Biochemical Methane Potential of Mechanically and Enzymatically Pretreated Solid Olive Mill Waste
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrate and Inoculum
2.2. Experimental Design
2.2.1. Enzymatic Pretreatments
2.2.2. Destoning by Horizontal Screw Press (HSP) and Centrifugation
2.3. Anaerobic Digestion
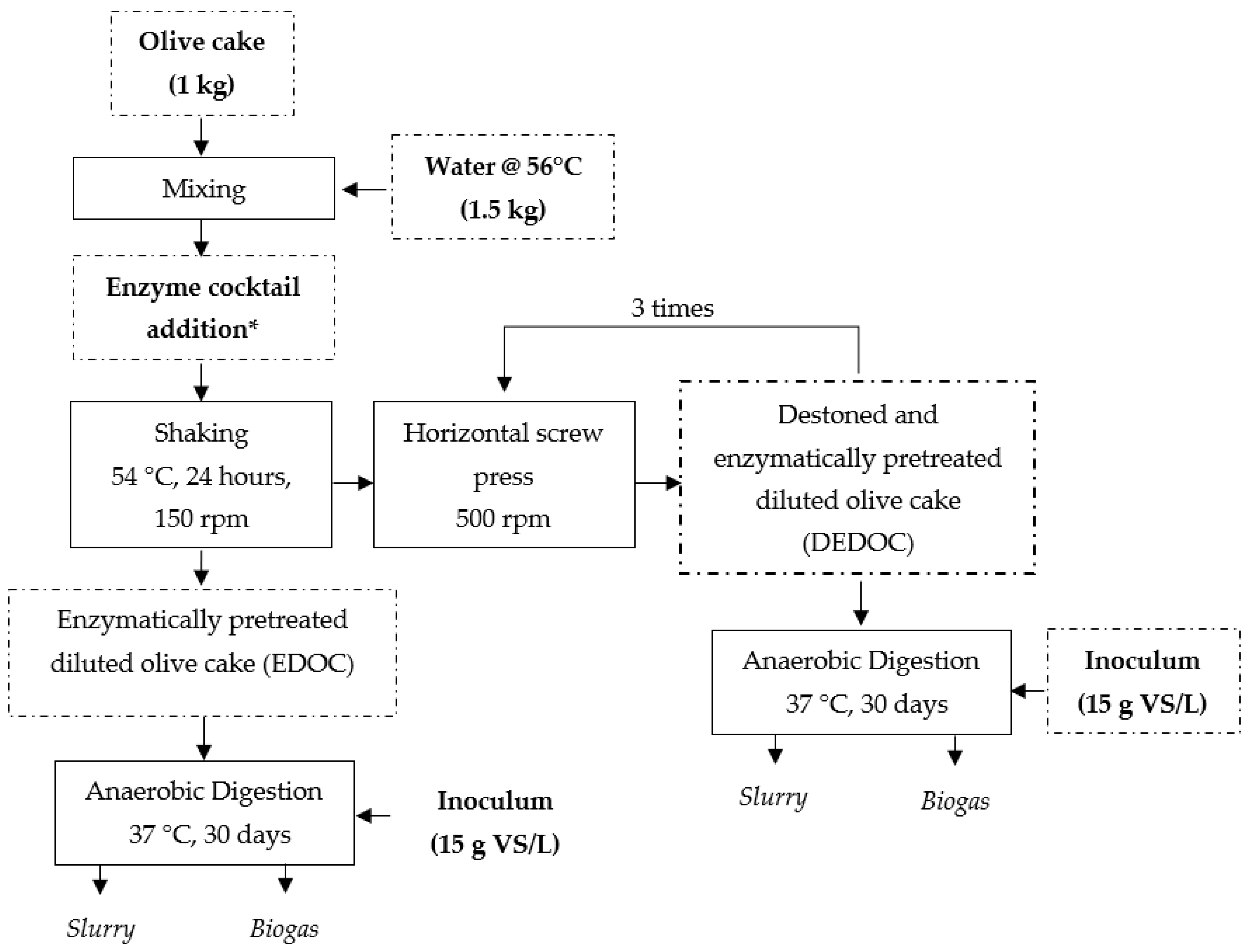
2.3.1. Mechanical and Enzymatic Pretreatment of Olive Cake
2.3.2. Olive Cake Characterization
2.3.3. Biochemical Methane Potential Substrate and Inoculum Loadings
2.3.4. Biogas Analysis
2.3.5. Carbohydrate Profile of Olive Cake and Digestate
2.3.6. Digestate Analysis
2.4. Calculations
2.5. Statistical Analysis
3. Results and Discussion
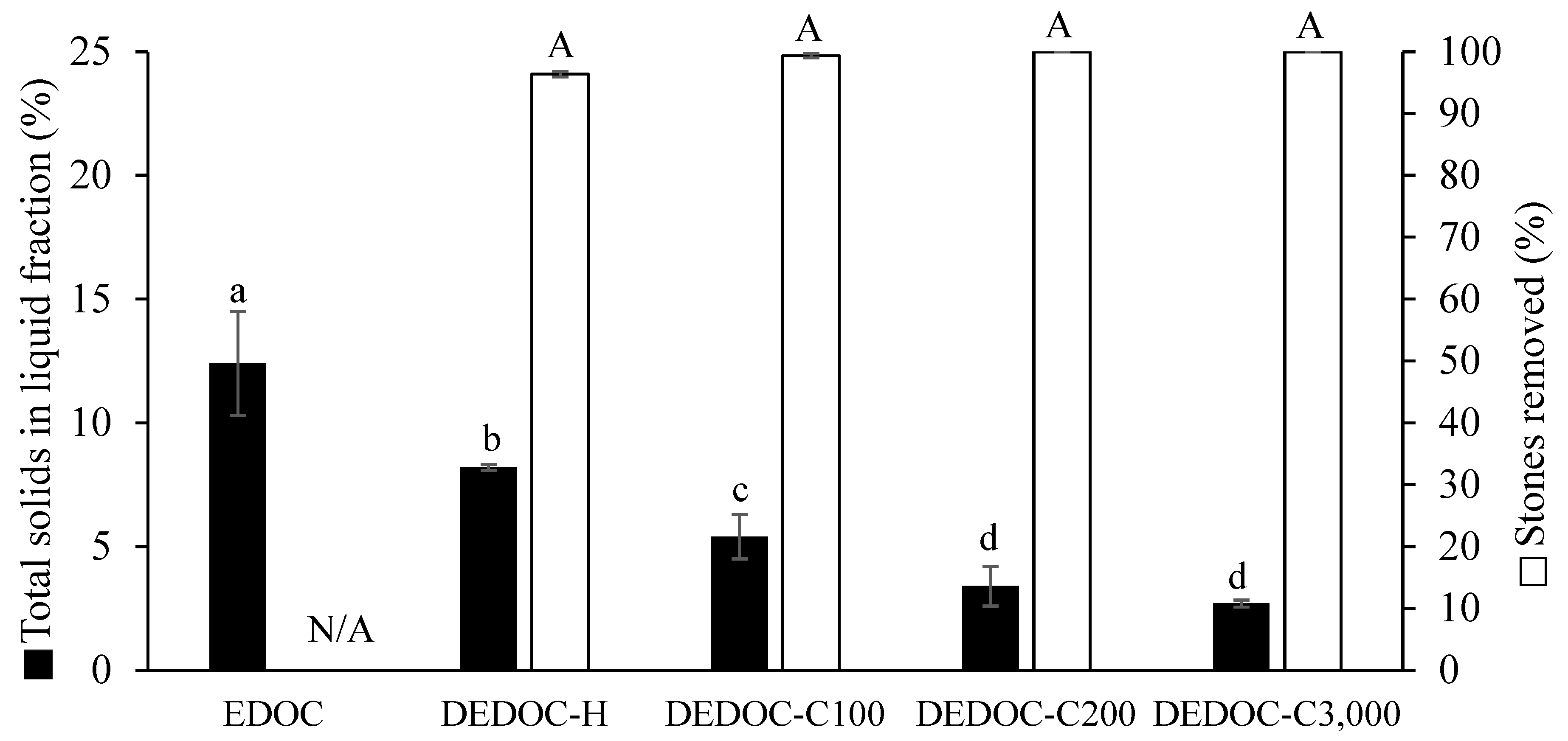
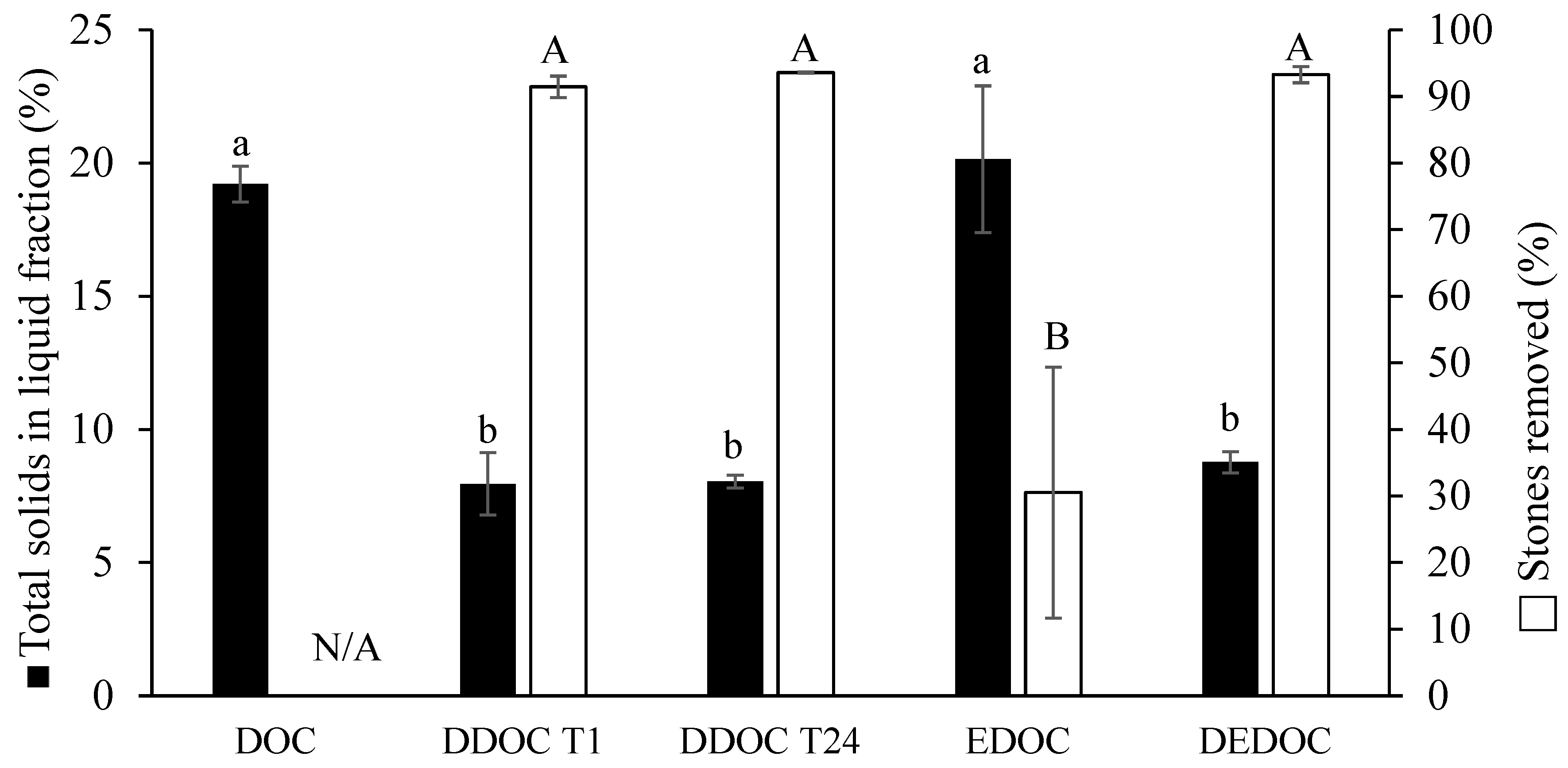
3.1. Stone Removal, and Total Solid Distribution with Horizontal Screw Press (HSP) or Centrifugation
3.2. Olive Cake Destoning Efficiency of a Horizontal Screw Press (HSP) Compared to Enzymatic Pretreatment
3.3. Impact of Pretreatment on Olive Cake Characteristics
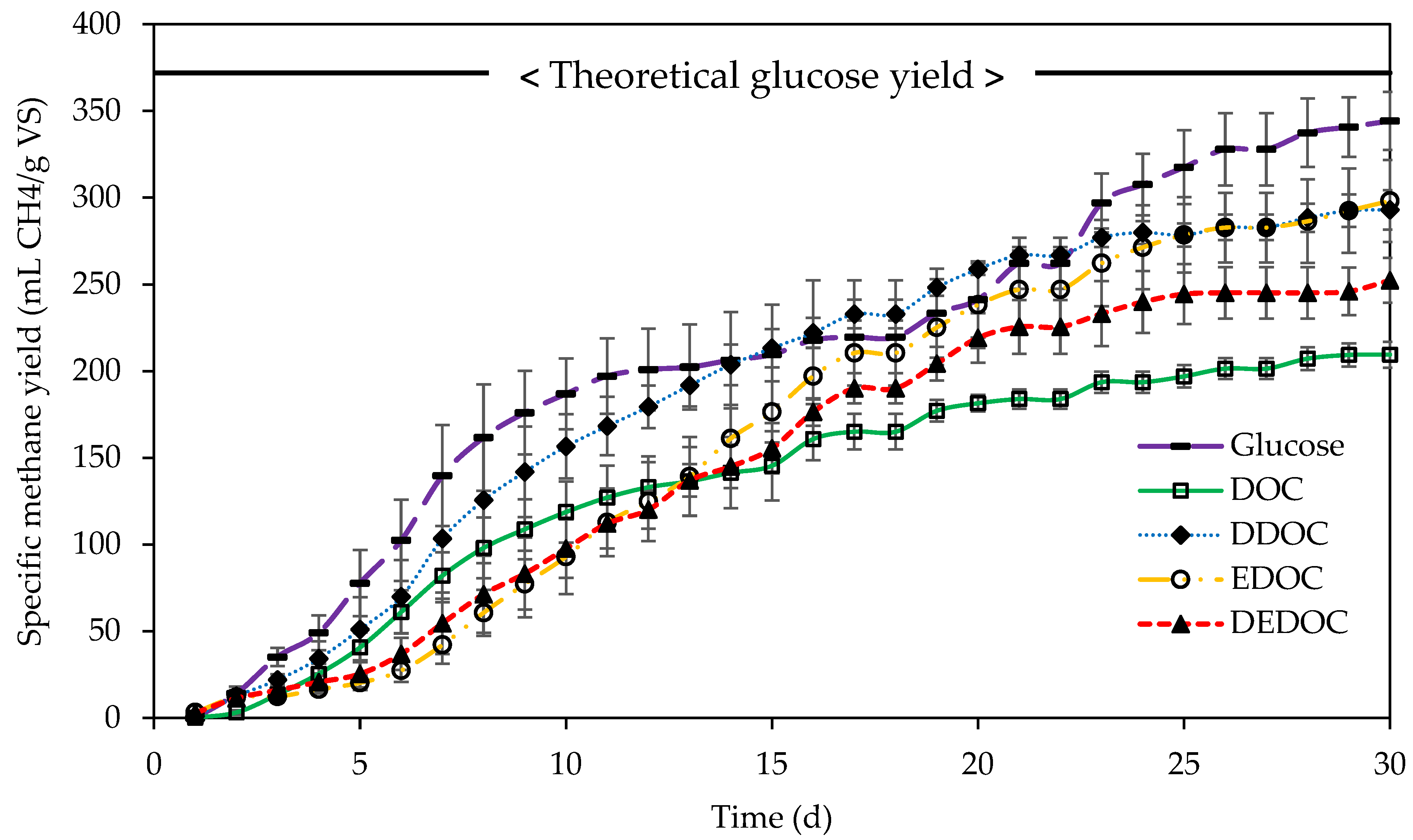
3.4. Methane Yields
3.5. Carbohydrate Content and Profile
3.6. Valorization Potential of the Olive Cake Digestate
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Olive Council. Olive Oil Dashboard. 2023. Available online: https://www.internationaloliveoil.org/wp-content/uploads/2021/12/IOC-Olive-Oil-Dashboard-1.html#production-2 (accessed on 14 February 2023).
- Guasch-Ferré, M.; Liu, G.; Li, Y.; Sampson, L.; Manson, J.E.; Salas-Salvadó, J.; Martínez-González, M.A.; Stampfer, M.J.; Willett, W.C.; Sun, Q.; et al. Olive Oil Consumption and Cardiovascular Risk in U.S. Adults. J. Am. Coll. Cardiol. 2020, 75, 1729–1739. [Google Scholar] [CrossRef] [PubMed]
- Al Afif, R.; Linke, B. Biogas Production from Three-Phase Olive Mill Solid Waste in Lab-Scale Continuously Stirred Tank Reactor. Energy 2019, 171, 1046–1052. [Google Scholar] [CrossRef]
- Dammak, I.; Neves, M.; Souilem, S.; Isoda, H.; Sayadi, S.; Nakajima, M. Material Balance of Olive Components in Virgin Olive Oil Extraction Processing. FSTR 2015, 21, 193–205. [Google Scholar] [CrossRef] [Green Version]
- Hachicha, R.; Rigane, H.; Khodher, M.B.; Nasri, M.; Medhioub, K. Effects of Partial Stone Removal on the Co-composting of Olive-oil Processing Solid Residues with Poultry Manure and the Quality of Compost. Environ. Technol. 2003, 24, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Roig, A.; Cayuela, M.L.; Sánchez-Monedero, M.A. An Overview on Olive Mill Wastes and Their Valorisation Methods. Waste Manag. 2006, 26, 960–969. [Google Scholar] [CrossRef]
- Masghouni, M.; Hassairi, M. Energy Applications of Olive-Oil Industry by-Products: I. The Exhaust Foot Cake. Biomass Bioenergy 2000, 18, 257–262. [Google Scholar] [CrossRef]
- Wilkie, A.C. Biomethane from Biomass, Biowaste, and Biofuels. In Bioenergy; Wall, J.D., Harwood, C.S., Demain, A., Eds.; ASM Press: Washington, DC, USA, 2014; pp. 195–205. [Google Scholar]
- Alonso-Fariñas, B.; Oliva, A.; Rodríguez-Galán, M.; Esposito, G.; García-Martín, J.F.; Rodríguez-Gutiérrez, G.; Serrano, A.; Fermoso, F.G. Environmental Assessment of Olive Mill Solid Waste Valorization via Anaerobic Digestion Versus Olive Pomace Oil Extraction. Processes 2020, 8, 626. [Google Scholar] [CrossRef]
- Makádi, M.; Tomócsik, A.; Orosz, V. Digestate: A New Nutrient Source–Review. In Biogas; Kumar, S., Ed.; Intech: Rijeka, Croatia, 2012; pp. 295–310. [Google Scholar]
- Triolo, J.M.; Sommer, S.G.; Møller, H.B.; Weisbjerg, M.R.; Jiang, X.Y. A New Algorithm to Characterize Biodegradability of Biomass during Anaerobic Digestion: Influence of Lignin Concentration on Methane Production Potential. Bioresour. Technol. 2011, 102, 9395–9402. [Google Scholar] [CrossRef]
- Guo, Z.; Jia, X.; Zheng, Z.; Lu, X.; Zheng, Y.; Zheng, B.; Xiao, J. Chemical Composition and Nutritional Function of Olive (Olea europaea L.): A Review. Phytochem. Rev. 2018, 17, 1091–1110. [Google Scholar] [CrossRef]
- Miranda, I.; Simões, R.; Medeiros, B.; Nampoothiri, K.M.; Sukumaran, R.K.; Rajan, D.; Pereira, H.; Ferreira-Dias, S. Valorization of Lignocellulosic Residues from the Olive Oil Industry by Production of Lignin, Glucose and Functional Sugars. Bioresour. Technol. 2019, 292, 121936. [Google Scholar] [CrossRef]
- Fernández-Lobato, L.; López-Sánchez, Y.; Blejman, G.; Jurado, F.; Moyano-Fuentes, J.; Vera, D. Life Cycle Assessment of the Spanish Virgin Olive Oil Production: A Case Study for Andalusian Region. J. Clean. Prod. 2021, 290, 125677. [Google Scholar] [CrossRef]
- Barreca, F.; Fichera, C.R. Use of Olive Stone as an Additive in Cement Lime Mortar to Improve Thermal Insulation. Energy Build. 2013, 62, 507–513. [Google Scholar] [CrossRef]
- Fiol, N.; Villaescusa, I.; Martínez, M.; Miralles, N.; Poch, J.; Serarols, J. Sorption of Pb(II), Ni(II), Cu(II) and Cd(II) from Aqueous Solution by Olive Stone Waste. Sep. Purif. Technol. 2006, 50, 132–140. [Google Scholar] [CrossRef]
- Sánchez Moral, P.; Ruiz Méndez, M.V. Production of Pomace Olive Oil. Grasas y Aceites 2006, 57, 47–55. [Google Scholar] [CrossRef]
- Carlini, M.; Castellucci, S.; Moneti, M. Anaerobic Co-Digestion of Olive-Mill Solid Waste with Cattle Manure and Cattle Slurry: Analysis of Bio-Methane Potential. Energy Procedia 2015, 81, 354–367. [Google Scholar] [CrossRef] [Green Version]
- de la Lama-Calvente, D.; Fernández-Rodríguez, M.J.; Llanos, J.; Mancilla-Leytón, J.M.; Borja, R. Enhancing Methane Production from the Invasive Macroalga Rugulopteryx Okamurae through Anaerobic Co-Digestion with Olive Mill Solid Waste: Process Performance and Kinetic Analysis. J. Appl. Phycol. 2021, 33, 4113–4124. [Google Scholar] [CrossRef]
- Holliger, C.; Alves, M.; Andrade, D.; Angelidaki, I.; Astals, S.; Baier, U.; Bougrier, C.; Buffière, P.; Carballa, M.; de Wilde, V.; et al. Towards a Standardization of Biomethane Potential Tests. Water Sci. Technol. 2016, 74, 2515–2522. [Google Scholar] [CrossRef]
- Bailey, M.J.; Poutanen, K. Production of Xylanolytic Enzymes by Strains of Aspergillus. Appl. Microbiol. Biotechnol. 1989, 30, 5–10. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 16th ed.; American Public Health Association: Washington DC, USA, 1985. [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC International, 18th ed.; Association of Officiating Analytical Chemists: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Obied, H.K.; Bedgood, D.R.; Prenzler, P.D.; Robards, K. Bioscreening of Australian Olive Mill Waste Extracts: Biophenol Content, Antioxidant, Antimicrobial and Molluscicidal Activities. Food Chem. Toxicol. 2007, 45, 1238–1248. [Google Scholar] [CrossRef]
- Angelidaki, I.; Alves, M.; Bolzonella, D.; Borzacconi, L.; Campos, J.L.; Guwy, A.J.; Kalyuzhnyi, S.; Jenicek, P.; van Lier, J.B. Defining the Biomethane Potential (BMP) of Solid Organic Wastes and Energy Crops: A Proposed Protocol for Batch Assays. Water Sci. Technol. 2009, 59, 927–934. [Google Scholar] [CrossRef] [Green Version]
- Parr Instrument Company. 203M 1241 Oxygen Bomb Calorimeter Operating Instructions. 2013. Available online: https://www.parrinst.com/products/oxygen-bomb-calorimeters/previous-calorimeter-models/ (accessed on 29 March 2018).
- Yan, H.; Zhao, C.; Zhang, J.; Zhang, R.; Xue, C.; Liu, G.; Chen, C. Study on Biomethane Production and Biodegradability of Different Leafy Vegetables in Anaerobic Digestion. AMB Expr. 2017, 7, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felizón, B.; Fernández-Bolaños, J.; Heredia, A.; Guillén, R. Steam-Explosion Pretreatment of Olive Cake. J. Amer. Oil Chem. Soc. 2000, 77, 15–22. [Google Scholar] [CrossRef]
- Serrano, A.; Fermoso, F.G.; Alonso-Fariñas, B.; Rodríguez-Gutierrez, G.; Fernandez-Bolaños, J.; Borja, R. Olive Mill Solid Waste Biorefinery: High-Temperature Thermal Pre-Treatment for Phenol Recovery and Biomethanization. J. Clean. Prod. 2017, 148, 314–323. [Google Scholar] [CrossRef] [Green Version]
- Chiofalo, B.; Liotta, L.; Zumbo, A.; Chiofalo, V. Administration of Olive Cake for Ewe Feeding: Effect on Milk Yield and Composition. Small Rumin. Res. 2004, 55, 169–176. [Google Scholar] [CrossRef]
- Ferrer, P.; García-Rebollar, P.; Cerisuelo, A.; Ibáñez, M.A.; Rodríguez, C.A.; Calvet, S.; De Blas, C. Nutritional Value of Crude and Partially Defatted Olive Cake in Finishing Pigs and Effects on Nitrogen Balance and Gaseous Emissions. Anim. Feed Sci. Technol. 2018, 236, 131–140. [Google Scholar] [CrossRef] [Green Version]
- Sadeghi, H.; Yansari, A.T.; Ansari-Pirsarai, Z. Effects of Different Olive Cake by Products on Dry Matter Intake, Nutrient Digestibility and Performance of Zel Sheep. Int. J. Agric. Biol. 2009, 11, 6. [Google Scholar]
- Vlyssides, A.; Loizidou, M.; Gimouhopoulos, K.; Zorpas, A. Olive Oil Processing Wastes Production and Their Characteristics in Relation to Olive Oil Extraction Methods. Fresenius Enivr. Bull. 1998, 7, 308–311. [Google Scholar]
- Akassou, M.; Kaanane, A.; Crolla, A.; Kinsley, C. Statistical Modelling of the Impact of Some Polyphenols on the Efficiency of Anaerobic Digestion and the Co-Digestion of the Wine Distillery Wastewater with Dairy Cattle Manure and Cheese Whey. Water Sci. Technol. 2010, 62, 475–483. [Google Scholar] [CrossRef]
- Messineo, A.; Maniscalco, M.P.; Volpe, R. Biomethane Recovery from Olive Mill Residues through Anaerobic Digestion: A Review of the State of the Art Technology. Sci. Total Environ. 2020, 703, 135508. [Google Scholar] [CrossRef]
- Koch, K.; Hafner, S.; Astals, S.; Weinrich, S. Evaluation of Common Supermarket Products as Positive Controls in Biochemical Methane Potential (BMP) Tests. Water 2020, 12, 1223. [Google Scholar] [CrossRef]
- Yuan, J.-J.; Tu, J.-L.; Qin, F.G.F.; Xu, Y.-J.; Li, B. Phenolic Composition of Oleuropein Extract after Enzymatic Process by HPLC-MS and Their Antioxidant and Antibacterial Activities. J. Food Biochem. 2018, 42, e12517. [Google Scholar] [CrossRef]
- Bermúdez-Oria, A.; Rodríguez-Juan, E.; Rodríguez-Gutiérrez, G.; Fernández-Prior, Á.; Fernández-Bolaños, J. Effect of the Olive Oil Extraction Process on the Formation of Complex Pectin–Polyphenols and Their Antioxidant and Antiproliferative Activities. Antioxidants 2021, 10, 1858. [Google Scholar] [CrossRef] [PubMed]
- Tekin, A.R.; Dalgıç, A.C. Biogas Production from Olive Pomace. Resour., Conserv. Recycling 2000, 30, 301–313. [Google Scholar] [CrossRef]
- Chynoweth, D.P.; Turick, C.E.; Owens, J.M.; Jerger, D.E.; Peck, M.W. Biochemical Methane Potential of Biomass and Waste Feedstocks. Biomass Bioenergy 1993, 5, 95–111. [Google Scholar] [CrossRef]
- Pellera, F.-M.; Gidarakos, E. Effect of Substrate to Inoculum Ratio and Inoculum Type on the Biochemical Methane Potential of Solid Agroindustrial Waste. J. Environ. Chem. Eng. 2016, 4, 3217–3229. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, R.; Liu, G.; Chen, C.; He, Y.; Liu, X. Comparison of Methane Production Potential, Biodegradability, and Kinetics of Different Organic Substrates. Bioresour. Technol. 2013, 149, 565–569. [Google Scholar] [CrossRef]
- Ruggeri, B.; Battista, F.; Bernardi, M.; Fino, D.; Mancini, G. The Selection of Pretreatment Options for Anaerobic Digestion (AD): A Case Study in Olive Oil Waste Production. Chem. Eng. J. 2015, 259, 630–639. [Google Scholar] [CrossRef]
- Coimbra, M.A.; Waldron, K.W.; Selvendran, R.R. Isolation and Characterisation of Cell Wall Polymers from the Heavily Lignified Tissues of Olive (Olea Europaea) Seed Hull. Carbohydr. Polym. 1995, 27, 285–294. [Google Scholar] [CrossRef]
- Rodríguez, G.; Lama, A.; Rodríguez, R.; Jiménez, A.; Guillén, R.; Fernández-Bolaños, J. Olive Stone an Attractive Source of Bioactive and Valuable Compounds. Bioresour. Technol. 2008, 99, 5261–5269. [Google Scholar] [CrossRef]
- Pelaez-Samaniego, M.R.; Hummel, R.L.; Liao, W.; Ma, J.; Jensen, J.; Kruger, C.; Frear, C. Approaches for Adding Value to Anaerobically Digested Dairy Fiber. Renew. Sustain. Energy Rev. 2017, 72, 254–268. [Google Scholar] [CrossRef] [Green Version]
- Orive, M. Integrated Biorefinery Process for Olive Pomace Valorisation. Biomass Bioenergy 2021, 149, 106079. [Google Scholar] [CrossRef]
- Najafi, E.; Castro, E.; Karimi, K. Biorefining for Olive Wastes Management and Efficient Bioenergy Production. Energy Convers. Manag. 2021, 244, 114467. [Google Scholar] [CrossRef]

| Abbreviation | The Sequence of Pretreatments Performed |
|---|---|
| Destoning by horizontal screw press or centrifugation experiment | |
| EDOC | Tap water dilution, carbohydrase cocktail enzyme addition |
| DEDOC-H | Tap water dilution, carbohydrase cocktail enzyme addition, stone fragment removal by horizontal screw press |
| DEDOC-C100 | Tap water dilution, carbohydrase cocktail enzyme addition, stone fragment removal by centrifugation at 100× g |
| DEDOC-C200 | Tap water dilution, carbohydrase cocktail enzyme addition, stone fragment removal by centrifugation at 200× g |
| DEDOC-C3000 | Tap water dilution, carbohydrase cocktail enzyme addition, stone fragment removal by centrifugation at 3000× g |
| DDOC T1 | Tap water dilution, soak at 56 °C for 1 h, stone fragment removal by horizontal screw press |
| DDOC T24 | Tap water dilution, soak at 56 °C for 24 h, stone fragment removal by horizontal screw press |
| Anaerobic digestion experiment | |
| DOC | Tap water dilution |
| DDOC | Tap water dilution, stone fragment removal by horizontal screw press |
| EDOC | Tap water dilution, carbohydrase cocktail enzyme addition |
| DEDOC | Tap water dilution, carbohydrase cocktail enzyme, stone fragment removal by horizontal screw press |
| Characteristics | Inoculum | DOC 1 | DDOC 2 | EDOC 3 | DEDOC 4 |
|---|---|---|---|---|---|
| Total Solids (g/kg) | 26.1 ± 0.1 a | 192.0 ± 6.7 c | 80.0 ± 2.4 b | 201.0 ± 27.5 c | 87.0 ± 4.0 b |
| Kjeldahl Nitrogen (%TS) | N.D. | 0.8 ± 0.1 a | 1.4 ± 0.1 b | 0.9 ± 0.2 a | 1.6 ± 0.1 b |
| Crude Fat (%TS) | N.D. | 8.3 ± 0.3 a | 13.9 ± 0.5 c | 10.6 ± 1.0 b | 10.4 ± 0.8 b |
| Volatile Solids (%TS) | 62.4 ± 0.0 a | 97.0 ± 0.3 c | 92.0 ± 0.3 b | 97.0 ± 0.4 c | 92.0 ± 0.4 b |
| Ash (%TS) | 37.6 ± 0.2 c | 3.0 ± 0.4 a | 8.2 ± 0.3 b | 3.2 ± 0.5 a | 7.8 ± 0.4 b |
| pH | 07.9 ± 0.0 b | 4.3 ± 0.2 a | 4.3 ± 0.1 a | 4.2 ± 0.2 a | 4.1 ± 0.1 a |
| Hemicellulose (%TS) | N.D. | 22.9 ± 3.7 b | 16.3 ± 1.1 a | 22.0 ± 3.3 b | 15.1 ± 2.0 a |
| Cellulose (%TS) | N.D. | 15.0 ± 3.5 b | 12.0 ± 0.4 ab | 14.5 ± 2.5 b | 10.0 ± 1.5 a |
| Lignin (%TS) | N.D. | 29.0 ± 2.9 b | 12.1 ± 1.0 a | 24.4 ± 5.2 b | 14.0 ± 1.6 a |
| Total Phenolics (mg GAE/mL) | N.D. | 0.7 ± 0.1 a | 0.8 ± 0.2 a | 1.0 ± 0.1 a | 0.9 ± 0.2 a |
| Reducing Sugar (mg/mL) | N.D. | 10.0 ± 2.0 a | 11.8 ± 3.8 a | 027.9 ± 3.9 b | 27.3 ± 1.3 b |
| Substrate | Specific Methane Yield | References |
|---|---|---|
| (mL CH4/g VS) | ||
| DOC 1 | 209.5 ± 7.5 | N/A |
| EDOC 2 | 298.1 ± 23.7 | N/A |
| Carbohydrate (Theoretical) | 350 | [8] |
| Proteins (Leucine, Theoretical) | 570 | [8] |
| Fats (Lauric Acid, Theoretical) | 950 | [8] |
| Bamboo | 16 | [40] |
| Pine | 59 | [40] |
| Sorghum | 260–380 | [40] |
| Cellulose | 370 | [40] |
| Food Waste | 540 | [40] |
| 2POMW 3 | 213.1 | [41] |
| Cotton Gin Waste | 235.7 | [41] |
| Juice Waste | 446.0 | [41] |
| Winery Waste | 446.2 | [41] |
| Corn Stover | 241 | [42] |
| Vinegar Residue | 253 | [42] |
| Rice Straw | 281 | [42] |
| Chicken Manure | 295 | [42] |
| Substrate | Glucose | Xylose/Galactose | Arabinose | Total | |
|---|---|---|---|---|---|
| (mg/mL) | (mg/mL) | (mg/mL) | (mg/mL) | ||
| DOC 1 | Pre-digestion | 5.34 ± 1.50 c | 5.38 ± 1.49 c | n.d. | 10.72 ± 3.00 c |
| Day 0 | 0.18 ± 0.08 a | 0.17 ± 0.08 a | n.d. | 00.35 ± 0.16 a | |
| Day 15 | n.d. | n.d. | n.d. | n.d. | |
| Day 30 | n.d. | n.d. | n.d. | n.d. | |
| DDOC 2 | Pre-digestion | 6.80 ± 2.48 cd | 6.49 ± 2.33 c | n.d. | 13.29 ± 4.80 c |
| Day 0 | 0.44 ± 0.14 ab | 0.68 ± 0.19 b | n.d. | 01.12 ± 0.33 b | |
| Day 15 | n.d. | n.d. | n.d. | n.d. | |
| Day 30 | n.d. | n.d. | n.d. | n.d. | |
| EDOC 3 | Pre-digestion | 9.88 ± 0.48 d | 6.61 ± 1.90 c | 2.45 ± 0.21 b | 18.94 ± 2.35 c |
| Day 0 | 0.61 ± 0.15 b | 0.44 ± 0.10 ab | 0.18 ± 0.04 a | 01.23 ± 0.29 b | |
| Day 15 | n.d. | n.d. | n.d. | n.d. | |
| Day 30 | n.d. | n.d. | n.d. | n.d. | |
| DEDOC 4 | Pre-digestion | 10.35 ± 0.44 d | 6.23 ± 0.52 c | 2.53 ± 0.16 b | 19.11 ± 0.95 c |
| Day 0 | 0.63 ± 0.02 b | 0.47 ± 0.00 ab | 0.13 ± 0.02 a | 1.23 ± 0.01 b | |
| Day 15 | n.d. | n.d. | n.d. | n.d. | |
| Day 30 | n.d. | n.d. | n.d. | n.d. | |
| Characteristics | Inoculum | DDOC 1 | EDOC 2 |
|---|---|---|---|
| Total Solids (g/kg) | 21.35 ± 0.03 a | 26.55 ± 0.05 b | 29.40 ± 0.45 b |
| Kjeldahl Nitrogen (%TS) | 3.99 ± 0.05 a | 3.69 ± 0.07 b | 3.66 ± 0.01 b |
| Crude Fat (%TS) | 3.18 ± 0.19 a | 3.16 ± 0.31 a | 3.18 ± 0.33 a |
| Volatile Solids (%TS) | 59.08 ± 0.62 a | 66.94 ± 0.43 ab | 73.74 ± 7.78 b |
| Ash (%TS) | 40.92 ± 0.62 b | 33.05 ± 0.43 ab | 26.25 ± 7.78 a |
| pH | 8.59 ± 0.02 c | 7.52 ± 0.02 a | 7.73 ± 0.03 b |
| Hemicellulose (%TS) | 15.59 ± 1.19 a | 16.68 ± 1.20 b | 17.32 ± 1.55 b |
| Cellulose (%TS) | 2.02 ± 0.79 a | 4.64 ± 0.95 b | 4.62 ± 0.51 b |
| Lignin 3 (%TS) | 10.32 ± 0.38 a | 11.68 ± 0.71 b | 11.86 ± 0.61 b |
| Total Phenolics | 0.017 ± 0.001 a | 0.026 ± 0.001 a | 0.019 ± 0.003 a |
| (mg GAE/g) 4 | |||
| Gross Heat of Combustion | 3.11 ± 0.04 a | 3.31 ± 0.17 a | 3.65 ± 0.43 a |
| (kJ/g TS) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tai, P.; Spierling, R.; Carroll, J.; Jung, S. Biochemical Methane Potential of Mechanically and Enzymatically Pretreated Solid Olive Mill Waste. Processes 2023, 11, 865. https://doi.org/10.3390/pr11030865
Tai P, Spierling R, Carroll J, Jung S. Biochemical Methane Potential of Mechanically and Enzymatically Pretreated Solid Olive Mill Waste. Processes. 2023; 11(3):865. https://doi.org/10.3390/pr11030865
Chicago/Turabian StyleTai, Patrick, Ruth Spierling, Jennifer Carroll, and Stephanie Jung. 2023. "Biochemical Methane Potential of Mechanically and Enzymatically Pretreated Solid Olive Mill Waste" Processes 11, no. 3: 865. https://doi.org/10.3390/pr11030865
APA StyleTai, P., Spierling, R., Carroll, J., & Jung, S. (2023). Biochemical Methane Potential of Mechanically and Enzymatically Pretreated Solid Olive Mill Waste. Processes, 11(3), 865. https://doi.org/10.3390/pr11030865









