Optimal Alkalinity Model of Ladle Furnace Slag for Bearing Steel Production Based on Ion–Molecule Coexistence Theory
Abstract
:1. Introduction
2. Materials and Methods
2.1. Production Procedure and Materials
2.2. Establishment of the IMCT Model
- The structural units in CaO–SiO2–MgO–Al2O3–FeO–CaF2 at steelmaking temperature are composed of Ca2+, Mg2+, Fe2+ and F− as simple ions, and SiO2 and Al2O3 as simple molecules, silicates, aluminates, ferrites and so on as complex molecules.
- Complex molecules are formed by the reactions of simple ions and simple molecules under dynamic equilibrium.
- The chemical reactions obey the law of mass conservation.
- The activity of constituents in the slag equals the mass action concentration of the structural unit. The activity is relative to pure solid or liquid matter as the standard state according to the existing state at evaluated temperature.
2.3. Characterization of the Optimal Alkalinity
3. Results and Discussion
3.1. Impact of FeO
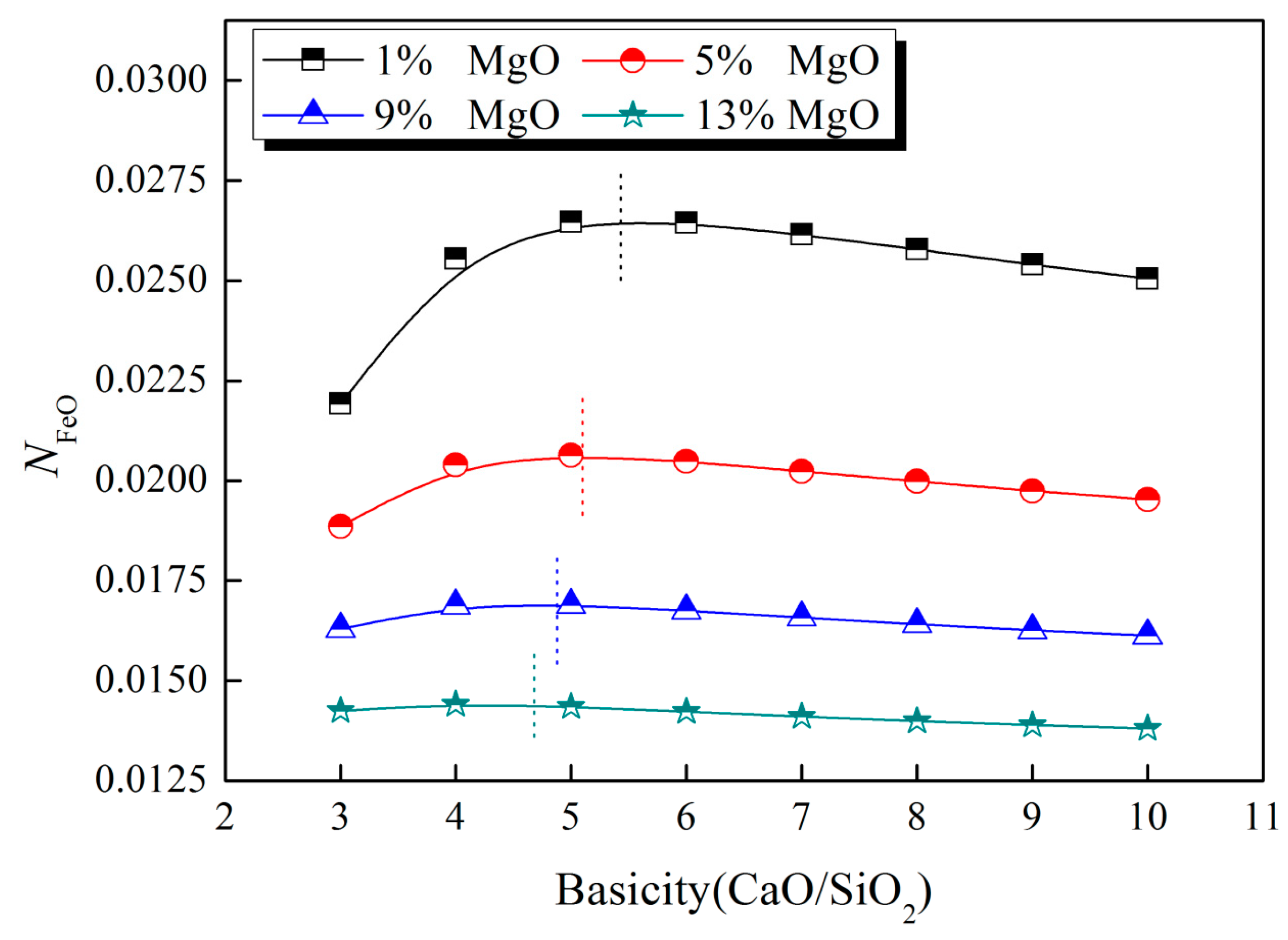
3.2. Impact of MgO
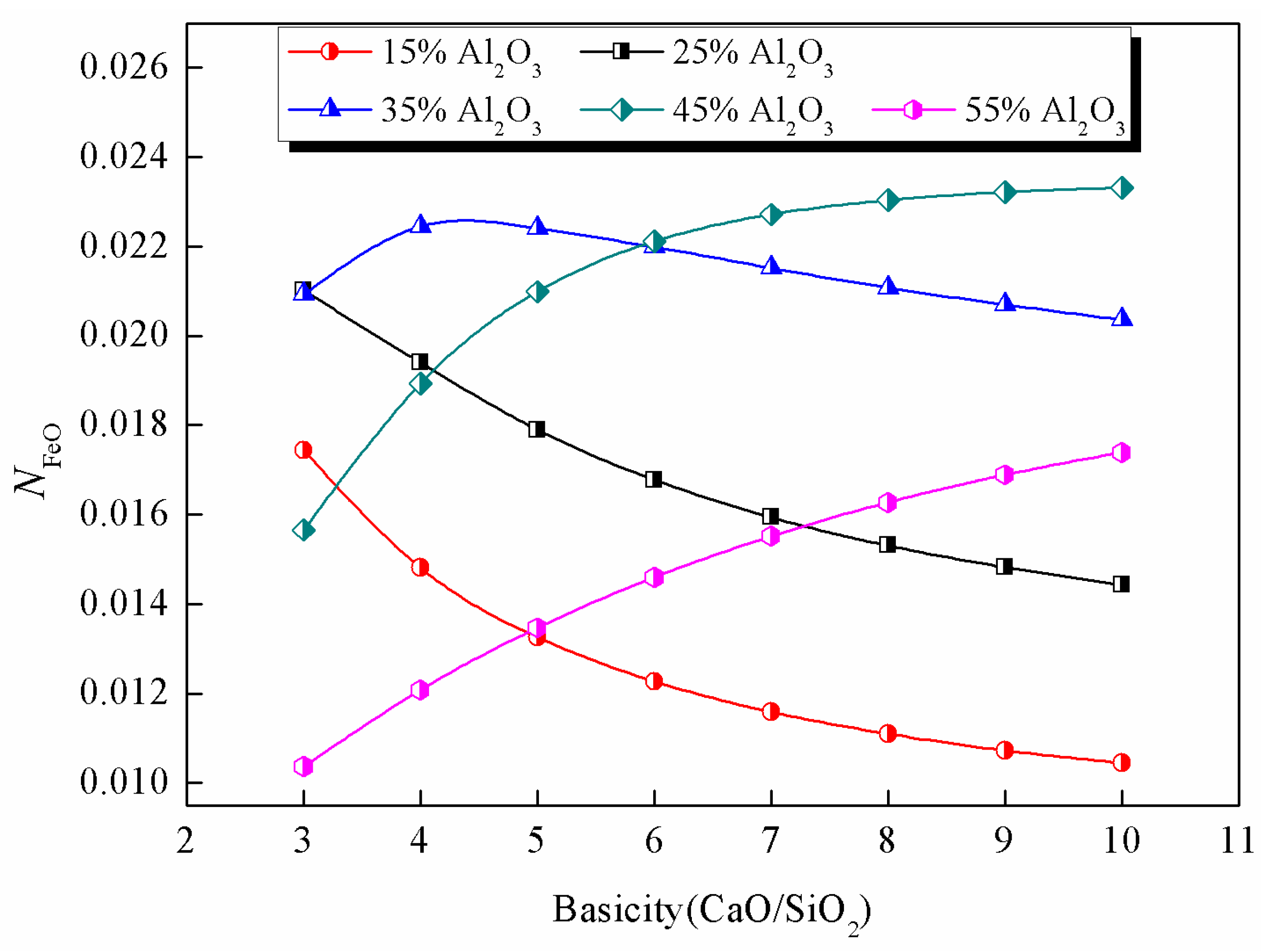
3.3. Impact of Al2O3
3.4. Impact of w(CaO)/w(Al2O3) Mass Ratio
3.5. Impact of CaF2
4. Conclusions
- The maximum oxidation capacity value of the refining slag occurs when the alkalinity is about five at varied FeO contents. The optimum alkalinity remains the same with changes in FeO content.
- The content of MgO has an obvious effect on the optimum alkalinity. The results show that the optimum alkalinity decreases with increasing MgO content. However, the value of against the higher alkalinity changes subtly at a given MgO content.
- Al2O3 content has a significant effect on the oxidation capacity of slag. Its effect on the optimum alkalinity is opposite to that of MgO. With increasing Al2O3 content, the optimal alkalinity obviously increased, while the maximum value of occurs when the Al2O3 content varied from 35 wt% to 45 wt% at higher alkalinity.
- The higher w(CaO)/w(Al2O3) mass ratio has an obvious effect on the value of against alkalinity. With the increase of w(CaO)/w(Al2O3) mass ratio, the value of decreases at a fixed alkalinity with a value greater than 4.5. In order to achieve a better refining effect, a lower w(CaO)/w(Al2O3) mass ratio is preferred.
- The effect of alkalinity on the value of is not obvious at a fixed CaF2 content; however, when the alkalinity remains the same, the value of increases slightly with increasing CaF2 content.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, X.; Zhang, Y.; Du, S. Preliminary research on response of GCr15 bearing steel under cyclic compression. Materials 2020, 13, 3443. [Google Scholar] [CrossRef]
- Nový, F.; Bokůvka, O.; Vicen, M.; Nikolić, R.; Činčala, M.; Medvecká, D. Fatigue damage initiation mechanisms in bearing steel. Mater. Today Proc. 2022, 62, 2637–2640. [Google Scholar] [CrossRef]
- Uesugi, T. Recent development of bearing steel in Japan. ISIJ Int. 2006, 28, 893–899. [Google Scholar] [CrossRef] [Green Version]
- Gu, C.; Bao, Y.P.; Gan, P.; Lian, J.H.; Münstermann, S. An experimental study on the impact of deoxidation methods on the fatigue properties of bearing steels. Steel Res. Int. 2018, 89, 1800129. [Google Scholar] [CrossRef]
- Wu, H.; Li, Q.; Wei, C.; Wang, Z. Study on the behaviour of DS-class inclusions in advanced bearing steel. Metall. Res. Technol. 2019, 116, 223. [Google Scholar] [CrossRef] [Green Version]
- Cheng, G.; Zhang, L.; Ren, Y. Characterization and evolution of non-Metallic inclusions in GCr15 bearing steels during cooling and solidification. Ironmak. Steelmak. 2020, 47, 1217–1225. [Google Scholar] [CrossRef]
- Ma, W.; Bao, Y.; Wang, M.; Zhao, L. Effect of Mg and Ca treatment on behavior and particle size of inclusions in bearing steels. ISIJ Int. 2014, 54, 536–542. [Google Scholar] [CrossRef] [Green Version]
- Liu, L. The key production technology of high quality special steel. Iron & Steel 2018, 53, 1–7. [Google Scholar]
- Zhang, Y.; Chen, W.; Yang, Y.; Mclean, A. Improved slags for ESR processing of high-carbon chromium bearing steel. ISIJ Int. 2017, 57, 322–328. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Jia, Y.; Hao, L.; Han, S.; Huang, F.; Yu, H.; Gao, X.; Ueda, S.; Kitamura, S.Y. Effects of slag composition and impurities of alloys on the inclusion transformation during industrial ladle furnace refining. Metals 2021, 11, 763. [Google Scholar] [CrossRef]
- Liu, Z.; Song, G.; Deng, Z.; Zhu, M. Effect of slag adjustment on inclusions in Si—Mn-Killed steel during ladle furnace (LF) refining process. Ironmak. Steelmak. 2021, 48, 893–900. [Google Scholar] [CrossRef]
- Tang, H.Y.; Wang, Y.; Wu, G.H.; Lan, P.; Zhang, J.Q. Inclusion evolution in 50CrVA spring steel by optimization of refining slag. J. Iron Steel Res. Int. 2017, 24, 879–887. [Google Scholar] [CrossRef]
- Guo, C.; Ling, H.; Zhang, L.; Yang, W.; Ren, Y.; Zhou, H. Effect of slag basicity adjusting on inclusions in tire cord steels during ladle furnace refining process. Metall. Res. Technol. 2017, 114, 602–608. [Google Scholar] [CrossRef]
- Chuiko, N.M. On the structural theory of metallurgical slags. Ferr. Met. 1959, 5, 3–10. [Google Scholar]
- Xie, S.L.; Wang, W.L.; Huang, D.Y.; Li, H.C.; Du, Y. Clarification of the dissolution of solid CaO and the phosphorus-enrichment capability of calcium silicates in the multiphase slag based on the ion and molecule coexistence theory. Steel Res. Int. 2018, 89, 1700317. [Google Scholar] [CrossRef]
- Zhang, J. Computational Thermodynamics of Metallurgical Melts and Solutions; Metallurgical Industry Press: Beijing, China, 2007. [Google Scholar]
- Huang, X.H. Metallurgy Principle of Iron and Steel; Metallurgical Industry Press: Beijing, China, 2013. [Google Scholar]
- Duan, S.C.; Guo, X.L.; Guo, H.J.; Guo, J. A manganese distribution prediction model for CaO-SiO2-FeO-MgO-MnO-Al2O3 slags based on IMCT. Ironmak. Steelmak. 2017, 44, 168–184. [Google Scholar] [CrossRef]
- Duan, S.C.; Li, C.; Guo, X.L.; Guo, H.J.; Guo, J.; Yang, W.S. A thermodynamic model for calculating manganese distribution ratio between CaO-SiO2-MgO-FeO-MnO-Al2O3-TiO2-CaF2 ironmaking slags and carbon saturated hot metal based on the IMCT. Ironmak. Steelmak. 2017, 45, 655–664. [Google Scholar] [CrossRef]
- Lei, J.L.; Zhao, D.N.; Feng, W.; Xue, Z.L. Titanium distribution ratio model of ladle furnace slags for tire cord steel production based on the ion-molecule coexistence theory at 1853 K. Processes 2019, 7, 788. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Li, L.; Guo, H.J.; Guo, J.; Duan, S.C.; Sun, W.X. A phosphorus distribution prediction model for CaO-SiO2-MgO-FeO-Fe2O3-Al2O3-P2O5 slags based on the IMCT. Ironmak. Steelmak. 2019, 47, 771–780. [Google Scholar] [CrossRef]
- Yang, X.M.; Li, J.Y.; Chai, G.M.; Duan, D.P.; Zhang, J. A thermodynamic model for predicting phosphorus partition between CaO-based slags and hot metal during hot metal dephosphorization pretreatment process based on the ion and molecule coexistence theory. Metall. Mater. Trans. B 2016, 47, 2279–2301. [Google Scholar] [CrossRef]
- Yang, X.M.; Zhang, M.; Chai, G.M.; Li, J.Y.; Liang, Q.; Zhang, J. Thermodynamic models for predicting dephosphorisation ability and potential of CaO-FeO-Fe2O3-Al2O3-P2O5 slags during secondary refining process of molten steel based on ion and molecule coexistence theory. Ironmak. Steelmak. 2016, 43, 663–687. [Google Scholar] [CrossRef]
- Yang, X.M.; Li, J.Y.; Zhang, M.; Yan, F.J.; Duan, D.P.; Zhang, J. A further evaluation of the coupling relationship between dephosphorization and desulfurization abilities or potentials for CaO-based slags: Influence of slag chemical composition. Metals 2018, 8, 1083. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.M.; Li, J.Y.; Zhang, M.; Chai, G.M.; Zhang, J. Prediction model of sulfide capacity for CaO-FeO-Fe2O3-Al2O3-P2O5 slags in a large variation range of oxygen potential based on the ion and molecule coexistence theory. Metall. Mater. Trans. B 2014, 45, 2118–2137. [Google Scholar] [CrossRef]
- Yang, X.M.; Li, J.Y.; Zhang, M.; Zhang, J. Prediction model of sulphur distribution ratio between CaO-FeO-Fe2O3-Al2O3-P2O5 slags and liquid iron over large variation range of oxygen potential during secondary refining process of molten steel based on ion and molecule coexistence theory. Ironmak. Steelmak. 2016, 43, 39–55. [Google Scholar] [CrossRef]
- Yang, X.M.; Zhang, M.; Zhang, J.L.; Li, P.C.; Li, J.Y.; Zhang, J. Representation of oxidation ability for metallurgical slags based on the ion and molecule coexistence theory. Steel Res. Int. 2014, 85, 347–375. [Google Scholar] [CrossRef]
- Kang, Y.B.; Pelton, A.D. Thermodynamic model and database for suldes dissolved in molten oxide slags. Metall. Mater. Trans. B 2009, 40, 979–994. [Google Scholar] [CrossRef] [Green Version]
- Kondratiev, A.; Jak, E. A quasi-chemical viscosity model for fully liquid slags in the Al2O3-CaO-FeO-SiO2 system. Metall. Mater. Trans. B 2005, 36, 623–638. [Google Scholar] [CrossRef]
- Turkdogan, E.T. Physical Chemistry of High Temperature Technology; Academic Press: New York, NY, USA, 1980; pp. 8–12. [Google Scholar]
- Ban-ya, S.; Chiba, A.; Hikosaka, A. Thermodynamics of FetO-MxOy(MxOy=CaO, SiO2, TiO2, and Al2O3) binary melts in equilibrium with solid iron. Tetsu-to-Hagane 1980, 66, 1484–1493. [Google Scholar] [CrossRef] [Green Version]
- Timucin, M.; Muan, A. Activity-composition relations in NiAl2O4-MnAl2O4 solid solutions and stabilities of NiAl2O4 and MnAl2O4 at 1300 °C and 1400 °C. J. Am. Ceram. Soc. 1992, 75, 1399–1406. [Google Scholar] [CrossRef]
- Barin, I.; Knacke, O.; Kubaschewski, O. Thermochemical Properties of Inorganic Substances (Supplement); Springer: New York, NY, USA, 1977; pp. 392–445. [Google Scholar]
- Kishimoto, T.; Hasegawa, M.; Ohnuki, K. The activities of FexO in CaO-SiO2-Al2O3-MgO-FexO slags at 1723 K. Steel Res. Int. 2005, 76, 341–347. [Google Scholar] [CrossRef]
- Yang, X.M.; Shi, C.B.; Zhang, M.; Zhang, J. A thermodynamic model for prediction of iron oxide activity in some FeO-containing slag systems. Steel Res. Int. 2012, 83, 244–258. [Google Scholar] [CrossRef]
- Basu, S.; Lahiri, A.K.; Seetharaman, S. Activity of iron oxide in steelmaking slag. Metall. Mater. Trans. B 2008, 39, 447–456. [Google Scholar] [CrossRef] [Green Version]
- Ottonello, G. Thermodynamic constraints arising from the polymeric approach to silicate slags: The system CaO-FeO-SiO2, as an example. J. Non-Cryst. Solids 2001, 282, 72–85. [Google Scholar] [CrossRef]
- Chen, C.; Jahanshahi, S. Thermodynamics of arsenic in FeOx-CaO-SiO2 slags. Metall. Mater. Trans. B 2010, 41, 1166–1174. [Google Scholar] [CrossRef]
- Li, Z.; Ma, G.J.; Liu, M.K.; Zou, J.J. Calculation model for activity of FeO in quaternary slag system SiO2-CaO-Al2O3-FeO. Metals 2018, 8, 714. [Google Scholar] [CrossRef] [Green Version]
- Li, P.C.; Li, J.Y.; Zhang, M.; Zhang, J.L.; Zhang, J.; Yang, X.M. Expression of oxidation ability for metallurgical slags based on the ion and molecule coexistence theory. Chin. J. Eng. 2013, 35, 1569–1579. [Google Scholar]
- Niu, H.Y.; Lei, Y. Produce test of ultra-low oxygen content steel. Gansu Metall. 2008, 30, 7–9. [Google Scholar]
- Gong, F.; Dong, D.X.; Liu, Y.; Yang, F.G.; Zhan, D.P.; Jiang, Z.H. Practice of production technology for bearing steel GCr15 with low oxygen content. Special Steel 2010, 31, 50–52. [Google Scholar]
- Ma, W.J.; Bao, Y.P.; Wang, M.; Zhao, D.W. Influence of slag composition on bearing steel cleanness. Ironmak. Steelmak. 2014, 41, 26–30. [Google Scholar] [CrossRef]
- Ma, W.J.; Lui, G.L.; Gao, P.; Luo, Y.Z.; Li, H.B.; Chen, B. Study on controlling inclusions and total oxygen of high-carbon steel. Mater. Sci. Technol. 2016, 32, 1119–1125. [Google Scholar] [CrossRef]
- Lei, J.L.; Zhu, H.Y.; Zhao, D.N.; Xue, Z.L. Generation mechanism of MgO and Al2O3 inclusions in 51CrV4 spring steel based on the ion-molecule coexistence theory. Metals 2019, 9, 830. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Xu, J.; Zhang, J.; Wang, X. Effect of Al2O3 content on metallurgical characteristics of refining slag. Ironmak. Steelmak. 2016, 43, 607–615. [Google Scholar] [CrossRef]
- Yoon, B.H.; Heo, K.H.; Kim, J.S.; Sohn, H.S. Improvement of steel cleanliness by controlling slag composition. Ironmak. Steelmak. 2002, 29, 215–218. [Google Scholar] [CrossRef]
- Zhao, S.; He, S.P.; Guo, Y.T.; Chen, G.J.; Lv, J.C. Effect on cleanliness of molten steel with different refining slag systems for low alloy ship plate. Ironmak. Steelmak. 2016, 43, 790–798. [Google Scholar] [CrossRef]
- Ma, W.J.; Bao, Y.P.; Wang, M.; Zhao, L. Key technologies of smelting in high cleanliness bearing steel with EAF. Steelmak. 2014, 30, 42–45. [Google Scholar]
- Shen, W.L.; Zhang, Z.C.; Luo, X.Y. A study on behavior of oxide inclusions in bearing steel GCr15 at refining end of a 60 t LF. Special Steel 2018, 39, 18–23. [Google Scholar]
- Shin, J.H.; Park, J.H. Effect of CaO/Al2O3 ratio of ladle slag on formation behavior of inclusions in Mn and V alloyed steel. ISIJ Int. 2018, 58, 88–97. [Google Scholar] [CrossRef] [Green Version]
- Ji, Y.Q.; Liu, C.Y.; Lu, Y.; Yu, H.X.; Huang, F.X.; Wang, X.H. Effects of FeO and CaO/Al2O3 ratio in slag on the cleanliness of Al-killed steel. Metall. Mater. Trans. B 2018, 49, 3127–3136. [Google Scholar] [CrossRef]
- Lin, L.H.; Zhang, L.Q.; Huang, X.L. Influence of CaF2 on the apparent viscosity of CaO-SiO2-MgO-Al2O3-TiO2 slags. Metall. Res. Technol. 2017, 114, 606–610. [Google Scholar] [CrossRef]
- Wang, X.J.; Jin, H.B.; Zhu, L.G.; Xu, Y.; Liu, R.; Piao, Z.L.; Qu, S. Effect of CaF2 on the viscosity and microstructure of CaO-SiO2-Al2O3 based continuous casting mold flux. Metals 2019, 9, 871. [Google Scholar] [CrossRef] [Green Version]



| Slag Systems | Applications | Ref. |
|---|---|---|
| CaO–SiO2–FeO–MgO–MnO–Al2O3 | A thermodynamic model for calculating and predicting manganese distribution ratio and manganese capacity in the slag system was built based on IMCT. | [18] |
| CaO–SiO2–MgO–FeO–MnO–Al2O3–TiO2– CaF2 | A thermodynamic model for predicting manganese distribution ratio between the slag and carbon-saturated liquid iron was established based on IMCT. | [19] |
| CaO–SiO2–Al2O3–MgO–FeO–MnO–TiO2 | A thermodynamic model for titanium distribution ratio calculation between the slag and liquid steel was developed based on IMCT combined with industrial measurements. | [20] |
| CaO–SiO2–MgO–FeO–Fe2O3–Al2O3–P2O5 | A thermodynamic model for calculating the phosphorus distribution ratio between the slag and liquid steel was built based on IMCT. | [21] |
| CaO–based slags | A thermodynamic model for calculating the phosphorus partition between CaO-based slags and hot metal during hot metal dephosphorization pretreatment process was developed based on IMCT. | [22] |
| CaO–FeO–Fe2O3–Al2O3–P2O5 | Thermodynamic models for predicting dephosphorization ability and potential of the slag during the secondary refining process was developed based on IMCT. | [23] |
| CaO–based slags | A further evaluation of the coupling relationship between dephosphorization and desulfurization abilities or potentials for CaO-based slags was built based on IMCT. | [24] |
| CaO–FeO–Fe2O3–Al2O3–P2O5 | A thermodynamic model for calculating sulfide capacity of slag in various oxygen potential ranges was established based on IMCT. | [25] |
| CaO–FeO–Fe2O3–Al2O3–P2O5 | Prediction model of sulfur distribution ratio between the slag and liquid iron over a large variation range of oxygen potential during secondary refining process was built based on IMCT. | [26] |
| CaO–SiO2–MgO–FeO–Fe2O3–MnO–Al2O3– P2O5 | Representation of oxidation ability for metallurgical slags based on IMCT was verified by comparing with the reported activity in the slag systems. | [27] |
| CaO | SiO2 | Al2O3 | MgO | FeO | CaF2 | Binary Alkalinity |
|---|---|---|---|---|---|---|
| 44.50 | 6.25 | 37.13 | 3.29 | 0.74 | 8.09 | 7.12 |
| Items | Constitutional Units | Balanced Mole Number | Mass Action Concentrations |
|---|---|---|---|
| Simple cations and anions | |||
| Simple molecules | |||
| Complex molecules | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, J.; Li, J.; Yang, L.; Zhang, Y. Optimal Alkalinity Model of Ladle Furnace Slag for Bearing Steel Production Based on Ion–Molecule Coexistence Theory. Processes 2023, 11, 763. https://doi.org/10.3390/pr11030763
Lei J, Li J, Yang L, Zhang Y. Optimal Alkalinity Model of Ladle Furnace Slag for Bearing Steel Production Based on Ion–Molecule Coexistence Theory. Processes. 2023; 11(3):763. https://doi.org/10.3390/pr11030763
Chicago/Turabian StyleLei, Jialiu, Jie Li, Ling Yang, and Yucheng Zhang. 2023. "Optimal Alkalinity Model of Ladle Furnace Slag for Bearing Steel Production Based on Ion–Molecule Coexistence Theory" Processes 11, no. 3: 763. https://doi.org/10.3390/pr11030763





