Exploration of the Potential Targets and Molecular Mechanism of Carthamus tinctorius L. for Liver Fibrosis Based on Network Pharmacology and Molecular Docking Strategy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Prediction of the Bioactive Components and Treatment Targets of HH
2.2. Collection of the Targets of Liver Fibrosis
2.3. The Construction of the Protein–Protein Interactive (PPI) Network
2.4. The analysis of Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathway Enrichment for Major Targets
2.5. The Identifition of Key Componets and Targets of HH in the Treatment of Liver Fibrosis
2.6. The Validation by Molecular Docking Experiments
3. Results
3.1. The Bioactive Components and Treating Targets of HH in the Treatment of Liver Fibrosis
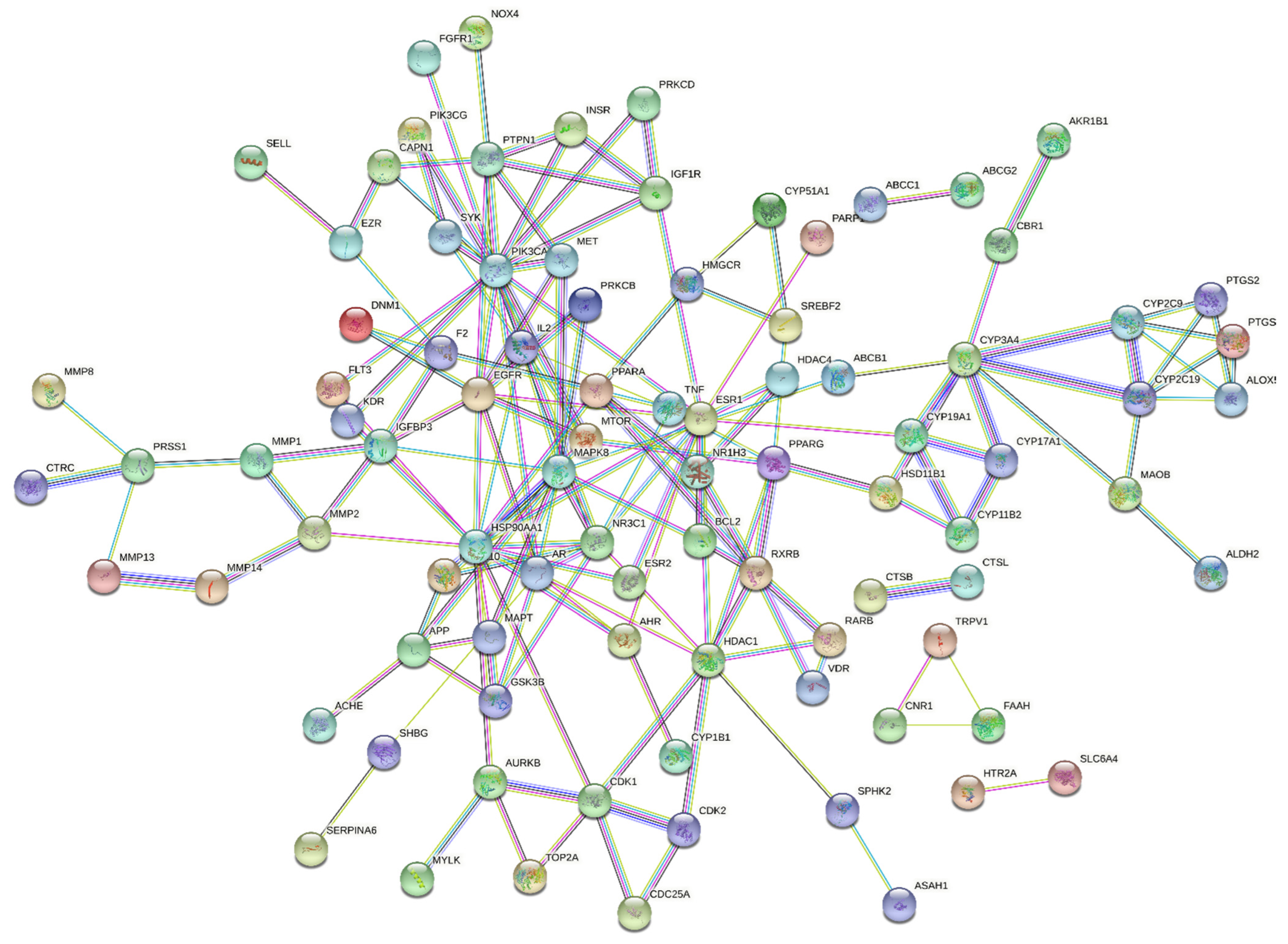
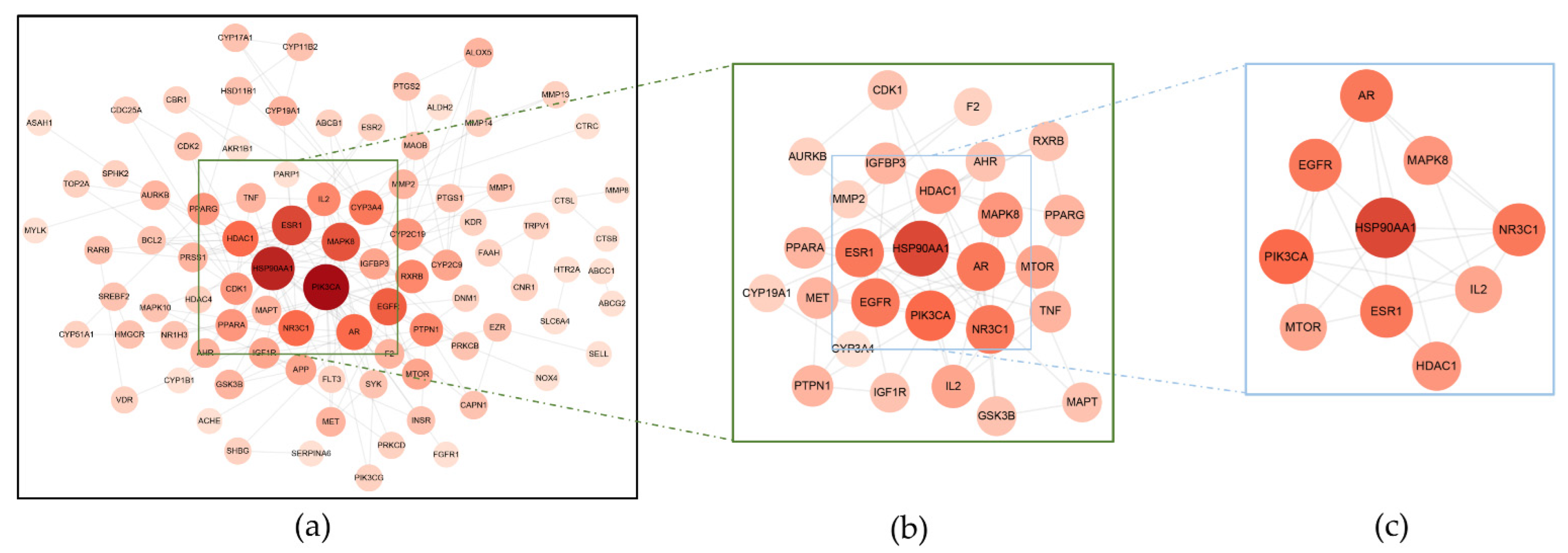
3.2. The PPI Network Constructed by STRING
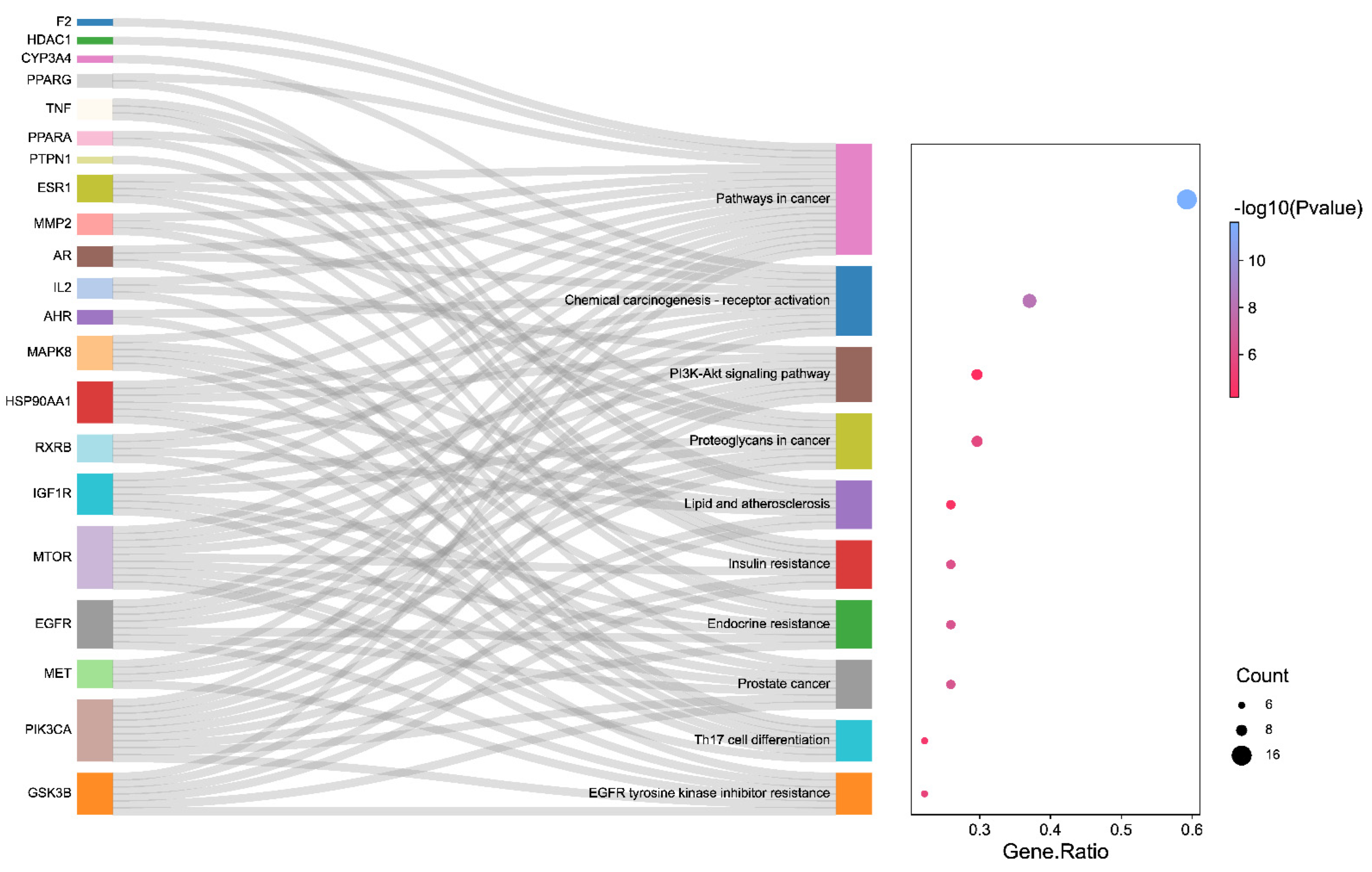
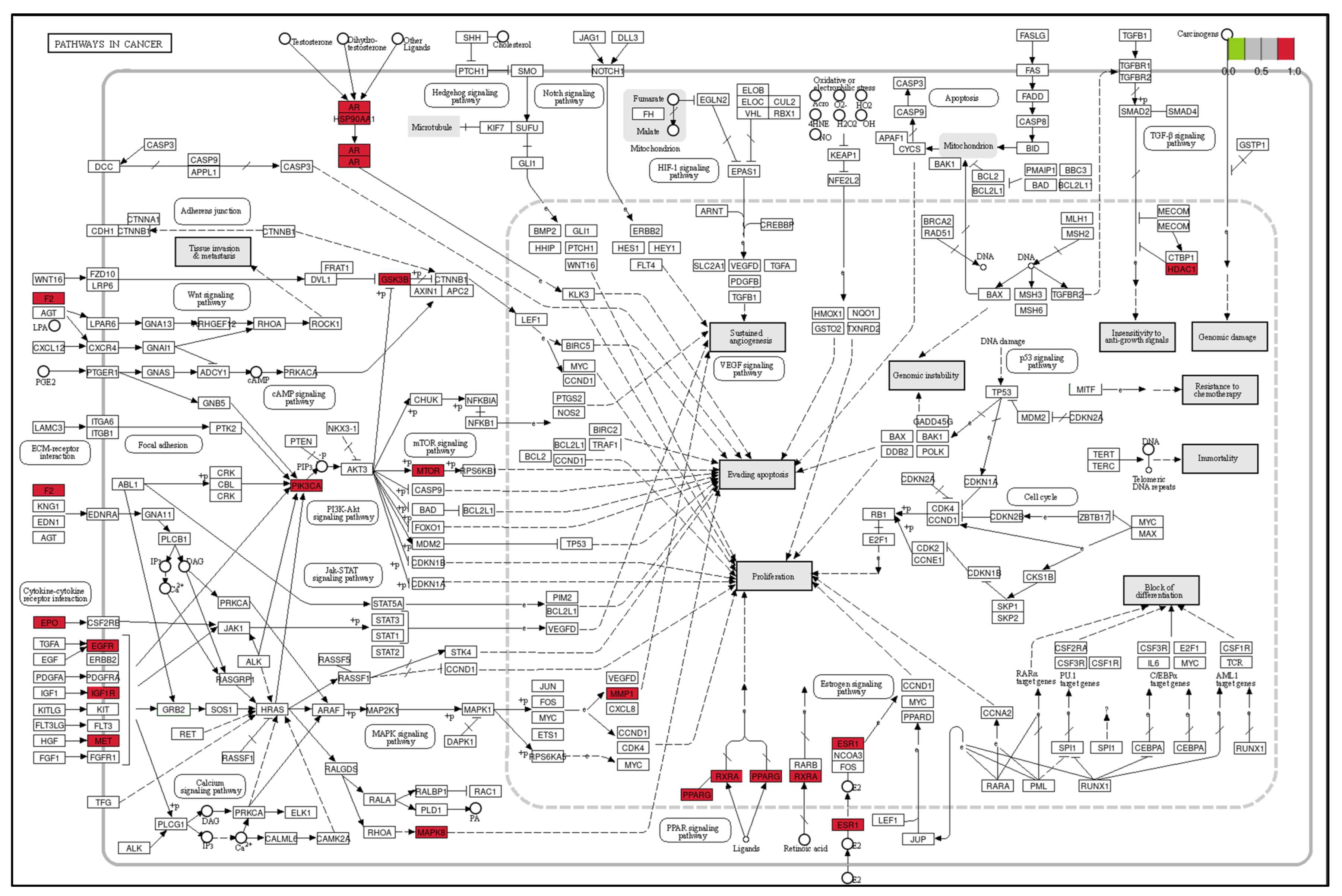
3.3. The Results of GO and KEGG Pathway Enrichment Analysis
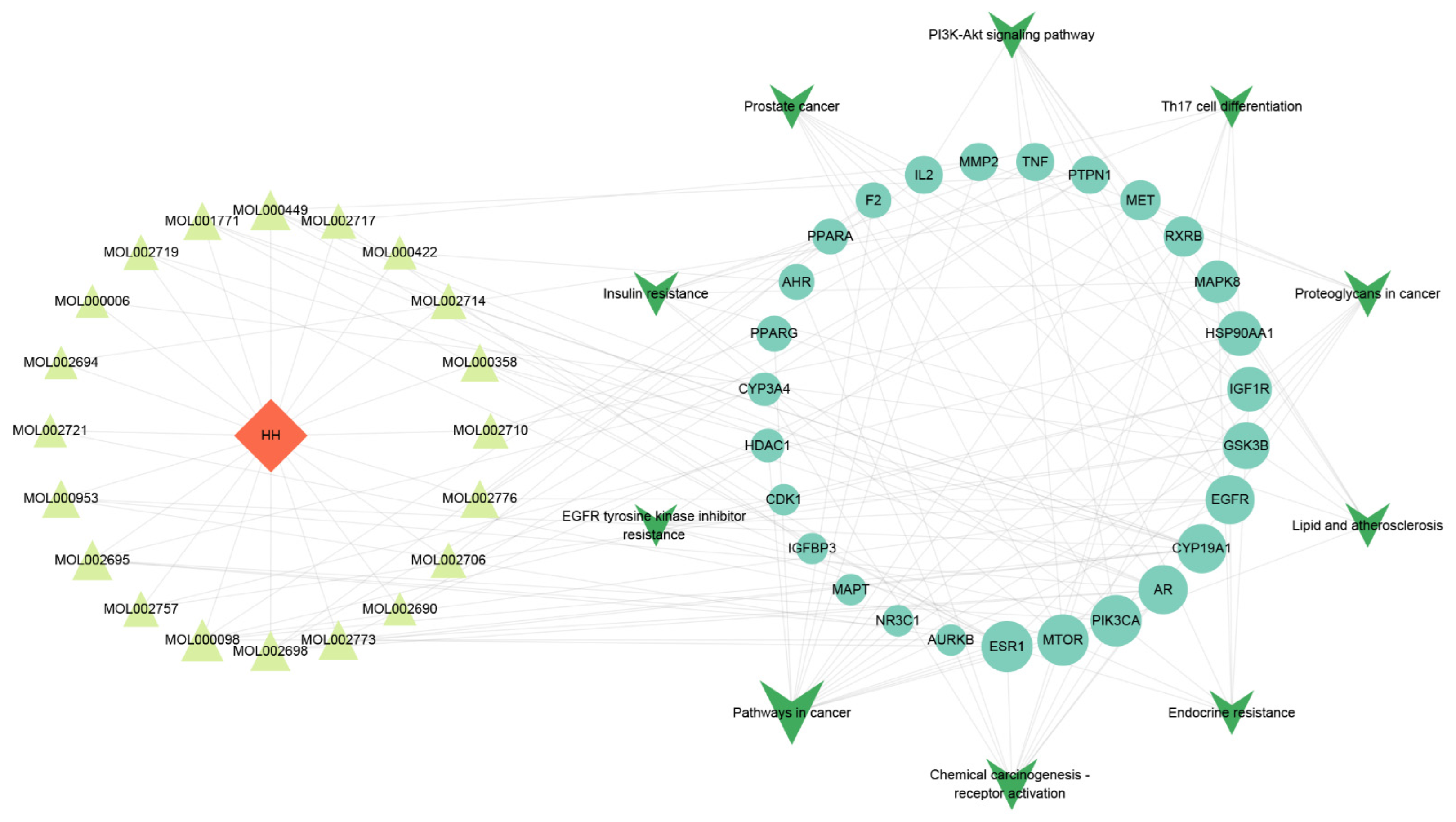
3.4. The Analysis Results of Drug–Component–Major Target–Pathway Interactive Network
3.5. Molecular Docking Experiments Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tan, Z.; Sun, H.; Xue, T.; Gan, C.; Liu, H.; Xie, Y.; Yao, Y.; Ye, T. Liver Fibrosis: Therapeutic Targets and Advances in Drug Therapy. Front. Cell Dev. Biol. 2021, 9, 730176. [Google Scholar] [CrossRef] [PubMed]
- Zoubek, M.E.; Trautwein, C.; Strnad, P. Reversal of Liver Fibrosis: From Fiction to Reality. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Vilar-Gomez, E.; Calzadilla-Bertot, L.; Wai-Sun Wong, V.; Castellanos, M.; Aller-de la Fuente, R.; Metwally, M.; Eslam, M.; Gonzalez-Fabian, L.; Alvarez-Quiñones Sanz, M.; Conde-Martin, A.F.; et al. Fibrosis Severity as a Determinant of Cause-Specific Mortality in Patients With Advanced Nonalcoholic Fatty Liver Disease: A Multi-National Cohort Study. Gastroenterology 2018, 155, 443–457.e17. [Google Scholar] [CrossRef]
- Liu, P.; Mao, Y.; Xie, Y.; Wei, J.; Yao, J. Stem Cells for Treatment of Liver Fibrosis/Cirrhosis: Clinical Progress and Therapeutic Potential. Stem Cell Res. Ther. 2022, 13, 356. [Google Scholar] [CrossRef] [PubMed]
- Caligiuri, A.; Gentilini, A.; Pastore, M.; Gitto, S.; Marra, F. Cellular and Molecular Mechanisms Underlying Liver Fibrosis Regression. Cells 2021, 10, 2759. [Google Scholar] [CrossRef]
- Cai, X.; Wang, J.; Wang, J.; Zhou, Q.; Yang, B.; He, Q.; Weng, Q. Intercellular Crosstalk of Hepatic Stellate Cells in Liver Fibrosis: New Insights into Therapy. Pharmacol. Res. 2020, 155, 104720. [Google Scholar] [CrossRef] [PubMed]
- Flores-Contreras, L.; Sandoval-Rodríguez, A.S.; Mena-Enriquez, M.G.; Lucano-Landeros, S.; Arellano-Olivera, I.; Alvarez-Álvarez, A.; Sanchez-Parada, M.G.; Armendáriz-Borunda, J. Treatment with Pirfenidone for Two Years Decreases Fibrosis, Cytokine Levels and Enhances CB2 Gene Expression in Patients with Chronic Hepatitis C. BMC Gastroenterol. 2014, 14, 131. [Google Scholar] [CrossRef] [PubMed]
- Neuschwander-Tetri, B.A.; Loomba, R.; Sanyal, A.J.; Lavine, J.E.; van Natta, M.L.; Abdelmalek, M.F.; Chalasani, N.; Dasarathy, S.; Diehl, A.M.; Hameed, B.; et al. Farnesoid X Nuclear Receptor Ligand Obeticholic Acid for Non-Cirrhotic, Non-Alcoholic Steatohepatitis (FLINT): A Multicentre, Randomised, Placebo-Controlled Trial. Lancet 2015, 385, 956–965. [Google Scholar] [CrossRef]
- Mu, M.; Zuo, S.; Wu, R.-M.; Deng, K.-S.; Lu, S.; Zhu, J.-J.; Zou, G.-L.; Yang, J.; Cheng, M.-L.; Zhao, X.-K. Ferulic Acid Attenuates Liver Fibrosis and Hepatic Stellate Cell Activation via Inhibition of TGF-β/Smad Signaling Pathway. Drug Des. Dev. Ther. 2018, 12, 4107–4115. [Google Scholar] [CrossRef]
- Chen, A.; Zheng, S. Curcumin Inhibits Connective Tissue Growth Factor Gene Expression in Activated Hepatic Stellate Cells in Vitro by Blocking NF-KappaB and ERK Signalling. Br. J. Pharmacol. 2008, 153, 557–567. [Google Scholar] [CrossRef] [Green Version]
- Nan, Y.; Su, H.; Lian, X.; Wu, J.; Liu, S.; Chen, P.; Liu, S. Pathogenesis of Liver Fibrosis and Its TCM Therapeutic Perspectives. Evid. Based Complement. Altern. Med. 2022, 2022, 5325431. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Guo, C.; Peng, C.; Li, Y. Advances of Natural Activators for Nrf2 Signaling Pathway on Cholestatic Liver Injury Protection: A Review. Eur. J. Pharmacol. 2021, 910, 174447. [Google Scholar] [CrossRef]
- Wu, X.; Cai, X.; Ai, J.; Zhang, C.; Liu, N.; Gao, W. Extraction, Structures, Bioactivities and Structure-Function Analysis of the Polysaccharides From Safflower (Carthamus tinctorius L.). Front. Pharmacol. 2021, 12, 767947. [Google Scholar] [CrossRef] [PubMed]
- Waki, T.; Terashita, M.; Fujita, N.; Fukuda, K.; Kato, M.; Negishi, T.; Uchida, H.; Aoki, Y.; Takahashi, S.; Nakayama, T. Identification of the Genes Coding for Carthamin Synthase, Peroxidase Homologs That Catalyze the Final Enzymatic Step of Red Pigmentation in Safflower (Carthamus tinctorius L.). Plant Cell Physiol. 2021, 62, 1528–1541. [Google Scholar] [CrossRef]
- Hong, H.; Lim, J.M.; Kothari, D.; Kwon, S.H.; Kwon, H.C.; Han, S.-G.; Kim, S.-K. Antioxidant Properties and Diet-Related α-Glucosidase and Lipase Inhibitory Activities of Yogurt Supplemented with Safflower (Carthamus tinctorius L.) Petal Extract. Food Sci. Anim. Resour. 2021, 41, 122–134. [Google Scholar] [CrossRef]
- Xi, S.; Yue, L.; Shi, M.; Peng, Y.; Xu, Y.; Wang, X.; Li, Q.; Kang, Z.; Li, H.; Wang, Y. The Effects of Taoren-Honghua Herb Pair on Pathological Microvessel and Angiogenesis-Associated Signaling Pathway in Mice Model of CCl4-Induced Chronic Liver Disease. Evid. Based Complement. Altern. Med. 2016, 2016, 2974256. [Google Scholar] [CrossRef]
- Zhang, Y.-B.; Dong, H.-Y.; Zhao, X.-M.; Fan, L.; Zou, Y.; Zhang, C.; Li, G.; Liu, J.-C.; Niu, Y.-C. Hydroxysafflor Yellow A Attenuates Carbon Tetrachloride-Induced Hepatic Fibrosis in Rats by Inhibiting ERK5 Signaling. Am. J. Chin. Med. 2012, 40, 481–494. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, C.Y.; Liu, Z.; Ma, X.S.; He, Y.H.; Chen, S.S.; Bai, X.Y. Hydroxysafflor Yellow A Suppresses Liver Fibrosis Induced by Carbon Tetrachloride with High-Fat Diet by Regulating PPAR-γ/P38 MAPK Signaling. Pharm. Biol. 2014, 52, 1085–1093. [Google Scholar] [CrossRef]
- Tao, Y.; Tian, K.; Chen, J.; Tan, D.; Liu, Y.; Xiong, Y.; Chen, Z.; Tian, Y. Network Pharmacology-Based Prediction of the Active Compounds, Potential Targets, and Signaling Pathways Involved in Danshiliuhao Granule for Treatment of Liver Fibrosis. Evid. Based Complement. Altern. Med. 2019, 2019, 2630357. [Google Scholar] [CrossRef]
- Fan, C.; Wu, F.R.; Zhang, J.F.; Jiang, H. A Network Pharmacology Approach to Explore the Mechanisms of Shugan Jianpi Formula in Liver Fibrosis. Evid Based Complement. Alternat. Med. 2020, 2020, 4780383. [Google Scholar] [CrossRef]
- Williams, J.; Siramshetty, V.; Nguyễn, Ð.-T.; Padilha, E.C.; Kabir, M.; Yu, K.-R.; Wang, A.Q.; Zhao, T.; Itkin, M.; Shinn, P.; et al. Using in Vitro ADME Data for Lead Compound Selection: An Emphasis on PAMPA PH 5 Permeability and Oral Bioavailability. Bioorg. Med. Chem. 2022, 56, 116588. [Google Scholar] [CrossRef] [PubMed]
- Bitew, M.; Desalegn, T.; Demissie, T.B.; Belayneh, A.; Endale, M.; Eswaramoorthy, R. Pharmacokinetics and Drug-Likeness of Antidiabetic Flavonoids: Molecular Docking and DFT Study. PLoS ONE 2021, 16, e0260853. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated Data and New Features for Efficient Prediction of Protein Targets of Small Molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Morris, J.H.; Cook, H.; Kuhn, M.; Wyder, S.; Simonovic, M.; Santos, A.; Doncheva, N.T.; Roth, A.; Bork, P.; et al. The STRING Database in 2017: Quality-Controlled Protein-Protein Association Networks, Made Broadly Accessible. Nucleic Acids Res. 2017, 45, D362–D368. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING V11: Protein-Protein Association Networks with Increased Coverage, Supporting Functional Discovery in Genome-Wide Experimental Datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef]
- Liu, J.; Liu, J.; Tong, X.; Peng, W.; Wei, S.; Sun, T.; Wang, Y.; Zhang, B.; Li, W. Network Pharmacology Prediction and Molecular Docking-Based Strategy to Discover the Potential Pharmacological Mechanism of Huai Hua San Against Ulcerative Colitis. Drug Des. Dev. Ther. 2021, 15, 3255–3276. [Google Scholar] [CrossRef] [PubMed]
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of Liver Diseases in the World. J. Hepatol. 2019, 70, 151–171. [Google Scholar] [CrossRef]
- Kisseleva, T.; Brenner, D. Molecular and Cellular Mechanisms of Liver Fibrosis and Its Regression. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 151–166. [Google Scholar] [CrossRef]
- Andres, S.; Pevny, S.; Ziegenhagen, R.; Bakhiya, N.; Schäfer, B.; Hirsch-Ernst, K.I.; Lampen, A. Safety Aspects of the Use of Quercetin as a Dietary Supplement. Mol. Nutr. Food Res. 2018, 62, 1700447. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhang, H.; Wang, Y.; Song, F.; Yuan, Y. Inhibitory Effects of Quercetin on the Progression of Liver Fibrosis through the Regulation of NF-KB/IkBα, P38 MAPK, and Bcl-2/Bax Signaling. Int. Immunopharmacol. 2017, 47, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Feng, J.; Lu, X.; Yao, Z.; Liu, Q.; Lv, Y.; Han, Y.; Deng, J.; Zhou, Y. Isorhamnetin Inhibits Liver Fibrosis by Reducing Autophagy and Inhibiting Extracellular Matrix Formation via the TGF-Β1/Smad3 and TGF-Β1/P38 MAPK Pathways. Mediat. Inflamm. 2019, 2019, 6175091. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, J.; Deng, Y.; Liao, L.; Zhou, M.; Peng, C.; Li, Y. Quercetin as a Protective Agent for Liver Diseases: A Comprehensive Descriptive Review of the Molecular Mechanism. Phytother. Res. 2021, 35, 4727–4747. [Google Scholar] [CrossRef]
- Bohn, T.; Desmarchelier, C.; El, S.N.; Keijer, J.; van Schothorst, E.; Rühl, R.; Borel, P. β-Carotene in the Human Body: Metabolic Bioactivation Pathways—From Digestion to Tissue Distribution and Excretion. Proc. Nutr. Soc. 2019, 78, 68–87. [Google Scholar] [CrossRef]
- Marcelino, G.; Machate, D.J.; Freitas, K.d.C.; Hiane, P.A.; Maldonade, I.R.; Pott, A.; Asato, M.A.; Candido, C.J.; Guimarães, R. de C.A. β-Carotene: Preventive Role for Type 2 Diabetes Mellitus and Obesity: A Review. Molecules 2020, 25, 5803. [Google Scholar] [CrossRef]
- Liu, X.; Shen, H.; Chen, M.; Shao, J. Clinical Relevance of Vitamins and Carotenoids With Liver Steatosis and Fibrosis Detected by Transient Elastography in Adults. Front. Nutr. 2021, 8, 760985. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ding, C.; Zeng, F.; Zhu, H. Low Levels of Serum β-Carotene and β-Carotene/Retinol Ratio Are Associated with Histological Severity in Nonalcoholic Fatty Liver Disease Patients. Ann. Nutr. Metab. 2019, 74, 156–164. [Google Scholar] [CrossRef]
- El-Baz, F.K.; Salama, A.; Ali, S.I.; Elgohary, R. Haematococcus Pluvialis Carotenoids Enrich Fractions Ameliorate Liver Fibrosis Induced by Thioacetamide in Rats: Modulation of Metalloproteinase and Its Inhibitor. BioMed Res. Int. 2021, 2021, 6631415. [Google Scholar] [CrossRef]
- Latief, U.; Ahmad, R. β-Carotene Inhibits NF-ΚB and Restrains Diethylnitrosamine-Induced Hepatic Inflammation in Wistar Rats. Int. J. Vitam. Nutr. Res. 2020, 20, 1–10. [Google Scholar] [CrossRef]
- Pan, X.; Ma, X.; Jiang, Y.; Wen, J.; Yang, L.; Chen, D.; Cao, X.; Peng, C. A Comprehensive Review of Natural Products against Liver Fibrosis: Flavonoids, Quinones, Lignans, Phenols, and Acids. Evid Based Complement. Altern. Med. 2020, 2020, 7171498. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, H.; Cao, Y.; Li, Y.; Sun, S.; Zhang, J.; Zhang, G. Schisandrin B Attenuates CCl4-Induced Liver Fibrosis in Rats by Regulation of Nrf2-ARE and TGF-β/Smad Signaling Pathways. Drug Des. Dev. Ther. 2017, 11, 2179–2191. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Ju, B.; Zhang, X.; Zhu, Y.; Nie, Y.; Xu, Y.; Lei, Q. Magnolol Attenuates Concanavalin A-Induced Hepatic Fibrosis, Inhibits CD4+ T Helper 17 (Th17) Cell Differentiation and Suppresses Hepatic Stellate Cell Activation: Blockade of Smad3/Smad4 Signalling. Basic Clin. Pharmacol. Toxicol. 2017, 120, 560–570. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, J.; Dong, H.; Zhao, X.; Zhou, L.; Li, X.; Liu, J.; Niu, Y. Hydroxysafflor Yellow A Protects against Chronic Carbon Tetrachloride-Induced Liver Fibrosis. Eur. J. Pharmacol. 2011, 660, 438–444. [Google Scholar] [CrossRef]
- Li, Y.; Shi, Y.; Sun, Y.; Liu, L.; Bai, X.; Wang, D.; Li, H. Restorative Effects of Hydroxysafflor Yellow A on Hepatic Function in an Experimental Regression Model of Hepatic Fibrosis Induced by Carbon Tetrachloride. Mol. Med. Rep. 2017, 15, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.-H.; Guo, H.; Xie, B. Structural Modifications of Matrine-Type Alkaloids. Mini Rev. Med. Chem. 2018, 18, 730–744. [Google Scholar] [CrossRef] [PubMed]
- Haas, M.J.; Onstead-Haas, L.M.; Szafran-Swietlik, A.; Kojanian, H.; Davis, T.; Armstrong, P.; Wong, N.C.W.; Mooradian, A.D. Induction of Hepatic Apolipoprotein A-I Gene Expression by the Isoflavones Quercetin and Isoquercetrin. Life Sci. 2014, 110, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Gao, C.; Yao, P.; Gong, Z. Quercetin Alleviates High-Fat Diet-Induced Oxidized Low-Density Lipoprotein Accumulation in the Liver: Implication for Autophagy Regulation. BioMed Res. Int. 2015, 2015, 607531. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Shoorei, H.; Khanbabapour Sasi, A.; Taheri, M.; Ayatollahi, S.A. The Impact of the Phytotherapeutic Agent Quercetin on Expression of Genes and Activity of Signaling Pathways. Biomed. Pharmacother. 2021, 141, 111847. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Y.; Pan, Z.; Yang, C.; Chen, L.; Wang, Y.; Xu, D.; Xia, H.; Wang, S.; Chen, S.; et al. Potential “Therapeutic” Effects of Tocotrienol-Rich Fraction (TRF) and Carotene “Against” Bleomycin-Induced Pulmonary Fibrosis in Rats via TGF-β/Smad, PI3K/Akt/MTOR and NF-ΚB Signaling Pathways. Nutrients 2022, 14, 1904. [Google Scholar] [CrossRef]
- Roehlen, N.; Crouchet, E.; Baumert, T.F. Liver Fibrosis: Mechanistic Concepts and Therapeutic Perspectives. Cells 2020, 9, 875. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Wang, D.; Xu, Y.; Dong, R.; Yang, Y.; Lv, Q.; Chen, X.; Zhang, Z. The Upstream Pathway of MTOR-Mediated Autophagy in Liver Diseases. Cells 2019, 8, 1597. [Google Scholar] [CrossRef]
- Reif, S.; Lang, A.; Lindquist, J.N.; Yata, Y.; Gabele, E.; Scanga, A.; Brenner, D.A.; Rippe, R.A. The Role of Focal Adhesion Kinase-Phosphatidylinositol 3-Kinase-Akt Signaling in Hepatic Stellate Cell Proliferation and Type I Collagen Expression. J. Biol. Chem. 2003, 278, 8083–8090. [Google Scholar] [CrossRef]
- Parsons, C.J.; Takashima, M.; Rippe, R.A. Molecular Mechanisms of Hepatic Fibrogenesis. J. Gastroenterol. Hepatol. 2007, 22 (Suppl. S1), S79–S84. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Kim, S.M.; Hur, W.; Kang, B.-Y.; Lee, H.L.; Nam, H.; Yoo, S.H.; Sung, P.S.; Kwon, J.H.; Jang, J.W.; et al. Tenofovir Disoproxil Fumarate Directly Ameliorates Liver Fibrosis by Inducing Hepatic Stellate Cell Apoptosis via Downregulation of PI3K/Akt/MTOR Signaling Pathway. PLoS ONE 2021, 16, e0261067. [Google Scholar] [CrossRef] [PubMed]
- Thoen, L.F.R.; Guimarães, E.L.M.; Dollé, L.; Mannaerts, I.; Najimi, M.; Sokal, E.; van Grunsven, L.A. A Role for Autophagy during Hepatic Stellate Cell Activation. J. Hepatol. 2011, 55, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.-H.; Chen, D.-Q.; Wang, Y.-N.; Feng, Y.-L.; Cao, G.; Vaziri, N.D.; Zhao, Y.-Y. New Insights into TGF-β/Smad Signaling in Tissue Fibrosis. Chem. Biol. Interact. 2018, 292, 76–83. [Google Scholar] [CrossRef]
- Dewidar, B.; Meyer, C.; Dooley, S.; Meindl-Beinker, A.N. TGF-β in Hepatic Stellate Cell Activation and Liver Fibrogenesis-Updated. Cells 2019, 8, 1419. [Google Scholar] [CrossRef]
- Derynck, R.; Budi, E.H. Specificity, Versatility, and Control of TGF-β Family Signaling. Sci. Signal 2019, 12, 570. [Google Scholar] [CrossRef]
- Dong, H.; Liu, Y.; Zou, Y.; Li, C.; Li, L.; Li, X.; Zhao, X.; Zhou, L.; Liu, J.; Niu, Y. Alteration of the ERK5 Pathway by Hydroxysafflor Yellow A Blocks Expression of MEF2C in Activated Hepatic Stellate Cells in Vitro: Potential Treatment for Hepatic Fibrogenesis. Pharm. Biol. 2013, 54, 435–443. [Google Scholar] [CrossRef]
- Fougerat, A.; Montagner, A.; Loiseau, N.; Guillou, H.; Wahli, W. Peroxisome Proliferator-Activated Receptors and Their Novel Ligands as Candidates for the Treatment of Non-Alcoholic Fatty Liver Disease. Cells 2020, 9, 1638. [Google Scholar] [CrossRef]
- Choudhary, N.S.; Kumar, N.; Duseja, A. Peroxisome Proliferator-Activated Receptors and Their Agonists in Nonalcoholic Fatty Liver Disease. J. Clin. Exp. Hepatol. 2019, 9, 731–739. [Google Scholar] [CrossRef] [Green Version]
- Musso, G.; Cassader, M.; Paschetta, E.; Gambino, R. Thiazolidinediones and Advanced Liver Fibrosis in Nonalcoholic Steatohepatitis: A Meta-Analysis. JAMA Intern. Med. 2017, 177, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Yaşar, P.; Ayaz, G.; User, S.D.; Güpür, G.; Muyan, M. Molecular Mechanism of Estrogen-Estrogen Receptor Signaling. Reprod. Med. Biol. 2017, 16, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Klair, J.S.; Yang, J.D.; Abdelmalek, M.F.; Guy, C.D.; Gill, R.M.; Yates, K.; Unalp-Arida, A.; Lavine, J.E.; Clark, J.M.; Diehl, A.M.; et al. A Longer Duration of Estrogen Deficiency Increases Fibrosis Risk among Postmenopausal Women with Nonalcoholic Fatty Liver Disease. Hepatology 2016, 64, 85–91. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, C.-G.; Ji, L.-H.; Zhao, G.; Wu, Z.-Y. Estrogen Receptor β Selective Agonist Ameliorates Liver Cirrhosis in Rats by Inhibiting the Activation and Proliferation of Hepatic Stellate Cells. J. Gastroenterol. Hepatol. 2018, 33, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Teschke, R.; Zhang, L.; Long, H.; Schwarzenboeck, A.; Schmidt-Taenzer, W.; Genthner, A.; Wolff, A.; Frenzel, C.; Schulze, J.; Eickhoff, A. Traditional Chinese Medicine and Herbal Hepatotoxicity: A Tabular Compilation of Reported Cases. Ann. Hepatol. 2015, 14, 7–19. [Google Scholar] [CrossRef]
- Namjoo, A.; Nasri, H.; Talebi-Juneghani, A.; Baradaran, A.; Rafieian-Kopaei, M. Safety Profile of Carthamus Tinctorius L. in Lactation: Brain, Renal and Hepatotoxicity. Pak. J. Med. Sci. 2013, 29, 378–383. [Google Scholar] [CrossRef]
- Mirhoseini, M.; Mohamadpour, M.; Khorsandi, L. Toxic Effects of Carthamus tinctorius L. (Safflower) Extract on Mouse Spermatogenesis. J. Assist. Reprod. Genet. 2012, 29, 457–461. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, R.; Pu, X.; Sun, Y.; Zhao, X. Evaluation of the Sub-Chronic Toxicity of a Standardized Flavonoid Extract of Safflower in Rats. Regul. Toxicol. Pharmacol. 2017, 85, 98–107. [Google Scholar] [CrossRef]
- Lewin, G.; Joel, M.; Peter, B.; Lützow, M. Food Grade Safflower Concentrate: No Evidence for Reproduction and Early Developmental Toxicity. Reprod. Toxicol. 2021, 104, 155–165. [Google Scholar] [CrossRef]
- Fan, X.; Wang, X.; Lian, J.; Pei, Z.; Jiang, M.; Bai, M. Flos Carthami Exerts Hepatoprotective Action in a Rat Model of Alcoholic Liver Injury via Modulating the Metabolomics Profile. Evid. Based Complement. Altern. Med. 2022, 2022, 8158699. [Google Scholar] [CrossRef]





| Components | MOLID | Pubchem CID | Components | MOLID | Pubchem CID |
|---|---|---|---|---|---|
| Poriferast-5-en-3beta-ol | MOL001771 | 457801 | 6-Hydroxynaringenin | MOL002719 | 188308 |
| Flavoxanthin | MOL002680 | 5281238 | Quercetagetin | MOL002721 | 5281680 |
| 4-[(E)-4-(3,5-dimethoxy-4-oxo-1-cyclohexa-2,5-dienylidene)but-2-enylidene]-2,6-dimethoxycyclohexa-2,5-dien-1-one | MOL002694 | 10237057 | 7,8-dimethyl-1H-pyrimido [5,6-g]quinoxaline-2,4-dione | MOL002757 | 21786815 |
| Lignan | MOL002695 | 261166 | Beta-carotene | MOL002773 | 5280489 |
| Lupeol-palmitate | MOL002698 | 162847783 | Baicalin | MOL002776 | 64982 |
| Phytoene | MOL002706 | 5280784 | Beta-sitosterol | MOL000358 | 222284 |
| Phytofluene | MOL002707 | 6436722 | Kaempferol | MOL000422 | 5280863 |
| Pyrethrin II | MOL002710 | 5281555 | Stigmasterol | MOL000449 | 5280794 |
| 6-Hydroxykaempferol | MOL002712 | 5281638 | Luteolin | MOL000006 | 5280445 |
| Baicalein | MOL002714 | 5281605 | CLR | MOL000953 | 5997 |
| Qt_carthamone | MOL002717 | 131833009 | Quercetin | MOL000098 | 5280343 |
| Hydroxysafflor Yellow A | MOL002690 | 6443665 |
| Target Gene | Uniprot ID | Degree | Target Gene | Uniprot ID | Degree |
|---|---|---|---|---|---|
| PIK3CA | P42336 | 17 | PPARG | P37231 | 6 |
| HSP90AA1 | P07900 | 15 | IGFBP3 | P17936 | 5 |
| ESR1 | P03372 | 12 | MTOR | P42345 | 5 |
| MAPK8 | P45983 | 11 | IGF1R | P08069 | 5 |
| EGFR | P00533 | 10 | AHR | P35869 | 4 |
| AR | P10275 | 9 | GSK3B | P49841 | 4 |
| NR3C1 | P04150 | 9 | MAPT | P10636 | 4 |
| HDAC1 | Q13547 | 9 | AURKB | Q96GD4 | 4 |
| CYP3A4 | P08684 | 8 | CYP19A1 | P11511 | 4 |
| RXRB | P28702 | 7 | F2 | P00734 | 4 |
| PTPN1 | P18031 | 7 | MET | P08581 | 4 |
| IL2 | P60568 | 7 | MMP2 | P08253 | 4 |
| CDK1 | P06493 | 6 | TNF | P01375 | 4 |
| PPARA | Q07869 | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Lan, Y.; Ran, Q.; Gan, Q.; Tang, S.; Huang, W. Exploration of the Potential Targets and Molecular Mechanism of Carthamus tinctorius L. for Liver Fibrosis Based on Network Pharmacology and Molecular Docking Strategy. Processes 2022, 10, 1735. https://doi.org/10.3390/pr10091735
Hu Y, Lan Y, Ran Q, Gan Q, Tang S, Huang W. Exploration of the Potential Targets and Molecular Mechanism of Carthamus tinctorius L. for Liver Fibrosis Based on Network Pharmacology and Molecular Docking Strategy. Processes. 2022; 10(9):1735. https://doi.org/10.3390/pr10091735
Chicago/Turabian StyleHu, Yu, Yunxi Lan, Qiqi Ran, Qianrong Gan, Songqi Tang, and Wei Huang. 2022. "Exploration of the Potential Targets and Molecular Mechanism of Carthamus tinctorius L. for Liver Fibrosis Based on Network Pharmacology and Molecular Docking Strategy" Processes 10, no. 9: 1735. https://doi.org/10.3390/pr10091735






