Thermal Stability for the Continuous Production of γ-Valerolactone from the Hydrogenation of N-Butyl Levulinate in a CSTR
Abstract
1. Introduction
2. Materials and Methods
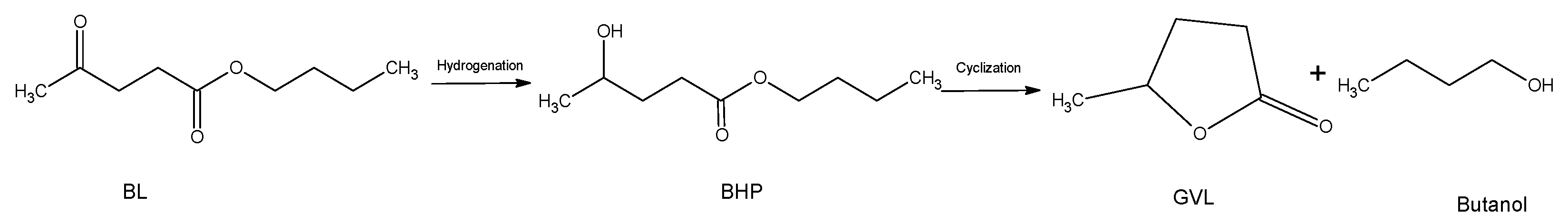
2.1. Kinetics
2.2. Mass and Energy Balances
2.3. Operating Conditions
2.4. Thermal Stability Criterion
2.5. Simulation and Parametric Sensitivity
3. Results and Discussion
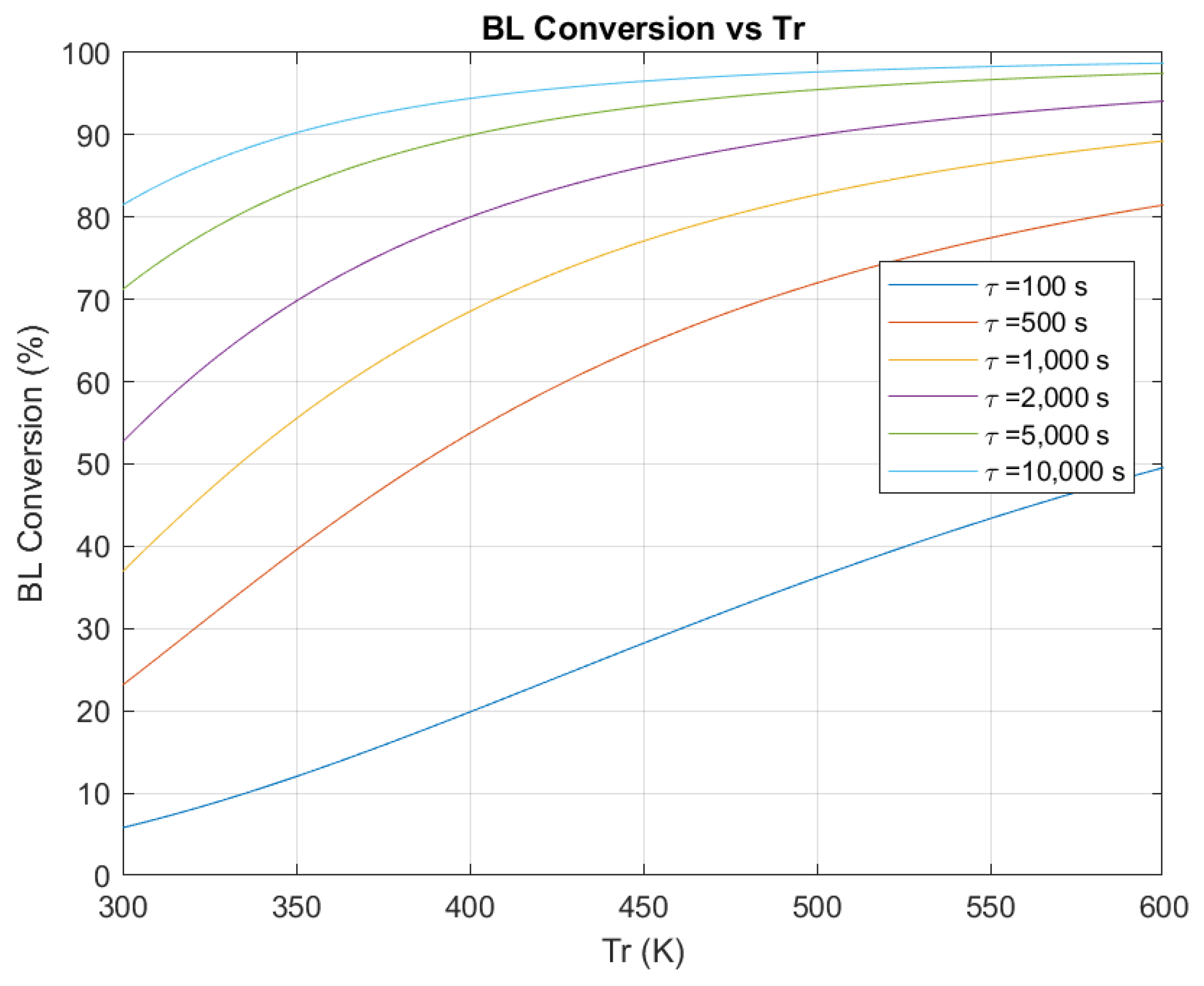
3.1. Effect of Space-Time on Conversion
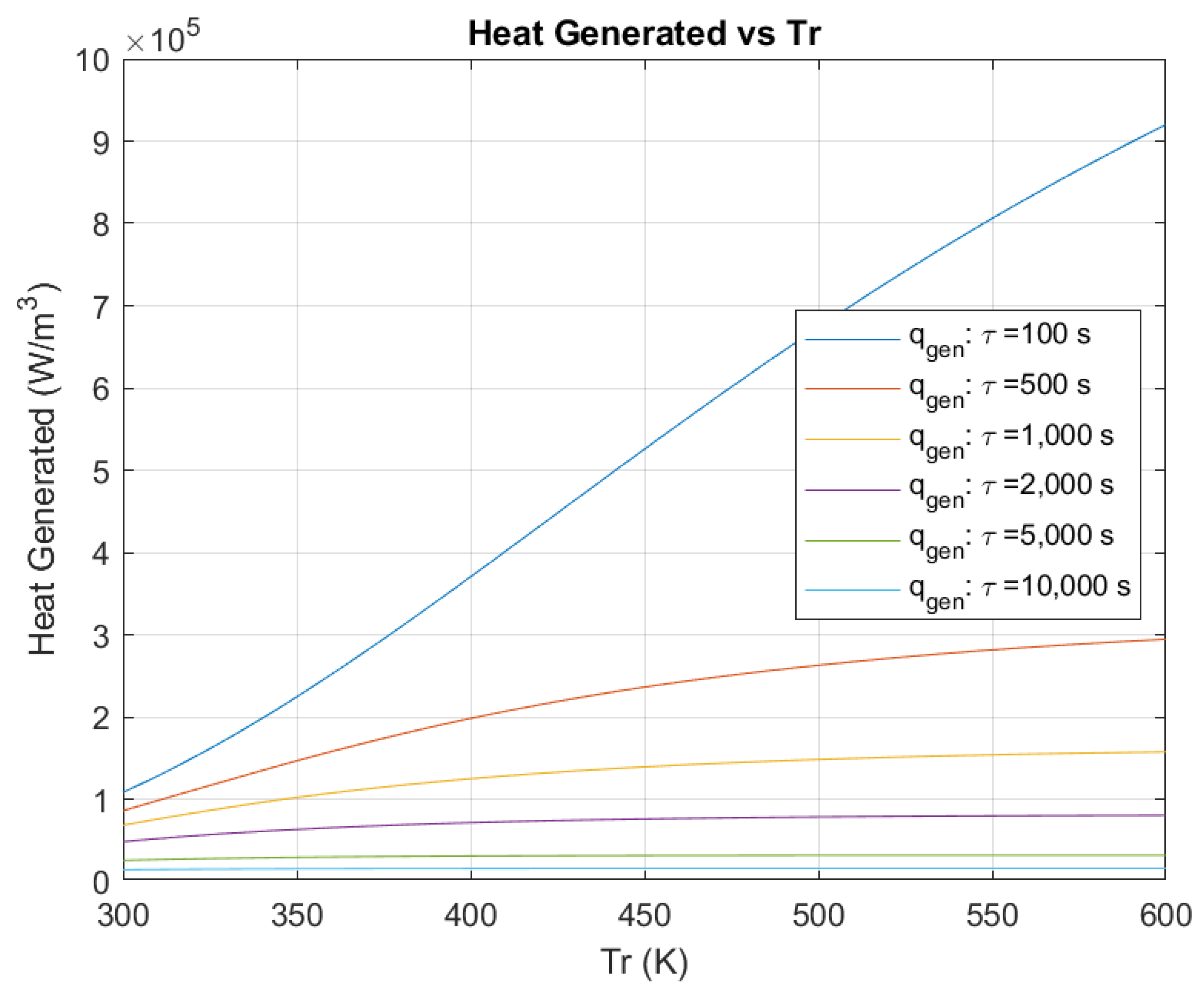
3.2. Comparison of Heat Flow Rate Exchange Due to Chemical Reactions
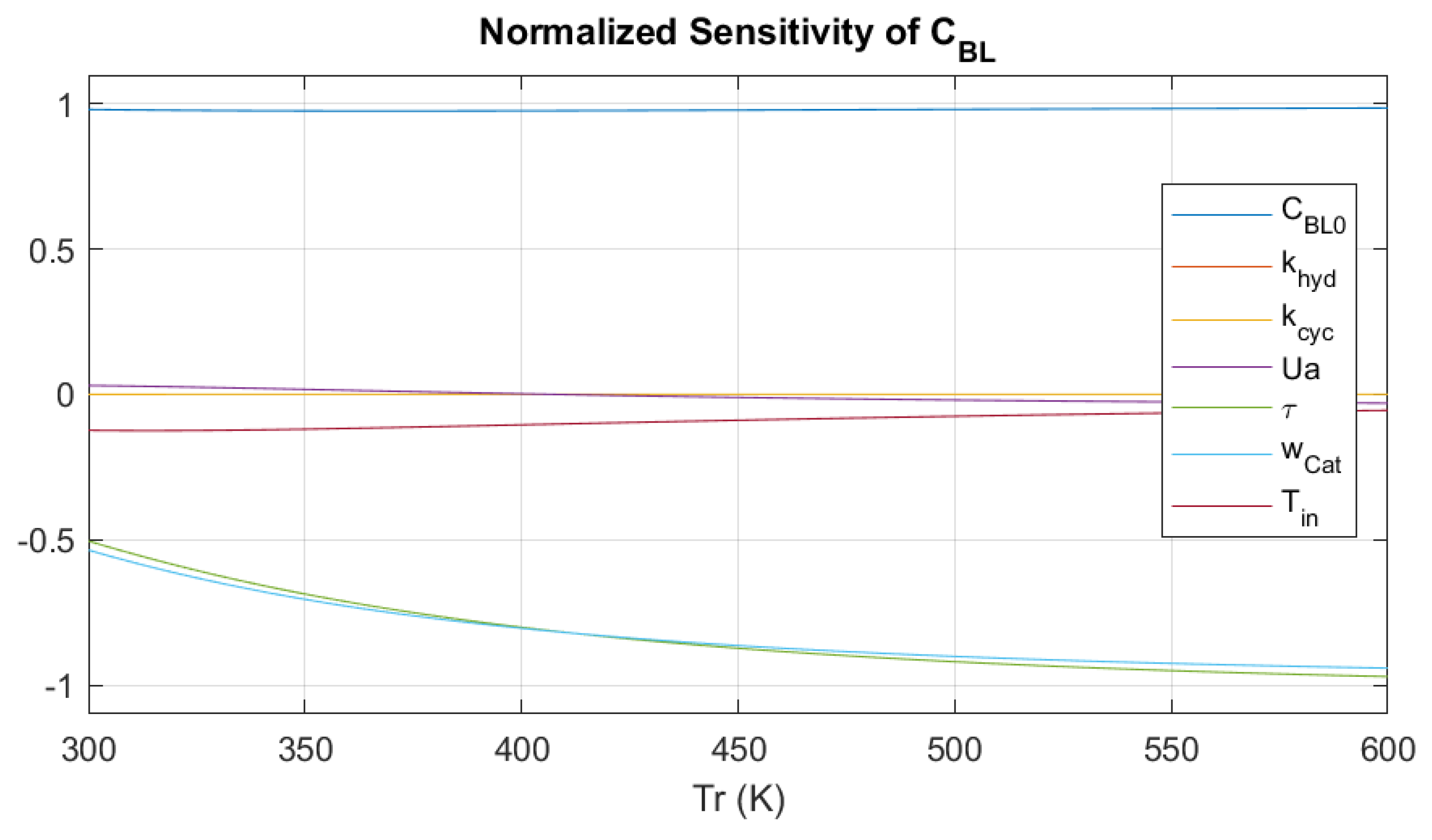
3.3. Parametric Sensitivity
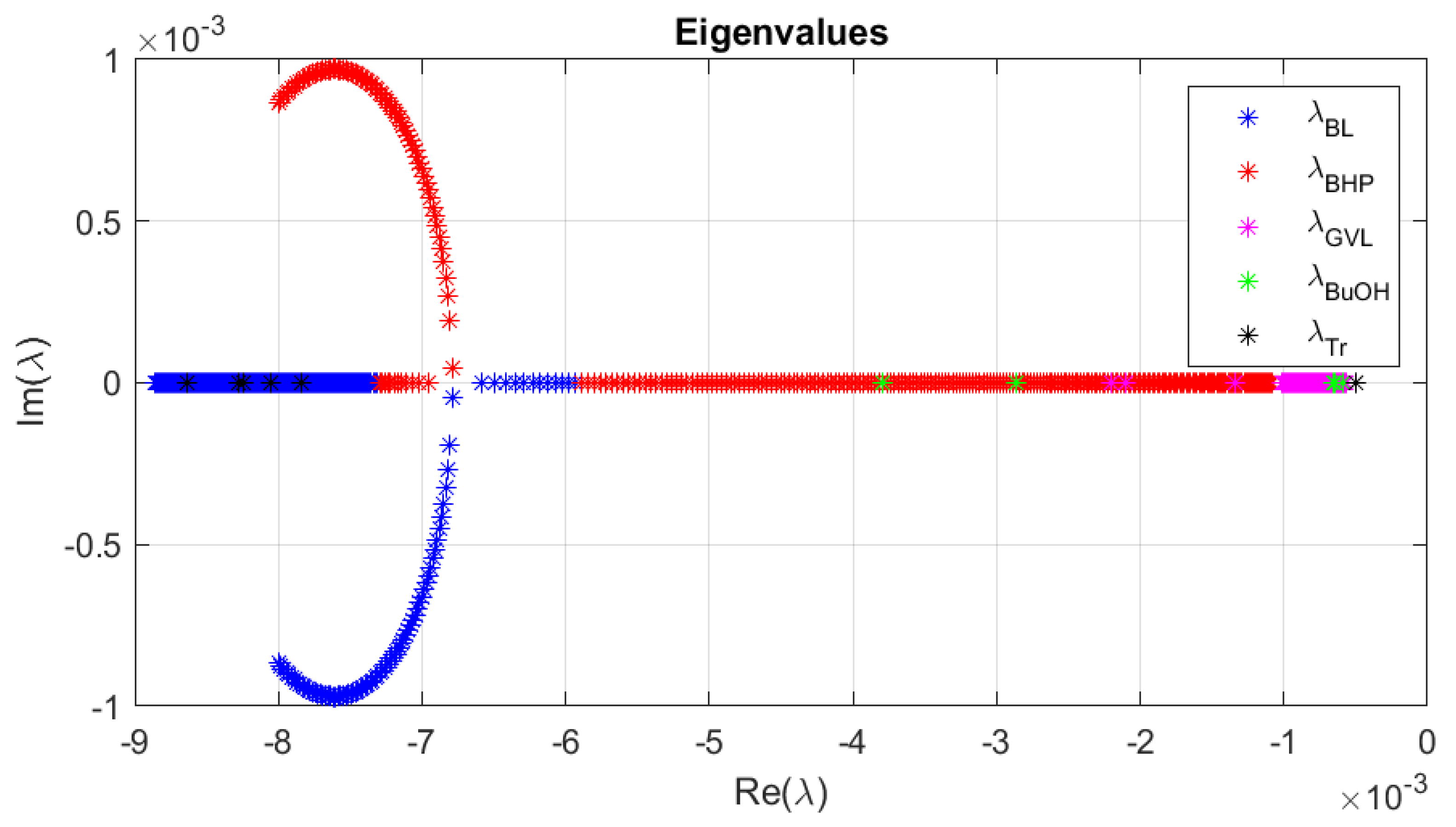
3.4. Dynamic Thermal Stability
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Filip, O.; Janda, K.; Kristoufek, L.; Zilberman, D. Food versus fuel: An updated and expanded evidence. Energy Econ. 2019, 82, 152–166. [Google Scholar] [CrossRef]
- Timilsina, G.R.; Shrestha, A. How much hope should we have for biofuels? Energy 2011, 36, 2055–2069. [Google Scholar] [CrossRef]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic biomass: A sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef]
- Gunasekaran, M.; Kumar, G.; Karthikeyan, O.P.; Varjani, S. Lignocellulosic biomass as an optimistic feedstock for the production of biofuels as valuable energy source: Techno-economic analysis, Environmental Impact Analysis, Breakthrough and Perspectives. Environ. Technol. Innov. 2021, 24, 102080. [Google Scholar] [CrossRef]
- Nahak, B.K.; Preetam, S.; Sharma, D.; Shukla, S.K.; Syväjärvi, M.; Toncu, D.C.; Tiwari, A. Advancements in net-zero pertinency of lignocellulosic biomass for climate neutral energy production. Renew. Sustain. Energy Rev. 2022, 161, 112393. [Google Scholar] [CrossRef]
- Melero, J.A.; Iglesias, J.; Garcia, A. Biomass as renewable feedstock in standard refinery units. Feasibility, opportunities and challenges. Energy Environ. Sci. 2012, 5, 7393–7420. [Google Scholar] [CrossRef]
- Di Menno Di Bucchianico, D.; Wang, Y.; Buvat, J.C.; Pan, Y.; Casson Moreno, V.; Leveneur, S. Production of levulinic acid and alkyl levulinates: A process insight. Green Chem. 2022, 24, 614–646. [Google Scholar] [CrossRef]
- Ye, L.; Han, Y.; Feng, J.; Lu, X. A review about GVL production from lignocellulose: Focusing on the full components utilization. Ind. Crops Prod. 2020, 144, 112031. [Google Scholar] [CrossRef]
- Xu, R.; Liu, K.; Du, H.; Liu, H.; Cao, X.; Zhao, X.; Qu, G.; Li, X.; Li, B.; Si, C. Falling Leaves Return to Their Roots: A Review on the Preparation of γ-Valerolactone from Lignocellulose and Its Application in the Conversion of Lignocellulose. ChemSusChem 2020, 13, 6461–6476. [Google Scholar] [CrossRef]
- Alonso, D.M.; Wettstein, S.G.; Dumesic, J.A. Gamma-valerolactone, a sustainable platform molecule derived from lignocellulosic biomass. Green Chem. 2013, 15, 584–595. [Google Scholar] [CrossRef]
- Tang, X.; Zeng, X.; Li, Z.; Hu, L.; Sun, Y.; Liu, S.; Lei, T.; Lin, L. Production of γ-valerolactone from lignocellulosic biomass for sustainable fuels and chemicals supply. Renew. Sustain. Energy Rev. 2014, 40, 608–620. [Google Scholar] [CrossRef]
- Fábos, V.; Lui, M.Y.; Mui, Y.F.; Wong, Y.Y.; Mika, L.T.; Qi, L.; Cséfalvay, E.; Kovács, V.; Szucs, T.; Horváth, I.T. Use of Gamma-Valerolactone as an Illuminating Liquid and Lighter Fluid. ACS Sustain. Chem. Eng. 2015, 3, 1899–1904. [Google Scholar] [CrossRef]
- Kerkel, F.; Markiewicz, M.; Stolte, S.; Müller, E.; Kunz, W. The green platform molecule gamma-valerolactone—Ecotoxicity, biodegradability, solvent properties, and potential applications. Green Chem. 2021, 23, 2962–2976. [Google Scholar] [CrossRef]
- Di Menno Di Bucchianico, D.; Buvat, J.C.; Mignot, M.; Casson Moreno, V.; Leveneur, S. Role of solvent the production of butyl levulinate from fructose. Fuel 2022, 318, 123703. [Google Scholar] [CrossRef]
- Luo, Y.; Li, Z.; Zuo, Y.; Su, Z.; Hu, C. A Simple Two-Step Method for the Selective Conversion of Hemicellulose in Pubescens to Furfural. ACS Sustain. Chem. Eng. 2017, 5, 8137–8147. [Google Scholar] [CrossRef]
- Yuan, C.; Shi, W.; Chen, P.; Chen, H.; Zhang, L.; Hu, G.; Jin, L.; Xie, H.; Zheng, Q.; Lu, S. Dissolution and transesterification of cellulose in γ-valerolactone promoted by ionic liquids. New J. Chem. 2019, 43, 330–337. [Google Scholar] [CrossRef]
- Xue, Z.; Zhao, X.; Sun, R.C.; Mu, T. Biomass-derived γ-valerolactone-based solvent systems for highly efficient dissolution of various lignins: Dissolution behavior and mechanism study. ACS Sustain. Chem. Eng. 2016, 4, 3864–3870. [Google Scholar] [CrossRef]
- Motagamwala, A.H.; Won, W.; Maravelias, C.T.; Dumesic, J.A. An engineered solvent system for sugar production from lignocellulosic biomass using biomass derived γ-valerolactone. Green Chem. 2016, 18, 5756–5763. [Google Scholar] [CrossRef]
- Di Menno Di Bucchianico, D.; Cipolla, A.; Buvat, J.C.; Mignot, M.; Casson Moreno, V.; Leveneur, S. Kinetic Study and Model Assessment for n-Butyl Levulinate Production from Alcoholysis of 5-(Hydroxymethyl)furfural over Amberlite IR-120. Ind. Eng. Chem. Res. 2022, 61, 10818–10836. [Google Scholar] [CrossRef]
- Yan, K.; Yang, Y.; Chai, J.; Lu, Y. Catalytic reactions of gamma-valerolactone: A platform to fuels and value-added chemicals. Appl. Catal. B Environ. 2015, 179, 292–304. [Google Scholar] [CrossRef]
- Bond, J.Q.; Alonso, D.M.; Wang, D.; West, R.M.; Dumesic, J.A. Integrated catalytic conversion of γ-valerolactone to liquid alkenes for transportation fuels. Science 2010, 327, 1110–1114. [Google Scholar] [CrossRef]
- Al-Naji, M.; Puértolas, B.; Kumru, B.; Cruz, D.; Bäumel, M.; Schmidt, B.V.K.J.; Tarakina, N.V.; Pérez-Ramírez, J. Sustainable Continuous Flow Valorization of γ-Valerolactone with Trioxane to α-Methylene-γ-Valerolactone over Basic Beta Zeolites. ChemSusChem 2019, 12, 2628–2636. [Google Scholar] [CrossRef]
- Manzer, L.E. Catalytic synthesis of α-methylene-γ-valerolactone: A biomass-derived acrylic monomer. Appl. Catal. A Gen. 2004, 272, 249–256. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Kaburagi, W.; Osada, Y.; Fujitani, T.; Yamashita, H. Catalytic transfer hydrogenation of biomass-derived levulinic acid and its esters to γ-valerolactone over ZrO2 catalyst supported on SBA-15 silica. Catal. Today 2017, 281, 418–428. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Kango, H.; Yamashita, H. Catalytic Transfer Hydrogenation of Biomass-Derived Levulinic Acid and Its Esters to γ-Valerolactone over Sulfonic Acid-Functionalized UiO-66. ACS Sustain. Chem. Eng. 2017, 5, 1141–1152. [Google Scholar] [CrossRef]
- Liu, M.; Li, S.; Fan, G.; Yang, L.; Li, F. Hierarchical Flower-like Bimetallic NiCu catalysts for Catalytic Transfer Hydrogenation of Ethyl Levulinate into γ-Valerolactone. Ind. Eng. Chem. Res. 2019, 58, 10317–10327. [Google Scholar] [CrossRef]
- Oklu, N.K.; Makhubela, B.C.E. Highly selective and efficient solvent-free transformation of bio-derived levulinic acid to γ-valerolactone by Ru(II) arene catalyst precursors. Inorg. Chim. Acta 2018, 482, 460–468. [Google Scholar] [CrossRef]
- Chalid, M.; Broekhuis, A.A.; Heeres, H.J. Experimental and kinetic modeling studies on the biphasic hydrogenation of levulinic acid to γ-valerolactone using a homogeneous water-soluble Ru-(TPPTS) catalyst. J. Mol. Catal. A Chem. 2011, 341, 14–21. [Google Scholar] [CrossRef]
- Agirrezabal-Telleria, I.; Hemmann, F.; Jäger, C.; Arias, P.L.; Kemnitz, E. Functionalized partially hydroxylated MgF2 as catalysts for the dehydration of d-xylose to furfural. J. Catal. 2013, 305, 81–91. [Google Scholar] [CrossRef]
- Han, Y.; Ye, L.; Gu, X.; Zhu, P.; Lu, X. Lignin-based solid acid catalyst for the conversion of cellulose to levulinic acid using γ-valerolactone as solvent. Ind. Crops Prod. 2019, 127, 88–93. [Google Scholar] [CrossRef]
- Li, F.; France, L.J.; Cai, Z.; Li, Y.; Liu, S.; Lou, H.; Long, J.; Li, X. Catalytic transfer hydrogenation of butyl levulinate to Γ-valerolactone over zirconium phosphates with adjustable Lewis and Brønsted acid sites. Appl. Catal. B Environ. 2017, 214, 67–77. [Google Scholar] [CrossRef]
- Guo, H.; Ding, S.; Zhang, H.; Wang, C.; Peng, F.; Xiong, L.; Chen, X.; Ouyang, X. Improvement on the catalytic performances of butyl levulinate hydrogenation to γ-valerolactone over self-regenerated CuNiCoB/Palygorskite catalyst. Mol. Catal. 2021, 504, 111483. [Google Scholar] [CrossRef]
- Delgado, J.; Vasquez Salcedo, W.N.; Bronzetti, G.; Casson Moreno, V.; Mignot, M.; Legros, J.; Held, C.; Grénman, H.; Leveneur, S. Kinetic model assessment for the synthesis of γ-valerolactone from n-butyl levulinate and levulinic acid hydrogenation over the synergy effect of dual catalysts Ru/C and Amberlite IR-120. Chem. Eng. J. 2022, 430, 133053. [Google Scholar] [CrossRef]
- Capecci, S.; Wang, Y.; Casson Moreno, V.; Held, C.; Leveneur, S. Solvent effect on the kinetics of the hydrogenation of n-butyl levulinate to γ-valerolactone. Chem. Eng. Sci. 2021, 231, 116315. [Google Scholar] [CrossRef]
- Wang, Y.; Plazl, I.; Vernières-Hassimi, L.; Leveneur, S. From calorimetry to thermal risk assessment: γ-Valerolactone production from the hydrogenation of alkyl levulinates. Process Saf. Environ. Prot. 2020, 144, 32–41. [Google Scholar] [CrossRef]
- Wang, Y.; Vernières-Hassimi, L.; Casson-Moreno, V.; Hébert, J.P.; Leveneur, S. Thermal Risk Assessment of Levulinic Acid Hydrogenation to γ-Valerolactone. Org. Process Res. Dev. 2018, 22, 1092–1100. [Google Scholar] [CrossRef]
- Fei, Y.; Sun, B.; Zhang, F.; Xu, W.; Shi, N.; Jiang, J. Inherently safer reactors and procedures to prevent reaction runaway. Chin. J. Chem. Eng. 2018, 26, 1252–1263. [Google Scholar] [CrossRef]
- Aguilar-López, R.; Mata-Machuca, J.L.; Godinez-Cantillo, V. A tito control strategy to increase productivity in uncertain exothermic continuous chemical reactors. Processes 2021, 9, 873. [Google Scholar] [CrossRef]
- Abusrafa, A.E.; Challiwala, M.S.; Wilhite, B.A.; Elbashir, N.O. Thermal assessment of a micro fibrous fischer tropsch fixed bed reactor using computational fluid dynamics. Processes 2020, 8, 1213. [Google Scholar] [CrossRef]
- Jayakumar, N.S.; Agrawal, A.; Hashim, M.A.; Sahu, J.N. Experimental and theoretical investigation of parametric sensitivity and dynamics of a continuous stirred tank reactor for acid catalyzed hydrolysis of acetic anhydride. Comput. Chem. Eng. 2011, 35, 1295–1303. [Google Scholar] [CrossRef]
- Gómez García, M.Á.; Dobrosz-Gómez, I.; Ojeda Toro, J.C. Thermal stability and dynamic analysis of the acetic anhydride hydrolysis reaction. Chem. Eng. Sci. 2016, 142, 269–276. [Google Scholar] [CrossRef]
- Nguyen, S.T.; Hoang, N.H.; Hussain, M.A. Analysis of the Steady-State Multiplicity Behavior for Polystyrene Production in the CSTR. Chem. Prod. Process Model. 2017, 12, 20170027. [Google Scholar] [CrossRef]
- Schweitzer, J.M.; López-García, C.; Ferré, D. Thermal runaway analysis of a three-phase reactor for LCO hydrotreatment. Chem. Eng. Sci. 2010, 65, 313–321. [Google Scholar] [CrossRef]
- Wang, Y.; Cipolletta, M.; Vernières-Hassimi, L.; Casson-Moreno, V.; Leveneur, S. Application of the concept of Linear Free Energy Relationships to the hydrogenation of levulinic acid and its corresponding esters. Chem. Eng. J. 2019, 374, 822–831. [Google Scholar] [CrossRef]
- Ariba, H.; Wang, Y.; Devouge-Boyer, C.; Stateva, R.P.; Leveneur, S. Physicochemical Properties for the Reaction Systems: Levulinic Acid, Its Esters, and γ-Valerolactone. J. Chem. Eng. Data 2020, 65, 3008–3020. [Google Scholar] [CrossRef]
- Lu, X.; Wang, Y.; Estel, L.; Kumar, N.; Grénman, H.; Leveneur, S. Evolution of specific heat capacity with temperature for typical supports used for heterogeneous catalysts. Processes 2020, 8, 911. [Google Scholar] [CrossRef]
- Aris, R. On stability criteria of chemical reaction engineering. Chem. Eng. Sci. 1969, 24, 149–169. [Google Scholar] [CrossRef]
- Van Heerden, C. Autothermic Processes. Ind. Eng. Chem. 1953, 45, 1242–1247. [Google Scholar] [CrossRef]
- Kummer, A.; Varga, T. What do we know already about reactor runaway?—A review. Process Saf. Environ. Prot. 2021, 147, 460–476. [Google Scholar] [CrossRef]
- Varma, A.; Morbidelli, M.; Wu, H. Parametric Sensitivity in Chemical Systems; Cambridge University Press: Cambridge, UK, 1999; ISBN 0521019842. [Google Scholar]




| Values | Units | |
|---|---|---|
| k1 (T = 403.15 K) | 3.09·10−6 | m6·mol−1·kg−1·s−1 |
| Ea1 | 9.68 | kJ·mol−1 |
| ΔHR1 | −38.66 | kJ·mol−1 |
| k2 (T = 403.15 K) | 1.88·10−4 | s−1 |
| Ea2 | 10.25 | kJ·mol−1 |
| ΔHR2 | 6.50 | kJ·mol−1 |
| Inlet Parameters | Values | Units |
|---|---|---|
| 4840 | mol·m−3 | |
| 0 | mol·m−3 | |
| 2080 | mol·m−3 | |
| 0 | mol·m−3 | |
| 10 | kg·m−3 | |
| 300 to 600 | K | |
| 333.15 | K | |
| 25 | bar | |
| 2000 | s | |
| 17,000 | W·m−3·K−1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vásquez Salcedo, W.N.; Renou, B.; Leveneur, S. Thermal Stability for the Continuous Production of γ-Valerolactone from the Hydrogenation of N-Butyl Levulinate in a CSTR. Processes 2023, 11, 237. https://doi.org/10.3390/pr11010237
Vásquez Salcedo WN, Renou B, Leveneur S. Thermal Stability for the Continuous Production of γ-Valerolactone from the Hydrogenation of N-Butyl Levulinate in a CSTR. Processes. 2023; 11(1):237. https://doi.org/10.3390/pr11010237
Chicago/Turabian StyleVásquez Salcedo, Wenel Naudy, Bruno Renou, and Sébastien Leveneur. 2023. "Thermal Stability for the Continuous Production of γ-Valerolactone from the Hydrogenation of N-Butyl Levulinate in a CSTR" Processes 11, no. 1: 237. https://doi.org/10.3390/pr11010237
APA StyleVásquez Salcedo, W. N., Renou, B., & Leveneur, S. (2023). Thermal Stability for the Continuous Production of γ-Valerolactone from the Hydrogenation of N-Butyl Levulinate in a CSTR. Processes, 11(1), 237. https://doi.org/10.3390/pr11010237







