Targeted Myocardial Restoration with Injectable Hydrogels—In Search of The Holy Grail in Regenerating Damaged Heart Tissue
Abstract
1. Introduction
1.1. “Stem Cells Are the Future of Heart Treatment, and They Will Always Be” Norman Shumway
1.2. The Unique and Complex Structure of a Healthy and Injured Myocardium
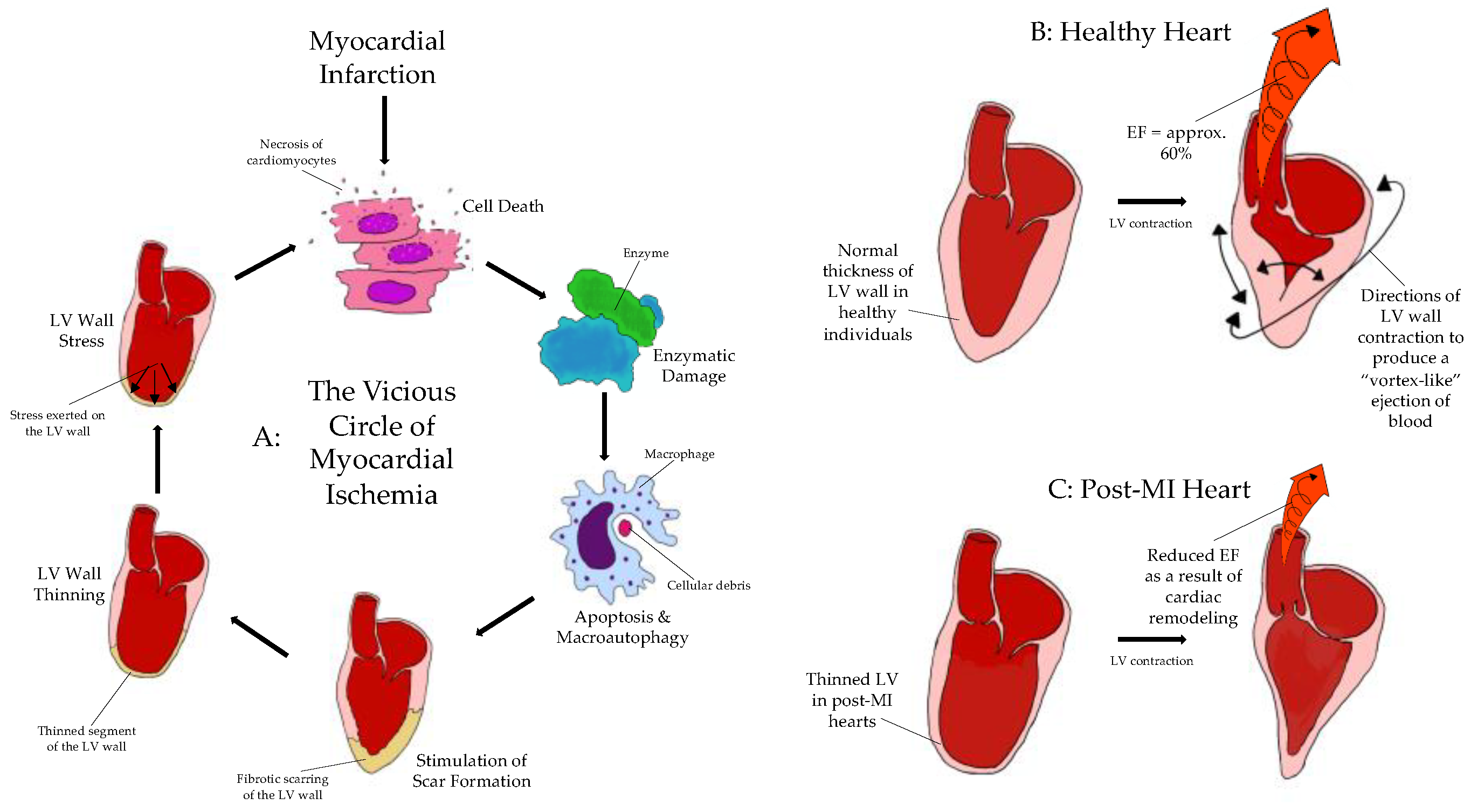
1.3. The Vicious Circle of Myocardial Ischemia and the Mechanics of Remodeling
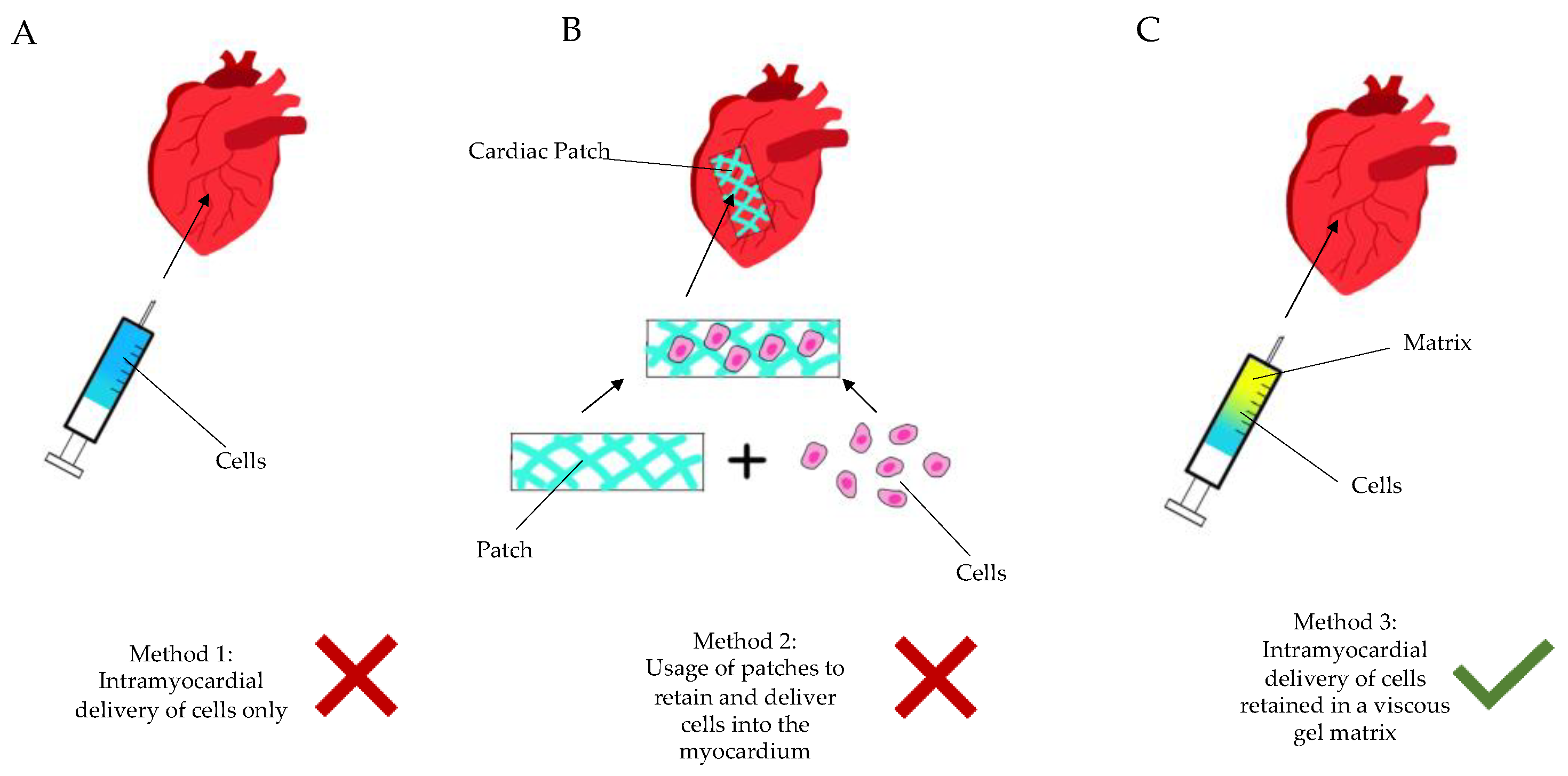
1.4. Cell-Based Therapy: Unfulfilled Hopes or Misguided Expectations? Why Not Only Cells?
- Injectable, hence minimally invasive, administration
- Autologous material, not of stem cell nature, to be derived simply during treatment
- A polytherapy approach to address concomitant aspects of the vicious circle of myocardial ischemia (antioxidants, purine metabolism blockers/anti-inflammatory drugs)
- Easy adoption and clinical penetration in the horizon.
2. Materials and Methods
3. Findings
3.1. Treatment with Hydrogel Improves Systolic and Diastolic Cardiac Function
3.2. Treatment with Hydrogel Attenuated LV Remodeling
3.3. Treatment with Hydrogel Reduces Cardiac Fibrosis
3.4. Treatment with Hydrogel Supports Angiogenesis Post-Infarction
4. Discussion
4.1. Post-MI Survivability
4.2. Injectable Hydrogel-Based Approach for Cardiac Tissue Engineering
4.3. Less Invasive Administration Modes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AA | Ascorbic Acid |
| AE | Arterial Elastance |
| AGHM | Acidic Gelatin Hydrogel Microspheres |
| BDNF | Brain-Derived Neurotrophic Factor |
| BNP | B-type Natriuretic Peptide |
| bFGF | Basic Fibroblast Growth Factor |
| BNDF | Brain-Derived Neurotrophic Factor |
| BV | Blood Vessel |
| BZ | Border Zone |
| CACs | Circulating Angiogenic Cells |
| CB-MNC | Cord Blood Mononuclear Cells |
| CH | Chitosan Hydrogel |
| CI | Cardiac Index = SV x (heart rate)/BSA/10 |
| CO | Cardiac Output |
| cTnT | Cardiac Troponin-T |
| DAPI | 4’,6-diamidino-2-phenylindole |
| DC | Dual-Crosslinking Hydrogel |
| +Dp/Dt | Measure of Systolic Function |
| -Dp/Dt | Measure of Diastolic Function |
| E/A | E-wave to A-wave Ratio |
| EC | Endothelial Cell |
| ECM | Extracellular Matrix |
| EDV | End-Diastolic Volume |
| EDP | End Diastolic Pressure |
| ELRs | Elastin-Like Recombinamers |
| Emax | Maximum Chamber Elasticity |
| ES | End-Systolic |
| ESP | End-Systolic Pressure |
| ESPVR | End-Systolic Pressure-Volume Relationship |
| ESV | End-Systolic Volume |
| FAC | Fractional Area Change |
| FAS | Fractional Area Shortening |
| FL-MMP-2 | Full Length Matrix Metalloproteinase 2 |
| GF | Growth Factor |
| GH | Guest-Host Hydrogel |
| HA | Hyaluronic Acid |
| HAMMPS | Matrix Metalloproteinase-Sensitive Hyaluronic Acid Gel |
| hBMCs | Human Bone Marrow Derived Mesenchymal Cells |
| hCDCs | Human Cardiosphere-Derived Cells |
| HEMA | Hydroyethyl (Methacrylate) |
| HEMA-HA | Hyaluronic Acid Macromers with HEMA Group Modification |
| HF | Heart Failure |
| HGF | Hepatocyte Growth Factor |
| HG | Hydrogel |
| hUMSC | Human Umbilical Mesenchymal Stem Cells |
| IGF-1 | Insulin-Like Growth Factor 1 |
| IVS | Interventricular Septum |
| LAD | Left Anterior Descending Artery |
| LCx | Left Circumflex Artery |
| LV | Left Ventricle |
| LV EDd | Left Ventricular End-Diastolic Dimension |
| LVEDP | Left Ventricular End Diastolic Pressure |
| LV ESV | Left Ventricular End-Systolic Volume |
| LVEF | Left Ventricular Ejection Fraction |
| LVMV | Left Ventricle Mass Volume |
| MAPLA | Methacrylate Polylactide |
| MBF | Myocardial Blood Flow |
| MeHA | Methacrylated Hyaluronic Acid |
| MFR | Myocardial Flow Reserve |
| MI | Myocardial Infarction |
| miR | microRNA |
| miR-NC | microRNA-Negative Control |
| MLWHFQ | Minnesota Living With Heart Failure Questionnaire |
| MMP | Matrix Metalloproteinase |
| MNC | Mononuclear Cells |
| MSN | Mesoporous Silica Nanoparticles |
| NF | Nanofibres |
| NIPAAm | N-Isopropyl Acrylamide |
| NRG | Neuregulin |
| NS | Normal Saline |
| NRG | Neuregulin |
| NYHA | New York Heart Association |
| PBS | Phosphate-Buffered Saline |
| PCI | Percutaneous Coronary Intervention |
| PCWP | Pulmonary Artery Wedge Pressure |
| PEG | Polyethylene Glycol |
| PRP | Platelet-Rich Plasma |
| PRSW | Preload Recruitable Stroke Work |
| PSV | Peak of Systolic Velocity |
| ROS | Reactive Oxygen Species |
| RWM | Regional Wall Motion |
| rTIMP-3 | Tissue Inhibitors of Metalloproteinase Recombinant Protein |
| STEMI | ST-Segment Elevated Myocardial Infraction |
| SV | Stroke Volume |
| T | Time constant of Left Ventricular Pressure Decay |
| TMJR | Transmyocardial Jet Revascularization |
| UPy | Ureidopyrimidinone |
| VEGF | Vascular Endothelial Growth Factor |
| WMSI | Wall Motion Score Index |
References
- Buckberg, G.D. Basic science review: The helix and the heart. J. Thorac. Cardiovasc. Surg. 2002, 124, 863–883. [Google Scholar] [CrossRef] [PubMed]
- Vu, D.T.; Martinez, E.C. Myocardial restoration: Is it the cell or the architecture or both? Cardiol. Res. Pract. 2012, 2012, 240497. [Google Scholar] [CrossRef] [PubMed]
- McCord, J.M.; Roy, R.S. Free radicals and myocardial ischemia. The role of xanthine oxidase. Adv. Myocardiol. 1985, 5, 183–189. [Google Scholar]
- Yin, F.C.P. Ventricular wall stress. Invited review. Circ. Res. 1981, 49, 829–842. [Google Scholar] [CrossRef]
- Di Napoli, P.; Taccardi, A.A. Left ventricular wall stress as a direct correlate of cardiomyocyte apoptosis in patients with severe dilated cardiomyopathy. Am. Heart J. 2003, 146, 1105–1111. [Google Scholar] [CrossRef]
- Kofidis, T.; Lee, C.N. From vision to mission in myocardial restoration. Asian Cardiovasc. Thorac. Ann. 2008, 16, 91–92. [Google Scholar] [CrossRef] [PubMed]
- Menasche, P.; Alfieri, O. The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial: First randomized placebo-controlled study of myoblast transplantation. Circulation 2008, 117, 1189–1200. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Lu, K. Stem cell therapy for ischemic heart diseases. Br. Med. Bull. 2017, 121, 135–154. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- Zhou, D.; Xiong, L. Effects of transmyocardial jet revascularization with chitosan hydrogel on channel patency and angiogenesis in canine infarcted hearts. J. Biomed. Mater. Res. Part A 2013, 101A, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, L. Application of bFGF and BDNF to Improve Angiogenesis and Cardiac Function. J. Surg. Res. 2006, 136, 85–91. [Google Scholar] [CrossRef]
- Leor, J.; Tuvia, S. Intracoronary Injection of In Situ Forming Alginate Hydrogel Reverses Left Ventricular Remodeling After Myocardial Infarction in Swine. J. Am. Coll. Cardiol. 2009, 54, 1014–1023. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Zhu, Y. Intramyocardial injection of a fully synthetic hydrogel attenuates left ventricular remodeling post myocardial infarction. Biomaterials 2019, 217, 119289. [Google Scholar] [CrossRef]
- Contessotto, P.; Orbanić, D. Elastin-like recombinamers-based hydrogel modulates post-ischemic remodeling in a non-transmural myocardial infarction in sheep. Sci. Transl. Med. 2021, 13, eaaz5380. [Google Scholar] [CrossRef]
- Ifkovits, J.L.; Tous, E. Injectable hydrogel properties influence infarct expansion and extent of postinfarction left ventricular remodeling in an ovine model. Proc. Natl. Acad. Sci. USA 2010, 107, 11507–11512. [Google Scholar] [CrossRef]
- Rodell, C.B.; Lee, M.E. Injectable Shear-Thinning Hydrogels for Minimally Invasive Delivery to Infarcted Myocardium to Limit Left-Ventricular Remodeling. Circ. Cardiovasc. Interv. 2016, 9, e004058. [Google Scholar] [CrossRef] [PubMed]
- Spaulding, K.A.; Zhu, Y. Myocardial injection of a thermoresponsive hydrogel with reactive oxygen species scavenger properties improves border zone contractility. J. Biomed. Mater. Res. A. 2020, 108, 1736–1746. [Google Scholar] [CrossRef] [PubMed]
- Traverse, J.H.; Henry, T.D. First-in-Man Study of a Cardiac Extracellular Matrix Hydrogel in Early and Late Myocardial Infarction Patients. Am. Coll. Cardiol. Basic Trans. Sci. 2019, 4, 659–669. [Google Scholar] [CrossRef]
- Giordano, C.; Stephanie, L. Preclinical Evaluation of Biopolymer-Delivered Circulating Angiogenic Cells in a Swine Model of Hibernating Myocardium. Circ. Cardiovasc. Imaging 2013, 6, 982–991. [Google Scholar] [CrossRef]
- Wang, Q.; He, X. Injectable collagen scaffold promotes swine myocardial infarction recovery by long-term local retention of transplanted human umbilical cord mesenchymal stem cells. Sci. China Life Sci. 2021, 64, 269–281. [Google Scholar] [CrossRef]
- Yamamoto, T.; Suto, N. Intramyocardial delivery of basic fibroblast growth factor-impregnated gelatin hydrogel microspheres enhances collateral circulation to infarcted canine myocardium. Jpn. Circ. J. 2001, 65, 439–444. [Google Scholar] [CrossRef]
- Cohen, J.E.; Goldstone, A.B. A Bioengineered Neuregulin-Hydrogel Therapy Reduces Scar Size and Enhances Post-Infarct Ventricular Contractility in an Ovine Large Animal Model. J. Cardiovasc. Dev. Dis. 2020, 7, 53. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, X. Injectable hydrogel with MSNs/microRNA-21-5p delivery enables both immunomodification and enhanced angiogenesis for myocardial infarction therapy in pigs. Sci. Adv. 2021, 7, eabd6740. [Google Scholar] [CrossRef]
- Purcell, B.P.; Barlow, S.C. Delivery of a matrix metalloproteinase-responsive hydrogel releasing TIMP-3 after myocardial infarction: Effects on left ventricular remodeling. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H814–H825. [Google Scholar] [CrossRef]
- Chang, M.Y.; Huang, T.T. Injection of Human Cord Blood Cells With Hyaluronan Improves Postinfarction Cardiac Repair in Pigs. Stem Cells Transl. Med. 2016, 5, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Koudstaal, S.; Bastings, M.M.C. Sustained Delivery of Insulin-Like Growth Factor-1/Hepatocyte Growth Factor Stimulates Endogenous Cardiac Repair in the Chronic Infarcted Pig Heart. J. Cardiovasc. Trans. Res. 2014, 7, 232–241. [Google Scholar] [CrossRef]
- Li, Y.D.; Chang, M.Y. Injection of Peptide nanogels preserves postinfarct diastolic function and prolongs efficacy of cell therapy in pigs. Tissue Eng. Part A. 2015, 21, 1662–1671. [Google Scholar]
- Chang, M.Y.; Chang, C.H. The time window for therapy with peptide nanofibers combined with autologous bone marrow cells in pigs after acute myocardial infarction. PLoS ONE 2015, 10, e0115430. [Google Scholar] [CrossRef]
- Takehara, N.; Tsutsumi, Y. Controlled Delivery of Basic Fibroblast Growth Factor Promotes Human Cardiosphere-Derived Cell Engraftment to Enhance Cardiac Repair for Chronic Myocardial Infarction. J. Am. Coll. Cardiol. 2008, 52, 1858–1865. [Google Scholar] [CrossRef]
- Vu, T.D.; Pal, S.N. An autologous platelet-rich plasma hydrogel compound restores left ventricular structure, function and ameliorates adverse remodeling in a minimally invasive large animal myocardial restoration model: A translational approach: Vu and Pal “Myocardial Repair: PRP, Hydrogel and Supplements”. Biomaterials 2015, 45, 27–35. [Google Scholar]
- Ezekowitz, J.A.; Kaul, P. Declining in-hospital mortality and increasing heart failure incidence in elderly patients with first myocardial infarction. J. Am. Coll. Cardiol. 2009, 53, 13–20. [Google Scholar] [CrossRef]
- Barasa, A.; Schaufelberger, M. Heart failure in young adults: 20-year trends in hospitalization, aetiology, and case fatality in Sweden. Eur. Heart J. 2014, 35, 5–32. [Google Scholar] [CrossRef]
- Mamas, M.A.; Sperrin, M. Do patients have worse outcomes in heart failure than in cancer? A primary care-based cohort study with 10-year follow-up in Scotland. Eur. J. Heart Fail. 2017, 19, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.; Ekman, I. Population impact of heart failure and the most common forms of cancer: A study of 1 162 309 hospital cases in Sweden (1988 to 2004). Circ. Cardiovasc. Qual. Outcomes 2010, 3, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Siminiak, T.; Fiszer, D. Percutaneous trans-coronary-venous transplantation of autologous skeletal myoblasts in the treatment of post-infarction myocardial contractility impairment: The POZNAN trial. Eur. Heart J. 2005, 26, 1188–1195. [Google Scholar] [CrossRef] [PubMed]
| Author | Species | Sex/Age | Weight (kg) | Number | Grouping | Aim of the Study |
|---|---|---|---|---|---|---|
| Giordano, C et al., 2013 [19] | Swine, Yorkshire Pig | F/− | 8–10 | 32 | Control: 10; CAC: 8; CAC+Matrix: 7; Death: 7 | To investigate the effects of biopolymer-supported delivery of circulating angiogenic cells. |
| Leor, J et al., 2009 [12] | Swine, Domestic | F/- | 50–60 | 58 | Death: 22; Exclusion: 1; Control: 16; Intervention: 19 | To investigate whether a selective intracoronary injection of alginate solution would result in localised gelation as scaffold in the infarcted tissue. |
| Matsumura, Y et al., 2019 [13] | Swine, Yorkshire Pig | F/4–5 months | 20–30 | 12 | Control: 6; Treatment: 6 | To investigate whether the injection of a fully synthetic hydrogel designed for MI treatment was effective in attenuating post-MI LV remodeling. |
| Qiang Wang et al., 2021 [20] | Chinese Pama Minipig | M/6 months | 15–20 | 45 | Control: 15; hUMSC: 15; hUMSC with Collagen: 15 | To investigate whether an injectable collagen scaffold promoted the long-term retention of transplanted stem cells. |
| Yamamoto, T et al., 2001 [21] | Canine, Mongrel | −/Adult | 20.3 ± 0.6 | 28 | AGHM-bFGF: 13; bGFG only: 9; AGHM only: 6 | To find out if bFGF-impregnated AGHM would enhance collateral development to the infarct area. |
| Zhou, D et al., 2012 [10] | Canine, Mongrel | −/− | 9–14 | 32 | MI: 5; MI + NS: 6; CH: 6; CH + GF: 7; Death: 8 | Determine whether TMJR with chitosan scaffolds retained channel patency and enhanced angiogenesis. |
| Cohen, J.E et al., 2020 [22] | Ovine, Dorset Sheep | M/6–7 months | 35–40 | 21 | Saline: 6; HG: 4; NRG: 4; NRG-HG: 7 | To evaluate the effectiveness of an NRG-HG therapy to enhance cardiac function. |
| Contessotto, P et al., 2021 [14] | Ovine, Romanov Sheep | M/8 months | 30–40 | 39 | MI only (7 day): 6; MI only (28 day): 8; PBS: 6; ELRs-Hydrogel: 6; Death: 12; Exclusion: 1 | To evaluate the effectiveness of an ECM-mimicking hydrogel in modulating post-ischemic. |
| Li, Y et al., 2021 [23] | Swine, Yucatan mini pigs | M/- | 45–50 | 25 | Sham: 3; Saline: 5; agomiR-21-5p: 5; Gel@MSN/miR-NC: 6; Gel@MSN/miR-21-5p: 6 | To demonstrate that a microRNA-21-5p delivery system enables both immuno-modification and enhanced angiogenesis for myocardial infarction. |
| Purcell, B.P et al., 2013 [24] | Swine, Yorkshire Pig | M/− | 20 | 26 | Sham:5; MI/Saline: 7; MI/HAMMPS: 7; MI/HAMMPS/rTIMP-3: 7 | To investigate whether the localized delivery of an MMP-sensitive biomaterial that releases a recombinant TIMP held promise as a means to interrupt adverse post-MI remodeling. |
| Chang, M.Y et al., 2016 [25] | Swine, Lanyu Minipigs | M/5 months | 22.26 ± 0.78 | 34 | Sham; MI + NS; MI + CB-MNC; MI + HA;
MI + CB-MNC/HA | To investigate whether the injection of CB-MNCs combined with hyaluronan hydrogel improved cell therapy efficacy. |
| Ifkovits, J. L et al., 2010 [15] | Ovine, Dorset Sheep | M/Adult | 35–40 | 21 | Control: 9; MeHA high: 7;
MeHA low: 5 | To compare the effects of two injectable MeHA formulations that exhibit similar degradation but have differential moduli. |
| Koudstaal, S et al., 2014 [26] | Swine, Dalland Landrace | F/6 months | ~70 | 18 | Control (GF): 5; Death: 4; Hydrogel: 4; Hydrogel + GF: 5 | To investigate whether the effect of IGF-1/HFG therapy was also effective in the post-MI heart. |
| Lin, Y.D et al., 2015 [27] | Swine, Minipigs | −/5 months | - | 27 | 5-6 per group: Sham, NS, NF only, MNC only, MNC + NF; Death: 1 | To test whether the benefits of an injection of peptide nanofibers continued to persist as the material degraded. |
| Liu, Y et al., 2006 [11] | Canine, Mongrel | −/− | 15–20 | 18 | Control: 6; bFGF alone: 6; bFGF + BDNF: 6 | To assess whether the simultaneous application of bFGF- and BDNF-incorporating gelatin hydrogels improved angiogenesis. |
| Yao Chang, M et al., 2005 [28] | Porcine, Lanyu Minipigs | −/5 months | 23.88 (mean) | 45 | Sham, MI+NS, MI+NF (1 day), MI+MNC (1 day), MI+NF/MNC (1 day), MI+NF/MNC (4 days), MI+NF/MNC (7 days) | To evaluate the therapeutic time window for NF/MNC therapy in acute myocardial infarction. |
| Rodell, C.B et al., 2016 [16] | Ovine, Dorset | M/Adult | 45 | 22 | Saline, GH Hydrogel, DC Hydrogel | To investigate whether soft hydrogels with in-vivo stiffening enhanced therapeutic efficacy to limit LV remodeling and heart failure. |
| Spaulding, K.A et al., 2020 [17] | Ovine, Dorset Cross-Breed | M, castrated/− | − | 14 | Control: 7; Treatment: 7 | To find out if an injection of a thermoresponsive hydrogel, with ROS scavenging properties, into the MI would decrease ROS. |
| Takehara, N et al., 2008 [29] | Swine, Yorkshire pigs, | F/- | − | 60 | Placebo: 15; Gelatin hydrogel: 6; hBMCs: 6; hCDCs: 9; Death: 17; Exclusion: 7 | To determine whether the controlled release of bFGF might improve hCDC therapy. |
| Vu, T.D et al., 2011 [30] | Swine, Yorkshire pig | F/- | 65–70 | 36 | Sham: 6; Control: 6; Hydrogel only: 6; PRP-only: 6; Hydrogel + AA: 6; Hydrogel + AA + PRP: 6 | To evaluate whether hyaluronic acid-based hydrogel, coupled with PRP, improved host-cell viability. |
| Traverse, J.H et al., 2019 [18] | First in Human Study | M/F (12/3), 59.6 ± 8.8 years | − | 15 patients | Early group (2–12 months post-STEMI): 7; Late group (1–3 years post STEMI): 8 | To evaluate the safety and feasibility of transendocardial injections of VentriGel, a cardiac extracellular matrix hydrogel, in early and late post-MI patients with LV dysfunction. |
| Author | Echo | MRI | CT | PET | Ventricular catheterization | Immunohistochemistry | Masson’s Trichrome Staining | Other |
|---|---|---|---|---|---|---|---|---|
| Giordano, C et al., 2013 [19] | LVEF ↑ WMSI ↓ | MBF during: Rest ↓, Stress ↑, MFR ↑ | BV amount ↑ | |||||
| Leor, J et al., 2009 [12] | ES area ↓, LV mass ↓ | Wall thickness ↑ | ||||||
| Matsumura, Y et al., 2019 [13] | ESV ↓, EDV ~, LVEF ↑, FAC ↑, Scar size ↓, SV ↑ | Angiotensin II ↑ | Cardiac fibrosis ↓ | Stiffness ↑ (Biaxial mechanical) | ||||
| Qiang Wang et al., 2021 [20] | LVEF ↑ CO ↑, SV ↑, ESV ↓, EDV ↓ | LVEF ↑ CO ↑, SV ↑ ESV ↓, EDV ↓ | Scar size ↓ Infarct size ↓ LVMV ↓ | Cell retention ↑, Arteriole density ↑, Island-/strip- shaped cTnT-positive cells ↑ | ||||
| Yamamoto, T et al., 2001 [21] | LVEF ~, LVEDP ~, Antegrade flow ↑, Wall motion ~, MBF in ischemic region ↑ | BV density ↑ | ||||||
| Zhou, D et al., 2012 [10] | Endothelization ↑, Arteriole and small vessel density ↑, Larger arteriole density ↑ | Cardiac fibrosis ~ | Size of infarct region ~ (via weighing) | |||||
| Cohen, J.E et al., 2020 [22] | LVEF ↑, Mean arterial pressure ↑ EDV ↓, ESV ↓, ESPVR ↑ | |||||||
| Contessotto, P et al., 2021 [14] | LVEF ↑ | Wall thickness ↑, Collagen fibers ↓ BV density ↑, Cardiomyocyte preservation ↑ | Cardiac fibrosis ↓ | |||||
| Li, Y et al., 2021 [23] | LVEF ↑ EDV ↓, ESV ↓ LV EDd ↑ | Scar size ↓ Wall thickness ↑ | BV density ↑, BV volume ↑, Infarct size ↓ Immunomodulatory effect ↓ | Cardiac fibrosis ↓ | ||||
| Purcell, B.P et al., 2013 [24] | LVEF ↑, EDV ↓, ESV ↓, Wall thickness ↑, LV mass ↓ | Transcriptional activity of myofibroblasts and profibrotic pathways ↓ (mRNA profiling) | ||||||
| Chang, M.Y et al., 2016 [25] | LVEF ↑ IVS thickness ↑ | +dp/dt ↑, -dp/dt ↑ LV EDP ↓, EDV ↓ | Cell retention ↑ BV density ↑ EC differentiation ↑ | Scar size ↓ Wall thickness ↑ (Via gross cross-section) | ||||
| Ifkovits, J. L et al., 2010 [15] | LVEF ↑, ESV ↑, EDV ↑, CO ↑ | Wall thickness ↑ Infarct area ↓ | ||||||
| Koudstaal, S et al., 2014 [26] | LVEF ↑ EDV ↑, ESV ↑, FAS ↑, PRSW ↑ | Cardiomyocyte hypertrophy ↓, Cardiomyocyte proliferation ↑, Fibrosis extent ↓, BV density ↑, C-kit number ↑ | ||||||
| Lin, Y.D et al., 2015 [27] | LVEF ↑, PSV ↑, E/A ratio ↓ | SV ↑, AE ↑, +dP/dt ↑, -dP/dt ↑, PRSW ↑, Emax ↑, T ↓, ESV ↓, EDV ↓, ESP ↑, EDP ↓ | BV density ↑ | |||||
| Liu, Y et al., 2006 [11] | LVEF ↑ | MBF ↑ | BV density ↑, bFGF expression ↑ BDNF expression ↑, Distribution of bFGF and BDNF positive cells ~ | |||||
| Yao Chang, M et al., 2005 [28] | LVEF ↑ | IVS thickness ↑ Systolic function ↑ EDP ↑, EDV ↑, Emax ↑ | Infarct size ↓, Infarct length ratio ↓ BV density ↑, Blood flow ↑ | Cardiac fibrosis ↓ | Stem cell retention ↑ (Confocal microscopy with Dil and DAPI staining) | |||
| Rodell, C.B et al., 2016 [16] | LVEF ↑ EDV, ESV ↓ LV wall thickness ↑ | Myofiber stress reduction ↑ (Via FE simulation model, dimensions of model obtained via MRI) | ||||||
| Spaulding, K.A et al., 2020 [17] | LVEF ↑ (2 wk), LVEF ↓ (6 wk), EDV ↑ ESV ↑ | LV wall thickness ↑ Demembranated muscle force ↑ | Levels of ROS in BZ ↓ FL-MMP-2 ↓ | SV ↑ (2 weeks) SV ↓ (6 weeks) PCWP ↑ (Via Swan Ganz) | ||||
| Takehara, N et al., 2008 [29] | LVEF ↑, RWM ↑ Myocardial perfusion ↑ Infarct size ↓ | Stem cell retention ↑ | Myocyte conversion ↑ | |||||
| Vu, T.D et al., 2011 [30] | LVEF ↑ FAC ↑ EDV ↓ | LV mass ↓ LV collagen area fraction ↓ Scar size ↓ | BV density ↑ BV amount ↑ | |||||
| Traverse, J.H et al., 2019 [18] | LVEF ~, EDV ↓, ESV ↓ Scar size ~, Viable mass ↑ | BNP ↓, 6-min walk test distance ↑ NYHA class ↑, MLWHFQ score ↑ |
| Author | Method of MI Creation | Artery Involved | Cell Delivered | Type of Matrix | Method of Delivery to Myocardium |
|---|---|---|---|---|---|
| Giordano, C et al., 2013 [19] | Left thoracotomy with ameroid constrictor | Proximal LCx | CAC | Type-I rat tail collagen cross-linked with glutaraldehyde (BD Bioscience, Oakville, Canada) | Open; Intramyocardial |
| Leor, J et al., 2009 [12] | Balloon occlusion | Mid-LAD artery | Acellular | Sodium alginate (VLVG, NovaMatrix, FMC Biopolymers, Drammen, Norway) | Intracoronary |
| Matsumura, Y et al., 2019 [13] | Left thoracotomy with suture ligation | Between 1st and 2nd diagonal branches | Acellular | Synthetic Hydrogel: Poly (NIPAAm-co-HEMA-co-MAPLA) (Sigma-Aldrich, USA) | Open; Intramyocardial |
| Qiang Wang et al., 2021 [20] | Left thoracotomy with suture ligation | LAD distal to origin of 2nd branch | hUMSC | Bovine collagen | Open; Intramyocardial |
| Yamamoto, T et al., 2001 [21] | Left thoracotomy with suture ligation | LAD between 1st and 2nd diagonal branches | bFGF | AGHM | Open; Subepicardial implantation |
| Zhou, D et al., 2012 [10] | Left thoracotomy with suture ligation | LAD below 1st diagonal branch | VEGF165 | Temperature-responsive Chitosan hydrogel | Open; Transmyocardial jet revascularization |
| Cohen, J.E et al., 2020 [22] | Left thoracotomy with suture ligation | 2nd and 3rd diagonal branches of LAD | NRG (R&D Systems, Minneapolis, MN, USA) | HEMA-HA based hydrogel (Lifecore Biomedical Inc., Chaska, MN, USA) | Open; intramyocardial |
| Contessotto, P et al., 2021 [14] | Left thoracotomy with suture ligation | LAD from 1st diagonal branch, moving distally till apex | Acellular | ELRs hydrogel | Open; Intramyocardial |
| Li, Y et al., 2021 [23] | Left thoracotomy with suture ligation | 1st two obtuse marginal arteries of LCx | MSN/miR-21-5p complex | Injectable hydrogel matrix | Open; Intramyocardial |
| Purcell, B.P et al., 2013 [24] | Left thoracotomy with suture ligation | 1st two obtuse marginal arteries of LCx | Full-length rTIMP-3 | Hyaluronic acid-based hydrogel with MMP | Open; Intramyocardial |
| Chang, M.Y et al., 2016 [25] | Left thoracotomy with suture ligation | Mid-LAD | CB-MNC | Hyaluronic acid hydrogel | Open; Intramyocardial |
| Ifkovits, J. L et al., 2010 [15] | Left thoracotomy with suture ligation | LAD and 2nd diagonal coronary artery | Acellular | Methacrylated hyaluronic acid macromers (MeHA) hydrogel | Open; Intramyocardial |
| Koudstaal, S et al., 2014 [26] | 75 min intracoronary balloon occlusion | LCx | IGF-1/HGF | UPy hydrogel | Open; Intramyocardial |
| Lin, Y.D et al., 2015 [27] | Left thoracotomy with suture ligation | Mid-LAD | MNCs | Peptide nanofibers | Open; Intramyocardial |
| Liu, Y et al., 2006 [11] | Left thoracotomy with suture ligation | LAD distal to 1st diagonal branch | bFGF, BDNF | Gelatin hydrogel (Boster Bioengineering Company, Wuhan, China) | Open; Intramyocardial |
| Yao Chang, M et al., 2005 [28] | Left thoracotomy with suture ligation | Mid-LAD | Bone marrow MNC | Peptide nanofibers | Open; Intramyocardial |
| Rodell, C.B et al., 2016 [16] | Left thoracotomy with suture ligation | Selective ligation of obtuse marginal branches | Acellular | Guest-host hydrogels; Dual-crosslinking hydrogels | Open; Intramyocardial |
| Spaulding, K.A et al., 2020 [17] | Left thoracotomy with suture ligation | LAD and its diagonal branches | Acellular | NIPAAm-PEG1500 hydrogel (Sigma-Aldrich, USA) | Open; Intramyocardial |
| Takehara, N et al., 2008 [29] | 90 min intracoronary balloon occlusion, followed by reperfusion | LAD | bFGF (Kaken Pharmaceutical Co., Tokyo, Japan) | Gelatin hydrogel | Open; Intramyocardial |
| Vu, T.D et al., 2011 [30] | Left thoracotomy with suture ligation | Proximal LCx | Platelet-rich plasma | Hyaluronate Gelatin (Glycosan BioSystems Inc, Salt Lake City, UT, USA) | Open; Intramyocardial |
| Traverse, J.H et al., 2019 [18] | Patients with 1st STEMI treated by PCI within past 60 days to 3 years with moderate LV dysfunction. | − | Acellular | VentriGelTM—ECM from decellularized porcine myocardium | Transcatheter delivery through endocardium into myocardium |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sazzad, F.; Kuzemczak, M.; Loh, E.; Wu, W.; Kofidis, T. Targeted Myocardial Restoration with Injectable Hydrogels—In Search of The Holy Grail in Regenerating Damaged Heart Tissue. Biomedicines 2021, 9, 595. https://doi.org/10.3390/biomedicines9060595
Sazzad F, Kuzemczak M, Loh E, Wu W, Kofidis T. Targeted Myocardial Restoration with Injectable Hydrogels—In Search of The Holy Grail in Regenerating Damaged Heart Tissue. Biomedicines. 2021; 9(6):595. https://doi.org/10.3390/biomedicines9060595
Chicago/Turabian StyleSazzad, Faizus, Michał Kuzemczak, Engracia Loh, Wellington Wu, and Theo Kofidis. 2021. "Targeted Myocardial Restoration with Injectable Hydrogels—In Search of The Holy Grail in Regenerating Damaged Heart Tissue" Biomedicines 9, no. 6: 595. https://doi.org/10.3390/biomedicines9060595
APA StyleSazzad, F., Kuzemczak, M., Loh, E., Wu, W., & Kofidis, T. (2021). Targeted Myocardial Restoration with Injectable Hydrogels—In Search of The Holy Grail in Regenerating Damaged Heart Tissue. Biomedicines, 9(6), 595. https://doi.org/10.3390/biomedicines9060595