Abstract
Bacterial infections and cancers are important issues in public health around the world. Currently, Western medicine is the most suitable approach when dealing with these issues. “Antibiotics” and “Corticosteroids” are the Western medicines used for bacterial infection. “Chemotherapy drugs”, “surgery”, and “radiotherapy” are common techniques used to treat cancer. These are conventional treatments with many side effects. PDT is a non-invasive and effective therapy for bacterial infection and cancer diseases. Methods: Nine electronic databases, namely WanFang Data, PubMed, Science Direct, Scopus, Web of Science, Springer Link, SciFinder, and China National Knowledge Infrastructure (CNKI), were searched to conduct this literature review, without any regard to language constraints. Studies focusing on the photodynamic actions of hydrogel and Resveratrol were included that evaluated the effect of PDT against bacteria and cancer. All eligible studies were analyzed and summarized in this review. Results: Resveratrol has antibacterial and anticancer effects. It can also act as PS in PDT or adjuvant but has some limitations. This is much better when combined with a hydrogel to enhance the effectiveness of PDT in the fight against bacteria and cancer. Conclusions: Resveratrol combined with hydrogel is possible for PDT treatment in bacteria and cancer. They are compatible and reinforce each other to increase the effectiveness of PDT. However, much more work is required, such as cytotoxicity safety assessments of the human body and further enhancing the effectiveness of PDT in different environments for future investigations.
1. Introduction
Bacterial infection and cancer disease are issues involved in public health around the world. Currently, Western medicine is the common approach to managing these conditions.
Antibiotics and corticosteroids are medicines that are used to fight bacterial infections. They either kill or stop bacterial growth by affecting self-reproduction and preventing mutation, gene recombination, and transfer. This allows the body’s natural defenses to eliminate the pathogens [1]. The negative impacts of antibiotics are increasing morbidity and mortality, leading to antimicrobial resistance (AMR), which poses a major threat to human health in the world [2].
Chemotherapy drugs, surgery, and radiotherapy are the general techniques used to fight against cancer, but these have side effects on the human body [3]. Therefore, photodynamic therapy (PDT) has become more popular in medical usage.
PDT is a non-invasive therapy, which is different from traditional surgery. This is a selective and repeatable treatment, unlike radiation therapy, which is harmful to normal cells or tissues. Compared with antibiotics and corticosteroid chemotherapy, PDT is more effective and has fewer side effects [4].
Resveratrol is a natural product that has been extracted from Chinese herbal plants [5], and it has been applied for the treatment of different diseases, such as bacterial infections and cancer. It also can act as a photosensitizer (PS) in PDT therapy or adjuvant. If it is a PS, a photo-induced chemical reaction of trans-Resveratrol occurs to generate singlet oxygen (1O2) during the PDT process [6].
PDT has been used for a long time in the treatment of bacterial infections and cancer. Antibacterial photodynamic therapy (aPDT) has the potential to kill multidrug-resistant pathogenic bacteria, such as Gram-positive bacteria (Staphylococcus aureus and Enteroccoccus fecalis) and Gram-negative bacteria (Escherichia coli, Proteus mirabilis, and Pseudomonas aeruginosa). It has a low tendency to induce drug resistance, and bacteria rapidly develop against traditional antibiotic therapy [7]. PDT enables the treatment of multifocal disease with the least amount of tissue damage in terms of cancer [8]. The mechanisms of PDT, either in bacterial infection or cancer, are quite similar and are involved in Types I and II to generate the singlet oxygen (1O2) and reactive oxygen species (ROS) in the electron-transfer reaction.
Resveratrol can act as a natural PS, but it has some limitations for PDT, for example, its short wavelength and poor bioavailability. It is usually combined with hydrogel to enhance the function of PDT. Resveratrol and hydrogel are complementary during the PDT process in terms of maximizing effectiveness. Recently, Gan and Liu et al. have published a similar review of hydrogel-based phototherapy and drug-delivery systems [9,10], but these do not describe the natural product Resveratrol.
Hence, this review article is divided into five parts, namely (1) the definition of hydrogel, (2) the theory and mechanisms of PDT, (3) natural PS, especially in the case of Resveratrol, (4) the photodynamic action of hydrogel, and (5) the possibility of combining hydrogel with Resveratrol for PDT against bacteria and cancers.
1.1. Hydrogels
Hydrogels are three-dimensional (3D) network structures with crosslinked polymer chains that form ionic or covalent bonds for linking one polymer chain to another [11]. Glutaraldehyde [12,13] is the most common compound for crosslinking a hydrogel, and other crosslinking compounds include Formaldehyde [14], Epoxy [15], etc. (Figure 1). The crosslinking changes a liquid polymer into a solid or gel by restricting its ability to move. Since the crosslinking structure of a hydrogel can absorb relatively large amounts of water, its properties are soft and resemble living tissues in humans [16].
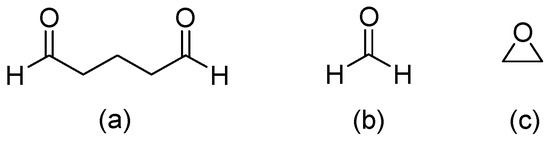
Figure 1.
Crosslinking compounds of hydrogel: (a) Glutaraldehyde, (b) Formaldehyde, and (c) Epoxy.
Hydrogels are porous, with spaces available between adjacent crosslinking structures in the polymer network [17]. There are some development theories for porous hydrogels, including the kinetics of swelling, equilibrium swelling, the structure–stiffness relationship, and solute diffusion in dense hydrogels.
The swelling of a hydrogel is the kinetic process of coupling mass transport and mechanical deformation that includes linear and non-linear poroelasticity. These theories are consistent within the linear regime under small perturbations from an isotropically swollen hydrogel state, such as a change in the volume of a hydrogel as it absorbs a compatible solvent or water content [18]. Hydrogel also causes equilibrium swelling depending on the pH of an environment. It acts as a polyampholyte polyelectrolyte when immersed in an ionic solution, producing hydrogen and hydroxide ions in a reaction [19].
This is the soft or weak material with a structure–stiffness relationship. Water content and mechanical properties of the hydrogel are adjusted over a wide range through the dissociation and reformation of hydrogen bonds by solvent exchange and the heating process [20]. Diffusion of solutes within the hydrogel occurs through nano-to-microscopic open spaces or dynamic free volumes between the aqueous solution and the liquid-filled polymer fibers, which includes sub-nanometer-scale cavities between molecules [21].
1.2. Examples of Antibacterial and Anticancer Applications
Hydrogels are naturally derived from alginate and chitosan or synthetic modification from polyacrylamide. Fasiku et al. reported a chitosan-based hydrogel for dual delivery with hydrogen peroxide of antimicrobial peptide against bacterial methicillin-resistant Staphylococcus aureus biofilm-infected wounds that were prepared through the Michael addition technique (Figure 2) [22]. In 2022, Abbasalizadeh et al. developed a curcumin-chrysin-alginate-chitosan hydrogel that was prepared through the ionic gelation mechanism utilizing CaCl2 to treat breast (T47D) and lung cancers (A549) (Figure 3) [23]. Lu et al. identified the polyacrylamides hydrogel causing Staphylococcus aureus and Escherichia coli differentiation upon visible light irradiation (Figure 4) [24]. Andrade et al. indicated that stimuli-responsive hydrogel was able to change its physical state from liquid to gel according to external factors such as temperature, pH, light, ionic strength, and magnetic field for cancer treatment (Figure 5) [25].
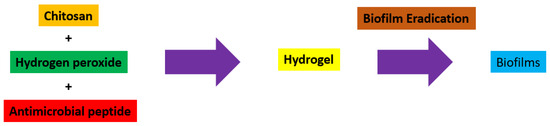
Figure 2.
Synthetic diagram for the synthesis of a chitosan-based hydrogel with hydrogen peroxide of antimicrobial peptide against Staphylococcus aureus.
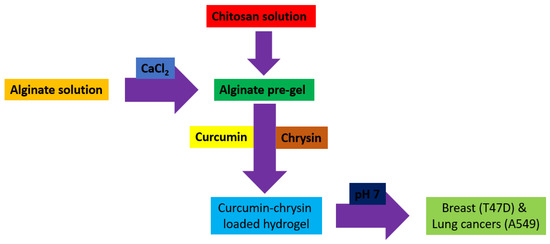
Figure 3.
Synthetic diagram for the synthesis of a curcumin-chrysin-alginate-chitosan hydrogel against breast (T47D) and lung cancers (A549).
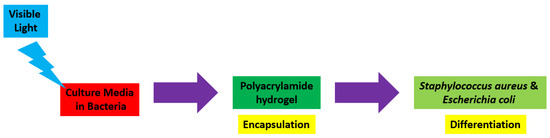
Figure 4.
Synthetic diagram for the synthesis of a polyacrylamide hydrogel against Staphylococcus aureus and Escherichia coli.
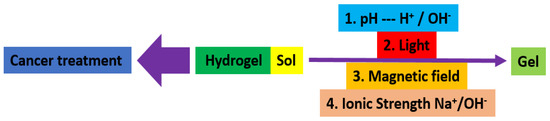
Figure 5.
Synthetic diagram for the synthesis of a stimuli−responsive hydrogel for cancer treatment.
1.3. Drug-Delivery System
There are some development theories of hydrogels to the drug-delivery approach. First, hydrogel establishes scaffolds to address easily inadequate local drug availability and delivery sites. These have a highly hydrated mesh network formed from natural, synthetic, or semi-synthetic polymers with physically or covalently crosslinked (Table 1) [26]. They provide high biocompatibility [27], drug protection [28], spatiotemporal control of the drug release [29], and physicochemical tailorability [30].

Table 1.
Classification of hydrogel for the formation of crosslinking (modified from [10]).
Biocompatibility is the ability of a biomaterial (e.g., hydrogel) to perform with an appropriate host response in the specific application [27]. Hydrogels also serve as a platform on which various physiochemical interactions with the encapsulated drugs occur to control drug release [28]. This provides a wide range of drug–carrier interface modifications to maximize drug-delivery needs through physical (electric field, magnetic field, light), mechanical (ultrasound, mechanical strain), or chemical (pH, redox gradient, enzymes), and without side effects [29].
Moreover, the physicochemical tailorability sparks through the inherent bioactivity of gelatin in tissue engineering. Spatial and temporal control of hydrogel crosslinking and its rheological behavior enables deposition via a variety of manufacturing-related techniques. Hydrogels can tailor to the remodeling rate of the target tissue to produce viable well-defined 3D structures or long-term cellular performance in non-biofabricated structures [30]. Therefore, hydrogel is a suitable material as a drug-delivery system applied in PDT.
1.4. Reasons for Hydrogel Use in PDT
In general, hydrogel has several competitive advantages, including good biocompatibility and low cytotoxicity, enhanced antitumor effect, reduced toxic and side effects, as well as maintaining a high concentration and archive the selective of a drug during the PDT process. These characteristics of hydrogel overcome the limitations of PDT and enhance its effectiveness, which has been discussed in drug-delivery systems. Previous studies have investigated a novel hydrogel shell on cancer cells that were prepared through in situ photopolymerization of polyethyleneglycol diacrylate (PEGDA) using methylene blue (MB)-sensitized mesoporous titanium oxide (TiO2) nanocrystal for enhancing the effectiveness of PDT. TiO2 was the PS and activated the formation of hydrogel, which protected the MB and acted as a significant photosensitive additive to improve the treatment of PDT. MB would be eliminated and inactivated after undergoing the PDT process [31]. Thus, there is a relationship between the principle of PDT and hydrogel.
2. Principle of PDT
The basic principle of PDT is a dynamic interaction between the photosensitizer (PS) and light with a specific wavelength that generates the reactive oxygen species (ROS), such as singlet oxygen (1O2) and superoxide anion (O2−) that causes irreversible oxidative damage to the cell membrane and DNA, leading to the selective destruction of target tissue, bacteria, and cancer [32].
Conventional PDT with the advantages of direct targeting, low invasiveness, remarkable curative, and fewer side effects [33]. However, it has limited or weak light-penetration depth, a short release distance, and a lifetime of ROS to fight against bacteria [34]. This also exists alongside poor selectivity and oxygen dependence for the therapeutic effectiveness of solid tumors [35]. Table 2 summarizes the representative cases of recent progress in PDT against bacteria and cancer over five years.

Table 2.
Recent progress in PDT against bacteria and cancer.
2.1. Mechanisms
In general, PDT causes the activation of PS through visible or ultraviolet light, which generates the 1O2 and free radicals. The electrons of the PS are promoted from the ground to an excited state by the light activation. These excited singlet-state electrons from the PS are unstable and release the excess energy backing to the ground state by emitting fluorescence. Its excited singlet-state electrons can also change and shift to the triplet state through the intersystem crossing system [42].
There are two types of pathways for the triplet state of PS that react with the substrate [43]. Type-I and II pathways involve the electron transfer from triplet state PS. The difference between these two pathways is the generation of final products. Type-I pathway consists of free radicals, and these interact with oxygen at a molecular level to form ROS, such as superoxide (O2•−), hydroxyl radicals (•OH), and hydrogen peroxide (H2O2). Type II pathway contains 1O2 only generated by the electron transfer between the excited PS and the ground-state molecular oxygen [44]. Type-I pathway operates in an oxygen (O2)-independent manner, and the type II pathway relies on the presence of molecular oxygen (Figure 6).
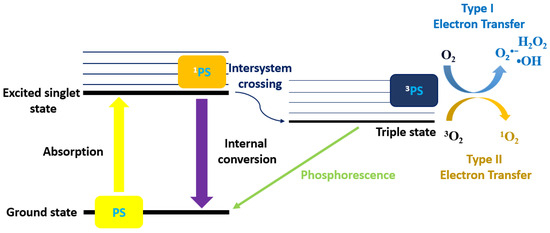
Figure 6.
Schematic diagram of the PDT Types I and II mechanisms.
These Type-I and II pathways of antimicrobial photodynamic therapy (aPDT) are employed for killing Gram-positive bacteria (Staphylococcus aureus, Enteroccoccus fecalis) and Gram-negative bacteria (Escherichia coli, Proteus mirabilis, and Pseudomonas aeruginosa) bacteria [42], which causes irreversible oxidative damage to the cell membrane and DNA, leading to bacterial cell death [45]. Both Type-I and II pathways are also applied in the treatment of cancers that can trigger different cell death mechanisms and are directly cytotoxic to the cancer cells resulting in apoptosis or necrosis [46]. The percentage or ratio of the final products for Type-I and II pathways depends on the nature or type of PS.
2.2. Natural Photosensitizer (PS)
Typically, PS molecules absorb light with a suitable wavelength that initiates the activation processes for the selective destruction of cancer cells [47]. Many natural PS from traditional Chinese medicinal plants are applied in PDT, including Pheophorbide a (Pa) [48], Hypocrellin B (HB) [49], and Curcumin (Cur) (Figure 7) [50], since these are phytochemical compounds with less- or non-toxic side effects [51].
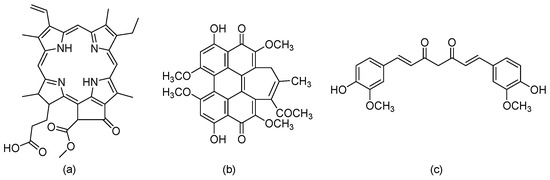
Figure 7.
Chemical structures of (a) Pheophorbide a (Pa), (b) Hypocrellin B (HB), and (c) Curcumin (Cur).
They usually act as antibacterial and anticancer agents of natural products origin [52]. PS can selectively target microbes and leave out normal tissue, making it more efficient against the bacterial infection site when as an antibacterial agent. If the PS is an anticancer agent, it reduces toxicity to healthy tissues and has a lower incidence of side effects [53]. Some representative examples of natural PS for PDT against bacteria and cancer are shown in Table 3. Resveratrol as a PS will be discussed in the next section.

Table 3.
Natural PS for PDT against bacteria and cancer.
2.2.1. Resveratrol (3,5,4′-Trihydroxystilbene) (RSV)
This is a plant compound with a natural polyphenol stilbene structure that is isolated from the root of Veratrum grandiflorum [59] or darakchasava, or manakka for medicinal purposes [60]. Its molecular weight of 228.25 g/mol consists of two phenolic rings formed by a double styrene bond. Thus, there are two isomeric cis- and trans-forms (Figure 8).
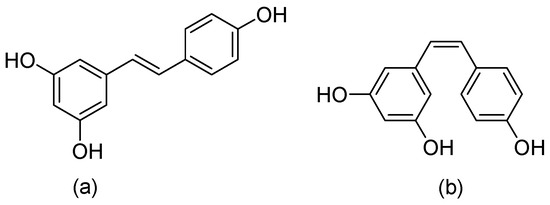
Figure 8.
Chemical structures of (a) trans-Resveratrol, and (b) cis-Resveratrol.
The cis- and trans- forms of Resveratrol can co-exist in a variety of fruits, including grapes (Vitaceae) [61] and blueberries (Vaccinium spp.), blackberries (Morus spp.), and peanuts (Arachis hypogaea) [62,63], and red wine. Trans-Resveratrol is the predominant form and is more stable than cis-Resveratrol in nature [64,65]. It is a bioactive compound with a wide range of pharmacology properties, e.g., immunomodulatory [66], glucose and lipid regulatory [67], neuroprotective [68], cardiovascular protective effects [69], antioxidant [70], anti-inflammatory [71], antibacterial [72,73,74,75,76,77], and anticancer (Table 4) [78,79,80,81,82,83,84,85,86,87].

Table 4.
Antibacterial and anticancer effects of Resveratrol.
Table 4 shows the investigation of an antibacterial and anticancer effect on Resveratrol. The antibacterial mechanisms of Resveratrol consist of DNA damage [88], cell division impairment [89], oxidative membrane damage [90], and metabolic enzyme inhibition [91].
Resveratrol increases the expression of genes from a SOS–stress regulon, which causes DNA fragmentation, cell cycle arrest, and DNA impairment in bacteria, such as Escherichia coli [72,88]. It can also affect the prokaryotic cell division protein (FtsZ) and GTPase activity. This protein is highly conserved throughout eubacteria, which may cause cell division impairment in bacteria [92].
The effect of Resveratrol on E. coli is not through diffusible ROS. It is mediated site-specifically as the primary event for oxidative damage of the cell membrane [90]. It also inhibits ATPase and the synthesis of ATP bound in a hydrophobic pocket between the C-terminal tip of the gamma subunit and the hydrophobic interior of the surrounding torus, a region critical for gamma-subunit rotation [91].
Resveratrol has several anticancer mechanisms, including initiating the apoptosis of mitochondria in the cytoplasm, enhancing oxidative stress, and interfering with energy metabolism in cancer cells. The generation of ROS causes cancer cell damage and death [93].
Mitochondria is an intrinsic apoptotic pathway causing stimulation through the activation of the proapoptotic Bcl-2 family (Bak, Bax). By suppressing the anti-apoptotic proteins Bcl-xL, Bcl-2, and Mcl-1, it diminished the potential of mitochondrial outer membrane permeability (MOMP). The cytochrome c then binds to this cytosolic apoptotic protease activating factor-1 (Apaf-1) [94], which recruits the initiator procaspase-9 activating downstream executor caspase-3, -6, and -7 for cleavage of cytoplasm leading to the cell apoptosis [95].
The intervention is an extrinsic apoptotic pathway involving tumor necrosis factor (TNF) to interact with cell surface receptors such as TRAILR1 (DR4)-TRAIL, TRAILR2 (DR5)-TRAIL, TNFR1-TNFα and FAS (CD95, APO-1)-FasL [96]. It causes apoptosis by the activation of caspase-3, -6, and -7, which leads to the disassembly of cell survival and death [97].
Meanwhile, Resveratrol inhibits the neurogenic locus notch homolog protein 1 to increase the generation of ROS. These signal transductions upregulate the phosphatase tensin homolog and down-regulate AKT Serine/Threonine Kinase 1. As a result, caspase-3 cleavage by decreasing phospho-Akt induced cell death [98]. Growing evidence has indicated that trans-Resveratrol has anticancer properties. It is a PS applied to the PDT to enhance its functions against bacteria and cancer.
2.2.2. Photodynamic Action of Resveratrol
Trans-Resveratrol has been reported to have significant photodynamic activity. The generation of singlet oxygen by trans-Resveratrol after light irradiation is used as PS for the photooxidation of ergosterol via a [4+2] cycloaddition (Figure 9) [99].
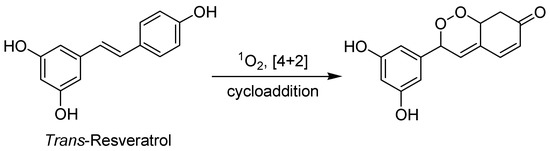
Figure 9.
Trans-Resveratrol photooxidation of ergosterol via a [4+2] cycloaddition.
Singlet oxygen is a ROS generated by ground-state (triplet) oxygen excitation. This is the energy transfer from a photoexcited PS in the PDT process [100]. Resveratrol quinone is the main source for quenching 1O2. Its mechanism is based on the resorcinol moiety and the carbon–carbon double bond [101]. It has an excellent triplet-quenching activity in a lower concentration range of 52 μM [102].
Regarding trans-Resveratrol with antibacterial and anticancer functions, the ROS causes the inactivation of bacteria, which oxidizes proteins or lipids, leading to bacteria death [103]. It also inhibits the proliferation of cancer cells due to increased metabolic rate, gene mutation, and relative hypoxia [104], as well as suppressing cell growth accompanied by apoptosis evoked through increased intracellular ROS levels in mitochondria [105]. Some examples of Resveratrol as a PS for PDT against bacteria and cancer are shown in Table 5.

Table 5.
Photodynamic action of Resveratrol against bacteria and cancer.
3. Photodynamic Action of Hydrogel
Hydrogels are an effective way to release drugs and antibacterial agents that greatly improve the utilization of antibacterial agents in bacteria and reduce the toxic effects on normal cells [111,112]. It is also a potent novel medication that targets cancer cells and reduces damage to normal tissues during PDT treatment. They act as a powerful drug-delivery capacity for a precisely controlled drug release because hydrogels can be loaded for many therapeutic agents, such as chemotherapeutic agents, radionuclides, and immunosuppressants, to induce a cascade of multiple therapeutic modalities. This can operate as a carrier and release system of PS for PDT, which also enhances the light-penetration depth and enables an integrated sequence of cancer treatment [113].
Hydrogels have a variety of sizes, from macrogels, microgels (0.5 to 10 μm), and nanogels (less than 200 nm) during the PDT treatment, which allows precise access to the cancer site and provides continuous as well as controlled drug delivery for the selectivity of tumors. This also minimizes the usage of drugs and reduces systemic toxicity to the corresponding tissues [114]. The hydrogel system is highly dependent on internal and external environmental stimulations, such as pH, temperature, redox potential, and reactive oxygen concentration. Meanwhile, oxygen dependence is an important factor for PDT treatment. Thus, the hydrogel system can target cancer cells and release drugs to improve the therapeutic effectiveness of solid tumors [115]. Some examples of hydrogel for PDT against bacteria and cancer (Table 6).
PDT can lead to additional crosslinking within the hydrogel network, which reacts with polymer chains, altering the mechanical properties and swelling behavior of the hydrogel. The incorporation of photosensitizers into hydrogels greatly enhances their localized concentration and biocompatibility. It prolongs their residence time to generate ROS against bacteria and cancer during the PDT process [9]. However, PDT may also cause the degradation of hydrogel to release a drug (e.g., Resveratrol) since ROS breaks down polymer chains at the same time, resulting in fragmentation and affecting overall stability. The drug-delivery system is the most important point for the hydrogel applications on antibacterial and anticancer functions [116].

Table 6.
Photodynamic action of hydrogel against bacteria and cancer.
Table 6.
Photodynamic action of hydrogel against bacteria and cancer.
| Bacteria | |||||
|---|---|---|---|---|---|
| Study | Photosensitizer and Dosage | Usage of Light and Energy (J) | Consequences | Reference | |
| 1 | Photosensitizer-loaded hydrogels for photodynamic inactivation of multiresistant bacteria in wounds | 326 μM of tetrakis(1 methylpyridinium-4-yl)porphyrin p-toluenesulfonate (TMPyP) and 242 μM of tetrahydroporphyrin—p toluenesulfonate (THPTS) on Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumonia, Acinetobacter baumannii, Pseudomonas aeruginosa, Escherichia coli, and Achromobacter xylosoxidans. | Irradiated with red light at 760 nm in 18 W/cm2, and fluence 20 J/cm2 for 36 to 90 min. | TMPyP-loaded hydrogels were more effective than those loaded with THPTS, which displayed effectivity against all investigated bacteria strains, improving the treatment of wounds infected with problematic bacterial pathogens. | [117] |
| 2 | An injectable dipeptide–fullerene supramolecular hydrogel for photodynamic antibacterial therapy | 200 μM of dipeptide–fullerene supramolecular hydrogel on Staphylococcus aureus in wound healing. | Irradiated with white light at 400 nm in 0.1 W/cm2, and fluence 10 J/cm2 for 10 min. | Peptide fullerene hydrogels inhibited multi-antibiotic-resistant Staphylococcus aureus and promote wound healing. | [118] |
| 3 | Optimization of hydrogel containing toluidine blue O for photodynamic therapy in treating acne | 0.1 μM of toluidine blue O on Propionibacterium acnes, Staphylococcus aureus, and Escherichia coli. | Irradiated with red light at 630 nm in 0.4 W/cm2, and fluence 13 J/cm2 for 15 min. | Toluidine blue O hydrogel for PDT showed effective antibacterial activity for Propionibacterium acnes, Staphylococcus aureus, and Escherichia coli. | [119] |
| Cancer | |||||
| Study | Photosensitizer and Dosage | Usage of Light and Energy (J) | Consequences | Reference | |
| 1 | Synthesis and Characterization of Temperature-sensitive and Chemically Crosslinked Poly(N-isopropylacrylamide)/Photosensitizer Hydrogels for Applications in Photodynamic Therapy | 90 μM of Pheophorbide a-poly(N-isopropylacrylamide) nanohydrogel on Human Colorectal Adenocarcinoma, HT29. | Irradiated with white light at 681 nm in 110 W/cm2 and fluence 20 J/cm2 for 18 to 24 h. | Pheophorbide a-poly(N-isopropylacrylamide) nanohydrogel with reasonable biocompatibility and acceptable photocytotoxicity in the low μM range. | [120] |
| 2 | Alginate-Based Microcapsules with a Molecule Recognition Linker and Photosensitizer for the Combined Cancer Treatment | 30 μM of Ca-ALG (HB-lipid), and Ca-ALG-DOX-(HB-lipid) hydrogels on Immortal cells, HeLa. | Irradiated with blue light at 488 nm in 0.5 W/cm2, and fluence 30 J/cm2 for 36 h. | Ca-ALG (HB-lipid) and Ca-ALG-DOX-(HB-lipid) hydrogels are the co-delivery carriers with high efficiency in treating PDT against Immortal cells (HeLa). | [121] |
| 3 | Curcumin and silver nanoparticles carried out from polysaccharide-based hydrogels improved the photodynamic properties of curcumin through metal-enhanced singlet oxygen effect | 91.5 μM of CHT/CS/CUR-AgNPs hydrogel on Human Colon Cancer cells, Caco-2. | Irradiated with green light at 525 nm in 420 W/cm2, and fluence 50 J/cm2 for 24 h. | PDT selective illumination led to the inhibition of Human Colon Cancer cells (Caco-2) by the CHT/CS/CU R-AgNPs hydrogel, and CUR can work as a diagnostic fluorescence probe in this system. | [122] |
3.1. Resveratrol Combined with Hydrogel
Resveratrol has limitations in clinical studies, including poor bioavailability, low water solubility, and chemical instability in neutral and alkaline environments [123]. Hydrogel is a special material, and Resveratrol overcomes the above issue with the help of this. A hydrogel has several properties, such as high biocompatibility, drug protection, spatiotemporal control of the drug release, and physicochemical tailorability. Some examples are summarized in Table 7.

Table 7.
Hydrogel with Resveratrol against bacteria and cancers.
Mechanism of Combination
The mechanism for the combination of Resveratrol with hydrogel against bacteria and cancer is based on the antimicrobial peptide, antimicrobial agents, antibiotics, and polysaccharide, e.g., chitosan or cyclodextrin, e.g., (i) incorporation of Resveratrol–hydroxypropyl-β-cyclodextrin complexes into hydrogel formulation for wound treatment against Staphylococcus aureus, Escherichia coli, and Candida albicans (Figure 10) [126], and (ii) chitosan-based injectable in situ forming hydrogels containing dopamine-reduced graphene oxide and Resveratrol for breast cancer chemo-photothermal therapy (Figure 11) [130].

Figure 10.
Synthetic diagram for the incorporation of Resveratrol–hydroxypropyl–β-cyclodextrin complexes into hydrogel formulation against bacteria.

Figure 11.
Synthetic diagram for the chitosan-based hydrogels containing dopamine-reduced graphene oxide and Resveratrol against breast cancer.
Based on the above information, it is possible to have a photodynamic action of Resveratrol with hydrogel against bacteria and cancer since the hydrogel is a three-dimensional (3D) network structure with crosslinked polymer chains that forms the ionic or covalent bond for linking one polymer chain to another, which provides high biocompatibility, drug protection, spatiotemporal control of the drug release, and physicochemical tailorability.
Resveratrol has antibacterial and anticancer effects. It affects the changes in cell morphology and DNA contents [133]. It is also able to inhibit the carcinogenesis stages, including initiation, promotion, and progression [134,135,136]. These are the reasons for using Resveratrol as a PS or adjuvant.
There is potential for the photodynamic activity of Resveratrol for treating various types of bacteria and cancers, with the particular advantage of causing minimal toxic side effects. This may increase the sensitivity of some cancer cells against chemotherapy drugs and overcome one or more of the body’s mechanisms, which eliminate the side effects of chemotherapy, such as anorexia, fatigue, depression, nerve pain, and cognitive impairment [137].
Regarding the photodynamic activity of Resveratrol, it is much better combined with the hydrogel. This eliminates the clinical application problems of Resveratrol, such as poor bioavailability, low water solubility, and chemical instability in neutral and alkaline environments. It greatly enhances the effectiveness of PDT against bacteria and cancer.
Moreover, hydrogel has started to be used with nanoparticles in recent research. Yang et al. reported that Resveratrol induced apoptosis and inhibited tumor growth after being coated with gold nanoflowers. It also enriched the tumor sites and identified tumor sites by computed tomography (CT) [138]. Xiang et al. identified Resveratrol-loaded dual-function titanium disulfide nanosheets for photothermal-triggered tumor chemotherapy with no remarkable tissue toxicity. Titanium disulfide nanosheets with Resveratrol can target and accumulate in mitochondria when triggered by near-infrared light. It induces the upregulation of key intrinsic apoptotic factors such as cytochrome c and initiates the caspase cascade to achieve the chemotherapeutic effect [139]. Thus, hydrogel nanoparticles or nanocomposites should be used in further investigation of Resveratrol with PDT in the treatment of bacteria and cancer.
However, there is some toxicity, and adverse effects were reported for the consumption of Resveratrol. Therefore, extensive studies on long-term effects and the in vivo adverse effects of Resveratrol supplementation in humans are needed [140]. These include concerns about the dosage of Resveratrol and duration time for humans if it is used as a PS or adjuvant.
4. Conclusions
Resveratrol combined with hydrogel is suitable for PDT treatment to fight against bacteria and cancer. Hydrogel consists of three-dimensional (3D) network structures with natural, synthetic, or semi-synthetic polymers through physical or chemical crosslinked methods. It has several competitive advantages, including good biocompatibility and low cytotoxicity, enhanced antitumor effect, reduced toxic and side effects, as well as maintaining a high concentration and archive the selective of a drug during the PDT process.
Resveratrol is a natural polyphenol stilbene structure containing two isomeric cis- and trans-forms. Trans-Resveratrol is dominant and more stable with significant photodynamic activity, which acts as a PS to generate ROS during the PDT process. However, Trans-Resveratrol has some limitations, with poor water solubility the major issue for biological application. Thus, they are compatible and reinforce each other to increase the effectiveness of PDT against bacteria and cancer.
However, more work is required, especially for the cytotoxicity safety assessment of the human body. The selection of hydrogel and Resveratrol acting as PS or adjuvant to enhance the effectiveness of PDT is another important milestone in the future.
5. Future Aspects
How do we enhance the effectiveness and therapeutic effect of PDT with natural products against bacteria and cancer in the future? There are some strategies to improve photodynamic therapy efficacy, such as making good use of non-reactive oxygen carriers (microbubbles/nano-bubbles, hemoglobin, and perfluorocarbon), reactive oxygen carriers (PDT dependent/independent materials), and regulating the microenvironment (blood perfusion, target mitochondria, moderate the level of Hypoxia-inducible factor 1, and hypoxia-activated therapy) [141]. Nanotechnology is another useful approach including the applications of liposomes, nanoparticles, and quantum dots [142]. In 2022, Li et al. developed the nanocomposite AuNS@ZrTCPP-GA (AZG), containing gambogic acid (GA), heat-shock protein 90 (HSP90) inhibitor, and the gold nanostars (AuNS) coated with PEGylated liposome (LP) to increase the stability and biocompatibility for enhancing the anticancer effect of PDT [143].
Meanwhile, it is suggested that the combination of photodynamic (PD) and photothermal (PT) therapies harness light to eliminate cancer cells with spatiotemporal precision by either generating ROS or increasing temperature. This addresses the limitations of the PDT/PTT modality and enhances treatment safety as well as efficacy. However, the complicated preclinical assessment of PDT/PTT combinations and possible rationale or guidelines to elucidate the mechanisms underlying PDT/PTT interactions are required for further investigations [144].
Author Contributions
Conceptualization, S.K.L., C.W.S.T. and C.W.C.L.; writing—original draft preparation, S.K.L., C.W.S.T. and C.W.C.L.; writing—review and editing, S.K.L., C.W.S.T. and C.W.C.L.; supervision, S.K.L. and D.C.T.A.; funding acquisition, D.C.T.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Chinese Medicine and Culture Research Center, Research Matching Grant (RMG030a).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bernier, S.P.; Surette, M.G. Concentration-dependent activity of antibiotics in natural environments. Front. Microbiol. 2013, 4, 20. [Google Scholar] [CrossRef] [PubMed]
- Friedman, N.D.; Temkin, E.; Carmeli, Y. The negative impact of antibiotic resistance. Clin. Microbiol. Infect. 2016, 22, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Grin, M.; Suvorov, N.; Ostroverkhov, P.; Pogorilyy, V.; Kirin, N.; Popov, A.; Sazonova, A.; Filonenko, E. Advantages of combined photodynamic therapy in the treatment of oncological diseases. Biophys. Rev. 2022, 14, 941–963. [Google Scholar] [CrossRef]
- Gu, B.; Wang, B.; Li, X.; Feng, Z.; Ma, C.; Gao, L.; Yu, Y.; Zhang, J.; Zheng, P.; Wang, Y.; et al. Photodynamic therapy improves the clinical efficacy of advanced colorectal cancer and recruits immune cells into the tumor immune microenvironment. Front. Immunol. 2022, 13, 1050421. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Liu, J. Resveratrol: A review of plant sources, synthesis, stability, modification and food application. J. Sci. Food Agric. 2020, 100, 1392–1404. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Shi, M.; Ye, J.H.; Zheng, X.Q.; Lu, J.L.; Liang, Y.R. Photo-induced chemical reaction of trans-resveratrol. Food Chem. 2015, 171, 137–143. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, R.; Zaat, S.A.J.; Breukink, E.; Heger, M. Antibacterial photodynamic therapy: Overview of a promising approach to fight antibiotic-resistant bacterial infections. J. Clin. Transl. Res. 2015, 1, 140–167. [Google Scholar]
- Bhattacharya, D.; Mukhopadhyay, M.; Shivam, K.; Tripathy, S.; Patra, R.; Pramanik, A. Recent developments in photodynamic therapy and its application against multidrug resistant cancers. Biomed. Mater. 2023, 18, 062005. [Google Scholar] [CrossRef]
- Gan, S.; Wu, Y.; Zhang, X.; Zheng, Z.; Zhang, M.; Long, L.; Liao, J.; Chen, W. Recent Advances in Hydrogel-Based Phototherapy for Tumor Treatment. Gels 2023, 9, 286. [Google Scholar] [CrossRef]
- Liu, B.; Chen, K. Advances in Hydrogel-Based Drug Delivery Systems. Gels 2024, 10, 262. [Google Scholar] [CrossRef]
- Maitra, J.; Shukla, V.K. Cross-linking in Hydrogels—A Review. Am. J. Polym. Sci. 2014, 4, 25–31. [Google Scholar]
- Gulati, N.; Nagaich, U.; Sharma, V.K.; Khosa, R.L. Effect of Polymer and Cross Linking Agent on In Vitro Release of Quercetin from Microbeads. Asian J. Pharm. Sci. 2011, 1, 2231–4423. [Google Scholar]
- Denizli, B.K.; Can, H.K.; Rzaev, Z.M.O.; Guner, A. Preparation conditions and swelling equilibria of dextran hydrogels prepared by some crosslinked agents. Polymer 2004, 45, 6431–6435. [Google Scholar] [CrossRef]
- Zhu, J. Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering. Biomaterials 2010, 31, 4639–4656. [Google Scholar] [CrossRef]
- Ramamurthi, A.; Vesely, I. Ultraviolet light-induced modification of crosslinked hyaluronan gels. J. Biomed. Mater. Res. A 2003, 66, 317–329. [Google Scholar] [CrossRef]
- Ho, T.C.; Chang, C.C.; Chan, H.P.; Chung, T.W.; Shu, C.W.; Chuang, K.P.; Duh, T.H.; Yang, M.H.; Tyan, Y.C. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef]
- Foudazi, R.; Zowada, R.; Manas-Zloczower, I.; Feke, D.L. Porous Hydrogels: Present Challenges and Future Opportunities. Langmuir 2023, 39, 2092–2111. [Google Scholar] [CrossRef]
- Bouklas, N.; Huang, R. Swelling kinetics of polymer gels: Comparison of linear and nonlinear theories. Soft Matter 2012, 8, 8194–8203. [Google Scholar] [CrossRef]
- Yan, H.; Jin, B. Equilibrium swelling of a polyampholytic pH-sensitive hydrogel. Eur. Phys. J. E Soft Matter 2013, 36, 27. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, Y.; Cao, Y.; Xue, B. Tough Hydrogels with Different Toughening Mechanisms and Applications. Int. J. Mol. Sci. 2024, 25, 2675. [Google Scholar] [CrossRef]
- Axpe, E.; Chan, D.; Offeddu, G.S.; Chang, Y.; Merida, D.; Hernandez, H.L.; Appel, E.A. A Multiscale Model for Solute Diffusion in Hydrogels. Macromolecules 2019, 52, 6889–6897. [Google Scholar] [CrossRef] [PubMed]
- Fasiku, V.O.; Omolo, C.A.; Devnarain, N.; Ibrahim, U.H.; Rambharose, S.; Faya, M.; Mocktar, C.; Singh, S.D.; Govender, T. Chitosan-Based Hydrogel for the Dual Delivery of Antimicrobial Agents against Bacterial Methicillin-Resistant Staphylococcus aureus Biofilm-Infected Wounds. ACS Omega 2021, 6, 21994–22010. [Google Scholar] [CrossRef] [PubMed]
- Abbasalizadeh, F.; Alizadeh, E.; Bagher Fazljou, S.M.; Torbati, M.; Akbarzadeh, A. Anticancer Effect of Alginate-chitosan Hydrogel Loaded with Curcumin and Chrysin on Lung and Breast Cancer Cell Lines. Curr. Drug Deliv. 2022, 19, 600–613. [Google Scholar] [PubMed]
- Lu, H.; Huang, Y.; Lv, F.; Liu, L.; Ma, Y.; Wang, S. Living Bacteria-Mediated Aerobic Photoinduced Radical Polymerization for in Situ Bacterial Encapsulation and Differentiation. CCS Chem. 2021, 3, 1296–1305. [Google Scholar] [CrossRef]
- Andrade, F.; Roca-Melendres, M.M.; Durán-Lara, E.F.; Rafael, D.; Schwartz, S., Jr. Stimuli-Responsive Hydrogels for Cancer Treatment: The Role of pH, Light, Ionic Strength and Magnetic Field. Cancers 2021, 13, 1164. [Google Scholar] [CrossRef]
- Nairon, K.G.; DePalma, T.; Sivakumar, H.; Skardal, A. Tunable Hydrogel Systems for Delivery and Release of Cell-Secreted and Synthetic Therapeutic Products. In Controlled Drug Delivery Systems, 1st ed.; CRC Press: Boca Raton, FL, USA, 2020; Volume 3, pp. 29–53. [Google Scholar]
- Naahidi, S.; Jafari, M.; Logan, M.; Wang, Y.; Yuan, Y.; Bae, H.; Dixon, B.; Chen, P. Biocompatibility of hydrogel-based scaffolds for tissue engineering applications. Biotechnol. Adv. 2017, 35, 530–544. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Rahoui, N.; Jiang, B.; Taloub, N.; Huang, Y.D. Spatio-temporal control strategy of drug delivery systems based nano structures. J. Control. Release 2017, 255, 176–201. [Google Scholar] [CrossRef]
- Klotz, B.J.; Gawlitta, D.; Rosenberg, A.J.W.P.; Malda, J.; Melchels, F.P.W. Gelatin-Methacryloyl Hydrogels: Towards Biofabrication-Based Tissue Repair. Trends Biotechnol. 2016, 34, 394–407. [Google Scholar] [CrossRef]
- Chang, G.; Zhang, H.; Li, S.; Huang, F.; Shen, Y.; Xie, A. Effective photodynamic therapy of polymer hydrogel on tumor cells prepared using methylene blue sensitized mesoporous titania nanocrystal. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 99, 1392–1398. [Google Scholar] [CrossRef]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.; Wei, M.; Yang, B. Recent advances in nanomedicines for photodynamic therapy (PDT)-driven cancer immunotherapy. Theranostics 2022, 12, 434–458. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhang, H.; Wang, Y.; Shiu, B.C.; Lin, J.H.; Zhang, S.; Lou, C.W.; Li, T.T. Synergistic antibacterial strategy based on photodynamic therapy: Progress and perspectives. Chem. Eng. J. 2022, 3, 138129. [Google Scholar] [CrossRef]
- Jiang, W.; Liang, M.; Lei, Q.; Li, G.; Wu, S. The Current Status of Photodynamic Therapy in Cancer Treatment. Cancers 2023, 15, 585. [Google Scholar] [CrossRef]
- Jiang, J.; Lv, X.; Cheng, H.; Yang, D.; Xu, W.; Hu, Y.; Song, Y.; Zeng, G. Type I photodynamic antimicrobial therapy: Principles, progress, and future perspectives. Acta Biomater. 2024, 177, 1–19. [Google Scholar] [CrossRef]
- Yanten, N.; Vilches, S.; Palavecino, C.E. Photodynamic therapy for the treatment of Pseudomonas aeruginosa infections: A scoping review. Photodiagnosis Photodyn. Ther. 2023, 44, 103803. [Google Scholar] [CrossRef]
- Gourlot, C.; Gosset, A.; Glattard, E.; Aisenbrey, C.; Rangasamy, S.; Rabineau, M.; Ouk, T.S.; Sol, V.; Lavalle, P.; Gourlaouen, C.; et al. Antibacterial Photodynamic Therapy in the Near-Infrared Region with a Targeting Antimicrobial Peptide Connected to a π-Extended Porphyrin. ACS Infect. Dis. 2022, 8, 1509–1520. [Google Scholar] [CrossRef]
- He, L.; Yu, X.; Li, W. Recent Progress and Trends in X-ray-Induced Photodynamic Therapy with Low Radiation Doses. ACS Nano 2022, 16, 19691–19721. [Google Scholar] [CrossRef]
- Alsaab, H.O.; Alghamdi, M.S.; Alotaibi, A.S.; Alzhrani, R.; Alwuthaynani, F.; Althobaiti, Y.S.; Almalki, A.H.; Sau, S.; Iyer, A.K. Progress in Clinical Trials of Photodynamic Therapy for Solid Tumors and the Role of Nanomedicine. Cancers 2020, 12, 2793. [Google Scholar] [CrossRef]
- Penetra, M.; Arnaut, L.G.; Gomes-da-Silva, L.C. Trial watch: An update of clinical advances in photodynamic therapy and its immunoadjuvant properties for cancer treatment. Oncoimmunology 2023, 12, 2226535. [Google Scholar] [CrossRef]
- Huang, L.; Xuan, Y.; Koide, Y.; Zhiyentayev, T.; Tanaka, M.; Hamblin, M.R. Type I and Type II mechanisms of antimicrobial photodynamic therapy: An in vitro study on gram-negative and gram-positive bacteria. Lasers Surg. Med. 2012, 44, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Foote, C.S. Definition of type I and type II photosensitized oxidation. Photochem. Photobiol. 1991, 54, 659. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, J.; Rahban, D.; Aghamiri, S.; Teymouri, A.; Bahador, A. Photosensitizers in antibacterial photodynamic therapy: An overview. Laser Ther. 2018, 27, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Wang, J.; Zhuang, A.; Liu, Q.; Li, F.; Yuan, K.; Yang, Y.; Liu, Y.; Chang, H.; Liang, Y.; et al. Metallacage-based enhanced PDT strategy for bacterial elimination via inhibiting endogenous NO production. Proc. Natl. Acad. Sci. USA 2023, 120, e2218973120. [Google Scholar] [CrossRef] [PubMed]
- Mishchenko, T.; Balalaeva, I.; Gorokhova, A.; Vedunova, M.; Dmitri, V. Krysko. Which cell death modality wins the contest for photodynamic therapy of cancer? Cell Death Dis. 2022, 13, 455. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy—Mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef]
- Tang, P.M.; Bui-Xuan, N.H.; Wong, C.K.; Fong, W.P.; Fung, K.P. Pheophorbide a-Mediated Photodynamic Therapy Triggers HLA Class I-Restricted Antigen Presentation in Human Hepatocellular Carcinoma. Transl. Oncol. 2010, 3, 114–122. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, C.; Li, S.; Jiao, Y.; Qi, T.; Wei, G.; Han, G. Effects of Photodynamic Therapy Using Yellow LED-light with Concomitant Hypocrellin B on Apoptotic Signaling in Keloid Fibroblasts. Int. J. Biol. Sci. 2017, 13, 319–326. [Google Scholar] [CrossRef]
- Kah, G.; Chandran, R.; Abrahamse, H. Curcumin a Natural Phenol and Its Therapeutic Role in Cancer and Photodynamic Therapy: A Review. Pharmaceutics 2023, 15, 639. [Google Scholar] [CrossRef]
- Sulaiman, C.; George, B.P.; Balachandran, I.; Abrahamse, H. Photoactive Herbal Compounds: A Green Approach to Photodynamic Therapy. Molecules 2022, 27, 5084. [Google Scholar] [CrossRef]
- Harvey, A.L.; Edrada-Ebel, R.; Quinn, R.J. The Re-Emergence of Natural Products for Drug Discovery in the Genomics Era. Nat. Rev. Drug Discov. 2015, 14, 111–129. [Google Scholar] [CrossRef] [PubMed]
- Kubrak, T.P.; Kołodziej, P.; Sawicki, J.; Mazur, A.; Koziorowska, K.; Aebisher, D. Some Natural Photosensitizers and Their Medicinal Properties for Use in Photodynamic Therapy. Molecules 2022, 27, 1192. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Iriuchishima, T.; Aizawa, S.; Okano, T.; Goto, B.; Nagai, Y.; Horaguchi, T.; Ryu, J.; Saito, A. Bactericidal effect of photodynamic therapy using Na-pheophorbide a: Evaluation of adequate light source. Photomed. Laser Surg. 2009, 27, 849–853. [Google Scholar] [CrossRef] [PubMed]
- Otieno, W.; Liu, C.; Deng, H.; Li, J.; Zeng, X.; Ji, Y. Hypocrellin B-Mediated Photodynamic Inactivation of Gram-Positive Antibiotic-Resistant Bacteria: An In Vitro Study. Photobiomodulation Photomed. Laser Surg. 2020, 38, 36–42. [Google Scholar] [CrossRef]
- Ribeiro, I.P.; Pinto, J.G.; Souza, B.M.N.; Miñán, A.G.; Ferreira-Strixino, J. Antimicrobial photodynamic therapy with curcumin on methicillin-resistant Staphylococcus aureus biofilm. Photodiagnosis Photodyn. Ther. 2022, 37, 102729. [Google Scholar] [CrossRef]
- Jiang, Y.; Leung, A.W.; Wang, X.; Zhang, H.; Xu, C. Effect of photodynamic therapy with hypocrellin B on apoptosis, adhesion, and migration of cancer cells. Int. J. Radiat. Biol. 2014, 90, 575–579. [Google Scholar] [CrossRef]
- Ravera, S.; Pasquale, C.; Panfoli, I.; Bozzo, M.; Agas, D.; Bruno, S.; Hamblin, M.R.; Amaroli, A. Assessing the Effects of Curcumin and 450 nm Photodynamic Therapy on Oxidative Metabolism and Cell Cycle in Head and Neck Squamous Cell Carcinoma: An In Vitro Study. Cancers 2024, 16, 1642. [Google Scholar] [CrossRef]
- Takaoka, M.J. Of the phenolic substances of white hellebore (Veratrum grandiflorum Loes. fil.) J. Fac. Sci. Hokkaido Imp. Univ. 1940, 3, 1–16. [Google Scholar] [CrossRef]
- Paul, B.; Masih, I.; Deopujari, J.; Charpentier, C. Occurrence of resveratrol and pterostilbene in age-old darakchasava, an ayurvedic medicine from India. J. Ethnopharmacol. 1999, 68, 71–76. [Google Scholar] [CrossRef]
- Elíes-Gómez, J. Efectos de los Isómeros del Resveratrol Sobre la Homeostasis del Calcio y del Óxido Nítrico en Células Vasculares. Ph.D. Thesis, Universidade de Santiago de Compostela, Santiago de Compostela, Spain, 2009. [Google Scholar]
- Soleas, G.J.; Diamandis, E.P.; Goldberg, D.M. Resveratrol: A molecule whose time has come? And gone? Clin. Biochem. 1997, 30, 91–113. [Google Scholar] [CrossRef]
- Sato, M.; Maulik, G.; Bagchi, D.; Das, D.K. Myocardial protection by protykin, a novel extract of trans-resveratrol and emodin. Free. Radic. Res. 2000, 32, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Palomino, O.; Gómez-Serranillos, M.P.; Slowing, K.; Carretero, E.; Villar, A. Study of polyphenols in grape berries by reversed-phase high-performance liquid chromatography. J. Chromatogr. A 2000, 870, 449–451. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.; Yokota, T.; Ashihara, H.; Lean, M.E.J.; Crozier, A. Plant foods and herbal sources of resveratrol. J. Agric. Food Chem. 2002, 50, 3337–3340. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Xu, Y.X.; Janakiraman, N.; Chapman, R.A.; Gautam, S.C. Immunomodulatory activity of resveratrol: Suppression of lymphocyte proliferation, development of cell-mediated cytotoxicity, and cytokine production. Biochem. Pharmacol. 2001, 62, 1299–1308. [Google Scholar] [CrossRef] [PubMed]
- Mahjabeen, W.; Khan, D.A.; Mirza, S.A. Role of resveratrol supplementation in regulation of glucose hemostasis, inflammation and oxidative stress in patients with diabetes mellitus type 2: A randomized, placebo-controlled trial. Complement. Ther. Med. 2022, 66, 102819. [Google Scholar] [CrossRef]
- Gu, J.; Li, Z.; Chen, H.; Xu, X.; Li, Y.; Gui, Y. Neuroprotective Effect of Trans-Resveratrol in Mild to Moderate Alzheimer Disease: A Randomized, Double-Blind Trial. Neurol. Ther. 2021, 10, 905–917. [Google Scholar] [CrossRef]
- Bradamante, S.; Barenghi, L.; Villa, A. Cardiovascular protective effects of resveratrol. Cardiovasc. Drug Rev. 2004, 22, 169–188. [Google Scholar] [CrossRef]
- de la Lastra, C.A.; Villegas, I. Resveratrol as an antioxidant and pro-oxidant agent: Mechanisms and clinical implications. Biochem. Soc. Trans. 2007, 35, 1156–1160. [Google Scholar] [CrossRef]
- Meng, T.; Xiao, D.; Muhammed, A.; Deng, J.; Chen, L.; He, J. Anti-Inflammatory Action and Mechanisms of Resveratrol. Molecules 2021, 26, 229. [Google Scholar] [CrossRef]
- Hwang, D.; Lim, Y.H. Resveratrol antibacterial activity against Escherichia coli is mediated by Z-ring formation inhibition via suppression of FtsZ expression. Sci. Rep. 2015, 5, 10029. [Google Scholar] [CrossRef]
- Cebrián, R.; Li, Q.; Peñalver, P.; Belmonte-Reche, E.; Andrés-Bilbao, M.; Lucas, R.; de Paz, M.V.; Kuipers, O.P.; Morales, J.C. Chemically Tuning Resveratrol for the Effective Killing of Gram-Positive Pathogens. J. Nat. Prod. 2022, 85, 1459–1473. [Google Scholar] [CrossRef] [PubMed]
- Karameşe, M.; Dicle, Y. The Antibacterial and Antibiofilm Activities of Resveratrol on Gram-positive and Gram-negative Bacteria. Kafkas J. Med. Sci. 2022, 12, 201–206. [Google Scholar] [CrossRef]
- Liu, L.; Yu, J.; Shen, X.; Cao, X.; Zhan, Q.; Guo, Y.; Yu, F. Resveratrol enhances the antimicrobial effect of polymyxin B on Klebsiella pneumoniae and Escherichia coli isolates with polymyxin B resistance. BMC Microbiol. 2020, 20, 306. [Google Scholar] [CrossRef] [PubMed]
- Migliaccio, A.; Stabile, M.; Bagattini, M.; Triassi, M.; Berisio, R.; De Gregorio, E.; Zarrilli, R. Resveratrol Reverts Tolerance and Restores Susceptibility to Chlorhexidine and Benzalkonium in Gram-Negative Bacteria, Gram-Positive Bacteria and Yeasts. Antibiotics 2022, 11, 961. [Google Scholar] [CrossRef] [PubMed]
- Al Azzaz, J.; Rieu, A.; Aires, V.; Delmas, D.; Chluba, J.; Winckler, P.; Bringer, M.A.; Lamarche, J.; Vervandier-Fasseur, D.; Dalle, F.; et al. Resveratrol-Induced Xenophagy Promotes Intracellular Bacteria Clearance in Intestinal Epithelial Cells and Macrophages. Front. Immunol. 2019, 9, 3149. [Google Scholar] [CrossRef]
- Zhou, M.; Niu, H.; Cui, D.; Huang, G.; Li, J.; Tian, H.; Xu, X.; Liang, F.; Chen, R. Resveratrol triggers autophagy-related apoptosis to inhibit the progression of colorectal cancer via inhibition of FOXQ1. Phytother. Res. 2024, 38, 3218–3239. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, Y.J. Synergistic anticancer activity of resveratrol in combination with docetaxel in prostate carcinoma cells. Nutr. Res. Pract. 2021, 15, 12–25. [Google Scholar] [CrossRef]
- Fatehi, R.; Rashedinia, M.; Akbarizadeh, A.R.; Zamani, M.; Firouzabadi, N. Metformin enhances anti-cancer properties of resveratrol in MCF-7 breast cancer cells via induction of apoptosis, autophagy and alteration in cell cycle distribution. Biochem. Biophys. Res. Commun. 2023, 644, 130–139. [Google Scholar] [CrossRef]
- Ma, L.; Li, W.; Wang, R.; Nan, Y.; Wang, Q.; Liu, W.; Jin, F. Resveratrol enhanced anticancer effects of cisplatin on non-small cell lung cancer cell lines by inducing mitochondrial dysfunction and cell apoptosis. Int. J. Oncol. 2015, 47, 1460–1468. [Google Scholar] [CrossRef]
- Ivanova, D.; Zhelev, Z.; Semkova, S.; Aoki, I.; Bakalova, R. Resveratrol Modulates the Redox-status and Cytotoxicity of Anticancer Drugs by Sensitizing Leukemic Lymphocytes and Protecting Normal Lymphocytes. Anticancer. Res. 2019, 39, 3745–3755. [Google Scholar] [CrossRef]
- ALkharashi, N.A. Efficacy of resveratrol against breast cancer and hepatocellular carcinoma cell lines. Saudi Med. J. 2023, 44, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Can, G.; Cakir, Z.; Kartal, M.; Gunduz, U.; Baran, Y. Apoptotic effects of resveratrol, a grape polyphenol, on imatinib-sensitive and resistant K562 chronic myeloid leukemia cells. Anticancer. Res. 2012, 32, 2673–2678. [Google Scholar] [PubMed]
- Du, Q.; Hu, B.; An, H.; Shen, K.; Xu, L.; Deng, S.; Wei, M. Synergistic anticancer effects of curcumin and resveratrol in Hepa1-6 hepatocellular carcinoma cells. Oncol. Rep. 2013, 29, 1851–1858. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Shin, H.; Kim, J. In vivo Anti-Cancer Effects of Resveratrol Mediated by NK Cell Activation. J. Innate Immun. 2021, 13, 94–106. [Google Scholar] [CrossRef]
- Moreira, H.; Szyjka, A.; Grzesik, J.; Pelc, K.; Żuk, M.; Kulma, A.; Emhemmed, F.; Muller, C.D.; Gąsiorowski, K.; Barg, E. Celastrol and Resveratrol Modulate SIRT Genes Expression and Exert Anticancer Activity in Colon Cancer Cells and Cancer Stem-like Cells. Cancers 2022, 14, 1372. [Google Scholar] [CrossRef]
- Subramanian, M.; Soundar, S.; Mangoli, S. DNA damage is a late event in resveratrol-mediated inhibition of Escherichia coli. Free Radic. Res. 2016, 50, 708–719. [Google Scholar] [CrossRef]
- Haranahalli, K.; Tong, S.; Ojima, I. Recent advances in the discovery and development of antibacterial agents targeting the cell-division protein FtsZ. Bioorg. Med. Chem. 2016, 24, 6354–6369. [Google Scholar] [CrossRef]
- Subramanian, M.; Goswami, M.; Chakraborty, S.; Jawali, N. Resveratrol induced inhibition of Escherichia coli proceeds via membrane oxidation and independent of diffusible reactive oxygen species generation. Redox Biol. 2014, 2, 865–872. [Google Scholar] [CrossRef]
- Dadi, P.K.; Ahmad, M.; Ahmad, Z. Inhibition of ATPase activity of Escherichia coli ATP synthase by polyphenols. Int. J. Biol. Macromol. 2009, 45, 72–79. [Google Scholar] [CrossRef]
- Beall, B.; Lutkenhaus, J. FtsZ in Bacillus subtilis is required for vegetative septation and for asymmetric septation during sporulation. Genes. Dev. 1991, 5, 447–455. [Google Scholar] [CrossRef]
- Kursvietiene, L.; Kopustinskiene, D.M.; Staneviciene, I.; Mongirdiene, A.; Kubová, K.; Masteikova, R.; Bernatoniene, J. Anti-Cancer Properties of Resveratrol: A Focus on Its Impact on Mitochondrial Functions. Antioxidants 2023, 12, 2056. [Google Scholar] [CrossRef] [PubMed]
- Danial, N.N.; Korsmeyer, S.J. Cell death: Critical control points. Cell 2004, 116, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Van Opdenbosch, N.; Lamkanfi, M. Caspases in cell death, inflammation, and disease. Immunity 2019, 50, 1352–1364. [Google Scholar] [CrossRef] [PubMed]
- Pistritto, G.; Trisciuoglio, D.; Ceci, C.; Garufi, A.; D’Orazi, G. Apoptosis as anticancer mechanism: Function and dysfunction of its modulators and targeted therapeutic strategies. Aging 2016, 8, 603–619. [Google Scholar] [CrossRef]
- Degterev, A.; Boyce, M.; Yuan, J. A decade of caspases. Oncogene 2003, 22, 8543–8567. [Google Scholar] [CrossRef]
- Kim, T.H.; Park, J.H.; Woo, J.S. Resveratrol induces cell death through ROS-dependent downregulation of Notch1/PTEN/Akt signaling in ovarian cancer cells. Mol. Med. Rep. 2019, 19, 3353–3360. [Google Scholar] [CrossRef]
- Lagunes, I.; Trigos, Á. Photo-oxidation of ergosterol: Indirect detection of antioxidants photosensitizers or quenchers of singlet oxygen. J. Photochem. Photobiol. B Biol. 2015, 145, 30–34. [Google Scholar] [CrossRef]
- Monsour, C.G.; Tadle, A.C.; Tafolla-Aguirre, B.J.; Lakshmanan, N.; Yoon, J.H.; Sabio, R.B.; Selke, M. Singlet Oxygen Quenching by Resveratrol Derivatives. Photochem. Photobiol. 2023, 99, 672–679. [Google Scholar] [CrossRef]
- Jiang, L.Y.; He, S.; Jiang, K.Z.; Sun, C.R.; Pan, Y.J. Resveratrol and its oligomers from wine grapes are selective 1O2 quenchers: Mechanistic implication by high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry and theoretical calculation. J. Agric. Food Chem. 2010, 58, 9020–9027. [Google Scholar] [CrossRef]
- Angelé-Martínez, C.; Goncalves, L.C.P.; Premi, S.; Augusto, F.A.; Palmatier, M.A.; Amar, S.K.; Brash, D.E. Triplet-Energy Quenching Functions of Antioxidant Molecules. Antioxidants 2022, 11, 357. [Google Scholar] [CrossRef]
- Maisch, T.; Baier, J.; Franz, B.; Maier, M.; Landthaler, M.; Szeimies, R.M.; Bäumler, W. The role of singlet oxygen and oxygen concentration in photodynamic inactivation of bacteria. Proc. Natl. Acad. Sci. USA 2007, 104, 7223–7228. [Google Scholar] [CrossRef] [PubMed]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Arihara, Y.; Takada, K.; Kamihara, Y.; Hayasaka, N.; Nakamura, H.; Murase, K.; Ikeda, H.; Iyama, S.; Sato, T.; Miyanishi, K.; et al. Small molecule CP-31398 induces reactive oxygen species-dependent apoptosis in human multiple myeloma. Oncotarget 2017, 8, 65889–65899. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, M.; Uehara, S.; Yoshida, A.; Sakagami, H.; Masuda, Y. Photodynamic Therapy with Resveratrol and an Nd:YAG Laser for Enterococcus faecalis Elimination. In Vivo 2024, 38, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, D.P.; Soares Lopes, D.P.; de Moraes, R.C., Jr.; Gonçalves, C.V.; Rosa, L.P.; da Silva Rosa, F.C.; da Silva, R.A.A. Photoactivated resveratrol against Staphylococcus aureus infection in mice. Photodiagnosis Photodyn. Ther. 2019, 25, 227–236. [Google Scholar] [CrossRef]
- Tosato, M.G.; Schilardi, P.L.; de Mele, M.F.L.; Thomas, A.H.; Miñán, A.; Lorente, C. Resveratrol enhancement staphylococcus aureus survival under levofloxacin and photodynamic treatments. Int. J. Antimicrob. Agents 2018, 51, 255–259. [Google Scholar] [CrossRef]
- Laszló, I.P.; Laszló, M.R.; Popescu, T.; Toma, V.; Ion, R.M.; Moldovan, R.; Filip, G.A.; Cainap, C.; Clichici, S.; Muresan, A. The comparative effects of Resveratrol and Curcumin in combination with photodynamic therapy. Med. Pharm. Rep. 2022, 95, 165–178. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, X.; Kang, S.; Liu, C.; Hao, Y. Resveratrol enhances the effects of ALA-PDT on skin squamous cells A431 through p38/MAPK signaling pathway. Cancer Biomark. 2018, 21, 797–803. [Google Scholar] [CrossRef]
- Hu, J.; Quan, Y.; Lai, Y.; Zheng, Z.; Hu, Z.; Wang, X.; Dai, T.; Zhang, Q.; Cheng, Y. A smart aminoglycoside hydrogel with tunable gel degradation, on-demand drug release, and high antibacterial activity. J. Control Release 2017, 247, 145–152. [Google Scholar] [CrossRef]
- Mensah, A.; Rodgers, A.M.; Larrañeta, E.; McMullan, L.; Tambuwala, M.; Callan, J.F.; Courtenay, A.J. Treatment of periodontal infections, the possible role of hydrogels as antibiotic drug-delivery systems. Antibiotics 2023, 12, 1073. [Google Scholar] [CrossRef]
- Su, J.; Lu, S.; Jiang, S.; Li, B.; Liu, B.; Sun, Q.; Li, J.; Wang, F.; Wei, Y. Engineered Protein Photo-Thermal Hydrogels for Outstanding In Situ Tongue Cancer Therapy. Adv. Mater. 2021, 33, e2100619. [Google Scholar] [CrossRef] [PubMed]
- Kass, L.E.; Nguyen, J. Nanocarrier-hydrogel composite delivery systems for precision drug release. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022, 14, e1756. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wang, Y.; Li, Q.; Yu, C.; Chu, W. Natural polymer-based stimuli-responsive hydrogels. Curr. Med. Chem. 2020, 27, 2631–2657. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, S.; Huang, L.; Li, Y.; Lu, Y.; Li, H.; Chen, G.; Meng, F.; Liu, G.L.; Yang, X.; et al. Reactive oxygen species-responsive and Raman-traceable hydrogel combining photodynamic and immune therapy for postsurgical cancer treatment. Nat. Commun. 2022, 13, 4553. [Google Scholar] [CrossRef] [PubMed]
- Glass, S.; Kühnert, M.; Lippmann, N.; Zimmer, J.; Werdehausen, R.; Abel, B.; Eulenburg, V.; Schulze, A. Photosensitizer-loaded hydrogels for photodynamic inactivation of multirestistant bacteria in wounds. RSC Adv. 2021, 11, 7600–7609. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, H.; Zou, Q.; Xing, R.; Jiao, T.; Yan, X. An injectable dipeptide-fullerene supramolecular hydrogel for photodynamic antibacterial therapy. J. Mater. Chem. B 2018, 6, 7335–7342. [Google Scholar] [CrossRef]
- Zheng, Y.; Yu, E.; Weng, Q.; Zhou, L.; Li, Q. Optimization of hydrogel containing toluidine blue O for photodynamic therapy in treating acne. Lasers Med. Sci. 2019, 34, 1535–1545. [Google Scholar] [CrossRef]
- Belali, S.; Savoie, H.; O’Brien, J.M.; Cafolla, A.A.; O’Connell, B.; Karimi, A.R.; Boyle, R.W.; Senge, M.O. Synthesis and Characterization of Temperature-Sensitive and Chemically Cross-Linked Poly( N-isopropylacrylamide)/Photosensitizer Hydrogels for Applications in Photodynamic Therapy. Biomacromolecules 2018, 19, 1592–1601. [Google Scholar] [CrossRef]
- Du, C.; Zhao, J.; Fei, J.; Gao, L.; Cui, W.; Yang, Y.; Li, J. Alginate-Based Microcapsules with a Molecule Recognition Linker and Photosensitizer for the Combined Cancer Treatment. Chem. Asian J. 2013, 8, 736–742. [Google Scholar] [CrossRef]
- de Freitas, C.F.; Kimura, E.; Rubira, A.F.; Muniz, E.C. Curcumin and silver nanoparticles carried out from polysaccharide-based hydrogels improved the photodynamic properties of curcumin through metal-enhanced singlet oxygen effect. Mater. Sci. Eng. C 2020, 112, 110853. [Google Scholar] [CrossRef]
- Lorenzo, D.; Giorgio, C.; Giulia, B.; Francesco Paolo, B.; Giulio, D.; Alessandra, G.; Cristiana, I.; Matteo, N.; Enrico, T.; Chiara, T.; et al. Certainty and uncertainty in the biological activities of resveratrol. Food Front. 2024, 5, 849–854. [Google Scholar]
- Vivero-Lopez, M.; Muras, A.; Silva, D.; Serro, A.P.; Otero, A.; Concheiro, A.; Alvarez-Lorenzo, C. Resveratrol-Loaded Hydrogel Contact Lenses with Antioxidant and Antibiofilm Performance. Pharmaceutics 2021, 13, 532. [Google Scholar] [CrossRef] [PubMed]
- Jøraholmen, M.W.; Johannessen, M.; Gravningen, K.; Puolakkainen, M.; Acharya, G.; Basnet, P.; Škalko-Basnet, N. Liposomes-In-Hydrogel Delivery System Enhances the Potential of Resveratrol in Combating Vaginal Chlamydia Infection. Pharmaceutics 2020, 12, 1203. [Google Scholar] [CrossRef] [PubMed]
- Radeva, L.; Yordanov, Y.; Spassova, I.; Kovacheva, D.; Tibi, I.P.; Zaharieva, M.M.; Kaleva, M.; Najdenski, H.; Petrov, P.D.; Tzankova, V.; et al. Incorporation of Resveratrol-Hydroxypropyl-β-Cyclodextrin Complexes into Hydrogel Formulation for Wound Treatment. Gels 2024, 10, 346. [Google Scholar] [CrossRef] [PubMed]
- Comotto, M.; Saghazadeh, S.; Bagherifard, S.; Aliakbarian, B.; Kazemzadeh-Narbat, M.; Sharifi, F.; Mousavi Shaegh, S.A.; Arab-Tehrany, E.; Annabi, N.; Perego, P.; et al. Breathable hydrogel dressings containing natural antioxidants for management of skin disorders. J. Biomater. Appl. 2019, 33, 1265–1276. [Google Scholar] [CrossRef]
- Euba, B.; López-López, N.; Rodríguez-Arce, I.; Fernández-Calvet, A.; Barberán, M.; Caturla, N.; Martí, S.; Díez-Martínez, R.; Garmendia, J. Resveratrol therapeutics combines both antimicrobial and immunomodulatory properties against respiratory infection by nontypeable Haemophilus influenzae. Sci. Rep. 2017, 7, 12860. [Google Scholar] [CrossRef]
- Shin, G.R.; Kim, H.E.; Ju, H.J.; Kim, J.H.; Choi, S.; Choi, H.S.; Kim, M.S. Injectable click-crosslinked hydrogel containing resveratrol to improve the therapeutic effect in triple negative breast cancer. Mater. Today Bio 2022, 16, 100386. [Google Scholar] [CrossRef]
- Bruna, L.; Melo, R.; Cátia, G.A.; André, F.; Moreira, I.J.; Correia, I.J.; de Melo-Diogo, D. Chitosan-based injectable in situ forming hydrogels containing dopamine-reduced graphene oxide and resveratrol for breast cancer chemo-photothermal therapy. Biochem. Eng. J. 2022, 185, 108529. [Google Scholar]
- Kotta, S.; Aldawsari, H.M.; Badr-Eldin, S.M.; Nair, A.B.; Kaleem, M.; Dalhat, M.H. Thermosensitive Hydrogels Loaded with Resveratrol Nanoemulsion: Formulation Optimization by Central Composite Design and Evaluation in MCF-7 Human Breast Cancer Cell Lines. Gels 2022, 8, 450. [Google Scholar] [CrossRef]
- Hung, C.F.; Lin, Y.K.; Huang, Z.R.; Fang, J.Y. Delivery of resveratrol, a red wine polyphenol, from solutions and hydrogels via the skin. Biol. Pharm. Bull. 2008, 31, 955–962. [Google Scholar] [CrossRef]
- Paulo, L.; Ferreira, S.; Gallardo, E.; Queiroz, J.A.; Domingues, F. Antimicrobial activity and effects of resveratrol on human pathogenic bacteria. World J. Microbiol. Biotechnol. 2010, 26, 1533–1538. [Google Scholar] [CrossRef]
- Zykova, T.A.; Zhu, F.; Zhai, X.; Ma, W.Y.; Ermakova, S.P.; Lee, K.W.; Bode, A.M.; Dong, Z. Resveratrol directly targets COX-2 to inhibit carcinogenesis. Mol. Carcinog. 2008, 47, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Varoni, E.M.; Lo Faro, A.F.; Sharifi-Rad, J.; Iriti, M. Anticancer molecular mechanisms of resveratrol. Front. Nutr. 2016, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- Pezzuto, J.M. Resveratrol as an inhibitor of carcinogenesis. Pharm. Biol. 2008, 46, 443–573. [Google Scholar] [CrossRef]
- Chen, J.; Liu, H.; Zhao, C.; Qin, G.; Xi, G.; Li, T.; Wang, X.; Chen, T. One-step reduction and PEGylation of graphene oxide for photothermally controlled drug delivery. Biomaterials 2014, 35, 4986–4995. [Google Scholar] [CrossRef]
- Yang, T.; Ren, H.; Zhang, W.; Rong, L.; Zhang, D. Resveratrol-Coated Gold Nanoflowers for CT Imaging and Apoptosis/Photothermal Synergistic Therapy of Malignant Melanoma. ACS Omega 2023, 8, 34629–34639. [Google Scholar] [CrossRef]
- Xiang, S.; Zhang, K.; Yang, G.; Gao, D.; Zeng, C.; He, M. Mitochondria-Targeted and Resveratrol-Loaded Dual-Function Titanium Disulfide Nanosheets for Photothermal-Triggered Tumor Chemotherapy. Nanoscale Res. Lett. 2019, 14, 211. [Google Scholar] [CrossRef]
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K.; et al. Potential Adverse Effects of Resveratrol: A Literature Review. Int. J. Mol. Sci. 2020, 21, 2084. [Google Scholar] [CrossRef]
- Li, X.; Chen, L.; Huang, M.; Zeng, S.; Zheng, J.; Peng, S.; Wang, Y.; Cheng, H.; Li, S. Innovative strategies for photodynamic therapy against hypoxic tumor. Asian J. Pharm. Sci. 2023, 18, 100775. [Google Scholar] [CrossRef]
- Olszowy, M.; Nowak-Perlak, M.; Woźniak, M. Current Strategies in Photodynamic Therapy (PDT) and Photodynamic Diagnostics (PDD) and the Future Potential of Nanotechnology in Cancer Treatment. Pharmaceutics 2023, 15, 1712. [Google Scholar] [CrossRef]
- Li, R.T.; Zhu, Y.D.; Li, W.Y.; Hou, Y.K.; Zou, Y.M.; Zhao, Y.H.; Zou, Q.; Zhang, W.H.; Chen, J.X. Synergistic photothermal-photodynamic-chemotherapy toward breast cancer based on a liposome-coated core-shell AuNS@NMOFs nanocomposite encapsulated with gambogic acid. J. Nanobiotechnology 2022, 20, 212. [Google Scholar] [CrossRef]
- Overchuk, M.; Weersink, R.A.; Wilson, B.C.; Zheng, G. Photodynamic and Photothermal Therapies: Synergy Opportunities for Nanomedicine. ACS Nano 2023, 17, 7979–8003. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).