Abstract
Carbon dioxide (CO2) electroreduction offers an attractive pathway for converting CO2 to valuable fuels and chemicals. Despite the existence of some excellent electrocatalysts with superior selectivity for specific products, these reactions are conducted at low current densities ranging from several mA cm−2 to tens of mA cm−2, which are far from commercially desirable values. To extend the applications of CO2 electroreduction technology to an industrial scale, long-term operations under high current densities (over 200 mA cm−2) are desirable. In this paper, we review recent major advances toward higher current density in CO2 reduction, including: (1) innovations in electrocatalysts (engineering the morphology, modulating the electronic structure, increasing the active sites, etc.); (2) the design of electrolyzers (membrane electrode assemblies, flow cells, microchannel reactors, high-pressure cells, etc.); and (3) the influence of electrolytes (concentration, pH, anion and cation effects). Finally, we discuss the current challenges and perspectives for future development toward high current densities.
1. Introduction
Electrochemical reduction of carbon dioxide (CO2) to chemicals is considered to be a sustainable strategy in preventing our planet from global warming while keeping the growth of our economy. So far, commercially available CO2 electroreduction reaction (CO2ER) technologies are almost nonexistent [1]. A cost-competitive CO2 electrolysis requires a high current density (>200 mA·cm−2), a high selectivity, a low overpotential (<1 V), and a long-term operation (>8000 h or 1 year) [2,3]. Among these factors, the current density is a key indicator for evaluating the catalytic performance, because a higher current density represents a higher reaction rate. Therefore, technical developments regarding electrocatalyst, electrolyzer, electrolyte, and operational condition are greatly demanded in order to realize high current density.
CO2 electroreduction involves different numbers of electrons and protons to produce specific products. Elemental metallic catalysts can be classified into the following three categories according to their major products: (1) Au, Ag, Pd, and Zn to produce CO [4,5,6]; (2) Pb, Bi, Sn, In, and Hg to produce formic acid/formate [7,8,9]; (3) Cu to produce various hydrocarbons [10,11,12]. Although the above catalysts have demonstrated remarkable selectivity towards different products, they are still far away from industrial application, especially regarding the aspect of the current density. However, in order to have a sustainable impact on the environment and climate, industrially relevant research is urgently required [13].
Therefore, the integration of catalyst innovations and reactor designs is desirable. Traditional CO2ERs are conducted in an H-type cell, which is limited to relatively low current densities due to the limited solubility of CO2 in aqueous solution. To meet the requirements for industrialization, various reactor configurations such as the GDE-based flow cell, microchannel reactor, membrane electrode assembly, and high-pressure cell have been developed. Although perovskite catalysts in solid oxide electrolyzer cells (SOECs) can electrolyze CO2 at the gas–solid interface with a high current density [14,15,16], they are not discussed within the scope of this article, due to their restricted operational conditions and products. In an economically practicable electrolyzer, variables related to electrodes, electrolytes, and operations should also be taken into account.
In this review, we give a comprehensive summary of catalysts for various products under high current densities. The developments of advanced CO2ER technologies, including the electrolyzer design, electrode structure, electrolyte effect, and operating conditions are also discussed. We ultimately provide an overview of the development towards high current density by considering three aspects: the design of electrocatalysts, electrolyzers, and appropriate anode reaction coupling.
2. Mechanisms of CO2ER
CO2 electrochemical reduction can proceed through reduction pathways involving two to eighteen electrons to produce various products including formic acid (HCOOH)/formate (HCOO−), carbon monoxide (CO), methane (CH4), methanol (CH3OH), acetic acid (CH3COOH), ethanal (CH3CHO), ethanol (CH3CH2OH), ethylene (CH2CH2), and others. The most commonly reported reactions are listed below in Table 1.

Table 1.
Electrochemical reactions and their equilibrium potentials.
During CO2ER, a CO2 molecule is first adsorbed onto a vacant catalyst site, electron transfer is carried out to form various intermediates [17], and then the corresponding products are obtained [18]. Figure 1 shows the proposed reaction mechanisms for various CO2ER products. The generally accepted first step in CO2ER is the protonation process of the CO2 molecule on different atoms (C or O atom) to form *OCHO or *COOH. In order to produce HCOOH, a further proton and electron transfer is needed. The production of CO requires the formation of *COOH in the first step, followed by H+/e− transfer to the hydroxyl group and then the loss of H2O [19]. The weak bond of *CO with the surface of a metal catalyst such as Au or Ag promotes the desorption of CO. In order to obtain C2+ products, either *CO dimerization [20] or *CHO formation [21] is required. The generation of *CHO is thought to be the potential-determining step for generating methane and ethylene [22]. However, the rate-determining steps for methane and ethylene are distinct, with the first e− transfer for ethylene, and the second e− transfer for methane [23].
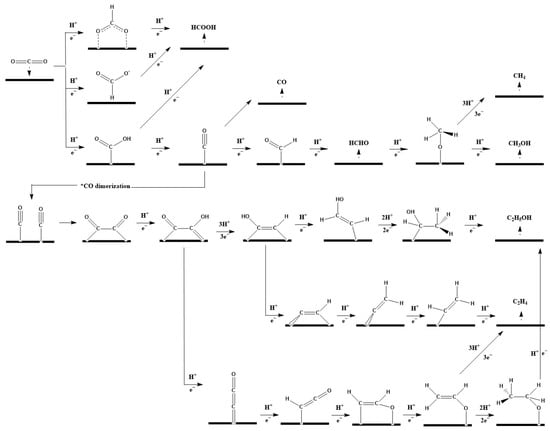
Figure 1.
Proposed reaction routes for CO2 electroreduction to various products such as CO, HCOOH, CH4, CH3OH, C2H4, and C2H5OH [18,24,25,26,27,28].
3. Electrocatalysts for CO2 Electroreduction
Elemental metallic catalysts for CO2 electroreduction are traditionally classified into four distinct groups depending on their major products, according to experimental data produced by Hori et al. [29] (Figure 2). In aqueous electrolytes, Au, Ag, and Zn catalysts mainly produce CO, whereas Cd, In, Sn, Hg, Tl, Pb, and Bi [30] catalysts favor the production of HCOOH. Cu is unique, and only Cu-based catalysts are able to yield large amounts of hydrocarbons, as they facilitate the formation of C-C bonds [31,32].
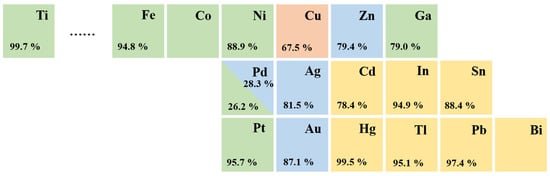
Figure 2.
Periodic table and Faradaic efficiencies of various metals depending on the major products in CO2 electroreduction: CO (blue); HCOOH (yellow); mixed hydrocarbons (red); H2 (green).
3.1. Metal-Based Catalysts
3.1.1. Noble Metals
- (I)
- Au
Currently, noble metal catalysts such gold (Au) and silver (Ag) show good performance for the electroreduction of CO2 to CO [33]. Among these, Au-based catalysts have been investigated extensively for their high CO selectivity at low overpotential, which is owing to the moderate adsorption of *COOH and *CO on the Au surface [34,35]. However, Au is confined to industrial applications with high costs. These costs can be mitigated by reducing the loading on the electrodes and modifying the surface morphologies.
The use of microporous or mesoporous supports with high surface areas represents a promising method of achieving lower precious metal loadings. Jhong et al. [36] reported that Au nanoparticles supported on poly(2,2′-(2,6-pyridine)-5,5′-bibenzimidazole) polymer (PyPBI) multiwall carbon nanotubes (MWNTs) on gas diffusion electrodes in microfluidic electrolysis cells can attain a partial current density for CO (jCO) of 160 mA cm−2 at −1.17 V (vs. RHE; all potentials correspond to this reference electrode unless otherwise specified). It is emphasized that the loading of Au nanoparticles was 0.17 mg cm−2. Verma et al. [37] further improved the synthesis method to reduce the Au loading from ~50% by weight to ~15% on PyPBI/MWNTs supports, and the synthesized Au nanoparticles in an alkaline flow electrolyzer led to a high jCO of 158 mA cm−2 at a cell overpotential (ηcell) of 0.94 V.
Alternatively, surface modification with polymer composites on metal could manipulate the electronic and geometric structures of the metal surface to promote CO2 adsorption, stabilize intermediates, or weaken the product binding energy. Ma et al. [38] reported polyvinyl alcohol (PVA)-modified Au NPs, which achieved a jCO of 98.6 mA cm−2 and 90% FECO in a two-component cell. Tafel analysis indicated that the improvement in the performance of PVA-modified Au NPs might be attributed to the hydrogen-bond network at the metal–polymer interface stabilizing the intermediate (*COOH).
- (II)
- Ag
As a precious metal, Ag can electrochemically convert CO2 to CO with high selectivity. The performance of noble metal catalysts in CO2 electroreduction has a size-dependent effect. To be specific, before the optimal size is reached, the catalytic activity dramatically increases with reducing nanoparticle size; however, when the diameter of the nanoparticles continues to drop, the activity reduces [34,39,40]. Density functional theory (DFT) calculations indicate that these trends are associated with the fact that the numbers of low-coordinated sites such as edges and corners increase for small nanoparticles [40]. Such low-coordinated sites can result in the reactants or intermediate products binding more strongly. For Ag nanoparticles, for example, the edges, which facilitate CO2 adsorption and stabilize the intermediate COOH*, serve as active sites for CO2ER leading to CO, and the corner sites of Ag serve as active sites for HER [41]. Thus, by controlling the sizes of the Ag particles, the ratio between edge and corner sites can be increased to promote CO production. Ma et al. [42] compared the activities of Ag nanoparticles supported on titanium dioxide (Ag/TiO2, 40 wt%) and on carbon black (Ag/C, 40 wt%). The jCO of the former catalyst reached 101 mA cm−2 in a flow cell, which was twice as high as the latter catalyst. Through structural characterization, the authors found that Ag particles with the optimal size were dispersed uniformly on the TiO2 carrier. Wang et al. [43] reported a layer-by-layer (LBL) growth and MOF-mediated approach for coating a Ag coordination polymer on a porous carbon-based microporous layer (MPL) to control the Ag loading. The obtained Ag gas diffusion electrodes (GDEs) displayed a peak jCO of 385 mA cm−2 in a gas-fed zero-gap flow electrolyzer.
As well as stabilizing and dispersing precious metal catalyst particles, support materials can also have a tremendous influence on electron conduction and mass transport. Carbon-based supports can enhance the intrinsic electrical properties through the synergetic effect of heteroatom (e.g., sulfur, boron, etc.) dopants. Chen et al. [44] reported Ag-decorated sulfur-doped graphitic carbon nitride/carbon nanotube nanocomposites (Ag-S-C3N4/CNT) for efficient CO2ER to CO, demonstrating a notable jCO of 303 mA cm−2 in a flow cell configuration. The experimental results benefited from the selective adsorption of CO2 and the complex oxygenated intermediates (e.g., *COOH, *CO) of graphitic carbon nitride (g-C3N4), and the improvement in the conductivity of S-CNT.
The above Ag nanoparticles were deposited on substrates or gas diffusion layers (GDL) for reduction. Few studies used support materials directly as co-catalysts to improve the performance of metal nanoparticle catalysts. Ma et al. [45] integrated MWCNTs with Ag in a “layered” or “mixed” structure, using an easy one-step method (Figure 3). In the former structure, the MWCNTs layer is covered with a Ag catalyst layer, and in the latter, the Ag nanoparticles and MWCNTs are merged homogeneously. The “mixed” structures attained a best jCO of 350 mA cm−2 in a flow reactor. These observed results may be due to the lower charge transfer resistance in the “mixed” structures.
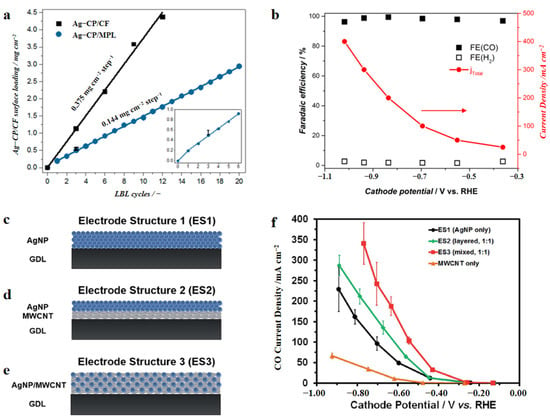
Figure 3.
(a) Surface loading of Ag coordination polymer vs. cycles of layer-by-layer deposition method. (b) FECO, FEH2, and total current density of Ag coordination polymer (3 cycles) on MPL vs. cathode potential in a gas-fed zero-gap flow electrolyzer. Reproduced with permission from Ref. [43]. Copyright 2019 American Chemical Society. The schematic diagram of three electrode structures: (c) AgNPs deposited on a GDL (ES1); (d) MWCNT layer deposited on a GDL and covered with an AgNP layer (ES2); (e) AgNPs and MWCNTs mixed uniformly and deposited on a GDL (ES3). (f) Values of jCO of ES1, ES2, ES3 vs. cathode potential in a flow cell. Reproduced with permission from Ref. [45]. Copyright 2016 the Royal Society of Chemistry.
- (III)
- Pd
Palladium (Pd)-based materials have been studied as potential catalysts for selectively reducing CO2 to CO and formate [46,47]. Zhu et al. [48] controlled the shape of Pd to investigate the effect of the crystalline facets on CO2 conversion. They synthesized Pd cubes (100) and Pd octahedra (111). The Pd octahedra (111) exhibited a high jCO of 220 mA cm−2, which was higher than that of the Pd cubes (100).
Pd shows different electrochemical performance at various overpotentials. In an electrolyte solution with a pH near 7, a Pd electrocatalyst mainly produces HCOOH at low overpotentials, while CO is the major product at high overpotentials. Theoretically, a weak CO binding energy over the electrocatalyst surface is beneficial for producing CO in CO2ER. Hence, reducing the CO binding energy over the surface of a Pd catalyst is an effective strategy for enhancing the selectivity for CO at low overpotentials. Molecular tuning by inducing functionalized organic molecules can be helpful in weakening the CO binding energy on the surface of Pd catalysts. Xia et al. [49] fabricated a polydiallyldimethyl ammonium (PDDA)-modified Pd catalyst, exhibiting a jCO of ~279 mA cm−2 at −0.65 V.
CO2 electroreduction performances of noble metal catalysts are summarized in Table 2.

Table 2.
CO2 electroreduction performances of noble metal catalysts.
3.1.2. Non-Noble Metals
- (I)
- Cu
To date, copper (Cu) is the only metal catalyst that can reduce CO2 to multicarbon (C2+) and hydrocarbon products. C2+ products such as ethylene (C2H4), ethanol (EtOH), and n-propanol (n-PrOH) are attractive in spite of the multistep and multielectron transfer reactions that make the design of the catalysts challenging. In order to promote C2+ production, effective strategies have been developed for manipulating the structures of Cu-based catalysts, such as modulating the nanostructure, controlling the facets, and promoting oxide-derived states.
Exploring appropriate synthesis methods to design the nanostructure of Cu with abundant active sites enables C2+ production. Metal–organic frameworks (MOF) and their derivatives are ideal platforms for increasing the catalyst’s active sites. Zhu et al. [50] synthesized a three-dimensional Cu dendrites electrocatalyst (d-Cu-1) derived from hollow Cu-MOF, which achieved a high jHCOOH of 100.3 mA cm−2 in a traditional H-cell. Yang et al. [51] presented porous cupric oxide nanowires (OD-Cu), derived from MOF using a controllable annealing method. These polycrystalline nanocatalysts demonstrated a jC2H4 of 141 mA cm−2 in a flow cell (Figure 4a,b). Yao et al. [52] also presented a Cu-MOF-derived Cu@CuxO core@shell structure, in which Cu+ can be formed rapidly and then Cu2+ can be transformed to Cu0 slowly (Figure 4c,d). The interfaces between Cu+ and Cu0 promote CO dimerization, leading to a jC2H4 of 150 mA cm−2 in a flow cell. Wang et al. [53] examined the activities of different shapes of Cu nanoparticles and found that Cu nanocubes with Cu(100) facets performed better than Cu nanospheres. The Cu nanocubes achieved a jC2H4 of 144 mA cm−2 in a flow cell (Figure 4e,f).
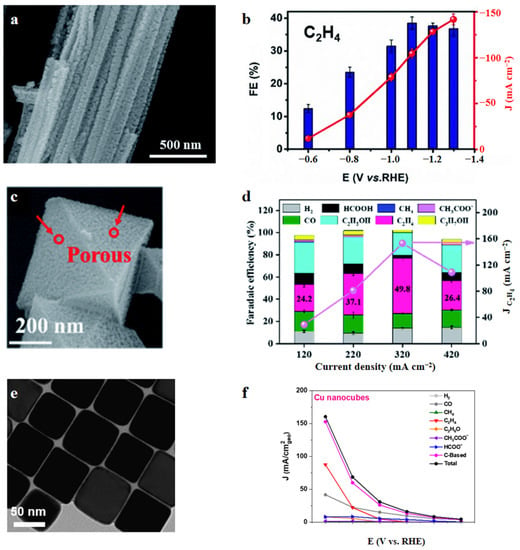
Figure 4.
(a) SEM image of CuO nanowires; (b) FE and jC2H4 of OD-Cu in a flow cell. Reproduced with permission from Ref. [51]. Copyright 2020 The Royal Society of Chemistry. (c) SEM image of a single octahedral particle; (d) FEproduct and jC2H4 vs. total current density in a flow cell. Reproduced with permission from Ref. [52]. Copyright 2020 The Royal Society of Chemistry. (e) TEM image of Cu nanocubes; (f) jproduct vs. potential for Cu nanocubes in a flow cell. Reproduced with permission from Ref. [53]. Copyright 2019 American Chemical Society.
In order to produce cost-effective catalysts, modifying metals in the form of homogeneous alloys or heterogeneous composites should be a promising approach. Recently, several studies have paid close attention to synergistic geometric and electronic effects in bimetallic catalysts to boost CO2 electrocatalysis by improving CO2 adsorption and C=O activation. Since Sn electrodes have the advantages of high catalytic activity, low cost, and low toxicity [54], alloying Sn with Cu is effective for selective CO2 reduction. Ju et al. [55] developed a Sn-decorated Cu-coated electrospun polyvinylidene fluoride (Sn/Cu-PVDF) nanofibers GDE, acting as a well-performing catalyst to attain a high jCO of above 100 mA cm−2. Xiang et al. [56] applied an in situ electrochemical spontaneous precipitation (ESP) method to synthesize Cu–In electrocatalysts with a GDE, and the optimum nanoscale “core–shell” structure of the Cu–In catalyst achieved a high jCO of ~173 mA cm−2 in a flow cell.
Nonetheless, due to the oxyphilic properties of metal in air and to the poor electrical conductivity of most metal oxides, controlling the surface structure of catalysts is a great challenge [57]. Preparing core–shell structured catalysts with a highly conductive metal core and a thin metal oxide shell is a feasible approach to solving the above problems. Ye et al. [58] reported a SnOx shell and Sn–Cu core for CO2ER, and the optimal Sn2.7Cu catalyst achieved a jC1 of ~397.88 mA cm−2. DFT calculations indicated that the interfaces of the reconstructed Sn and SnOx favored the formation of HCOOH via optimizing the binding of the HCOO* intermediate.
Alloying can not only promote CO2 adsorption and activation but also change the reduction pathway. Electrochemical reduction of CO2 to C2H4 or C2H5OH usually requires the same *C2H3O intermediate. Thus, in order to obtain ethanol rather than ethylene, it is crucial to stabilize and hydrogenate this intermediate to promote alcohol production. Li et al. [59] introduced Ag to a Cu catalyst (Ag0.14/Cu0.86) to destabilize the C2H4 intermediates, thereby promoting C2H5OH production. A very high jC2H5OH of 102.5 mA cm−2 was obtained in a flow cell.
Further studies have concentrated on modifying Cu catalysts with other non-metal materials (e.g., N, F), especially at high current densities. Ma et al. [60] reported a F-modified Cu catalyst (F−Cu) with an extremely high jC2+ of 1.28 A cm−2 (mainly C2H4 and C2H5OH) in a flow cell. Lee et al. [61] manufactured a self-formed tandem carbon nanofibers catalyst doped with N and Cu (Cu/N-CNF), using an oxygen-partial-pressure-controlled calcination method, exhibiting a jC2H4 of 372 mA cm−2. Chen et al. [62] discovered that N-doped graphene quantum dots (NGQ) on CuO-derived Cu nanorods (NGQ/Cu-nr) could achieve a jC2+alcohols of 147.8 mA cm−2.
Controlling the grain boundaries and microstrains formed in oxide-derived Cu, which can be proposed as catalytic sites, contributes to better catalytic performance. However, the annealing of GB-containing Cu catalysts could reduce the density of GBs [63]. Indeed, different cooling rates can result in different physical properties in microcrystalline materials. Yang et al. [64] tuned the grain boundaries and microstrains in CuO electrocatalysts by fast cooling with liquid nitrogen. Compared to samples with slower cooling rates, the fast-cooled CuO (CuO-FC) exhibited a high jC2 of 231 mA cm−2 (mainly C2H5OH).
The CO2 reduction reaction pathway is highly sensitive to the surface structure of Cu. Cu(110) favors the production of oxygenated hydrocarbons such as C2H5OH and CH3COOH [65,66]. Cu(100) and step facets such as Cu(211) preferentially produce C2+ products, due to the activity for CO dimerization [67,68]. In addition, Cu(111) is more selective toward CH4 as the major hydrocarbon product, while the Cu(100) facet is more favorable for C2H4 [69]. Wang et al. [70] proposed a method based on in situ electrodeposition of Cu (Cu-CO2), which could increase the ratio of Cu(100)/total facets by 70%, thus promoting the formation of C2+ products. They reported a jC2+ of 520 mA cm−2 in a flow cell.
Furthermore, Zhang et al. designed segmented gas diffusion electrodes (Cu/Fe-N-C s-GDE) to integrate the CO2-to-CO and CO-to-C2+ steps on two sites, achieving an FEC2+ of 90% and a jC2+ of over 1 A cm−2 [71].
CO2 electroreduction performances of Cu-based catalysts are summarized in Table 3.

Table 3.
CO2 electroreduction performances of Cu-based catalysts.
- (II)
- Zn
Zn, as an earth-abundant metal, can also reduce CO2 to CO with relatively low cost compared with noble metal catalysts such as Au and Ag. However, bulk Zn catalysts suffer from low activity and CO selectivity. Nanostructured Zn catalysts have been synthesized to overcome these limitations.
Luo et al. [72] developed a facile electrodeposition method to fabricate porous-structured Zn electrodes to efficiently reduce CO2 to CO in a GDE. The jCO could be boosted to 168 mA cm−2 in a flow cell, which can be attributed to the enhanced surface area and the local pH effect. In previous work, the surface area of the electrocatalyst could be significantly increased by introducing Cu2+ at the time of electrodeposition of Zn, whereas the introduction of Cu2+ compromises the FECO [73]. Inspired by this, Lamaison et al. [74] introduced Ag+ during the electrodeposition of Zn to obtain an Ag–Zn alloy catalyst with a high surface area, with a jCO of 286 mA cm−2 at elevated pressure.
- (III)
- Cd
The current density in CO2 electroreduction can be considerably increased by increasing the local electric field at the tips of sharp metal nanostructures [35,75]. Since electrostatic repulsion exists, free electrons will migrate to the zones with sharpest curvature, so that the local electrostatic field in metal nanoneedles will be enhanced by an order of magnitude over that of conventional nanorods and nanoparticles. Gao et al. [76] reported a cadmium sulfide (CdS) nanoneedle (CdS needle) with high curvature, exhibiting a jCO of 212 mA cm−2 in a flow cell. This current density can be attributed to the enriched K+ concentration at the regions of high curvature of the CdS needles, caused by electric fields, as K+ can stabilize CO2 through noncovalent interaction.
- (IV)
- Sn
Formate, as an important liquid product of CO2 reduction, can be regarded as an ideal hydrogen carrier or liquid fuel for low-temperature fuel cells [77]. However, the formation of HCOOH is limited by the inert CO2 molecule, and effective catalysts are needed to activate the reaction process, such as some main-group metals (e.g., Sn, Pb, In, Tl) and transition metals (Cd, Hg) with a d10 electronic configuration. Sn is low in price, high in selectivity, and without toxicity compared with noble metals such as Pd and Au or toxic metals such as In.
Among the Sn-derived catalysts, Sn oxides (SnO, SnO2, and SnOx) are attractive, due to their appropriate orbital energy and electronic configuration; however, because of the relatively low intrinsic electrical conductivity, Sn oxides are not active enough for the formation of formate [78]. Löwe et al. [79] fabricated a SnO2-based GDE, achieving a jformate of 800 mA cm−2 at 50 °C in a semi-batch cell, which can be attributed to optimization of both the catalyst particle size and the dispersion, together with the impact of temperature on the solubility and diffusion coefficients of CO2 in the electrolyte. Xiang et al. [80] fabricated a carbon-black-supported SnO2 catalyst and found that the optimum SnO2/C mass ratio achieved a maximum jformate of ~211 mA cm−2 in a flow cell.
Introducing other metals to form Sn-based bimetallic materials is an alternative strategy for improving electrochemical performance. Cu foams have a large surface area and high conductivity, and thus can be an alternative catalyst carrier [81]. Wang et al. [82] deposited Sn on a Cu foam to form heterostructured Cu3Sn/Cu6Sn5 (CuSn–C). A jformate of 148 mA cm−2 was achieved in a flow cell. In the interface between the Cu6Sn5 and Cu3Sn intermetallics, the adsorption of the intermediate tended to favor HCOO* rather than COOH*, so that CO2 was selectively converted to HCOOH.
- (V)
- Bi
In addition to the previously mentioned transition metals with a d10 electronic configuration, Bi, which shows intrinsic inertness toward HER, is also considered to be an advanced electrocatalyst for CO2 conversion to formate. Díaz-Sainz et al. [83] studied Bi-GDEs working in a continuous mode in a filter press reactor to reduce CO2 to formate, and found a jformate of up to 210 mA cm−2. Deng et al. [84] reported the preparation of carbon-nanorods-encapsulated bismuth oxides (Bi2O3@C) prepared by a facile spatially confined pyrolysis method and exhibiting a jformate of above 200 mA cm−2 in a flow cell. These outstanding performances were attributed to the effects of the high formate selectivity of Bi2O3 and the ability of the carbon matrix to improve the current density. Xia et al. [85] developed an ultrathin two-dimensional Bi (2D-Bi) catalyst with abundant undercoordinated active Bi sites in solid electrolytes, with a maximum jformate of over 172.2 mA cm−2 in a flow cell. Yang et al. [86] proposed leafy Bi-MOF-derived bismuth nanosheets (Bi NSs) electrocatalysts. The jHCOOH could exceed 374 mA cm−2 in the flow cell configuration.
Most of the reported Bi-based electrocatalysts show poor conductivity and limited exposure of active sites; however, metallene, a new 2D material with a thickness of a few layers and abundant defective and unsaturated sites, is currently emerging. Cao et al. [87] developed atomically thin bismuthene (Bi-ene), which can deliver a jHCOOH of ~200 mA cm−2.
CO2 electroreduction performances of other non-noble catalysts are summarized in Table 4.

Table 4.
CO2 electroreduction performances of other non-noble catalysts.
3.2. Metal-Free Carbon Catalysts
Carbon-based electrocatalysts without any metal content have gained attention for CO2 reduction, due to their high abundance, low cost, large available surface area, and resistance to poisoning [88]. Different heteroatoms such as N, P, or other chalcogens with carbon can modulate the charge redistribution among carbon atoms to add active sites for catalysis and lower the free energy barrier for CO2ER [89].
Yang et al. [90] developed N and S co-doped, hierarchically porous carbon membranes (NSHCF) to achieve a jCO of 96.82 mA cm−2 in an H-cell. This performance can be ascribed not only to the co-doping of pyridinic N and carbon-bonded S atoms, which can significantly reduce the free energy barrier for the binding of the *COOH intermediate, but also to the well-developed hierarchically porous structures of NSHCF, providing sufficient channels. Chen et al. [91] fabricated a novel electrocatalyst involving N, P co-doped carbon aerogels (NPCA), achieving a jCO of 143.6 mA cm−2 in an H-type cell. The excellent results can be attributed to the pyridinic N and co-doped P, which were selective for CO and inhibited HER.
CO2 electroreduction performances of metal-free carbon catalysts are summarized in Table 5.

Table 5.
CO2 electroreduction performances of metal-free carbon catalysts.
3.3. Single-Atom Catalysts
Single-atom catalysts (SAC) have been widely investigated for their high atomic efficiency, superior activity, and selectivity. However, the low loading of 1–2 wt% limits the industrial application of single-atom metals [92]. Approaches to anchoring single atoms onto high-surface-area supports can increase atomic dispersion and density, to achieve an industrial-level current density [93]. Yang et al. [94] designed a well-distributed Ni single-atom/porous carbon fiber membrane catalyst (NiSA/PCFM) with excellent mechanical strength via the electrospinning method, yielding a jCO of 308.4 mA cm−2 in a flow cell. They also applied the same method for single-atom Co sited on a high-yield carbon nanofibers membrane (CoSA/HCNFs) with a continuous porous structure, which led to a jCO of 211 mA cm−2 in a flow cell [95].
Recently, transition metal−nitrogen-carbon (M−N−C) catalysts have exhibited outstanding catalytic activity as electrocatalysts for CO2 electroreduction. M−N−C refers to an N-coordinated single-atom transition metal (M−Nx) supported on a carbon matrix, such as a single atom anchored on an N-doped graphene matrix [96]. Note that different N species (such as graphitic N, pyrrolic N, and pyridinic N) can exist on carbon supports.
As metallic Fe nanoparticles in Fe−N−C materials could reduce the overpotential, Fe–N–C catalysts synthesized with different support materials and precursors have been investigated [97,98,99,100]. However, the experimental results demonstrated limited current density. Fe3+ shows faster CO2 adsorption and weaker CO absorption than conventional Fe2+ sites. Gu et al. [101] reported a Fe3+–N–C catalyst for efficiently catalyzing CO2 with a jCO of 94 mA cm−2 in a flow cell.
Nevertheless, the Fe–Nx moiety may be poisoned by strong chemisorption of CO, compared to Ni–N–C, and thus the electrocatalytic performance of the former [102] is lower than that of the latter [103]. Zheng et al. [104] reported a Ni single-atom catalyst on commercial carbon black (Ni–NCB) employed in an anion membrane electrode assembly (MEA), giving a jCO of 130 mA cm−2. Jeong et al. [105] developed a Ni–SA–NCs catalyst using Si spheres as templates, yielding a jCO of around 380 mA cm−2 in an MEA cell.
Restraining the aggregation of metal precursors on substrates at high temperature is crucial for M–N–C catalysts. Zeolitic imidazolate frameworks (ZIFs) are attractive because of their many pores, large surface areas and adjustable composition. Wang et al. [106] reported a variety of cyano-substituted Ni-phthalocyanines-derived SACs in ZIFs (Ni-SAC(Pc)), which exhibited a superior jCO of 200 mA cm−2. Guo et al. [107] fabricated an ellipsoidal hierarchical nanoporous Ni−N−C electrocatalyst (Ni20−N−C) derived from a porphyrin-based porous Zr-MOF, that could achieve a very high jCO of 645 mA cm−2. The outstanding results can be attributed to micropores and interconnected mesopores leading to enhanced CO2 mass transfer (Figure 5).
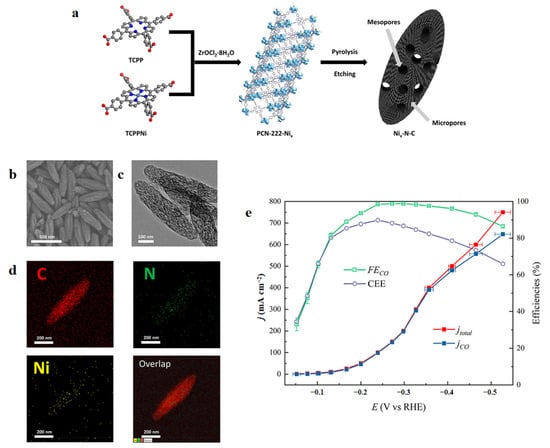
Figure 5.
(a) Fabrication procedure for Nix–N–C catalysts. SEM images of: (b) Ni20–N–C; (c) Ni100–N–C. (d) EDS mappings. (e) Electrocatalytic performance of Ni20–N–C in 6 M KOH. Reproduced with permission from Ref. [107]. Copyright 2020 American Chemical Society.
Zhang et al. [108] revealed that pyrolytic temperatures and oxygen-containing groups in the carbon substrate have an impact on the number of atomic metal active sites. With all factors optimized, Ni–N–C with the highest Ni loading of approximately 4.4 wt% exhibited a jCO of 152 mA cm−2 in a flow cell. Zhang et al. [109] engineered various pendant groups on phthalocyanine to form types of dispersed Ni phthalocyanine molecules supported on carbon nanotubes. The optimized catalyst with a methoxy group (NiPc–OMe MDE) could convert CO2 to CO with a high jCO of over 300 mA cm−2.
In addition, Wen et al. [110] regulated the local electronic environments of Ni species (Ni(NC)-1) to activate catalytically inert sites into active sites, achieving a considerable jCO of 158.4 mA cm−2 in a flow cell.
CO2 electroreduction performances of single-atom catalysts are summarized in Table 6.

Table 6.
CO2 electroreduction performances of single-atom catalysts.
Meanwhile, Co–N–C [111,112], Cu–N–C [113,114], Ce–N–C [113], and Pr–N–C [113], have the potential for current or selectivity improvements in CO2 reduction to CO and should be further explored.
3.4. Molecular Catalysts
Pyridine/pyridinium (py/pyH+) species have been revealed to be effective co-catalysts, not only for the aforementioned M–N–C catalysts but also for molecular catalysts. Molecular catalysts are highly selective to CO in CO2ER as they have a more tunable ligand structure of the primary and secondary coordination spheres than solid state catalysts, improving catalytic efficiency. Ren et al. [115] developed a cobalt phthalocyanine (CoPc1) catalyst to convert CO2 to CO, obtaining a jCO of 175 mA cm−2 in a zero-gap membrane flow reactor (Figure 6a). Cobalt phthalocyanine with a trimethyl ammonium group connecting up the phthalocyanine macrocycle (CoPc2) can show great durability at the highest jCO of 165 mA cm−2 in a flow cell [116], which may be attributed to the through-space reciprocities between the O atoms in CO2 (partial negative charge) and the trimethyl ammonium substituent (positive charge). These through-space interactions can promote CO2 molecule reduction coordinated with the Co metal center (Figure 6b,c).
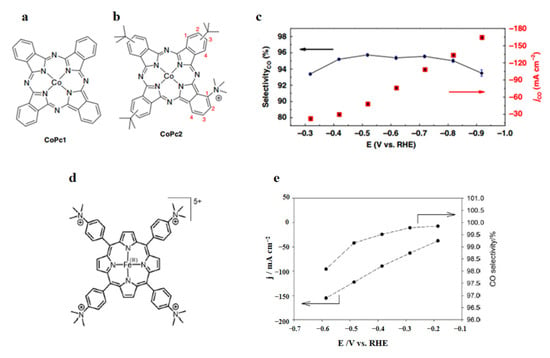
Figure 6.
Structure of: (a) cobalt phthalocyanine catalyst CoPc1; (b) CoPc2. (c) FECO and jCO of CoPc2 vs. potential. Reproduced with permission from Ref. [116]. Copyright 2019 Springer Nature Limited. (d) Structure of FeP; (e) jCO and FECO of FeP vs. potential. Reproduced with permission from Ref. [117]. Copyright 2020 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
In addition, depositing a molecular catalyst onto carbon supports with many pores, such as carbon powder, carbon nanotubes, and graphene, can be a promising way to improve catalyst activity. Torbensen et al. [117] developed an Fe porphyrin (FeP) and carbon black mixture loaded on carbon paper as a GDE in a flow cell, achieving a jCO of 152 mA cm−2 (Figure 6d,e).
CO2 electroreduction performances of molecular catalysts are summarized in Table 7.

Table 7.
CO2 electroreduction performances of molecular catalysts.
In order to provide the reader with a comprehensive overview, we summarize the catalysts which can electrochemically reduce CO2 to different products with high current densities in Figure 7. CO is the simplest product of CO2ER, involving a two electron/proton process. As shown in Figure 7, the products of CO are closest to industrial application. Tests of different catalysts for reducing CO2 to CO under various current densities have been explored, and the selectivities are generally around 90%. HCOOH is also a kinetically viable product of CO2 electrolysis. The selectivity for HCOOH is high but at a relatively low current density. As the product molecules become more complex, the reaction selectivity drops dramatically at high current density, especially for C2H5OH formation.
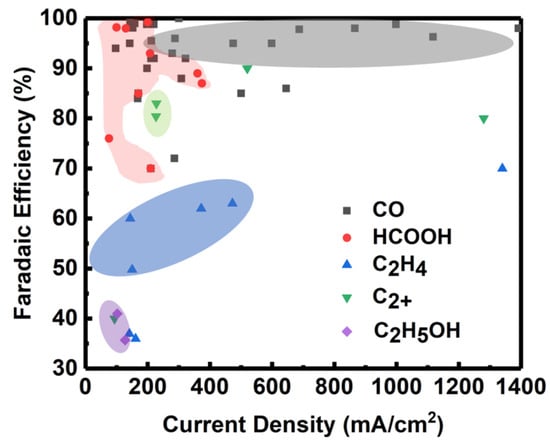
Figure 7.
Faradaic efficiency and corresponding current density for different CO2ER products. [43,48,49,50,51,52,53,59,60,61,63,64,70,72,74,76,79,83,84,86,87,90,91,94,95,103,107,110,115,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134].
4. Electrolyzer Design
4.1. Electrolyzer Types
Five main architectures have emerged for CO2 electrolyzers: H-type cells, microchannel reactors, liquid-phase electrolyzers, membrane electrode assemblies, and high-pressure cells (Figure 8). To date, H-type cells are still used for evaluating most of the catalysts for CO2 electroreduction. The catalyst is completely submerged, and CO2 is usually bubbled into the electrolyte in these H-type cells. Therefore, the low solubility of CO2 in aqueous electrolyte sets a limit on the current density of CO2 electroreduction and makes it difficult to reach industrial scales (>100 mA cm−2). In order to surmount these barriers, a series of investigations has been conducted on electrochemical reactors [122].
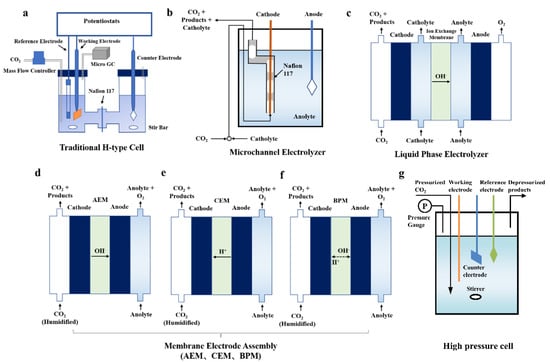
Figure 8.
Illustrations of different electrolyzer types. (a) H-type cell. Reproduced with permission from Ref. [135]. Copyright 2017 The Royal Society of Chemistry. (b) Microchannel electrolyzer. Reproduced with permission from Ref. [136]. Copyright 2020 American Chemical Society. (c) Liquid-phase electrolyzer; (d–f) membrane electrode assembly (AEM, CEM, BPM) electrolyzers; (g) high-pressure cell. Reproduced with permission from Ref. [137]. Copyright 2019 Elsevier Inc.
4.1.1. H-Type Cell
The fundamental exploration of CO2ER is commonly carried out in an H-type cell, which contains an anode and a cathode compartment. The two compartments are divided by an ion-exchange membrane (e.g., Nafion 117) to provide proton conductivity and mitigate the crossover of liquid-phase products from the working electrode to the counter electrode. In the cell, CO2 gas is bubbled into and dissolved in the liquid electrolyte. The configuration of the H-type cell is simple; thus, investigations into reaction mechanisms of catalysts are usually carried out in this reactor. However, the cell suffers mass transfer limitations due to the low CO2 solubility in liquid electrolyte, so the current density is usually below 30 mA cm−2. Interestingly, Chen et al. [91] achieved a significant current density (143.6 mA cm−2) in an H-type cell. The catalyst they used was N,P co-doped carbon aerogel carbonized at 900 °C (NPCA900), and the electrolyte was 0.5 M [Bmim]PF6/MeCN.
4.1.2. Microchannel Electrolyzer
Compared with conventional equipment, the interface area of the microchannel reactor is large. Due to the rapid rate of mixing, the discrete phase size in the microchannel is greatly reduced and the mass/heat transfer resistance is weakened [138,139,140,141]. Therefore, researchers who are focusing on CO2 electroreduction have used the microreactor as an efficient electrolyzer for process intensification [142,143,144,145].
Due to the low CO2 solubility in aqueous electrolyte, the electroreduction of CO2 is limited by mass transfer at high current density. A cylindrical microchannel electrochemical reactor was built to enhance mass transfer. The reactor consists of a pre-mixing section (5 m circular microchannels with an inner diameter of 1 mm used to pre-saturate the electrolyte with CO2) and a reaction section (cylindrical cation-exchange membrane tube, Nafion 117). The working electrode is located in the center of the Nafion 117 membrane tube (Figure 9) [136].
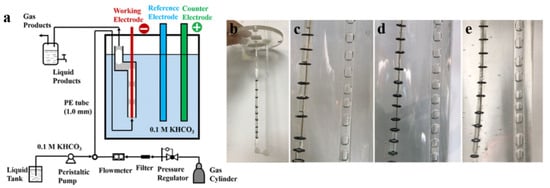
Figure 9.
(a) Schematic diagram of the microchannel electrolyzer. (b–e) Optical images of cylindrical cation-exchange membrane tube. Reproduced with permission from Ref. [136]. Copyright 2020 American Chemical Society.
Increasing the microchannel number is one convenient and secure way to make it possible for the microreactor to achieve industrial scale-up [142,146]. Zhang et al. [136] developed a reactor with multiple microchannels in series to increase the CO2 conversion (Figure 10a). When the number of microchannels in series increased from one to four, the conversion rate of CO2 was correspondingly increased by four times. Compared with the traditional H-type cell, the CO2 conversion rate in the microchannel showed an increase of 70.9%, due to the prolonged contact time between the gaseous CO2 and the surface of the cathode (Figure 10b). The drawbacks of the GDE, such as the extremely low CO2 conversion rate due to the radial flow, the complicated system setup, and carbonate salt formation and flooding, can be overcome by using a microchannel electrolyzer. Furthermore, Zhang et al. [136] also developed a reactor with multiple microchannels in parallel to enhance the yield in CO2 electroreduction (Figure 10c). The CO FE values were all over 95.0%, regardless of the number of the microchannels, indicating that industrial-scale amplification of the microchannel reactor can easily be achieved by simply increasing the number of microchannels in parallel (Figure 10d).
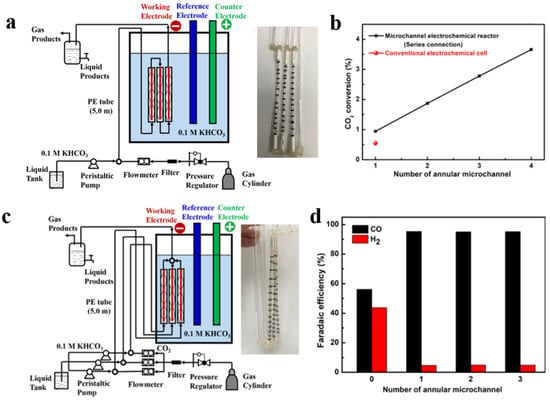
Figure 10.
(a) Diagram of the microchannel reactor with three microchannels in series. (b) CO2 conversion vs. number of annular microchannels. (c) Diagram of the microchannel reactor with three microchannels in parallel. (d) FECO, FEH2 vs. number of annular microchannels. Reproduced with permission from Ref. [136]. Copyright 2020 American Chemical Society.
4.1.3. Liquid-Phase Electrolyzer
Liquid-phase electrolyzers have drawn significant attention worldwide due to their ability to be scaled up and achieve an industrially viable process. They are typically composed of three flow channels, which are used for the gaseous CO2, catholyte, and anolyte, respectively. The gas and catholyte channels are separated by a GDE, while an ion-exchange membrane separates the catholyte and anolyte channels. In this liquid-phase configuration, the diffusion layer thickness of CO2 is much less than in the H-type cell. Target products such as CO [44,56,76,95,107,110,116,117,123,147], formate [79,83,84], and multicarbon hydrocarbons/oxygenates [59,70,148] can be obtained at high rates (current densities). Most of the studies mentioned above were carried out in the liquid-phase configuration.
As well as lab-scale tests, pilot-scale CO2ER is being developed using a GDE-based liquid-phase configuration. Evonik and Siemens [126] used a commercial Ag-based GDE in an industrial-scale chlorine–alkaline electrolyzer at a high current density of 300 mA cm−2, with an operational duration of over 1200 h. The Kopernikus project P2X [149] conducted CO2ER at a gas diffusion electrode with a 10 cm2 cell size at 30 bar, at up to 300 mA cm−2, achieving an FECO of above 90% over 1500 h. Furthermore, the first scaling step up to 300 cm2 was accomplished, and the rated power of the cell was around 300 W.
4.1.4. Membrane Electrode Assembly (MEA)
An MEA is composed of a cathode and an anode separated by an ion-exchange membrane, including a cation-exchange membrane (CEM), an anion-exchange membrane (AEM) [48,55,104,105,115,128,132,150], and a bipolar membrane (BPM) [151]. To maintain the membrane hydration during operation, the inlet CO2 gas must be humidified. There are three advantages of MEAs compared with their liquid-phase counterparts. Firstly, the MEA needs fewer electrolyte pumps, due to the removal of the catholyte, which eliminates multiple sources of instability such as electrolyte impurity deposition onto the catalysts, electrolyte flooding with GDE, and the formation of bicarbonate/carbonate salts. Secondly, it can be pressurized easily. Thirdly, it reduces ohmic losses. Larrazábal et al. [152] developed an MEA composed of a porous Ag membrane cathode and an IrO2/C anode with a Sustainion AEM separating the two electrodes, achieving a high jCO of around 200 mA cm−2 at 3.3 V (applied potential). Lee et al. [128] reported a gas-fed MEA consisting of carbon-supported Pd and Ag catalysts as a GDE cathode, and AEM and Ti felt as the anode. This MEA achieved a jtotal of above 200 mA cm−2 with an FECO of over 95% at a cell potential of −3.0 V. Using carbon-supported noble metal catalysts can help to reduce the amount of noble metal required and promote long-term stability. Furthermore, increasing the flow rate can further boost the yield of CO.
Nevertheless, liquid products may accumulate in the GDE and hinder gas diffusion. To obtain the target concentrated liquid product stream and maintain stability, timely extraction of liquid products from the GDE is necessary. Furthermore, the reactions between K+ and OH− result in the formation of potassium bicarbonate crystals on the cathode side, which can hinder the CO2 flow and reduce the yield of the MEA [115]. The performance of the MEA can be recovered by washing off these crystals.
Another disadvantage is the significant CO2 crossover through the AEM, mostly appearing in the form of CO32− but partly appearing in the form of HCOO−, which can cause ineffective conversion of CO2 and overestimated catalytic performance. Hence, it is necessary to treat membrane crossover as an important factor when evaluating the electrochemical performance of an MEA electrolyzer at high current densities, as well as the conventional activity and selectivity. Unlike typical alkaline liquid electrolytes (e.g., KHCO3, KOH), a type of high-ionic-conductivity alkaline polymer electrolyte (APE) was applied in an MEA with a common Au/C catalyst, achieving 500 mA cm−2 at 3 V (cell voltage) at an operational temperature of 60 °C (Figure 11d–f) [132].
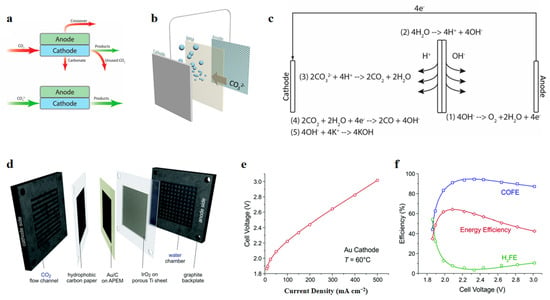
Figure 11.
(a) Carbon loss mechanisms. (b) Diagram of CO2 formation in the BPM through acid–base equilibrium reaction of H+ and CO32−. (c) Reaction mechanism using CO32− as feed and BPM in electrolyzer. Reproduced with permission from Ref. [151]. Copyright 2019 American Chemical Society. (d) Schematic images of MEA using alkaline polymer electrolytes. (e) I–V curves for operation at 60 °C; (f) FEs of CO and H2 production and energy efficiency. Reproduced with permission from Ref. [132]. Copyright 2019 The Royal Society of Chemistry.
To achieve highly effective electroreduction of CO2 in a gas-fed MEA, an appropriate number of protons are needed, which usually originate from the H2O molecules in the aqueous electrolyte. Supplying too few H2O molecules will starve the cathode and make the CO2 reduction reaction sluggish, while too much H2O will reduce the ability of CO2 to reach the surface of the catalyst (i.e., flooding) and reduce the energy efficiency and Faradaic efficiency of CO2ER production. Reyes et al. [150] researched the effect of cathode flooding on electrocatalytic performance and found a 37% drop in jCO and a 450 mV enhancement in cell voltage (Ecell). By coupling a hydrophobic cathode and a microporous thin film (≤40 μm), cathode flooding can be effectively alleviated, making it feasible to meet commercial requirements (jCO ≥ 100 mA cm−2 and Ecell < 3 V).
4.1.5. High-Pressure Cell
In order to realize the industrialization of CO2 electroreduction, the available amounts of CO2 near the surface of the electrode urgently require enhancement. The supply of CO2 to reaction sites in aqueous media is limited, due to poor solubility at ambient temperature and pressure. Thus, strategies for elevating the CO2 partial pressure could be one of the most feasible methods of addressing this issue. According to Henry’s law [153], the CO2 gas dissolved in the aqueous solution is proportional to the pressure of CO2. Significant efforts to explore optimal high-pressure cells have been made since the 1990s [154,155,156].
A typical high-pressure cell is a stainless-steel autoclave (Figure 8g), equipped with a pressurized CO2 inlet and a depressurized product outlet. Before the process of electrolysis, CO2 can be regulated to the operative pressure through a pressure gauge and a pressure relief valve, and then introduced into the electrolyte. The products are depressurized to 1 atm in preparation for further analysis. Recently, experiments have been conducted in a one-chamber high-pressure cell at relatively lower pressure, as shown in Table 8 [74,157,158,159].

Table 8.
CO2 electroreduction performances under high pressure.
In addition, the incorporation of GDEs into the flow cell systems and pressurization have been used to test the CO2 reduction capability and ease the transportation for further downstream processing [125,160].
In order to provide the reader with a comprehensive overview of electrolyzers, we summarize CO2ER conducted in various reactors at high current densities in Figure 12. Due to the mass transfer limitation of CO2 in aqueous solution, the current density of the H-type cell is generally less than 30 mA cm−2. Although Figure 12 shows several experimental results in H-cells where the partial current densities were over 100 mA cm−2, the applied electrolytes were high-cost ionic liquids. The liquid-phase electrolyzer is one of the most widely studied reactors. As far as is known, CO2ER processes with the highest current densities have been conducted in MEAs. Compared with their liquid-phase counterparts, MEAs need less electrolyte, eliminating electrolyte flooding and the formation of bicarbonate/carbonate salts. The selectivities of high-pressure reactors are relatively low.
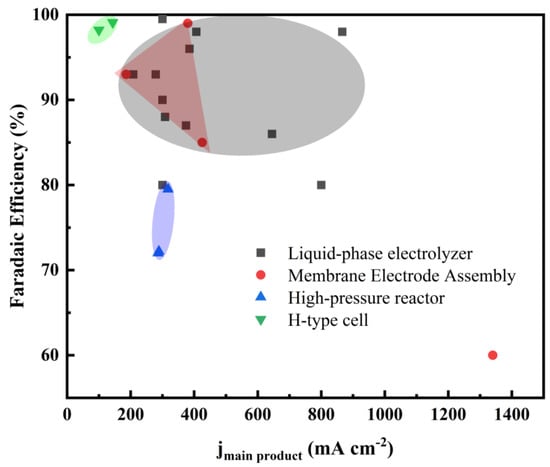
Figure 12.
Faradaic efficiency and corresponding current density for CO2ER in different reactors.
4.2. Gas Diffusion Electrodes (GDE)
4.2.1. Typical GDE
In recent years, GDE-based flow cells have attracted extensive attention, as they can reduce the CO2 mass transfer limitation in aqueous electrolyte [133]. A typical GDE consists of a macroporous, a microporous, and a catalyst layer (Figure 13). CO2 gas is directly delivered to the back side of the catalyst layer through the macroporous and microporous layers, which are both porous and hydrophobic. The front side of the catalyst layer is in close contact with the liquid electrolyte. The main function of the microporous layer is to provide a stable framework, which facilitates electronic contact and CO2 gas passage. The microporous layer enhances further electronic contact between interfaces and effectively prevents flooding.
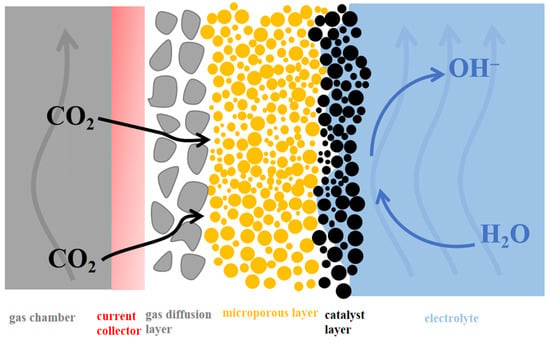
Figure 13.
Illustration of catalyst layer on GDE.
Catalysts are usually applied to the GDL via electrochemical deposition, drop-casting [44], electromagnetic sputtering, or airbrushing. One major difference between the GDE and the H-cell is that the thickness of the CO2 diffusion layer in the former (~50 nm) is less than 1/1000 of that of the latter (~50 μm). Hence, using a GDE can significantly improve current densities [161].
Recently, an inert material layer has been introduced on top of the catalyst layer, which functions as a current collector and protects the active catalyst from the deposition of electrolyte contaminants (e.g., Fe, Co, Ni, favoring H2 production) as well as catalyst restructuring [162,163,164].
4.2.2. Integrated GDE
One drawback of the traditional GDE is that the link between the catalysts and the substrate is loose, which means that the catalysts split away easily. Therefore, the electrochemical performance and long-term durability of CO2 electrocatalysis will be adversely affected. To avoid this problem, He et al. [94,95,114] developed an integrated strategy to fabricate GDE without adhesives (i.e., combining GDLs), forming a highly stable CO2–electrolyte–catalyst three-phase interface for CO2 electrocatalysis under high current densities (Figure 14).
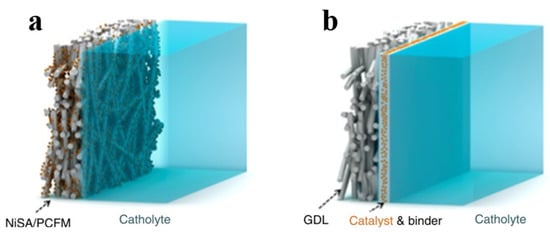
Figure 14.
(a) Typical GDE loaded with catalyst via polymer binder. (b) Ni single-atom/porous carbon fiber membrane used as integrated GDE. Reproduced with permission from Ref. [94]. Copyright 2020 Springer Nature Limited.
Interestingly, there are two completely opposing views on whether CO2ER is conducted at a three-phase interface (namely, the CO2 gas–aqueous electrolyte–electrocatalyst interface) or at a two-phase interface (namely, the dissolved CO2–electrocatalyst interface). The former is widely used to describe the mechanism of devices with a GDE. Burdyny et al. argued that the CO2 in the GDL reacted in the liquid phase during electrocatalysis, rather than in the gas form, which can be evidenced by the interesting experimental phenomenon that stable CO2ER could be maintained when the GDE was flooded. The results indicated that the three-phase interface does not exist [161,165].
4.3. Hydrophobic Electrode Design
Important advances in GDE-based electrolyzers have been made due to a breakthrough in the CO2 mass transport limitation where the diffusion layer thickness cannot be reduced. However, the stability of GDLs remains a challenge in flow cells. One major reason is that the catalyst layer in GDLs is hydrophilic. After a long period of exposure to the electrolyte, a liquid film is formed surrounding the catalyst particles, which blocks CO2 diffusion.
Recently, arrangements of the local environment of the gas/liquid/solid interface have gradually attracted widespread interest in CO2ER. These methods include hydrophobic engineering of the surface of the catalyst, employed to trap more CO2 to increase the local CO2 concentration in proximity to the catalyst [166,167,168,169]. Niu et al. [168] reported a hydrophobic hierarchical Cu catalyst which mimicked the structure of the leaves of Setaria. This hydrophobic Cu structure exhibited a maximum jC2+ of 255 ± 5.7 mA cm–2 in a flow cell. Xing et al. [167] showed that polytetrafluoroethylene (PTFE) added to commercial Cu led to the achievement of a partial current density of over 250 mA cm−2. This improvement can be attributed to the enhancement of the CO2 supply and the suppression of H+. DFT calculations showed that the local environment of the hydrophobic electrode could increase the energy barrier of H* desorption, which depresses HER and facilitates CO2ER [166].
4.4. Flow Pattern
To tackle the problem of the CO2 mass transfer limitation, different flow patterns have been examined for comparison. Flow patterns can be divided into two configurations: “flow-through” and “flow-by” configurations. The distinction relates to the flow mode in the GDL, which is convective for “flow-through” and diffusive for “flow-by” configurations (Figure 15). The “flow-through” configuration can perform better at limited current densities than the “flow-by” reactor, for the same FE [123].
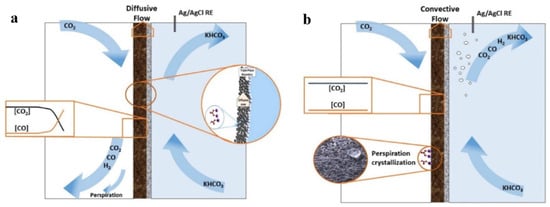
Figure 15.
Illustration of two flow patterns: (a) flow-by pattern and (b) flow-through pattern. Reproduced with permission from Ref. [123]. Copyright 2019 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
In the “flow-through” configuration, the gaseous CO2 is forced to pass through the pores of the GDE to reduce the thickness of the boundary layer between the GDE surface and the electrolyte, which accordingly alleviates the CO2 mass transport limitation. Vedharathinam et al. [170] demonstrate a 73-fold increase in jCO using a 3D porous “flow-through” electrode. However, the alkaline environment resulted in carbonate precipitation in the cathode flow channels, partly blocking the CO2 flow in “flow-through” configurations [43]. Hence, “flow-by” configurations are more common for long-term operations.
Compared with the “flow-through” electrolyzer, CO2 in a “flow-by” electrolyzer is not pushed through the pores but enters the gas compartment at the top of the cell. The CO2 gas only accesses the pores by diffusion, and therefore there are no gas bubbles in the catholyte flow channel, and the resistance of the cell is reduced. Nevertheless, in this “flow-by” mode, a pressure difference exists between the two sides of the GDE. The electrolyte may penetrate [134] and ultimately block the pores, preventing CO2 diffusion, which reduces the active area of the GDE. However, as the pressure difference increases to prevent perspiration, crystallized salt accumulation on the gas side is observed. So indeed, the phenomenon of perspiration has both positive and detrimental effects. The key points are to control the differential pressure across the GDE and manage the perspiration rate to avoid flooding. Jeanty et al. [165] controlled the pressure difference at the GDE by recirculation, to maintain an FECO of approximately 60% at 150 mA cm−2 for hundreds of hours.
5. Electrolyte
Combining catalyst materials modification with varying the local environment can notably reduce the energy barrier for CO2 reduction processes [161]. Aqueous electrolytes (e.g., KHCO3 and NaHCO3) can facilitate H+ transport and offer a good reaction environment, and therefore most CO2ER studies are conducted in aqueous solutions. However, the solubility of CO2 in water is approximately 34 mM at ambient temperature and pressure. In order to enhance the CO2 solubility, studies on liquid electrolytes with mixed components (e.g., ionic liquids [50,171]) have been undertaken in recent years. Ionic liquids are a promising absorbent for CO2. However, their high cost makes them unsuitable for industrial applications. In recent years, electrolyte design optimization has been widely investigated in terms of electrolyte concentration, pH, cation and anion composition, etc. In this section, we will focus on the above four important effects.
5.1. Concentration
The effect of electrolyte concentration on the reaction rate mainly originates from OH−, regardless of the type of cation [172]. Higher concentrations of electrolyte lead to a higher concentration of OH− adsorbed on the catalyst surface, thereby decreasing the charge transfer resistance (Rct) and the thickness of the electrical double layer (EDL) [119]. Verma et al. [172] found that when the electrolyte solution concentration increased from 0.5 M to 3.0 M, the jCO improved by several times. In addition, HER can be significantly inhibited by improving the electrolyte concentration. It was shown that FECO could be increased by 22% when the KOH concentration changed from 0.1 to 0.5 M. Using a Ag catalyst, it was observed that the FECO increased linearly with KOH concentration, which is due to the increase in the concentration of K2CO3 produced by the capture [151].
However, the range of influence of the electrolyte concentration is narrow, due to the mass transport limitation [76]. Kenis et al. [117] found that when the KOH concentration exceeded 2 M, there was no major increase in jCO, even though the concentration continued to increase. Therefore, from an industrial point of view, lower concentrations of aqueous electrolytes are more cost-efficient.
5.2. pH
The pH of the electrolyte plays a crucial part in its selectivity and overpotential. Since the local pH at the surface of the catalyst can increase as CO2 electroreduction proceeds, there may be a huge pH difference between the local cathode and the bulk solution [135]. The key equilibrium reactions of the CO2/bicarbonate system are as follows [173,174,175,176]:
When pH > 7, the above can be written as:
The rates of deprotonation reactions (2b, 3, 5) are very high, so it can be assumed that CO32− and HCO3− are always in balance [175]. However, the rate of the CO2 hydration reaction is very low, and thus the concentration of H2CO3 is extremely low [174,175]. In addition, the rate of the reaction between CO2 and OH− (Reaction 4) is higher than that of Reaction 2 when pH > 10 [176].
CO2ER products have high pH dependencies. Early studies showed that the production of C2H4 is largely unrelated to pH in H-type cells, unlike the pH-dependent production of CH4 [177]. In recent years, higher C2H4 selectivity has been achieved with a high-pH alkaline electrolyte in flow cells [122]. Gabardo et al. [125] found that alkaline conditions can lead to reduced overpotentials and H2 production, facilitating CO generation on the Ag catalyst in liquid-phase electrolyzers, while the selectivity for CO decreases as pH increases, which benefits HCOOH formation. A C–C coupling process occurs with high concentrations of OH− at the catalyst interface, with high energy input [133]. According to the above studies, we can draw the conclusion that higher pH values have kinetic benefits for CO2ER and can effectively reduce overpotentials and inhibit the hydrogen evolution side reaction, to obtain higher selectivity in CO2 electroreduction. However, the concentration of CO2 will inevitably decline due to the high pH in an H-type cell, so it is challenging to find a balance between the reaction kinetics and mass transfer. For flow cells, this problem can be solved successfully by optimizing the GDE design and employing continuous electrolyte flow. In addition, CO2ER in acidic media provides an alternative method of eliminating the formation of bicarbonate/carbonate salts. Sargent et al. [178] reported concentrating K+ near the active sites of the catalyst to promote CO2ER on Cu in acid conditions (pH < 1). They achieved a single-pass CO2 utilization of 77% at a j of 1.2 A cm−2. The presence of K+ suppresses HER. With an increase in K+ concentration, the selectivity for CO2ER increased, while the HER selectivity decreased.
5.3. Cation Effects
With regard to cation effects, cation identity is a critical factor for CO2ER. The hydration tendency of an ion in aqueous solution has a negative correlation with its radius, i.e., the larger the ionic radius, the higher the electrode adsorption. Therefore, large ions such as Cs+ can repel H+ ions from the cathode [179]. Furthermore, small hydrated cations experience smaller repulsion near the electrode, facilitating CO2 adsorption [178]. Thorson et al. [179] showed that a large-radius cation, specifically Cs+, in an electrochemical flow reactor, could achieve a partial current density of 72 mA cm−2 at a cathode potential of −1.4 V.
Saeki et al. [171] found that the cation of the supporting electrolyte played an important role in CO2ER in tetrabutylammonium tetrafluoroborate (TBABF4), performed at 200 mA cm−2 (20 °C) or 333 mA cm−2 (25 °C) under 40 atm, with a CO2 and methanol medium (the mole fraction of CO2 was about 33%), yielding CO as the main product. The TBA ion promoted CO2 reduction to CO2−•, which may be stabilized by forming an ion pair, {TBA+-CO2−•} and/or by being directly adsorbed on the catalyst as . Then, reacted with CO2 to produce CO. In addition, the TBA ion offered a hydrophobic environment around the catalyst, which can also benefit CO formation. Ma et al. [60] found that when the cation changed from Na+ to K+, the formation rate of C2H4 on an F-modified Cu catalyst increased significantly from 428 to 721 μmol h−1 cm−2, while the C2H4 formation rate decreased due to the increased production of HCOOH when the cation was changed to Cs+. This indicates that excessive H2O activation capacity is not conducive to the generation of C2H4 but is conducive to the production of HCOOH on the F-modified Cu.
5.4. Anion Effects
Anions were found to have a major influence on decreasing the onset potential. Among the available anions, OH− has outstanding benefits, as HER can be significantly suppressed in an alkaline environment. Moreover, a solution including OH−, such as a KOH electrolyte can induce high conductivity, which reduces ohmic losses compared with KHCO3 electrolytes [125]. However, extremely highly alkaline environments are harmful to product selectivity, though this can be offset by higher pressure. Edwards et al. [180] reported a pressurized alkaline electrolyzer with 50 bar of pressurization in 5 M KOH, which demonstrated a full cell EE of 67% at a current density of 200 mA cm−2. As well as alkaline solutions, alkaline polymer electrolytes (APEs) without the addition of an alkaline solution were used as a high-performance CO2 electrolyzer, achieving an excellent current density of 500 mA cm−2 at 3 V (cell voltage) at 60 °C, which can be explained by lower gas permeability leading to a minimized gap between the electrodes and a small ohmic loss [132].
It can be observed that jCO is related to the anion in an aqueous solution, and the influence degree follows the order from the biggest to the smallest: OH−, CO32−, HCO3−, and Cl−. Furthermore, FECO also changes significantly with the anion, following the sequence: OH− ≥ HCO3− > CO32− ≈ Cl− [119]. Specifically, the local environment and the reduction reaction influence each other. The local environment can directly affect the CO2ER path and dynamics, while CO2ER also highly affects the local environment [161]. Bhargave et al. [119] proposed that a high jCO was more likely to be obtained under the conditions of a high-concentration CsOH electrolyte with a large flow rate. They performed CO2 electroreduction on ordinary Ag nanoparticles, achieving a jCO of 417 mA cm−2 and an FECO of 100% at −2.5 V (cell potential).
6. Conclusions and Outlook
This paper systematically summarized the major research conducted at relatively high current densities in order to meet the requirements of industrial applications of CO2ER. Explorations of metal catalysts, especially the innovations of novel nanostructures and composite materials, are major fields for researchers to develop. In addition, the optimal design of reactors and the arrangement of the reaction microenvironment are also being investigated to improve the activity. Despite remarkable advances having been made in various aspects, long-term experiments at high current densities (>1 A cm−2) have not yet been carried out stably. On the basis of recent progress, we would like to emphasize four directions for future development:
- (1)
- The design of cost-efficient catalysts. Novel and cheap catalysts should be developed to replace or reduce the use of noble metals. Tailoring the morphology, crystal structure, and electronic distributions are three important strategies to optimize the usage of the active sites. By introducing heteroatoms (e.g., N, P, or other chalcogens), other metals, or specific functional groups, the lattice defects of metal catalysts such as vacancies and grain boundaries can be regulated.
- (2)
- Innovations in electrolyzers. Progress is also needed in the design of cheaper electrolyzers with higher efficiency. The facility should also be flexible enough to adapt to different CO2 resources such as CO2 captured from flue gas and biogas. At the same time, the use of a GDE (e.g., carbon matrix, PTFE) for stable and large-scale CO2 conversion should be optimized. In the future, better GDEs with excellent conductivity, hydrophobicity, and appropriate ventilation will be an intriguing development direction.
- (3)
- Research into non-OER anode reactions. Although the anodic OER reaction is green, it does not yield economic benefits. Coupling CO2ER with an anode oxidation reaction with more commercial value could be another industrially accessible approach. In this manner, CO2 electrolyzers could be easily integrated into other industrial processes in which the main product is formed on the anode. The existing challenge is proper product separation.
- (4)
- The exploration of complicated mechanisms. The electroreduction of CO2, especially to C2+ products, involves various electron transfer processes and the formation of intermediates. Theoretical calculations can provide new insights into the structure–property relationship and the rational design of catalysts. Remarkable effort has been dedicated to obtaining a better mechanistic understanding through DFT calculations and operando/in situ techniques. However, computational models are simplified and limited at present and require further development.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sánchez, O.G.; Birdja, Y.Y.; Bulut, M.; Vaes, J.; Breugelmans, T.; Pant, D. Recent Advances in Industrial CO2 Electroreduction. Curr. Opin. Green Sustain. Chem. 2019, 16, 47–56. [Google Scholar] [CrossRef]
- Verma, S.; Kim, B.; Jhong, H.; Ma, S.C.; Kenis, P.J.A. A Gross-Margin Model for Defining Technoeconomic Benchmarks in the Electroreduction of CO2. Chemsuschem 2016, 9, 1972–1979. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Guo, J.; Li, X.; Patel, P.; Seifitokaldani, A. Electrochemical Reactors for CO2 Conversion. Catalysts 2020, 10, 473. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, Y.J.; Zhang, H.; Lv, H.; Li, Q.; Michalsky, R.; Peterson, A.A.; Sun, S. Active and Selective Conversion of CO2 to CO on Ultrathin Au Nanowires. J. Am. Chem. Soc. 2014, 136, 16132–16135. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liu, M.; Sharma, P.P.; Yadav, R.M.; Ma, L.; Yang, Y.; Zou, X.; Zhou, X.D.; Vajtai, R.; Yakobson, B.I.; et al. Incorporation of Nitrogen Defects for Efficient Reduction of CO2 via Two-Electron Pathway on Three-Dimensional Graphene Foam. Nano Lett. 2016, 16, 466–470. [Google Scholar] [CrossRef]
- Gao, D.; Zhang, Y.; Zhou, Z.; Cai, F.; Zhao, X.; Huang, W.; Li, Y.; Zhu, J.; Liu, P.; Yang, F.; et al. Enhancing CO2 Electroreduction with the Metal-Oxide Interface. J. Am. Chem. Soc. 2017, 139, 5652–5655. [Google Scholar] [CrossRef]
- Kortlever, R.; Peters, I.; Koper, S.; Koper, M.T.M. Electrochemical CO2 Reduction to Formic Acid at Low Overpotential and with High Faradaic Efficiency on Carbon-Supported Bimetallic Pd-Pt Nanoparticles. ACS Catal. 2015, 5, 3916–3923. [Google Scholar] [CrossRef]
- Zhang, X.; Lei, T.; Liu, Y.Y.; Qiao, J.L. Enhancing CO2 electrolysis to formate on facilely synthesized Bi catalysts at low overpotential. Appl. Catal. B 2017, 218, 46–50. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Wan, Y.; Xie, Y.; Zhu, J.; Pan, H.; Zheng, X.; Xia, C. Perovskite Oxyfluoride Electrode Enabling Direct Electrolyzing Carbon Dioxide with Excellent Electrochemical Performances. Adv. Energy Mater. 2019, 9, 1803156. [Google Scholar] [CrossRef]
- Ren, D.; Deng, Y.L.; Handoko, A.D.; Chen, C.S.; Malkhandi, S.; Yeo, B.S. Selective Electrochemical Reduction of Carbon Dioxide to Ethylene and Ethanol on Copper(I) Oxide Catalysts. ACS Catal. 2015, 5, 2814–2821. [Google Scholar] [CrossRef]
- Ma, M.; Djanashvili, K.; Smith, W.A. Controllable Hydrocarbon Formation from the Electrochemical Reduction of CO2 over Cu Nanowire Arrays. Angew. Chem. Int. Ed. 2016, 55, 6680–6684. [Google Scholar] [CrossRef] [PubMed]
- Weng, Z.; Jing, J.B.; Wu, Y.S.; Wu, Z.S.; Guo, X.T.; Materna, K.L.; Liu, W.; Batista, V.S.; Brudvig, G.W.; Wang, H.L. Electrochemical CO2 Reduction to Hydrocarbons on a Heterogeneous Molecular Cu Catalyst in Aqueous Solution. J. Am. Chem. Soc. 2016, 138, 8076–8079. [Google Scholar] [CrossRef] [PubMed]
- Birdja, Y.Y.; Vaes, J. Towards a Critical Evaluation of Electrocatalyst Stability for CO2 Electroreduction. ChemElectroChem 2020, 7, 4713–4717. [Google Scholar] [CrossRef]
- Hou, Y.T.; Wang, L.J.; Bian, L.Z.; Wang, Y.D.; Chou, K.C.; Kumar, R.V. High-performance La0.3Sr0.7Fe0.9Ti0.1O3-delta as fuel electrode for directly electrolyzing CO2 in solid oxide electrolysis cells. Electrochim. Acta 2020, 342. [Google Scholar] [CrossRef]
- Hu, X.L.; Xie, K. Active and stable Ni/Cr2O3-delta cathodes for high temperature CO2 electrolysis. J. Power Sources 2019, 430, 20–24. [Google Scholar] [CrossRef]
- Wu, M.X.; Zhou, X.L.; Xu, J.; Li, S.; Pan, L.; Zhang, N.Q. Electrochemical performance of La0.3Sr0.7Ti0.3Fe0.7O3-delta/CeO2 composite cathode for CO2 reduction in solid oxide electrolysis cells. J. Power Sources 2020, 451, 227334. [Google Scholar] [CrossRef]
- Handoko, A.D.; Wei, F.; Jenndy; Yeo, B.S.; Seh, Z.W. Understanding heterogeneous electrocatalytic carbon dioxide reduction through operando techniques. Nat. Catal. 2018, 1, 922–934. [Google Scholar]
- Kortlever, R.; Shen, J.; Schouten, K.J.; Calle-Vallejo, F.; Koper, M.T. Catalysts and Reaction Pathways for the Electrochemical Reduction of Carbon Dioxide. J. Phys. Chem. Lett. 2015, 6, 4073–4082. [Google Scholar] [CrossRef]
- Peterson, A.A.; Norskov, J.K. Activity Descriptors for CO2 Electroreduction to Methane on Transition-Metal Catalysts. J. Phys. Chem. Lett. 2012, 3, 251–258. [Google Scholar] [CrossRef]
- Montoya, J.H.; Shi, C.; Chan, K.; Norskov, J.K. Theoretical Insights into a CO Dimerization Mechanism in CO2 Electroreduction. J. Phys. Chem. Lett. 2015, 6, 2032–2037. [Google Scholar] [CrossRef]
- Schouten, K.J.P.; Qin, Z.S.; Gallent, E.P.; Koper, M.T.M. Two Pathways for the Formation of Ethylene in CO Reduction on Single-Crystal Copper Electrodes. J. Am. Chem. Soc. 2012, 134, 9864–9867. [Google Scholar] [CrossRef] [PubMed]
- Peterson, A.A.; Abild-Pedersen, F.; Studt, F.; Rossmeisl, J.; Norskov, J.K. How copper catalyzes the electroreduction of carbon dioxide into hydrocarbon fuels. Energy Environ. Sci. 2010, 3, 1311–1315. [Google Scholar] [CrossRef]
- Schouten, K.J.P.; Kwon, Y.; van der Ham, C.J.M.; Qin, Z.; Koper, M.T.M. A new mechanism for the selectivity to C1 and C2 species in the electrochemical reduction of carbon dioxide on copper electrodes. Chem. Sci. 2011, 2, 1902–1909. [Google Scholar] [CrossRef]
- Kim, C.; Jeon, H.S.; Eom, T.; Jee, M.S.; Kim, H.; Friend, C.M.; Min, B.K.; Hwang, Y.J. Achieving Selective and Efficient Electrocatalytic Activity for CO2 Reduction Using Immobilized Silver Nanoparticles. J. Am. Chem. Soc. 2015, 137, 13844–13850. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Eom, T.; Jee, M.S.; Jung, H.; Kim, H.; Min, B.K.; Hwang, Y.J. Insight into Electrochemical CO2 Reduction on Surface-Molecule-Mediated Ag Nanoparticles. ACS Catal. 2016, 7, 779–785. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, C.; Liu, Y.; MacFarlane, D.R.; Wallace, G.G. Engineering Surface Amine Modifiers of Ultrasmall Gold Nanoparticles Supported on Reduced Graphene Oxide for Improved Electrochemical CO2 Reduction. Adv. Energy Mater. 2018, 8, 1801400. [Google Scholar] [CrossRef] [Green Version]
- Nam, D.H.; De Luna, P.; Rosas-Hernandez, A.; Thevenon, A.; Li, F.; Agapie, T.; Peters, J.C.; Shekhah, O.; Eddaoudi, M.; Sargent, E.H. Molecular enhancement of heterogeneous CO2 reduction. Nat. Mater. 2020, 19, 266–276. [Google Scholar] [CrossRef]
- Han, Z.; Kortlever, R.; Chen, H.Y.; Peters, J.C.; Agapie, T. CO2 Reduction Selective for C≥2 Products on Polycrystalline Copper with N-Substituted Pyridinium Additives. ACS Cent. Sci. 2017, 3, 853–859. [Google Scholar] [CrossRef] [Green Version]
- Hori, Y.; Vayenas, C.G. Modern Aspects of Electrochemistry; Vayenas, C.G., White, R.E., Gamboa-Aldeco, M.E., Eds.; Springer: New York, NY, USA, 2008; Volume 42, pp. 89–190. [Google Scholar]
- Wu, M.; Xu, B.; Zhang, Y.; Qi, S.; Ni, W.; Hu, J.; Ma, J. Perspectives in emerging bismuth electrochemistry. Chem. Eng. J. 2020, 381, 122558. [Google Scholar] [CrossRef]
- Huang-Fu, Z.C.; Song, Q.T.; He, Y.H.; Wang, J.J.; Ye, J.Y.; Zhou, Z.Y.; Sun, S.G.; Wang, Z.H. Electrochemical CO2 reduction on Cu and Au electrodes studied using in situ sum frequency generation spectroscopy. Phys. Chem. Chem. Phys. 2019, 21, 25047–25053. [Google Scholar] [CrossRef]
- Roy, S.; Sharma, B.; Peaut, J.; Simon, P.; Fontecave, M.; Tran, P.D.; Derat, E.; Artero, V. Molecular Cobalt Complexes with Pendant Amines for Selective Electrocatalytic Reduction of Carbon Dioxide to Formic Acid. J. Am. Chem. Soc. 2017, 139, 3685–3696. [Google Scholar] [CrossRef] [PubMed]
- Hori, Y.; Murata, A.; Kikuchi, K.; Suzuki, S. Electrochemical Reduction of Carbon Dioxide to Carbon Monoxide at a Gold Electrode in Aqueous Potassium Hydrogen Carbonate. J. Chem. Soc. Chem. Commun. 1987, 10, 728–729. [Google Scholar] [CrossRef]
- Zhu, W.; Michalsky, R.; Metin, Ö.; Lv, H.; Guo, S.; Wright, C.J.; Sun, X.; Peterson, A.A.; Sun, S. Monodisperse Au Nanoparticles for Selective Electrocatalytic Reduction of CO2 to CO. J. Am. Chem. Soc. 2013, 135, 16833–16836. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Pang, Y.; Zhang, B.; De Luna, P.; Voznyy, O.; Xu, J.; Zheng, X.; Dinh, C.T.; Fan, F.; Cao, C.; et al. Enhanced Electrocatalytic CO2 Reduction via Field-induced Reagent Concentration. Nature 2016, 537, 382–386. [Google Scholar] [CrossRef]
- Jhong, H.M.; Tornow, C.E.; Kim, C.; Verma, S.; Oberst, J.L.; Anderson, P.S.; Gewirth, A.A.; Fujigaya, T.; Nakashima, N.; Kenis, P.J.A. Gold Nanoparticles on Polymer-Wrapped Carbon Nanotubes: An Efficient and Selective Catalyst for the Electroreduction of CO2. Chemphyschem 2017, 18, 3274–3279. [Google Scholar] [CrossRef] [Green Version]
- Verma, S.; Hamasaki, Y.; Kim, C.; Huang, W.; Lu, S.; Jhong, H.-R.M.; Gewirth, A.A.; Fujigaya, T.; Nakashima, N.; Kenis, P.J.A. Insights into the Low Overpotential Electroreduction of CO2 to CO on a Supported Gold Catalyst in an Alkaline Flow Electrolyzer. ACS Energy Lett. 2017, 3, 193–198. [Google Scholar] [CrossRef]
- Ma, L.; Hu, W.; Pan, Q.; Zou, L.; Zou, Z.; Wen, K.; Yang, H. Polyvinyl Alcohol-modified Gold Nanoparticles with Record-high Activity for Electrochemical Reduction of CO2 to CO. J. CO2 Util. 2019, 34, 108–114. [Google Scholar] [CrossRef]
- Salehi-Khojin, A.; Jhong, H.-R.M.; Rosen, B.A.; Zhu, W.; Ma, S.; Kenis, P.J.A.; Masel, R.I. Nanoparticle Silver Catalysts That Show Enhanced Activity for Carbon Dioxide Electrolysis. J. Phys. Chem. C 2013, 117, 1627–1632. [Google Scholar] [CrossRef]
- Mistry, H.; Reske, R.; Zeng, Z.; Zhao, Z.J.; Greeley, J.; Strasser, P.; Cuenya, B.R. Exceptional size-dependent activity enhancement in the electroreduction of CO2 over Au nanoparticles. J. Am. Chem. Soc. 2014, 136, 16473–16476. [Google Scholar] [CrossRef]
- Liu, S.; Tao, H.; Zeng, L.; Liu, Q.; Xu, Z.; Liu, Q.; Luo, J.-L. Shape-Dependent Electrocatalytic Reduction of CO2 to CO on Triangular Silver Nanoplates. J. Am. Chem. Soc. 2017, 139, 2160–2163. [Google Scholar] [CrossRef]
- Ma, S.; Lan, Y.; Perez, G.M.; Moniri, S.; Kenis, P.J. Silver Supported on Titania as an Active Catalyst for Electrochemical Carbon Dioxide Reduction. ChemSusChem 2014, 7, 866–874. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Haspel, H.; Pustovarenko, A.; Dikhtiarenko, A.; Russkikh, A.; Shterk, G.; Osadchii, D.; Ould-Chikh, S.; Ma, M.; Smith, W.A.; et al. Maximizing Ag Utilization in High-Rate CO2 Electrochemical Reduction with a Coordination Polymer-Mediated Gas Diffusion Electrode. ACS Energy Lett. 2019, 4, 2024–2031. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Z.; Lee, H.; Mao, J.; Grimes, C.A.; Liu, C.; Zhang, M.; Lu, Z.; Chen, Y.; Feng, S.P. Efficient electroreduction of CO2 to CO by Ag-decorated S-doped g-C3N4/CNT nanocomposites at industrial scale current density. Mater. Today Phys. 2020, 12, 100176. [Google Scholar] [CrossRef]
- Ma, S.; Luo, R.; Gold, J.I.; Yu, A.Z.; Kim, B.; Kenis, P.J.A. Carbon nanotube containing Ag catalyst layers for efficient and selective reduction of carbon dioxide. J. Mater. Chem. A 2016, 4, 8573–8578. [Google Scholar] [CrossRef]
- Gao, D.; Zhou, H.; Cai, F.; Wang, J.; Wang, G.; Bao, X. Pd-Containing Nanostructures for Electrochemical CO2 Reduction Reaction. ACS Catal. 2018, 8, 1510–1519. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, X.G.; Jiang, K.; Wu, D.Y.; Cai, W.B. Boosting Formate Production in Electrocatalytic CO2 Reduction over Wide Potential Window on Pd Surfaces. J. Am. Chem. Soc. 2018, 140, 2880–2889. [Google Scholar] [CrossRef]
- Zhu, W.; Kattel, S.; Jiao, F.; Chen, J.G. Shape-Controlled CO2 Electrochemical Reduction on Nanosized Pd Hydride Cubes and Octahedra. Adv. Energy Mater. 2019, 9, 1802840. [Google Scholar] [CrossRef]
- Xia, R.; Zhang, S.; Ma, X.; Jiao, F. Surface-functionalized Palladium Catalysts for Electrochemical CO2 Reduction. J. Mater. Chem. A 2020, 8, 15884–15890. [Google Scholar] [CrossRef]
- Zhu, Q.; Yang, D.; Liu, H.; Sun, X.; Chen, C.; Bi, J.; Liu, J.; Wu, H.; Han, B. Hollow Metal-Organic-Framework-Mediated In Situ Architecture of Copper Dendrites for Enhanced CO2 Electroreduction. Angew Chem. Int. Ed. Engl. 2020, 59, 8896–8901. [Google Scholar] [CrossRef]
- Yang, F.; Deng, P.; Wang, Q.; Zhu, J.; Yan, Y.; Zhou, L.; Qi, K.; Liu, H.; Park, H.S.; Xia, B.Y. Metal-organic framework-derived cupric oxide polycrystalline nanowires for selective carbon dioxide electroreduction to C2 valuables. J. Mater. Chem. A 2020, 8, 12418–12423. [Google Scholar] [CrossRef]
- Yao, K.; Xia, Y.; Li, J.; Wang, N.; Han, J.; Gao, C.; Han, M.; Shen, G.; Liu, Y.; Seifitokaldani, A.; et al. Metal-organic Framework Derived Copper Catalysts for CO2 to Ethylene Conversion. J. Mater. Chem. A 2020, 8, 11117–11123. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, H.; Livi, K.J.T.; Raciti, D.; Zong, H.; Gregg, J.; Onadeko, M.; Wan, Y.; Watson, A.; Wang, C. Copper Nanocubes for CO2 Reduction in Gas Diffusion Electrodes. Nano Lett. 2019, 19, 8461–8468. [Google Scholar] [CrossRef] [PubMed]
- Baruch, M.F.; Pander, J.E.; White, J.L.; Bocarsly, A.B. Mechanistic Insights into the Reduction of CO2 on Tin Electrodes using in Situ ATR-IR Spectroscopy. ACS Catal. 2015, 5, 3148–3156. [Google Scholar] [CrossRef]
- Ju, W.; Jiang, F.; Ma, H.; Pan, Z.; Zhao, Y.-B.; Pagani, F.; Rentsch, D.; Wang, J.; Battaglia, C. Electrocatalytic reduction of gaseous CO2 to CO on SnCu-nanofiber-based gas diffusion electrodes. Adv. Energy Mater. 2019, 9, 1901514. [Google Scholar] [CrossRef]
- Xiang, H.; Rasul, S.; Hou, B.; Portoles, J.; Cumpson, P.; Yu, E.H. Copper-Indium Binary Catalyst on a Gas Diffusion Electrode for High-Performance CO2 Electrochemical Reduction with Record CO Production Efficiency. ACS Appl. Mater. Interfaces 2020, 12, 601–608. [Google Scholar] [CrossRef]
- Luc, W.; Collins, C.; Wang, S.; Xin, H.; He, K.; Kang, Y.; Jiao, F. Ag-Sn Bimetallic Catalyst with a Core-Shell Structure for CO2 Reduction. J. Am. Chem. Soc. 2017, 139, 1885–1893. [Google Scholar] [CrossRef] [PubMed]
- Ye, K.; Zhou, Z.; Shao, J.; Lin, L.; Gao, D.; Ta, N.; Si, R.; Wang, G.; Bao, X. In Situ Reconstruction of a Hierarchical Sn-Cu/SnOx Core/Shell Catalyst for High-Performance CO2 Electroreduction. Angew Chem. Int. Ed. Engl. 2020, 59, 4814–4821. [Google Scholar] [CrossRef]
- Li, Y.C.; Wang, Z.; Yuan, T.; Nam, D.H.; Luo, M.; Wicks, J.; Chen, B.; Li, J.; Li, F.; de Arquer, F.P.G.; et al. Binding Site Diversity Promotes CO2 Electroreduction to Ethanol. J. Am. Chem. Soc. 2019, 141, 8584–8591. [Google Scholar] [CrossRef]
- Ma, W.; Xie, S.; Liu, T.; Fan, Q.; Ye, J.; Sun, F.; Jiang, Z.; Zhang, Q.; Cheng, J.; Wang, Y. Electrocatalytic Reduction of CO2 to Ethylene and Ethanol through Hydrogen-assisted C–C Coupling over Fluorine-modified Copper. Nat. Catal. 2020, 3, 478–487. [Google Scholar] [CrossRef]
- Lee, J.-C.; Kim, J.-Y.; Joo, W.-H.; Hong, D.; Oh, S.-H.; Kim, B.; Lee, G.-D.; Kim, M.; Oh, J.; Joo, Y.-C. Thermodynamically Driven Self-formation of Copper-embedded Nitrogen-doped Carbon Nanofiber Catalysts for a Cascade Electroreduction of Carbon Dioxide to Ethylene. J. Mater. Chem. A 2020, 8, 11632–11641. [Google Scholar] [CrossRef]
- Chen, C.; Yan, X.; Liu, S.; Wu, Y.; Wan, Q.; Sun, X.; Zhu, Q.; Liu, H.; Ma, J.; Zheng, L.; et al. Highly Efficient Electroreduction of CO2 to C2+ Alcohols on Heterogeneous Dual Active Sites. Angew Chem. Int. Ed. Engl. 2020, 59, 16459–16464. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Jiang, K.; Fan, S.; Kanan, M.W. A Direct Grain-Boundary-Activity Correlation for CO Electroreduction on Cu Nanoparticles. ACS Cent. Sci. 2016, 2, 169–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.; Shen, H.; Guan, A.; Liu, J.; Li, T.; Ji, Y.; Al-Enizi, A.M.; Zhang, L.; Qian, L.; Zheng, G. Fast Cooling Induced Grain-boundary-rich Copper Oxide for Electrocatalytic Carbon Dioxide Reduction to Ethanol. J. Colloid Interface Sci. 2020, 570, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Hori, Y.; Takahashi, I.; Koga, O.; Hoshi, N. Electrochemical Reduction of Carbon Dioxide at Various Series of Copper Single Crystal Electrodes. J. Mol. Catal. A Chem. 2003, 199, 39–47. [Google Scholar] [CrossRef]
- Schouten, K.J.P.; Pérez Gallent, E.; Koper, M.T.M. Structure Sensitivity of the Electrochemical Reduction of Carbon Monoxide on Copper Single Crystals. ACS Catal. 2013, 3, 1292–1295. [Google Scholar] [CrossRef]
- Perez-Gallent, E.; Figueiredo, M.C.; Calle-Vallejo, F.; Koper, M.T. Spectroscopic Observation of a Hydrogenated CO Dimer Intermediate During CO Reduction on Cu(100) Electrodes. Angew Chem. Int. Ed. Engl. 2017, 56, 3621–3624. [Google Scholar] [CrossRef]
- Roberts, F.S.; Kuhl, K.P.; Nilsson, A. High Selectivity for Ethylene from Carbon Dioxide Reduction over Copper Nanocube Electrocatalysts. Angew Chem. Int. Ed. Engl. 2015, 54, 5179–5182. [Google Scholar] [CrossRef]
- Jiang, K.; Sandberg, R.B.; Akey, A.J.; Liu, X.; Bell, D.C.; Nørskov, J.K.; Chan, K.; Wang, H. Metal Ion Cycling of Cu Foil for Selective C–C Coupling in Electrochemical CO2 Reduction. Nat. Catal. 2018, 1, 111–119. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Dinh, C.-T.; Li, J.; Ozden, A.; Golam Kibria, M.; Seifitokaldani, A.; Tan, C.-S.; Gabardo, C.M.; Luo, M.; et al. Catalyst synthesis under CO2 electroreduction favours faceting and promotes renewable fuels electrosynthesis. Nat. Catal. 2019, 3, 98–106. [Google Scholar] [CrossRef]
- Zhang, T.; Bui, J.C.; Li, Z.; Bell, A.T.; Weber, A.Z.; Wu, J. Highly selective and productive reduction of carbon dioxide to multicarbon products via in situ CO management using segmented tandem electrodes. Nat. Catal. 2022, 5, 202–211. [Google Scholar] [CrossRef]
- Luo, W.; Zhang, J.; Li, M.; Züttel, A. Boosting CO Production in Electrocatalytic CO2 Reduction on Highly Porous Zn Catalysts. ACS Catal. 2019, 9, 3783–3791. [Google Scholar] [CrossRef] [Green Version]
- Lamaison, S.; Wakerley, D.; Montero, D.; Rousse, G.; Taverna, D.; Giaume, D.; Mercier, D.; Blanchard, J.; Tran, H.N.; Fontecave, M.; et al. Zn-Cu Alloy Nanofoams as Efficient Catalysts for the Reduction of CO2 to Syngas Mixtures with a Potential-Independent H2/CO Ratio. ChemSusChem 2019, 12, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Lamaison, S.; Wakerley, D.; Blanchard, J.; Montero, D.; Rousse, G.; Mercier, D.; Marcus, P.; Taverna, D.; Giaume, D.; Mougel, V.; et al. High-Current-Density CO2-to-CO Electroreduction on Ag-Alloyed Zn Dendrites at Elevated Pressure. Joule 2020, 4, 395–406. [Google Scholar] [CrossRef]
- Saberi Safaei, T.; Mepham, A.; Zheng, X.; Pang, Y.; Dinh, C.T.; Liu, M.; Sinton, D.; Kelley, S.O.; Sargent, E.H. High-Density Nanosharp Microstructures Enable Efficient CO2 Electroreduction. Nano Lett. 2016, 16, 7224–7228. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.Y.; Hu, S.J.; Zhang, X.L.; Zheng, Y.R.; Wang, H.J.; Niu, Z.Z.; Yang, P.P.; Bao, R.C.; Ma, T.; Dang, Z.; et al. High-Curvature Transition-Metal Chalcogenide Nanostructures with a Pronounced Proximity Effect Enable Fast and Selective CO2 Electroreduction. Angew Chem. Int. Ed. Engl. 2020, 59, 8706–8712. [Google Scholar] [CrossRef]
- Sordakis, K.; Tang, C.; Vogt, L.K.; Junge, H.; Dyson, P.J.; Beller, M.; Laurenczy, G. Homogeneous Catalysis for Sustainable Hydrogen Storage in Formic Acid and Alcohols. Chem. Rev. 2018, 118, 372–433. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, J.; Cheng, F.; Chen, J. Mn-doped atomic SnO2 layers for highly efficient CO2 electrochemical reduction. J. Mater. Chem. A 2019, 7, 19651–19656. [Google Scholar] [CrossRef]
- Löwe, A.; Rieg, C.; Hierlemann, T.; Salas, N.; Kopljar, D.; Wagner, N.; Klemm, E. Influence of Temperature on the Performance of Gas Diffusion Electrodes in the CO2 Reduction Reaction. ChemElectroChem 2019, 6, 4497–4506. [Google Scholar] [CrossRef] [Green Version]
- Xiang, H.; Miller, H.A.; Bellini, M.; Christensen, H.; Scott, K.; Rasul, S.; Yu, E.H. Production of Formate by CO2 Electrochemical Reduction and Its Application in Energy Storage. Sustain. Energy Fuels 2020, 4, 277–284. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, M.; Liu, J.; Zhou, J.; Cen, K. A Cu foam cathode used as a Pt–RGO catalyst matrix to improve CO2 reduction in a photoelectrocatalytic cell with a TiO2 photoanode. J. Mater. Chem. A 2015, 3, 12947–12957. [Google Scholar] [CrossRef]
- Wang, J.; Zou, J.; Hu, X.; Ning, S.; Wang, X.; Kang, X.; Chen, S. Heterostructured intermetallic CuSn catalysts high performance towards the electrochemical reduction of CO2 to formate. J. Mater. Chem. A 2019, 7, 27514–27521. [Google Scholar] [CrossRef]
- Díaz-Sainz, G.; Alvarez-Guerra, M.; Solla-Gullón, J.; García-Cruz, L.; Montiel, V.; Irabien, A. CO2 Electroreduction to Formate: Continuous Single-pass Operation in a Filter-press Reactor at High Current Densities Using Bi Gas Diffusion Electrodes. J. CO2 Util. 2019, 34, 12–19. [Google Scholar] [CrossRef]
- Deng, P.; Yang, F.; Wang, Z.; Chen, S.; Zhou, Y.; Zaman, S.; Xia, B.Y. Metal-Organic Framework-Derived Carbon Nanorods Encapsulating Bismuth Oxides for Rapid and Selective CO2 Electroreduction to Formate. Angew Chem. Int. Ed. Engl. 2020, 59, 10807–10813. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Zhu, P.; Jiang, Q.; Pan, Y.; Liang, W.; Stavitski, E.; Alshareef, H.N.; Wang, H. Continuous Production of Pure Liquid Fuel Solutions via Electrocatalytic CO2 Reduction Using Solid-electrolyte Devices. Nat. Energy 2019, 4, 776–785. [Google Scholar] [CrossRef]
- Yang, J.; Wang, X.; Qu, Y.; Wang, X.; Huo, H.; Fan, Q.; Wang, J.; Yang, L.M.; Wu, Y. Bi-Based Metal-Organic Framework Derived Leafy Bismuth Nanosheets for Carbon Dioxide Electroreduction. Adv. Energy Mater. 2020, 2001709. [Google Scholar] [CrossRef]
- Cao, C.; Ma, D.D.; Gu, J.F.; Xie, X.; Zeng, G.; Li, X.; Han, S.G.; Zhu, Q.L.; Wu, X.T.; Xu, Q. Metal-Organic Layers Leading to Atomically Thin Bismuthene for Efficient Carbon Dioxide Electroreduction to Liquid Fuel. Angew Chem. Int. Ed. Engl. 2020, 59, 15014–15020. [Google Scholar] [CrossRef]
- Han, P.; Yu, X.; Yuan, D.; Kuang, M.; Wang, Y.; Al-Enizi, A.M.; Zheng, G. Defective Graphene for Electrocatalytic CO2 Reduction. J. Colloid Interface Sci. 2019, 534, 332–337. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, J.; Zhang, F.; Zheng, L.; Mo, G.; Han, B.; Yang, G. Selenium-Doped Hierarchically Porous Carbon Nanosheets as an Effcient Metal-Free Electrocatalyst for CO2 Reduction. Adv. Funct. Mater. 2020, 30, 1906194. [Google Scholar] [CrossRef]
- Yang, H.; Wu, Y.; Lin, Q.; Fan, L.; Chai, X.; Zhang, Q.; Liu, J.; He, C.; Lin, Z. Composition Tailoring via N and S Co-doping and Structure Tuning by Constructing Hierarchical Pores: Metal-Free Catalysts for High-Performance Electrochemical Reduction of CO2. Angew Chem. Int. Ed. Engl. 2018, 57, 15476–15480. [Google Scholar] [CrossRef]
- Chen, C.; Sun, X.; Yan, X.; Wu, Y.; Liu, H.; Zhu, Q.; Bediako, B.B.A.; Han, B. Boosting CO2 Electroreduction on N, P-Co-doped Carbon Aerogels. Angew Chem. Int. Ed. Engl. 2020, 59, 11123–11129. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhao, S.; Johannessen, B.; Veder, J.P.; Saunders, M.; Rowles, M.R.; Cheng, M.; Liu, C.; Chisholm, M.F.; De Marco, R.; et al. Atomically Dispersed Transition Metals on Carbon Nanotubes with Ultrahigh Loading for Selective Electrochemical Carbon Dioxide Reduction. Adv. Mater. 2018, 30, 1706287. [Google Scholar] [CrossRef] [PubMed]
- Jhong, H.-R.M.; Brushett, F.R.; Kenis, P.J.A. The Effects of Catalyst Layer Deposition Methodology on Electrode Performance. Adv. Energy Mater. 2013, 3, 589–599. [Google Scholar] [CrossRef]
- Yang, H.; Lin, Q.; Zhang, C.; Yu, X.; Cheng, Z.; Li, G.; Hu, Q.; Ren, X.; Zhang, Q.; Liu, J.; et al. Carbon Dioxide Electroreduction on Single-atom Nickel Decorated Carbon Membranes with Industry Compatible Current Densities. Nat. Commun. 2020, 11, 593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Lin, Q.; Wu, Y.; Li, G.; Hu, Q.; Chai, X.; Ren, X.; Zhang, Q.; Liu, J.; He, C. Highly efficient utilization of single atoms via constructing 3D and free-standing electrodes for CO2 reduction with ultrahigh current density. Nano Energy 2020, 70, 104454. [Google Scholar] [CrossRef]
- Yang, H.B.; Hung, S.-F.; Liu, S.; Yuan, K.; Miao, S.; Zhang, L.; Huang, X.; Wang, H.-Y.; Cai, W.; Chen, R.; et al. Atomically dispersed Ni(i) as the active site for electrochemical CO2 reduction. Nat. Energy 2018, 3, 140–147. [Google Scholar] [CrossRef]
- Chen, Y.; Zou, L.; Liu, H.; Chen, C.; Wang, Q.; Gu, M.; Yang, B.; Zou, Z.; Fang, J.; Yang, H. Fe and N Co-Doped Porous Carbon Nanospheres with High Density of Active Sites for Efficient CO2 Electroreduction. J. Phys. Chem. C 2019, 123, 16651–16659. [Google Scholar] [CrossRef]
- Wu, S.; Lv, X.; Ping, D.; Zhang, G.; Wang, S.; Wang, H.; Yang, X.; Guo, D.; Fang, S. Highly exposed atomic Fe–N active sites within carbon nanorods towards electrocatalytic reduction of CO2 to CO. Electrochim. Acta 2020, 340, 135930. [Google Scholar] [CrossRef]
- Yan, C.; Ye, Y.; Lin, L.; Wu, H.; Jiang, Q.; Wang, G.; Bao, X. Improving CO2 Electroreduction over ZIF-derived Carbon Doped with Fe-N Sites by an Additional Ammonia Treatment. Catal. Today 2019, 330, 252–258. [Google Scholar] [CrossRef]
- Wang, X.; Pan, Y.; Ning, H.; Wang, H.; Guo, D.; Wang, W.; Yang, Z.; Zhao, Q.; Zhang, B.; Zheng, L.; et al. Hierarchically micro- and meso-porous Fe-N4O-doped carbon as robust electrocatalyst for CO2 reduction. Appl. Catal. B 2020, 266, 118630. [Google Scholar] [CrossRef]
- Gu, J.; Hsu, C.-S.; Bai, L.; Chen, H.M.; Hu, X. Atomically dispersed Fe3+ sitescatalyze efficient CO2 electroreduction to CO. Science 2019, 364, 1091–1094. [Google Scholar] [CrossRef]
- Hou, Y.; Liang, Y.-L.; Shi, P.-C.; Huang, Y.-B.; Cao, R. Atomically dispersed Ni species on N-doped carbon nanotubes for electroreduction of CO2 with nearly 100% CO selectivity. Appl. Catal. B 2020, 271, 118929. [Google Scholar] [CrossRef]
- Möller, T.; Ju, W.; Bagger, A.; Wang, X.; Luo, F.; Ngo Thanh, T.; Varela, A.S.; Rossmeisl, J.; Strasser, P. Efficient CO2 to CO Electrolysis on Solid Ni–N–C Catalysts at Industrial Current Densities. Energy Environ. Sci. 2019, 12, 640–647. [Google Scholar] [CrossRef]
- Zheng, T.; Jiang, K.; Ta, N.; Hu, Y.; Zeng, J.; Liu, J.; Wang, H. Large-Scale and Highly Selective CO2 Electrocatalytic Reduction on Nickel Single-Atom Catalyst. Joule 2019, 3, 265–278. [Google Scholar] [CrossRef] [Green Version]
- Jeong, H.-Y.; Balamurugan, M.; Choutipalli, V.S.K.; Jeong, E.-s.; Subramanian, V.; Sim, U.; Nam, K.T. Achieving Highly Efficient CO2 to CO Electroreduction Exceeding 300 mA cm−2 with Single-atom Nickel Electrocatalysts. J. Mater. Chem. A 2019, 7, 10651–10661. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, Z.; Zhang, X.; Niu, Z.; Zhou, Q.; Wang, X.; Li, H.; Lin, Z.; Zheng, H.; Liang, Y. Metal Phthalocyanine-Derived Single-Atom Catalysts for Selective CO2 Electroreduction under High Current Densities. ACS Appl. Mater. Interfaces 2020, 12, 33795–33802. [Google Scholar] [CrossRef]
- Guo, J.-H.; Zhang, X.-Y.; Dao, X.-Y.; Sun, W.-Y. Nanoporous Metal–Organic Framework-Based Ellipsoidal Nanoparticles for the Catalytic Electroreduction of CO2. ACS Appl. Nano Mater. 2020, 3, 2625–2635. [Google Scholar] [CrossRef]
- Zhang, T.; Lin, L.; Li, Z.; He, X.; Xiao, S.; Shanov, V.N.; Wu, J. Nickel Nitrogen Carbon Molecular Catalysts for High Rate CO2 Electro-reduction to CO On the Role of Carbon Substrate and Reaction Chemistry. ACS Appl. Nano Mater. 2020, 3, 1617–1626. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Gu, M.; Wang, M.; Zhang, Z.; Pan, W.; Jiang, Z.; Zheng, H.; Lucero, M.; Wang, H.; et al. Molecular engineering of dispersed nickel phthalocyanines on carbon nanotubes for selective CO2 reduction. Nat. Energy 2020, 5, 684–692. [Google Scholar] [CrossRef]
- Wen, C.F.; Mao, F.; Liu, Y.; Zhang, X.Y.; Fu, H.Q.; Zheng, L.R.; Liu, P.F.; Yang, H.G. Nitrogen-stabilized low-valent Ni motifs for efficient CO2 electrocatalysis. ACS Catal. 2020, 10, 1086–1093. [Google Scholar] [CrossRef]
- Hou, P.; Song, W.; Wang, X.; Hu, Z.; Kang, P. Well-Defined Single-Atom Cobalt Catalyst for Electrocatalytic Flue Gas CO2 Reduction. Small 2020, 16, 2001896. [Google Scholar] [CrossRef]
- Fujinuma, N.; Ikoma, A.; Lofland, S.E. Highly Efficient Electrochemical CO2 Reduction Reaction to CO with One-Pot Synthesized Co-Pyridine-Derived Catalyst Incorporated in a Nafion-Based Membrane Electrode Assembly. Adv. Energy Mater. 2020, 10, 2001645. [Google Scholar] [CrossRef]
- Roy, A.; Hursán, D.; Artyushkova, K.; Atanassov, P.; Janáky, C.; Serov, A. Nanostructured Metal-N-C Electrocatalysts for CO2 Reduction and Hydrogen Evolution Reactions. Appl. Catal. B 2018, 232, 512–520. [Google Scholar] [CrossRef]
- Yang, H.; Wu, Y.; Li, G.; Lin, Q.; Hu, Q.; Zhang, Q.; Liu, J.; He, C. Scalable Production of Efficient Single-Atom Copper Decorated Carbon Membranes for CO2 Electroreduction to Methanol. J. Am. Chem. Soc. 2019, 141, 12717–12723. [Google Scholar] [CrossRef]
- Ren, S.; Joulié, D.; Salvatore, D.; Torbensen, K.; Wang, M.; Robert, M.; Berlinguette, C.P. Molecular electrocatalysts can mediate fast, selective CO2 reduction in a flow cell. Science 2019, 365, 367–369. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Torbensen, K.; Salvatore, D.; Ren, S.; Joulie, D.; Dumoulin, F.; Mendoza, D.; Lassalle-Kaiser, B.; Isci, U.; Berlinguette, C.P.; et al. CO2 Electrochemical Catalytic Reduction with a Highly Active Cobalt Phthalocyanine. Nat. Commun. 2019, 10, 3602. [Google Scholar] [CrossRef] [PubMed]
- Torbensen, K.; Han, C.; Boudy, B.; von Wolff, N.; Bertail, C.; Braun, W.; Robert, M. Iron Porphyrin Allows Fast and Selective Electrocatalytic Conversion of CO2 to CO in a Flow Cell. Chemistry 2020, 26, 3034–3038. [Google Scholar] [CrossRef]
- Arquer, F.P.G.d.; Dinh, C.-T.; Ozden, A.; Wicks, J.; McCallum, C.; Kirmani, A.R.; Nam, D.-H.; Gabardo, C.; Seifitokaldani, A.; Wang, X.; et al. CO2 electrolysis to multicarbon products at activities greater than 1 A cm−2. Science 2020, 367, 661–666. [Google Scholar] [CrossRef]
- Bhargava, S.S.; Proietto, F.; Azmoodeh, D.; Cofell, E.R.; Henckel, D.A.; Verma, S.; Brooks, C.J.; Gewirth, A.A.; Kenis, P.J.A. System Design Rules for Intensifying the Electrochemical Reduction of CO2 to CO on Ag Nanoparticles. ChemElectroChem 2020, 7, 2001–2011. [Google Scholar] [CrossRef]
- Chen, L.; Xu, J.; Wang, X.; Xie, K. Sr2Fe1.5+xMo0.5O6-δ Cathode with Exsolved Fe Nanoparticles for Enhanced CO2 Electrolysis. Int. J. Hydrogen Energ. 2020, 45, 11901–11907. [Google Scholar] [CrossRef]
- De Luna, P.; Quintero-Bermudez, R.; Dinh, C.-T.; Ross, M.B.; Bushuyev, O.S.; Todorović, P.; Regier, T.; Kelley, S.O.; Yang, P.; Sargent, E.H. Catalyst Electro-redeposition Controls Morphology and Oxidation State for Selective Carbon Dioxide Reduction. Nat. Catal. 2018, 1, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Dinh, C.-T.; Burdyny, T.; Kibria, M.G.; Seifitokaldani, A.; Gabardo, C.M.; Arquer, F.P.G.d.; Kiani, A.; Edwards, J.P.; Luna, P.D.; Bushuyev, O.S.; et al. CO2 electroreduction to ethylene via hydroxide-mediated copper catalysis at an abrupt interface. Science 2018, 360, 783–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duarte, M.; De Mot, B.; Hereijgers, J.; Breugelmans, T. Electrochemical Reduction of CO2: Effect of Convective CO2 Supply in Gas Diffusion Electrodes. ChemElectroChem 2019, 6, 5596–5602. [Google Scholar] [CrossRef]
- Dufek, E.J.; Lister, T.E.; Stone, S.G.; McIlwain, M.E. Operation of a Pressurized System for Continuous Reduction of CO2. J. Electrochem. Soc. 2012, 159, F514–F517. [Google Scholar] [CrossRef]
- Gabardo, C.M.; Seifitokaldani, A.; Edwards, J.P.; Dinh, C.-T.; Burdyny, T.; Kibria, M.G.; O’Brien, C.P.; Sargent, E.H.; Sinton, D. Combined High Alkalinity and Pressurization Enable Efficient CO2 Electroreduction to CO. Energy Environ. Sci. 2018, 11, 2531–2539. [Google Scholar] [CrossRef]
- Haas, T.; Krause, R.; Weber, R.; Demler, M.; Schmid, G. Technical photosynthesis involving CO2 electrolysis and fermentation. Nat. Catal. 2018, 1, 32–39. [Google Scholar] [CrossRef]
- Huang, Z.; Qi, H.; Zhao, Z.; Shang, L.; Tu, B.; Cheng, M. Efficient CO2 electroreduction on a solid oxide electrolysis cell with La0.6Sr0.4Co0.2Fe0.8O3−δ-Gd0.2Ce0.8O2-δ infiltrated electrode. J. Power Sources 2019, 434, 226730. [Google Scholar] [CrossRef]
- Lee, J.; Lim, J.; Roh, C.-W.; Whang, H.S.; Lee, H. Electrochemical CO2 Reduction Using Alkaline Membrane Electrode Assembly on Various Metal Electrodes. J. CO2 Util. 2019, 31, 244–250. [Google Scholar] [CrossRef]
- Park, S.; Kim, Y.; Han, H.; Chung, Y.S.; Yoon, W.; Choi, J.; Kim, W.B. In Situ Exsolved Co Nanoparticles on Ruddlesden-Popper Material as Highly Active Catalyst for CO2 Electrolysis to CO. Appl. Catal B-Environ. 2019, 248, 147–156. [Google Scholar] [CrossRef]
- Tian, Y.F.; Zhang, L.L.; Jia, L.C.; Wang, X.; Yang, J.; Chi, B.; Pu, J.; Li, J. Novel Quasi-symmetrical Solid Oxide Electrolysis Cells with In-situ Exsolved Cathode for CO2 Electrolysis. J. CO2 Util. 2019, 31, 43–50. [Google Scholar] [CrossRef]
- Park, S.; Kim, Y.; Noh, Y.; Kim, T.; Han, H.; Yoon, W.; Choi, J.; Yi, S.H.; Leec, W.J.; Kim, W.B. A Sulfur-tolerant Cathode Catalyst Fabricated with in Situ Exsolved CoNi Alloy Nanoparticles Anchored on a Ruddlesden-Popper Support for CO2 Electrolysis. J. Mater. Chem. A 2020, 8, 138–148. [Google Scholar] [CrossRef]
- Yin, Z.; Peng, H.; Wei, X.; Zhou, H.; Gong, J.; Huai, M.; Xiao, L.; Wang, G.; Lu, J.; Zhuang, L. An Alkaline Polymer Electrolyte CO2 Electrolyzer Operated with Pure Water. Energy Environ. Sci. 2019, 12, 2455–2462. [Google Scholar] [CrossRef]
- Xiang, H.; Rasul, S.; Scott, K.; Portoles, J.; Cumpson, P.; Yu, E.H. Enhanced Selectivity of Carbonaceous Products from Electrochemical Reduction of CO2 in Aqueous Media. J. CO2 Util. 2019, 30, 214–221. [Google Scholar] [CrossRef]
- Mot, B.D.; Hereijgers, J.; Duarte, M.; Breugelmans, T. Influence of flow and pressure distribution inside a gas diffusion electrode on the performance of a flow-by CO2 electrolyzer. Chem. Eng. J. 2019, 378, 122224. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, B.; Zhong, J.; Cheng, Z. Selective electrochemical CO2 reduction over highly porous gold films. J. Mater. Chem. A 2017, 5, 21955–21964. [Google Scholar] [CrossRef]
- Zhang, F.; Jin, Z.; Chen, C.; Tang, Y.; Mahyoub, S.A.; Yan, S.; Cheng, Z. Electrochemical Conversion of CO2 to CO into a Microchannel Reactor System in the Case of Aqueous Electrolyte. Ind. Eng. Chem. Res. 2020, 59, 5664–5674. [Google Scholar] [CrossRef]
- Kibria, M.G.; Edwards, J.P.; Gabardo, C.M.; Dinh, C.T.; Seifitokaldani, A.; Sinton, D.; Sargent, E.H. Electrochemical CO2 Reduction into Chemical Feedstocks: From Mechanistic Electrocatalysis Models to System Design. Adv. Mater. 2019, 31, 1807166. [Google Scholar] [CrossRef]
- Tan, J.; Lu, Y.C.; Xu, J.H.; Luo, G.S. Mass transfer characteristic in the formation stage of gas–liquid segmented flow in microchannel. Chem. Eng. J. 2012, 185–186, 314–320. [Google Scholar] [CrossRef]
- Yue, J.; Chen, G.; Yuan, Q.; Luo, L.; Gonthier, Y. Hydrodynamics and mass transfer characteristics in gas–liquid flow through a rectangular microchannel. Chem. Eng. Sci. 2007, 62, 2096–2108. [Google Scholar] [CrossRef]
- Yue, J.; Luo, L.; Gonthier, Y.; Chen, G.; Yuan, Q. An experimental study of air–water Taylor flow and mass transfer inside square microchannels. Chem. Eng. Sci. 2009, 64, 3697–3708. [Google Scholar] [CrossRef]
- Chen, J.-F.; Chen, G.-Z.; Wang, J.-X.; Shao, L.; Li, P.-F. High-throughput microporous tube-in-tube microreactor as novel gas-liquid contactor: Mass transfer study. AIChE J. 2011, 57, 239–249. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, C.; Tang, Y.; Cheng, Z. CO2 reduction in a microchannel electrochemical reactor with gas-liquid segmented flow. Chem. Eng. J. 2020, 392, 124798. [Google Scholar] [CrossRef]
- Naqiuddin, N.H.; Saw, L.H.; Yew, M.C.; Yusof, F.; Ng, T.C.; Yew, M.K. Overview of micro-channel design for high heat flux application. Renew. Sust. Energy Rev. 2018, 82, 901–914. [Google Scholar] [CrossRef]
- Kamitani, A.; Morishita, S.; Kotaki, H.; Arscott, S. Improved Fuel Use Efficiency in Microchannel Direct Methanol Fuel Cells Using a Hydrophilic Macroporous Layer. J. Power Sources 2009, 187, 148–155. [Google Scholar] [CrossRef]
- Wu, G.; Constantinou, A.; Cao, E.; Kuhn, S.; Morad, M.; Sankar, M.; Bethell, D.; Hutchings, G.J.; Gavriilidis, A. Continuous Heterogeneously Catalyzed Oxidation of Benzyl Alcohol Using a Tube-in-Tube Membrane Microreactor. Ind. Eng. Chem. Res. 2015, 54, 4183–4189. [Google Scholar] [CrossRef] [Green Version]
- Miao, Y.; Siri-Nguan, N.; Sornchamni, T.; Jovanovic, G.N.; Yokochi, A.F. CO2 Reduction in Wet Ionic Liquid Solution in Microscale-based Electrochemical Reactor. Chem. Eng. J. 2018, 333, 300–309. [Google Scholar] [CrossRef]
- Zong, X.; Zhang, J.; Zhang, J.; Luo, W.; Züttel, A.; Xiong, Y. Synergistic Cu/CeO2 carbon nanofiber catalysts for efficient CO2 electroreduction. Electrochem. Commun. 2020, 114, 106716. [Google Scholar] [CrossRef]
- Zhang, J.; Luo, W.; Züttel, A. Self-supported Copper-based Gas Diffusion Electrodes for CO2 Electrochemical Reduction. J. Mater. Chem. A 2019, 7, 26285–26292. [Google Scholar] [CrossRef]
- Krause, R.; Reinisch, D.; Reller, C.; Eckert, H.; Hartmann, D.; Taroata, D.; Wiesner-Fleischer, K.; Bulan, A.; Lueken, A.; Schmid, G. Industrial Application Aspects of the Electrochemical Reduction of CO2 to CO in Aqueous Electrolyte. Chem. Ing. Tech. 2020, 92, 53–61. [Google Scholar] [CrossRef]
- Reyes, A.; Jansonius, R.P.; Mowbray, B.A.W.; Cao, Y.; Wheeler, D.G.; Chau, J.; Dvorak, D.J.; Berlinguette, C.P. Managing Hydration at the Cathode Enables Efficient CO2 Electrolysis at Commercially Relevant Current Densities. ACS Energy Lett. 2020, 5, 1612–1618. [Google Scholar] [CrossRef]
- Li, Y.C.; Lee, G.; Yuan, T.; Wang, Y.; Nam, D.-H.; Wang, Z.; García de Arquer, F.P.; Lum, Y.; Dinh, C.-T.; Voznyy, O.; et al. CO2 Electroreduction from Carbonate Electrolyte. ACS Energy Lett. 2019, 4, 1427–1431. [Google Scholar] [CrossRef]
- Larrazabal, G.O.; Strom-Hansen, P.; Heli, J.P.; Zeiter, K.; Therkildsen, K.T.; Chorkendorff, I.; Seger, B. Analysis of Mass Flows and Membrane Cross-over in CO2 Reduction at High Current Densities in an MEA-Type Electrolyzer. ACS Appl. Mater. Interfaces 2019, 11, 41281–41288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henry, W. III. Experiments on the Quantity of Gases Absorbed by Water, at Different Temperatures, and under Different Pressures. Philos. Trans. R. Soc. London 1803, 93, 14. [Google Scholar]
- Kohjiro Hara, A.K. Tadayoshi Sakata Electrochemical Reduction of Carbon Dioxide under High Pressure on Various Electrodes in an Aqueous Electrolyte. J. Electroanal. Chem. 1995, 391, 141–147. [Google Scholar] [CrossRef]
- Makoto Todoroki, K.H. Akihiko Kudo, Tadayoshi Sakata Electrochemical Reduction of High Pressure CO2 at Pb, Hg and In Electrodes in an Aqueous KHCO3 Solution. J. Electroanal. Chem. 1995, 394, 199–203. [Google Scholar] [CrossRef]
- Kohjiro Hara, T.S. Large Current Density CO2 Reduction under High Pressure Using Gas Diffusion Electrodes. Bull. Chem. Soc. Jpn. 1997, 70, 571–576. [Google Scholar] [CrossRef]
- Lin, J.; Yan, S.; Zhang, C.; Hu, Q.; Cheng, Z. Hydrophobic Electrode Design for CO2 Electroreduction in a Microchannel Reactor. ACS Appl. Mater. Interfaces 2022, 14, 8623–8632. [Google Scholar] [CrossRef]
- Yan, S.; Mahyoub, S.A.; Lin, J.; Zhang, C.; Hu, Q.; Zhong, J.; Chen, C.; Zhang, F.; Cheng, Z. Controllable Growth of Branched Silver Crystals over a Rod of the Same Material as an Efficient Electrode in CO2 Reduction at High Current Densities. J. Catal. 2022, 405, 224–235. [Google Scholar] [CrossRef]
- Mahyoub, S.A.; Qaraah, F.A.; Yan, S.; Hezam, A.; Zhong, J.; Cheng, Z. Rational design of low loading Pd-alloyed Ag nanocorals for high current density CO2-to-CO electroreduction at elevated pressure. Mater. Today Energy 2022, 24, 100923. [Google Scholar] [CrossRef]
- Fan, M.; Prabhudev, S.; Garbarino, S.; Qiao, J.; Botton, G.A.; Harrington, D.A.; Tavares, A.C.; Guay, D. Uncovering the nature of electroactive sites in nano architectured dendritic Bi for highly efficient CO2 electroreduction to formate. Appl. Catal. B 2020, 274, 119031. [Google Scholar] [CrossRef]
- Burdyny, T.; Smith, W.A. CO2 Reduction on Gas-diffusion Electrodes and Why Catalytic Performance must be assessed at Commercially-relevant Conditions. Energy Environ. Sci. 2019, 12, 1442–1453. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.C.; Zhou, D.; Yan, Z.; Gonçalves, R.H.; Salvatore, D.A.; Berlinguette, C.P.; Mallouk, T.E. Electrolysis of CO2 to Syngas in Bipolar Membrane-Based Electrochemical Cells. ACS Energy Lett. 2016, 1, 1149–1153. [Google Scholar] [CrossRef]
- Hori, Y.; Konishi, H.; Futamura, T.; Murata, A.; Koga, O.; Sakurai, H.; Oguma, K. “Deactivation of Copper Electrode” in Electrochemical Reduction of CO2. Electrochim. Acta 2005, 50, 5354–5369. [Google Scholar] [CrossRef]
- Wuttig, A.; Surendranath, Y. Impurity Ion Complexation Enhances Carbon Dioxide Reduction Catalysis. ACS Catal. 2015, 5, 4479–4484. [Google Scholar] [CrossRef] [Green Version]
- Jeanty, P.; Scherer, C.; Magori, E.; Wiesner-Fleischer, K.; Hinrichsen, O.; Fleischer, M. Upscaling and continuous operation of electrochemical CO2 to CO conversion in aqueous solutions on silver gas diffusion electrodes. J. CO2 Util. 2018, 24, 454–462. [Google Scholar] [CrossRef]
- Lv, K.L.; Teng, C.; Shi, M.H.; Yuan, Y.; Zhu, Y.; Wang, J.R.; Kong, Z.; Lu, X.Y.; Zhu, Y. Hydrophobic and Electronic Properties of the E-MoS2 Nanosheets Induced by FAS for the CO2 Electroreduction to Syngas with a Wide Range of CO/H2 Ratios. Adv. Funct. Mater. 2018, 28, 1802339. [Google Scholar] [CrossRef]
- Xing, Z.; Hu, L.; Ripatti, D.S.; Hu, X.; Feng, X.F. Enhancing carbon dioxide gas-diffusion electrolysis by creating a hydrophobic catalyst microenvironment. Nat. Commun. 2021, 12, 136. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.Z.; Gao, F.Y.; Zhang, X.L.; Yang, P.P.; Liu, R.; Chi, L.P.; Wu, Z.Z.; Qin, S.; Yu, X.X.; Gao, M.R. Hierarchical Copper with Inherent Hydrophobicity Mitigates Electrode Flooding for High-Rate CO2 Electroreduction to Multicarbon Products. J. Am. Chem. Soc. 2021, 143, 8011–8021. [Google Scholar] [CrossRef]
- Liu, S.Q.; Shahini, E.; Gao, M.R.; Gong, L.; Sui, P.F.; Tang, T.; Zeng, H.B.; Luo, J.L. Bi2O3 Nanosheets Grown on Carbon Nanofiber with Inherent Hydrophobicity for High-Performance CO2 Electroreduction in a Wide Potential Window. ACS Nano 2021, 15, 17757–17768. [Google Scholar] [CrossRef]
- Vedharathinam, V.; Qi, Z.; Horwood, C.; Bourcier, B.; Stadermann, M.; Biener, J.; Biener, M. Using a 3D Porous Flow-Through Electrode Geometry for High-Rate Electrochemical Reduction of CO2 to CO in Ionic Liquid. ACS Catal. 2019, 9, 10605–10611. [Google Scholar] [CrossRef]
- Saeki, T.; Hashimoto, K.; Kimura, N.; Omata, K.; Fujishima, A. Electrochemical reduction of CO2 with high current density in a CO2 + methanol medium II. CO formation promoted by tetrabutylammonium cation. J. Electroanal. Chem. 1995, 390, 77–82. [Google Scholar] [CrossRef]
- Verma, S.; Lu, X.; Ma, S.C.; Masel, R.I.; Kenis, P.J.A. The Effect of Electrolyte Composition on the Electroreduction of CO2 to CO on Ag Based Gas Diffusion Electrodes. Phys. Chem. Chem. Phys. 2016, 18, 7075–7084. [Google Scholar] [CrossRef] [PubMed]
- Nitopi, S.; Bertheussen, E.; Scott, S.B.; Liu, X.; Engstfeld, A.K.; Horch, S.; Seger, B.; Stephens, I.E.L.; Chan, K.; Hahn, C.; et al. Progress and Perspectives of Electrochemical CO2 Reduction on Copper in Aqueous Electrolyte. Chem. Rev. 2019, 119, 7610–7672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gattrell, M.; Gupta, N.; Co, A. A review of the aqueous electrochemical reduction of CO2 to hydrocarbons at copper. J. Electroanal. Chem. 2006, 594, 1–19. [Google Scholar] [CrossRef]
- Gupta, N.; Gattrell, M.; MacDougall, B. Calculation for the cathode surface concentrations in the electrochemical reduction of CO2 in KHCO3 solutions. J. Appl. Electrochem. 2005, 36, 161–172. [Google Scholar] [CrossRef] [Green Version]
- Pinsent, B.R.W.; Pearson, L.; Roughto, F.J.W. The kinetics of combination of carbon dioxide with hydroxide ions. Trans. Faraday Soc. 1956, 52, 1512–1520. [Google Scholar] [CrossRef]
- Varela, A.S.; Kroschel, M.; Reier, T.; Strasser, P. Controlling the Selectivity of CO2 Electroreduction on Copper: The Effect of the Electrolyte Concentration and the Importance of the Local pH. Catal. Today 2016, 260, 8–13. [Google Scholar] [CrossRef]
- Thorson, M.R.; Siil, K.I.; Kenis, P.J.A. Effect of Cations on the Electrochemical Conversion of CO2 to CO. J. Electrochem. Soc. 2013, 160, F69–F74. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.E.; Li, F.W.; Ozden, A.; Rasouli, A.S.; de Arquer, F.P.G.; Liu, S.J.; Zhang, S.Z.; Luo, M.C.; Wang, X.; Lum, Y.W.; et al. CO2 Electrolysis to Multicarbon Products in Strong Acid. Science 2021, 372, 1074–1078. [Google Scholar] [CrossRef]
- Edwards, J.P.; Xu, Y.; Gabardo, C.M.; Dinh, C.-T.; Li, J.; Qi, Z.; Ozden, A.; Sargent, E.H.; Sinton, D. Efficient electrocatalytic conversion of carbon dioxide in a low-resistance pressurized alkaline electrolyzer. Appl. Energy 2020, 261, 114305. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).