2013, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Hu, D.; Li, Q.; Xu, T.; Sui, Y.; Qu, Y. Protective effects of microRNA-181b on aged rats with sepsis-induced hippocampus injury in vivo. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2019, 31, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Zhang, Y.; Cui, Y.; Ren, Y.; Li, R.; Rong, Q. Inhibition of microRNA-155 relieves sepsis-induced liver injury through inactivating the JAK/STAT pathway. Mol. Med. Rep. 2015, 12, 6013–6018. [Google Scholar] [CrossRef]
- Maiese, A.; Scatena, A.; Costantino, A.; Chiti, E.; Occhipinti, C.; La Russa, R.; Di Paolo, M.; Turillazzi, E.; Frati, P.; Fineschi, V. Expression of MicroRNAs in Sepsis-Related Organ Dysfunction: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 9354. [Google Scholar] [CrossRef]
- Wang, H.-F.; Li, Y.; Wang, Y.-Q.; Li, H.-J.; Dou, L. MicroRNA-494-3p alleviates inflammatory response in sepsis by targeting TLR6. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 2971–2977. [Google Scholar] [CrossRef]
- Adamzik, M.; Schäfer, S.; Frey, U.H.; Becker, A.; Kreuzer, M.; Winning, S.; Frede, S.; Steinmann, J.; Fandrey, J.; Zacharowski, K.; et al. The NFKB1 Promoter Polymorphism (−94ins/delATTG) Alters Nuclear Translocation of NF-κB1 in Monocytes after Lipopolysaccharide Stimulation and Is Associated with Increased Mortality in Sepsis. Anesthesiology 2013, 118, 123–133. [Google Scholar] [CrossRef]
- Vasques-Nóvoa, F.; Laundos, T.L.M.; Cerqueira, R.J.; Quina-Rodrigues, C.; Soares-Dos-Reis, R.; Baganha, F.M.; Ribeiro, S.; Mendonça, L.; Gonçalves, F.; Reguenga, C.; et al. MicroRNA-155 Amplifies Nitric Oxide/cGMP Signaling and Impairs Vascular Angiotensin II Reactivity in Septic Shock. Crit. Care Med. 2018, 46, e945–e954. [Google Scholar] [CrossRef]
- Chen, S.-L.; Cai, G.-X.; Ding, H.-G.; Liu, X.-Q.; Wang, Z.-H.; Jing, Y.-W.; Han, Y.-L.; Jiang, W.-Q.; Wen, M.-Y. JAK/STAT signaling pathway-mediated microRNA-181b promoted blood-brain barrier impairment by targeting sphingosine-1-phosphate receptor 1 in septic rats. Ann. Transl. Med. 2020, 8, 1458. [Google Scholar] [CrossRef]
- Ma, X.-F.; Qin, J.; Guo, X.-H. MiR-181-5p protects mice from sepsis via repressing HMGB1 in an experimental model. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 9712–9720. [Google Scholar] [CrossRef]
- Liu, D.; Wang, Z.; Wang, H.; Ren, F.; Li, Y.; Zou, S.; Xu, J.; Xie, L. The protective role of miR-223 in sepsis-induced mortality. Sci. Rep. 2020, 10, 17691. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zong, X.; Cheng, Y.; Xu, J.; Deng, J.; Huang, Y.; Ma, C.; Fu, Q. miR-223-3p contributes to suppressing NLRP3 inflammasome activation in Streptococcus equi ssp. zooepidemicus infection. Veter. Microbiol. 2022, 269, 109430. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhao, L.; Zhang, L.; Qiao, L.; Gao, S. Downregulation of miR-574-5p inhibits HK-2 cell viability and predicts the onset of acute kidney injury in sepsis patients. Ren. Fail. 2021, 43, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Wang, J.; Li, Z.; Li, J.; Sang, M. MicroRNA-205-5b inhibits HMGB1 expression in LPS-induced sepsis. Int. J. Mol. Med. 2016, 38, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, P.; Goodwin, A.J.; Cook, J.A.; Halushka, P.V.; Chang, E.; Zingarelli, B.; Fan, H. Exosomes from endothelial progenitor cells improve outcomes of the lipopolysaccharide-induced acute lung injury. Crit. Care 2019, 23, 44. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, Y.; Hou, H.; Yao, Y.; Meng, H. MiR-150 predicts survival in patients with sepsis and inhibits LPS-induced inflammatory factors and apoptosis by targeting NF-κB1 in human umbilical vein endothelial cells. Biochem. Biophys. Res. Commun. 2018, 500, 828–837. [Google Scholar] [CrossRef]
- Möhnle, P.; Hirschberger, S.; Hinske, L.C.; Briegel, J.; Hübner, M.; Weis, S.; Dimopoulos, G.; Bauer, M.; Giamarellos-Bourboulis, E.J.; Kreth, S. MicroRNAs 143 and 150 in whole blood enable detection of T-cell immunoparalysis in sepsis. Mol. Med. 2018, 24, 54. [Google Scholar] [CrossRef]
- Karam, R.A.; Zidan, H.E.; Karam, N.A.; Rahman, D.M.A.; El-Seifi, O.S. Diagnostic and prognostic significance of serum miRNA-146-a expression in Egyptian children with sepsis in a pediatric intensive care unit. J. Gene Med. 2019, 21, e3128. [Google Scholar] [CrossRef]
- Lu, Z.; Yu, H.; Xu, Y.; Chen, K.; Lin, Y.; Lin, K.; Wang, Y.; Xu, K.; Fu, L.; Li, W.; et al. LNC-ZNF33B-2:1 gene rs579501 polymorphism is associated with organ dysfunction and death risk in pediatric sepsis. Front. Genet. 2022, 13, 947317. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, S.; Cao, Y.; Yang, Y. Altered miRNAs Expression Profiles and Modulation of Immune Response Genes and Proteins During Neonatal Sepsis. J. Clin. Immunol. 2014, 34, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Wu, P.; Li, J. miR-331 inhibits CLDN2 expression and may alleviate the vascular endothelial injury induced by sepsis. Exp. Ther. Med. 2020, 20, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, V.; Beard, R.S., Jr.; Reynolds, J.J.; Haines, R.; Guo, M.; Rubin, M.; Guido, J.; Wu, M.H.; Yuan, S.Y. MicroRNA-147b Regulates Vascular Endothelial Barrier Function by Targeting ADAM15 Expression. PLoS ONE 2014, 9, e110286. [Google Scholar] [CrossRef]
- Wu, M.; Gu, J.-T.; Yi, B.; Tang, Z.-Z.; Tao, G.-C. microRNA-23b regulates the expression of inflammatory factors in vascular endothelial cells during sepsis. Exp. Ther. Med. 2015, 9, 1125–1132. [Google Scholar] [CrossRef]
- Murugesapillai, D.; McCauley, M.J.; Maher, L.J., 3rd; Williams, M.C. Single-molecule studies of high-mobility group B architectural DNA bending proteins. Biophys. Rev. 2016, 9, 17–40. [Google Scholar] [CrossRef] [PubMed]
- Fatmi, A.; Chabni, N.; Cernada, M.; Vento, M.; González-López, M.; Aribi, M.; Pallardó, F.V.; García-Giménez, J.L. Clinical and immunological aspects of microRNAs in neonatal sepsis. Biomed. Pharmacother. 2021, 145, 112444. [Google Scholar] [CrossRef]
- Zeng, Z.; Lan, Y.; Chen, Y.; Zuo, F.; Gong, Y.; Luo, G.; Peng, Y.; Yuan, Z. LncRNA GAS5 suppresses inflammatory responses by inhibiting HMGB1 release via miR-155-5p/SIRT1 axis in sepsis. Eur. J. Pharmacol. 2023, 942, 175520. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, J.; Huang, Y.; Tong, J.; Zhang, L.; Zhang, Z.; Yu, W.; Qiu, Y. Accuracy of circulating microRNAs in diagnosis of sepsis: A systematic review and meta-analysis. J. Intensiv. Care 2020, 8, 84. [Google Scholar] [CrossRef]
- Qu, Y.-L.; Wang, H.-F.; Sun, Z.-Q.; Tang, Y.; Han, X.-N.; Yu, X.-B.; Liu, K. Up-regulated miR-155-5p promotes cell proliferation, invasion and metastasis in colorectal carcinoma. Int. J. Clin. Exp. Pathol. 2015, 8, 6988–6994. [Google Scholar] [PubMed]
- Chen, M.; Wang, F.; Xia, H.; Yao, S. MicroRNA-155: Regulation of Immune Cells in Sepsis. Mediat. Inflamm. 2021, 2021, 8874854. [Google Scholar] [CrossRef]
- O’Connell, R.M.; Taganov, K.D.; Boldin, M.P.; Cheng, G.; Baltimore, D. MicroRNA-155 is induced during the mac-rophage inflammatory response. Proc. Natl. Acad. Sci. USA 2007, 104, 1604–1609. [Google Scholar] [CrossRef] [PubMed]
- McCoy, C.E.; Sheedy, F.J.; Qualls, J.E.; Doyle, S.L.; Quinn, S.R.; Murray, P.J.; O’Neill, L.A. IL-10 Inhibits miR-155 Induction by Toll-like Receptors. J. Biol. Chem. 2010, 285, 20492–20498. [Google Scholar] [CrossRef] [PubMed]
- Formosa, A.; Turgeon, P.; dos Santos, C.C. Role of miRNA dysregulation in sepsis. Mol. Med. 2022, 28, 99. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, H.-C.; Chen, C.; Zeng, J.; Wang, Q.; Zheng, L.; Yu, H.-D. Differential expression of plasma miR-146a in sepsis patients compared with non-sepsis-SIRS patients. Exp. Ther. Med. 2013, 5, 1101–1104. [Google Scholar] [CrossRef]
- Doxaki, C.; Kampranis, S.C.; Eliopoulos, A.G.; Spilianakis, C.; Tsatsanis, C. Coordinated Regulation of miR-155 and miR-146a Genes during Induction of Endotoxin Tolerance in Macrophages. J. Immunol. 2015, 195, 5750–5761. [Google Scholar] [CrossRef]
- Xiao, W.; Mindrinos, M.N.; Seok, J.; Cuschieri, J.; Cuenca, A.G.; Gao, H.; Hayden, D.L.; Hennessy, L.; Moore, E.E.; Minei, J.P.; et al. A genomic storm in critically injured humans. J. Exp. Med. 2011, 208, 2581–2590. [Google Scholar] [CrossRef]
- Doerschug, K.C.; Delsing, A.S.; Schmidt, G.A.; Haynes, W.G. Impairments in microvascular reactivity are related to organ failure in human sepsis. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H1065–H1071. [Google Scholar] [CrossRef]
- Vincent, J.-L.; De Backer, D. Microvascular dysfunction as a cause of organ dysfunction in severe sepsis. Crit. Care 2005, 9 (Suppl. S4), S9–S12. [Google Scholar] [CrossRef]
- Ho, J.; Chan, H.; Wong, S.H.; Wang, M.H.T.; Yu, J.; Xiao, Z.; Liu, X.; Choi, G.; Leung, C.C.H.; Wong, W.T.; et al. The involvement of regulatory non-coding RNAs in sepsis: A systematic review. Crit. Care 2016, 20, 383. [Google Scholar] [CrossRef]
- Zhao, H.; Anand, A.R.; Ganju, R.K. Slit2–Robo4 Pathway Modulates Lipopolysaccharide-Induced Endothelial Inflammation and Its Expression Is Dysregulated during Endotoxemia. J. Immunol. 2014, 192, 385–393. [Google Scholar] [CrossRef]
- Cheng, H.S.; Sivachandran, N.; Lau, A.; Boudreau, E.; Zhao, J.L.; Baltimore, D.; Delgado-Olguin, P.; Cybulsky, M.I.; Fish, J.E. MicroRNA-146 represses endothelial activation by inhibiting pro-inflammatory pathways. EMBO Mol. Med. 2013, 5, 1017–1034. [Google Scholar] [CrossRef]
- Rajput, C.; Tauseef, M.; Farazuddin, M.; Yazbeck, P.; Amin, M.-R.; Br, V.A.; Sharma, T.; Mehta, D. MicroRNA-150 Suppression of Angiopoetin-2 Generation and Signaling Is Crucial for Resolving Vascular Injury. Arter. Thromb. Vasc. Biol. 2016, 36, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Zhu, J.; Cao, L.; Zhu, B.; Lin, L. Ruscogenin Attenuates Lipopolysaccharide-Induced Septic Vascular Endothelial Dysfunction by Modulating the miR-146a-5p/NRP2/SSH1 Axis. Drug Des. Dev. Ther. 2023, 16, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Asahara, T.; Murohara, T.; Sullivan, A.; Silver, M.; van der Zee, R.; Li, T.; Witzenbichler, B.; Schatteman, G.; Isner, J.M. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997, 275, 964–966. [Google Scholar] [CrossRef] [PubMed]
- Kalka, C.; Masuda, H.; Takahashi, T.; Kalka-Moll, W.M.; Silver, M.; Kearney, M.; Li, T.; Isner, J.M.; Asahara, T. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc. Natl. Acad. Sci. USA 2000, 97, 3422–3427. [Google Scholar] [CrossRef]
- Li, X.; Chen, C.; Wei, L.; Li, Q.; Niu, X.; Xu, Y.; Wang, Y.; Zhao, J. Exosomes derived from endothelial progenitor cells attenuate vascular repair and accelerate reendothelialization by enhancing endothelial function. Cytotherapy 2016, 18, 253–262. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.; Li, L.; Li, M.; Guo, C.; Yao, J.; Mi, S. Exosome and Exosomal MicroRNA: Trafficking, Sorting, and Function. Genom. Proteom. Bioinform. 2015, 13, 17–24. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, Y.; Lin, S.; Li, Y.; Hua, Y.; Zhou, K. Diagnostic significance of microRNAs in sepsis. PLoS ONE 2023, 18, e0279726. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Fan, M.; Tu, F.; Yang, K.; Ha, T.; Liu, L.; Kalbfleisch, J.; Williams, D.; Li, C. Endothelial HSPA12B Exerts Protection Against Sepsis-Induced Severe Cardiomyopathy via Suppression of Adhesion Molecule Expression by miR-126. Front. Immunol. 2020, 11, 566. [Google Scholar] [CrossRef]
- Qian, Y.; Song, J.; Ouyang, Y.; Han, Q.; Chen, W.; Zhao, X.; Xie, Y.; Chen, Y.; Yuan, W.; Fan, C. Advances in Roles of miR-132 in the Nervous System. Front. Pharmacol. 2017, 8, 770. [Google Scholar] [CrossRef]
- Wang, C.; Liang, G.; Shen, J.; Kong, H.; Wu, D.; Huang, J.; Li, X. Long Non-Coding RNAs as Biomarkers and Therapeutic Targets in Sepsis. Front. Immunol. 2021, 12, 722004. [Google Scholar] [CrossRef] [PubMed]
- Bhan, A.; Soleimani, M.; Mandal, S.S. Long Noncoding RNA and Cancer: A New Paradigm. Cancer Res. 2017, 77, 3965–3981. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, L.; Wang, C.; Xie, X.; Han, Y. Research Progress of Long Non-Coding RNA in Tumor Drug Resistance: A New Paradigm. Drug Des. Dev. Ther. 2024, 18, 1385–1398. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, H.; Niu, M.; Wang, Y.; Xu, R.; Guo, Y.; Zhang, C. Roles of long noncoding RNAs in human inflammatory diseases. Cell Death Discov. 2024, 10, 235. [Google Scholar] [CrossRef]
| Biomarker | Role | Use | Reference |
|---|---|---|---|
| SuPAR—soluble urokinase-type plasminogen activator receptor | Non-specific inflammation marker, yet with prognostic value as high levels correlate with increased deaths | Prognosis | Backes 2012 [13] |
| PCT—procalcitonin | Distinguishes sepsis from SIRS | Diagnosis | Wacker 2013 [14] |
| sCD14—Presepsin | Macrophage/monocyte receptor recognizes LPS, useful for Gram(+) bacterial infections | Identification of cause | Kyriazopoulou 2023 [15] |
| Proenkephalin A | Reliable marker for progression of sepsis into acute kidney injury | Prognosis | Depret 2020 [16] |
| IP-10—interferon gamma-induced protein-10 | Marker for progression into severe respiratory failure | Prognosis | Samaras 2023 [17] |
| CRP—C reactive protein | Frequently used marker for detection of bacterial infections, used in combination with IP-10, distinguishes between viral and bacterial infections | Identification of cause | Pool 2017 [18] |
| IL-6—Interleukin 6 | Elevated levels correlate with severity of sepsis, especially in neonatal sepsis | Prognosis | Harbarth 2001 [19], Hou 2015 [20] |
| MCP-1—Monocyte Chemoattractant Protein-1 | Cytokine with serum levels elevated in sepsis | Diagnosis | Holub 2018 [21] |
| sTREM-1—Soluble Triggering Receptor Expressed on Myeloid Cells-1 | High sensitivity and specificity values in septic patients due to Shigella infection | Identification of cause | Huang 2019 [22] |
| CD64 | Useful marker for early detection | Diagnosis | Wang 2012 [23] |
| MIR | Target | Tissue | Function | Reference |
|---|---|---|---|---|
| MIR-155 | Myocardium and plasma | miR up-regulated in human sepsis | Vasques-Nóvoa F 2018 [30] | |
| MIR-155 | JACK/STAT pathway | Septic mice liver | Increased septic AHI | Lv-X 2015 [26] |
| MIR-181b | NF-kB pathway | Hippocampus | miR down-regulated in septic rats | Dong 2019 [25] |
| MIR-181b | sphingosine-1-phosphate receptor 1 | Cerebral cortex and serum | Up-regulated miR in septic rats | Chen S-L 2020 [31] |
| MIR181-5p | HMGB1 | Kidney of septic mice | Decreased infl response and decreased renal and hepatic dysfunction | Ma XF 2020 [32] |
| MIR-223-5p | FOXO1 | White blood cells of septic patients | Inversely proportional expression with rate of apoptosis | Liu D 2020 [33] |
| MIR-223-5p | NLRP3 | Septic mice | Suppresses formation of inflammasomes | Li 2022 [34] |
| MIR-494-3p | TLR-6 | MIR lower levels, TOL6 increased levels | Wang H-F 2019 [28] | |
| MIR-574-5p | STAT1 | Increased in serum of septic patients | Decreased sepsis-induced AKI | Liu S 2021 [35] |
| MIR-2055b | HMGB1 | Increased in serum and vital organs in septic mice | Increased a-infl activity in late sepsis | Zou 2016 [36] |
| MIR-23b | E-selectin, I-CAM1 | Leukocytes | Expression correlated with disease severity | Zhou 2019 [37] |
| MIR-150 | NF-kB1 | HUVECs and sepsis mice | MIR over-expression protected from apoptosis | Ma Y 2018 [38] |
| MIR-150 and MIR 143 | Nf-kB | Purified T cells from septic patients | Both miRs down-regulated in sepsis in correlation with SOFA scores | Mohnle 2018 [39] |
| MIR-146a | NF-kB | Down-regulated in sepsis and even more in septic shock | Karam 2019 [40] | |
| MIR-27a-6p | GSDMD | Sepsis-induced lung injury | Sepsis progression rate of miR expression and miR regulation by LNC-ZNF33B-2.1 | Lu Z 2022 [41] |
| MIR-96 | NF-kB | Plasma fraction of septic neonatals | Down-regulated in sepsis produced by Gram (+) bacteria | Chen J 2014 [42] |
| MIR-101 | PTGS-2 | Plasma fraction of septic neonatals | Down-regulated in sepsis produced by Gram (−) bacteria | Chen J 2014 [42] |
| MIR-331 | CLDN2 | Peripheral blood of septic patients | Down-regulates CLDN2 activity and restores cellular function of endothelial cells | Kong 2020 [43] |
| MIR147b | ADAM15 | Human vascular endothelial cells | Degrades ADAM15 mRNA as a protective mechanism | Chatterjee 2014 [44] |
| MIR146a | VCAM-1 | mice | MIR-146a knock-down enhances inflammatory action of VCAM-1 | Wu 2015 [45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valsamaki, A.; Vazgiourakis, V.; Mantzarlis, K.; Stamatiou, R.; Makris, D. MicroRNAs in Sepsis. Biomedicines 2024, 12, 2049. https://doi.org/10.3390/biomedicines12092049
Valsamaki A, Vazgiourakis V, Mantzarlis K, Stamatiou R, Makris D. MicroRNAs in Sepsis. Biomedicines. 2024; 12(9):2049. https://doi.org/10.3390/biomedicines12092049
Chicago/Turabian StyleValsamaki, Asimina, Vasileios Vazgiourakis, Konstantinos Mantzarlis, Rodopi Stamatiou, and Demosthenes Makris. 2024. "MicroRNAs in Sepsis" Biomedicines 12, no. 9: 2049. https://doi.org/10.3390/biomedicines12092049





