Characterization of Cassava Starch Extruded Sheets Incorporated with Tucumã Oil Microparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microencapsulation of TO
2.2.1. Spray Drying
2.2.2. Spray Chilling
2.2.3. Spray Drying Followed by Spray Chilling
2.3. Total Carotenoid Content and Carotenoid Retention of Microparticles
2.4. Obtaining Cassava Starch-Based Sheets by Extrusion
2.5. Characterization of the Sheets
2.5.1. Opacity and Color Parameters
2.5.2. Thickness and Mechanical Properties
2.5.3. Moisture Content
2.5.4. Microstructure of Films
2.5.5. Water Solubility
2.6. Statistical Analyses
3. Results and Discussion
3.1. Characterization of TO Microparticles
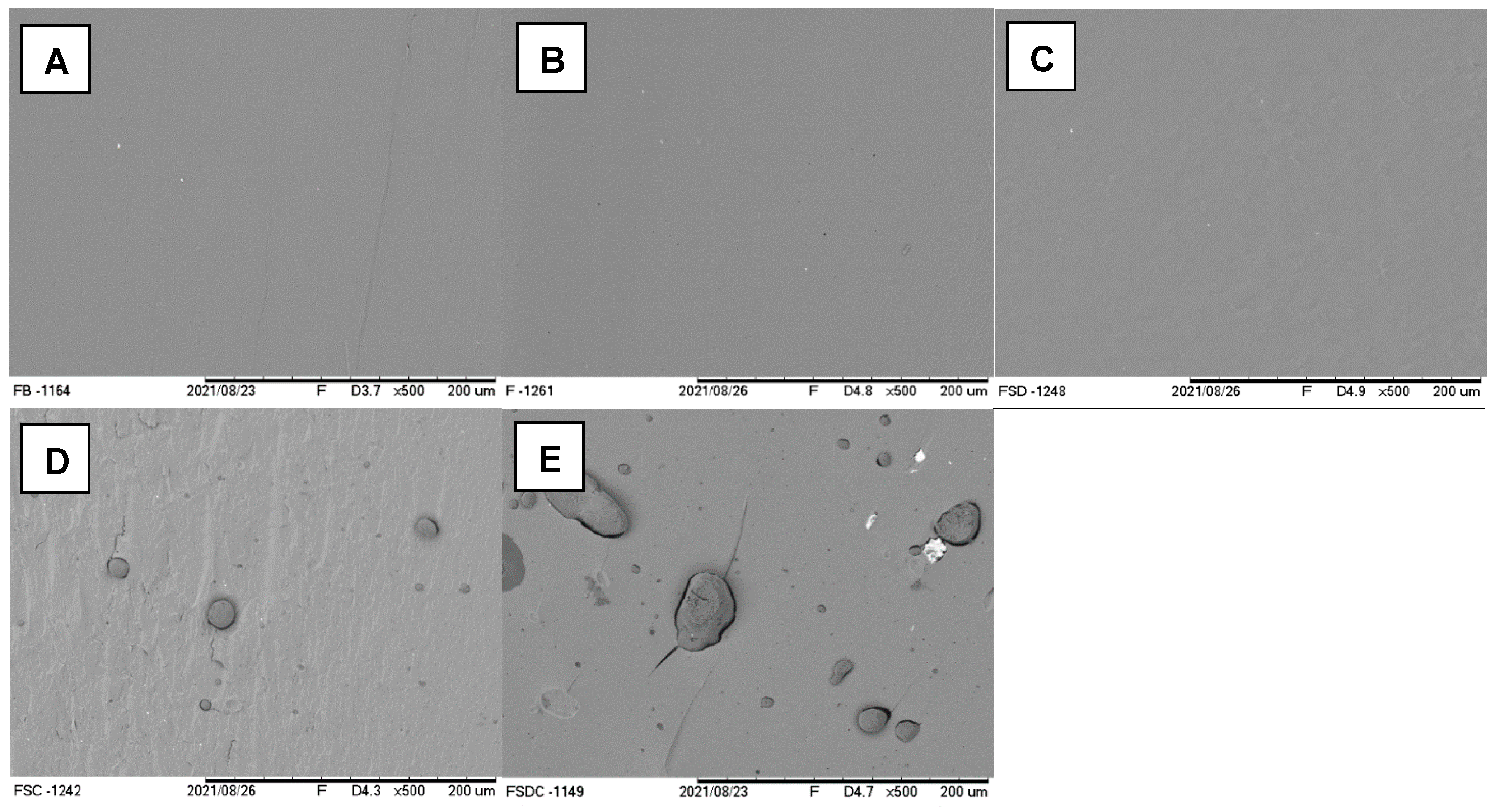
3.2. Characterization of Extruded Cassava Starch-Based Sheet
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brandelli, A.; Brum, L.F.W.; dos Santos, J.H.Z. Nanostructured Bioactive Compounds for Ecological Food Packaging. Environ. Chem. Lett. 2017, 15, 193–204. [Google Scholar] [CrossRef]
- Mackenzie, K.J. Film and Sheeting Materials. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 1–27. [Google Scholar]
- Khezerlou, A.; Tavassoli, M.; Sani, M.A.; Mohammadi, K.; Ehsani, A.; McClements, D.J. Application of Nanotechnology to Improve the Performance of Biodegradable Biopolymer-Based Packaging Materials. Polymers 2021, 13, 4399. [Google Scholar] [CrossRef] [PubMed]
- Mücke, N.; da Silva, T.B.V.; de Oliveira, A.; Moreira, T.F.M.; Venancio, C.D.S.; Marques, L.L.M.; Valderrama, P.; Gonçalves, O.H.; da Silva-Buzanello, R.A.; Marques, L.L.M.; et al. Use of Water-Soluble Curcumin in TPS/PBAT Packaging Material: Interference on Reactive Extrusion and Oxidative Stability of Chia Oil. Food Bioprocess Technol. 2021, 14, 471–482. [Google Scholar] [CrossRef]
- Piñeros-Hernandez, D.; Medina-Jaramillo, C.; López-Córdoba, A.; Goyanes, S. Edible Cassava Starch Films Carrying Rosemary Antioxidant Extracts for Potential Use as Active Food Packaging. Food Hydrocoll. 2017, 63, 488–495. [Google Scholar] [CrossRef]
- Assis, R.Q.; Pagno, C.H.; Costa, T.M.H.; Flôres, S.H.; Rios, A.d.O. Synthesis of Biodegradable Films Based on Cassava Starch Containing Free and Nanoencapsulated β— Carotene. Packag. Technol. Sci. 2018, 31, 157–166. [Google Scholar] [CrossRef]
- Laureanti, E.J.G.; Paiva, T.S.; de Souza Tasso, I.; Dallabona, I.D.; Helm, C.V.; de Matos Jorge, L.M.; Jorge, R.M.M. Development of Active Cassava Starch Films Reinforced with Waste from Industrial Wine Production and Enriched with Pink Pepper Extract. J. Appl. Polym. Sci. 2021, 138, 50922. [Google Scholar] [CrossRef]
- Vedove, T.M.A.R.D.; Maniglia, B.C.; Tadini, C.C. Production of Sustainable Smart Packaging Based on Cassava Starch and Anthocyanin by an Extrusion Process. J. Food Eng. 2021, 289, 110274. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, Y.; Yong, H.; Liu, J.; Zhang, X.; Liu, J. Preparation and Characterization of Active and Intelligent Packaging Films Based on Cassava Starch and Anthocyanins from Lycium Ruthenicum Murr. Int. J. Biol. Macromol. 2019, 134, 80–90. [Google Scholar] [CrossRef]
- Shah, U.; Gani, A.; Ashwar, B.A.; Shah, A.; Ahmad, M.; Gani, A.; Wani, I.A.; Masoodi, F.A. A Review of the Recent Advances in Starch as Active and Nanocomposite Packaging Films. Cogent Food Agric. 2015, 1, 1115640. [Google Scholar] [CrossRef]
- Rodrigues, R.; Patil, S.; Dhakane-Lad, J.; Nadanathangam, V.; Mahapatra, A. Effect of Green Tea Extract, Ginger Essential Oil and Nanofibrillated Cellulose Reinforcements in Starch Films on the Keeping Quality of Strawberries. J. Food Process. Preserv. 2022, 46, e16109. [Google Scholar] [CrossRef]
- Sartori, T.; Menegalli, F.C. Development and Characterization of Unripe Banana Starch Films Incorporated with Solid Lipid Microparticles Containing Ascorbic Acid. Food Hydrocoll. 2016, 55, 210–219. [Google Scholar] [CrossRef]
- Song, X.; Zuo, G.; Chen, F. Effect of Essential Oil and Surfactant on the Physical and Antimicrobial Properties of Corn and Wheat Starch Films. Int. J. Biol. Macromol. 2018, 107, 1302–1309. [Google Scholar] [CrossRef]
- Ghiasi, F.; Golmakani, M.T.; Eskandari, M.H.; Hosseini, S.M.H. A New Approach in the Hydrophobic Modification of Polysaccharide-Based Edible Films Using Structured Oil Nanoparticles. Ind. Crops Prod. 2020, 154, 112679. [Google Scholar] [CrossRef]
- Byun, Y.; Kim, Y.T.; Whiteside, S. Characterization of an Antioxidant Polylactic Acid (PLA) Film Prepared with α-Tocopherol, BHT and Polyethylene Glycol Using Film Cast Extruder. J. Food Eng. 2010, 100, 239–244. [Google Scholar] [CrossRef]
- Albert, K.; Matos, N.; Lima, D.P.; Paula, A.; Barbosa, P.; Mercadante, A.Z.; Chisté, R.C. Peels of Tucumã (Astrocaryum Vulgare) and Peach Palm (Bactris Gasipaes) Are by-Products Classified as Very High Carotenoid Sources. Food Chem. 2019, 272, 216–221. [Google Scholar] [CrossRef]
- Santos, P.D.d.F.; Rubio, F.T.V.; Balieiro, J.C.d.C.; Thomazini, M.; Favaro-Trindade, C.S. Application of Spray Drying for Production of Microparticles Containing the Carotenoid-Rich Tucumã Oil (Astrocaryum Vulgare Mart.). LWT Food Sci. Technol. 2021, 143, 111106. [Google Scholar] [CrossRef]
- Machado, A.P.d.F.; Nascimento, R.d.P.d.; Alves, M.d.R.; Reguengo, L.M.; Marostica Junior, M.R. Brazilian Tucumã-Do-Amazonas (Astrocaryum aculeatum) and Tucumã-Do-Pará (Astrocaryum vulgare) Fruits: Bioactive Composition, Health Benefits, and Technological Potential. Food Res. Int. 2022, 151, 110902. [Google Scholar] [CrossRef]
- Lino, R.C.; de Carvalho, S.M.; Noronha, C.M.; Sganzerla, W.G.; da Rosa, C.G.; Nunes, M.R.; D’Avila, R.F.; Zambiazi, R.C.; Barreto, P.L.M. Production of Methylcellulose Films Functionalized with Poly-ε-Caprolactone Nanocapsules Entrapped β-Carotene for Food Packaging Application. Food Res. Int. 2022, 160, 111750. [Google Scholar] [CrossRef]
- Juan-Polo, A.; Pérez, S.E.M.; Prieto, M.M.; Reig, C.S.; Tone, A.M.; Solana, N.H.; Sanahuja, A.B. Oxygen Scavenger and Antioxidant LDPE/EVOH/PET-Based Films Containing β-Carotene Intended for Fried Peanuts (Arachis Hypogaea L.) Packaging: Pilot Scale Processing and Validation Studies. Polymers 2022, 14, 3550. [Google Scholar] [CrossRef]
- Grune, T.; Lietz, G.; Palou, A.; Ross, A.C.; Stahl, W.; Tang, G.; Thurnham, D.; Yin, S.; Biesalski, H. β-Carotene Is an Important Vitamin A Source for Humans. J. Nutr. 2010, 140, 2268S–2285S. [Google Scholar] [CrossRef] [Green Version]
- Behjati, J.; Yazdanpanah, S. Nanoemulsion and Emulsion Vitamin D3 Fortified Edible Film Based on Quince Seed Gum. Carbohydr. Polym. 2021, 262, 117948. [Google Scholar] [CrossRef] [PubMed]
- Gahruie, H.H.; Eskandari, M.H.; Sadeghi, R.; Hosseini, S.M.H. Atmospheric Pressure Cold Plasma Modification of Basil Seed Gum for Fabrication of Edible Film Incorporated with Nanophytosomes of Vitamin D3 and Tannic Acid. Foods 2023, 12, 71. [Google Scholar] [CrossRef]
- Nascimento, K.; Copetti, P.M.; Fernandes, A.; Klein, B.; Fogaça, A.; Zepka, L.Q.; Wagner, R.; Ourique, A.F.; Sagrillo, M.R.; da Silva, J.E.P. Phytochemical Analysis and Evaluation of the Antioxidant and Antiproliferative Effects of Tucumã Oil Nanocapsules in Breast Adenocarcinoma Cells (MCF-7). Nat. Prod. Res. 2021, 35, 2060–2065. [Google Scholar] [CrossRef]
- Ochoa-Yepes, O.; Di Giogio, L.; Goyanes, S.; Mauri, A.; Famá, L. Influence of Process (Extrusion/Thermo-Compression, Casting) and Lentil Protein Content on Physicochemical Properties of Starch Films. Carbohydr. Polym. 2019, 208, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Balan, G.C.; Paulo, A.F.S.; Correa, L.G.; Alvim, I.D.; Ueno, C.T.; Coelho, A.R.; Ströher, G.R.; Yamashita, F.; Sakanaka, L.S.; Shirai, M.A. Production of Wheat Flour/PBAT Active Films Incorporated with Oregano Oil Microparticles and Its Application in Fresh Pastry Conservation. Food Bioprocess Technol. 2021, 14, 1587–1599. [Google Scholar] [CrossRef]
- Arenas-Jal, M.; Suñé-Negre, J.M.; García-Montoya, E. An Overview of Microencapsulation in the Food Industry: Opportunities, Challenges, and Innovations. Eur. Food Res. Technol. 2020, 246, 1371–1382. [Google Scholar] [CrossRef]
- de Freitas Santos, P.D.; Rubio, F.T.V.; da Silva, M.P.; Pinho, L.S.; Favaro-Trindade, C.S. Microencapsulation of Carotenoid-Rich Materials: A Review. Food Res. Int. 2021, 147, 110571. [Google Scholar] [CrossRef]
- Rubio, F.T.V.; Haminiuk, C.W.I.; Santos, P.D.d.F.; Martelli-Tosi, M.; Thomazini, M.; Balieiro, J.C.d.C.; Makimori, G.Y.F.; Fávaro-Trindade, C.S. Investigation of Brewer’s Spent Yeast as a Bio-Vehicle for Encapsulation of Natural Colorants from Pumpkin (Cucurbita Moschata) Peels. Food Funct. 2022, 13, 10096–10109. [Google Scholar] [CrossRef]
- Gharsallaoui, A.; Roudaut, G.; Chambin, O.; Voilley, A.; Saurel, R. Applications of Spray-Drying in Microencapsulation of Food Ingredients: An Overview. Food Res. Int. 2007, 40, 1107–1121. [Google Scholar] [CrossRef]
- Silva, M.P.; Tulini, F.L.; Matos-Jr, F.E.; Oliveira, M.G.; Thomazini, M.; Fávaro-Trindade, C.S. Application of Spray Chilling and Electrostatic Interaction to Produce Lipid Microparticles Loaded with Probiotics as an Alternative to Improve Resistance under Stress Conditions. Food Hydrocoll. 2018, 83, 109–117. [Google Scholar] [CrossRef]
- Sá, S.H.G.; Mazzocato, M.C.; Saliba, A.S.M.C.; Alencar, S.M.; Favaro-trindade, C.S. Evaluation of the Release, Stability and Antioxidant Activity of Brazilian Red Propolis Extract Encapsulated by Spray-Drying, Spray-Chilling and Using the Combination of Both Techniques. Food Res. Int. 2023, 164, 112423. [Google Scholar] [CrossRef]
- Lima, P.M.d.; Dacanal, G.C.; Pinho, L.S.; Sá, H.S.G.d.; Thomazini, M.; Favaro-Trindade, C.S. Combination of Spray-Chilling and Spray-Drying Techniques to Protect Carotenoid-Rich Extracts from Pumpkin (Cucurbita Moschata) Byproducts, Aiming at the Production of a Powdered Natural Food Dye. Molecules 2022, 27, 7530. [Google Scholar] [CrossRef]
- Llanquileo, L.; Gamboa, A.; Abugoch, L.; Tapia, C. Manufacture of β-Chitin Nano- and Microparticles from Jumbo Squid Pen (Dosidicus Gigas) and Evaluation of Their Effect on Mechanical Properties and Water Vapour Permeability of Polyvinyl Alcohol/Chitosan Films. J. Food Eng. 2021, 290, 110230. [Google Scholar] [CrossRef]
- Perazzo, K.K.N.C.L.; Conceição, A.C.D.V.; Dos Santos, J.C.P.; Assis, D.D.J.; Souza, C.O.; Druzian, J.I. Properties and Antioxidant Action of Actives Cassava Starch Films Incorporated with Green Tea and Palm Oil Extracts. PLoS ONE 2014, 9, e105199. [Google Scholar] [CrossRef] [Green Version]
- Pelissari, J.R.; Souza, V.B.; Pigoso, A.A.; Tulini, F.L.; Thomazini, M.; Rodrigues, C.E.C.; Urbano, A.; Favaro-Trindade, C.S. Production of Solid Lipid Microparticles Loaded with Lycopene by Spray Chilling: Structural Characteristics of Particles and Lycopene Stability. Food Bioprod. Process. 2016, 98, 86–94. [Google Scholar] [CrossRef]
- Carmona, P.A.O.; Garcia, L.C.; Antônio, J.; Ribeiro, D.A.; Valadares, L.F.; Marçal, A.D.F.; França, L.F.D.; Mendonça, S. Effect of Solids Content and Spray-Drying Operating Conditions on the Carotenoids Microencapsulation from Pressed Palm Fiber Oil Extracted with Supercritical CO2. Food Bioprocess Technol. 2018, 11, 1703–1718. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. A Guide to Carotenoid Analysis in Foods; ILSI Press: Washington, DC, USA, 2001; ISBN 1578810728. [Google Scholar]
- McLellan, M.R.; Lind, L.R.; Kime, R.W. Hue Angle Determinations and Statistical Analysis for Multiquadrant Hunter L,a,b Data. J. Food Qual. 1995, 18, 235–240. [Google Scholar] [CrossRef]
- Liu, Y.; Cai, Y.; Jiang, X.; Wu, J.; Le, X. Molecular Interactions, Characterization and Antimicrobial Activity of Curcumin-Chitosan Blend Films. Food Hydrocoll. 2016, 52, 564–572. [Google Scholar] [CrossRef]
- Mei, J.; Yuan, Y.; Wu, Y.; Li, Y. Characterization of Edible Starch-Chitosan Film and Its Application in the Storage of Mongolian Cheese. Int. J. Biol. Macromol. 2013, 57, 17–21. [Google Scholar] [CrossRef]
- Samsalee, N.; Sothornvit, R. Characterization of Food Application and Quality of Porcine Plasma Protein–Based Films Incorporated with Chitosan or Encapsulated Turmeric Oil. Food Bioprocess Technol. 2020, 13, 488–500. [Google Scholar] [CrossRef]
- Ferreira, M.J.A.; Mota, M.F.S.; Mariano, R.G.B.; Freitas, S.P. Evaluation of Liquid-Liquid Extraction to Reducing the Acidity Index of the Tucuma (Astrocaryum Vulgare Mart.) Pulp Oil. Sep. Purif. Technol. 2021, 257, 117894. [Google Scholar] [CrossRef]
- Menezes, E.G.O.; Barbosa, J.R.; Pires, F.C.S.; Ferreira, M.C.R.; de Souza e Silva, A.P.; Siqueira, L.M.M.; de Carvalho Junior, R.N. Development of a New Scale-up Equation to Obtain Tucumã-of-Pará (Astrocaryum Vulgare Mart.) Oil Rich in Carotenoids Using Supercritical CO2 as Solvent. J. Supercrit. Fluids 2022, 181, 105481. [Google Scholar] [CrossRef]
- Vulić, J.; Šeregelj, V.; Kalušević, A.; Lević, S.; Nedović, V.; Šaponjac, V.T.; Čanadanović-Brunet, J.; Ćetković, G. Bioavailability and Bioactivity of Encapsulated Phenolics and Carotenoids Isolated from Red Pepper Waste. Molecules 2019, 24, 2837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoll, L.; Rech, R.; Flôres, S.H.; Nachtigall, S.M.B.; de Oliveira Rios, A. Poly(Acid Lactic) Films with Carotenoids Extracts: Release Study and Effect on Sunflower Oil Preservation. Food Chem. 2019, 281, 213–221. [Google Scholar] [CrossRef]
- Stoll, L.; Domenek, S.; Flôres, S.H.; Nachtigall, S.M.B.; Rios, A.d.O. Polylactide Films Produced with Bixin and Acetyl Tributyl Citrate: Functional Properties for Active Packaging. J. Appl. Polym. Sci. 2021, 138, 50302. [Google Scholar] [CrossRef]
- Chen, H.; Li, L.; Ma, Y.; Mcdonald, T.P.; Wang, Y. Development of Active Packaging Film Containing Bioactive Components Encapsulated in β-Cyclodextrin and Its Application. Food Hydrocoll. 2019, 90, 360–366. [Google Scholar] [CrossRef]
- Tupuna-Yerovi, D.S.; Paese, K.; Flôres, S.H.; Guterres, S.S.; Rios, A. Addition of Norbixin Microcapsules Obtained by Spray Drying in an Isotonic Tangerine Soft Drink as a Natural Dye. J. Food Sci. Technol. 2020, 57, 1021–1031. [Google Scholar] [CrossRef]
- Maia, P.D.D.S.; dos Santos Baião, D.; da Silva, V.P.F.; de Araújo Calado, V.M.; Queiroz, C.; Pedrosa, C.; Valente-Mesquita, V.L.; Pierucci, A.P.T.R. Highly Stable Microparticles of Cashew Apple (Anacardium Occidentale L.) Juice with Maltodextrin and Chemically Modified Starch. Food Bioprocess Technol. 2019, 12, 2107–2119. [Google Scholar] [CrossRef]
- Estevez-Areco, S.; Guz, L.; Famá, L.; Candal, R.; Goyanes, S. Bioactive Starch Nanocomposite Films with Antioxidant Activity and Enhanced Mechanical Properties Obtained by Extrusion Followed by Thermo-Compression. Food Hydrocoll. 2019, 96, 518–528. [Google Scholar] [CrossRef]
- Kurek, M.; Benbettaieb, N.; Ščetar, M.; Chaudy, E.; Repajić, M.; Klepac, D.; Valić, S.; Debeaufort, F.; Galić, K. Characterization of Food Packaging Films with Blackcurrant Fruit Waste as a Source of Antioxidant and Color Sensing Intelligent Material. Molecules 2021, 26, 2569. [Google Scholar] [CrossRef]
- Yousuf, B.; Wu, S.; Gao, Y. Characteristics of Karaya Gum Based Films: Amelioration by Inclusion of Schisandra Chinensis Oil and Its Oleogel in the Film Formulation. Food Chem. 2021, 345, 128859. [Google Scholar] [CrossRef]
- Silva, M.d.F.; Lopes, P.S.; Da Silva, C.F.; Yoshida, C.M.P. Active Packaging Material Based on Buriti Oil—Mauritia Flexuosa L.f. (Arecaceae) Incorporated into Chitosan Films. J. Appl. Polym. Sci. 2016, 133, 1–9. [Google Scholar] [CrossRef]
- Wang, B.; Sui, J.; Yu, B.; Yuan, C.; Guo, L.; Abd El-Aty, A.M.; Cui, B. Physicochemical Properties and Antibacterial Activity of Corn Starch-Based Films Incorporated with Zanthoxylum Bungeanum Essential Oil. Carbohydr. Polym. 2021, 254, 117314. [Google Scholar] [CrossRef]
- Halimatul, M.J.; Sapuan, S.M.; Jawaid, M.; Ishak, M.R.; Ilyas, R.A. Water Absorption and Water Solubility Properties of Sago Starch Biopolymer Composite Films Filled with Sugar Palm Particles. Polimery 2019, 64, 27–35. [Google Scholar] [CrossRef] [Green Version]
- Herniou-Julien, C.; Mendieta, J.R.; Gutiérrez, T.J. Characterization of Biodegradable/Non-Compostable Films Made from Cellulose Acetate/Corn Starch Blends Processed under Reactive Extrusion Conditions. Food Hydrocoll. 2019, 89, 67–79. [Google Scholar] [CrossRef]
- McHugh, T.H.; Avena-Bustillos, R.; Krochta, J.M. Hydrophilic Edible Films: Modified Procedure for Water Vapor Permeability and Explanation of Thickness Effects. J. Food Sci. 1993, 58, 899–903. [Google Scholar] [CrossRef]
- Gutiérrez, T.J.; Tapia, M.S.; Pérez, E.; Famá, L. Structural and Mechanical Properties of Edible Films Made from Native and Modified Cush-Cush Yam and Cassava Starch. Food Hydrocoll. 2015, 45, 211–217. [Google Scholar] [CrossRef]
- Luchese, C.L.; Abdalla, V.F.; Spada, J.C.; Tessaro, I.C. Evaluation of Blueberry Residue Incorporated Cassava Starch Film as pH Indicator in Different Simulants and Foodstuffs. Food Hydrocoll. 2018, 82, 209–218. [Google Scholar] [CrossRef]
- Alasfar, R.H.; Ahzi, S.; Barth, N.; Kochkodan, V.; Khraisheh, M.; Koç, M. A Review on the Modeling of the Elastic Modulus and Yield Stress of Polymers and Polymer Nanocomposites: Effect of Temperature, Loading Rate and Porosity. Polymers 2022, 14, 360. [Google Scholar] [CrossRef]
- Zanela, J.; Casagrande, M.; Radaelli, J.C.; Dias, A.P.; Wagner Júnior, A.; Malfatti, C.R.M.; Yamashita, F. Active Biodegradable Packaging for Foods Containing Baccharis Dracunculifolia Leaf as Natural Antioxidant. Food Bioprocess Technol. 2021, 14, 1301–1310. [Google Scholar] [CrossRef]
| Sample | Total Carotenoids (µg/g) | Carotenoid Retention (%) |
|---|---|---|
| TO | 2679.1 ± 217.1 a | - |
| SD | 366.0 ± 4.0 b | 91 ± 1 a |
| SC | 338.9 ± 9.9 b | 84 ± 2 b |
| SDC | 55.0 ± 0.7 c | 91 ± 1 a |
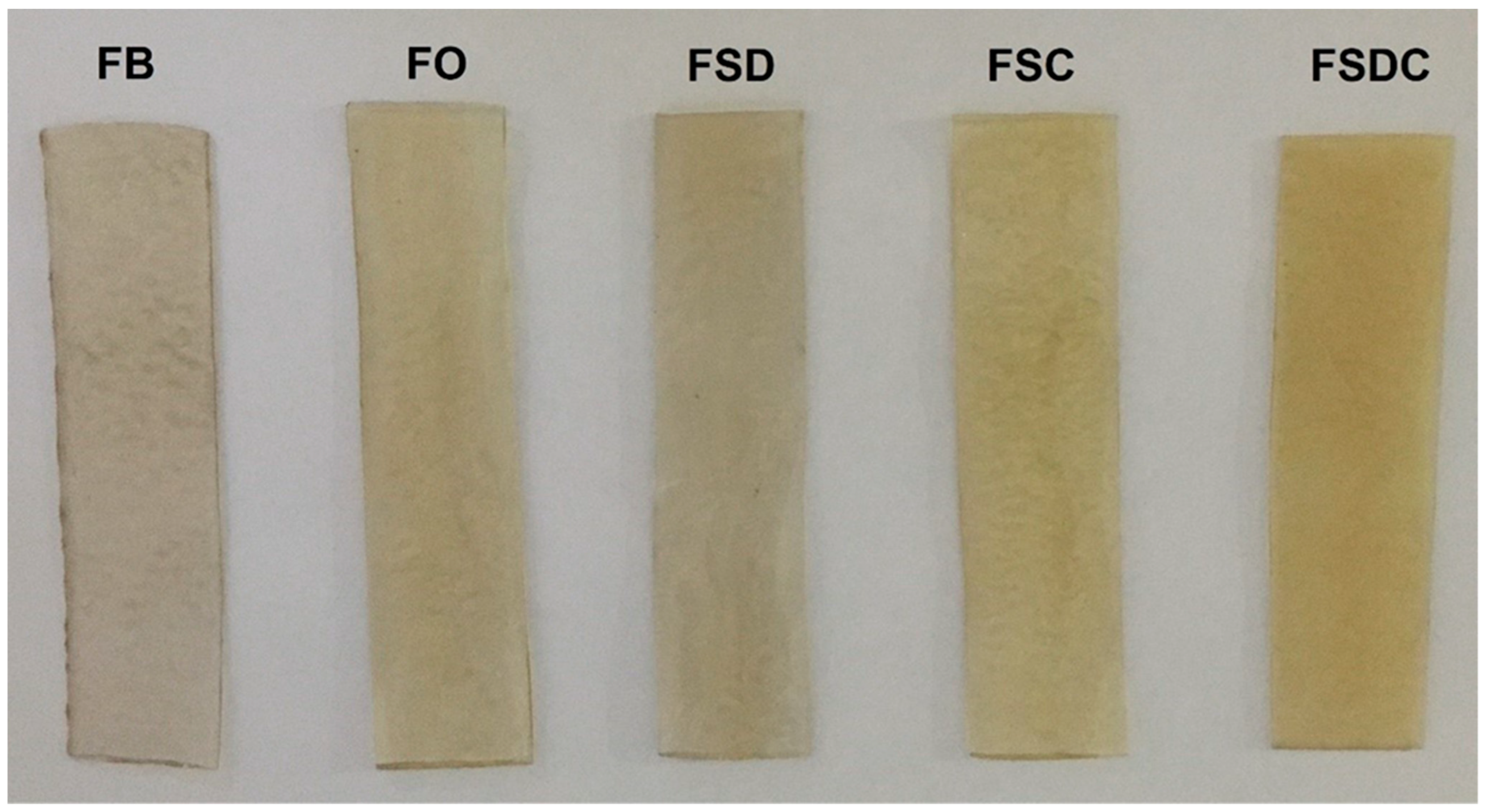
| Sheet | Opacity (%) | H° | C* | ΔE |
|---|---|---|---|---|
| FB | 6.3 ± 0.7 c | 51.45 ± 7.12 b | 1.7 ± 0.2 d | - |
| FO | 11.6 ± 1.0 b | 91.79 ± 0.18 a | 16.1 ± 0.3 c | 10.3 ± 2.9 a |
| FSD | 11.7 ± 0.8 b | 90.78 ± 0.31 a | 21.9 ± 1.0 b | 11.4 ± 1.0 a |
| FSC | 13.8 ± 1.0 b | 89.35 ± 0.51 a | 24.8 ± 1.4 a | 12.6 ± 0.5 a |
| FSDC | 25.3 ± 2.6 a | 86.33 ± 0.08 a | 23.2 ± 0.3 ab | 3.8 ± 1.5 b |
| Sheet | MC (%) | WS (%) |
|---|---|---|
| FB | 18.0 ± 0.3 b | 27.11 ± 0.30 a |
| FO | 19.5 ± 0.4 ab | 24.87 ± 0.11 c |
| FSD | 21.2 ± 0.7 a | 24.67 ± 0.28 c |
| FSC | 19.3 ± 0.6 ab | 25.54 ± 0.34 b |
| FSDC | 19.4 ± 1.6 ab | 24.71 ± 0.05 c |
| Sheet | Thickness (mm) | TS (MPa) | E (%) | YM (MPa) |
|---|---|---|---|---|
| FB | 1.4 ± 0.1 b | 2.4 ± 0.1 a | 74 ± 4 a | 0.260 ± 0.019 a |
| FO | 1.7 ± 0.5 a | 2.4 ± 0.2 a | 57 ± 6 b | 0.236 ± 0.033 a |
| FSD | 1.8 ± 0.4 a | 2.1 ± 0.1 ab | 65 ± 7 ab | 0.247 ± 0.025 a |
| FSC | 1.3 ± 0.2 b | 2.3 ± 0.1 a | 59 ± 7 ab | 0.231 ± 0.016 a |
| FSDC | 1.3 ± 0.1 b | 1.8 ± 0.1 b | 31 ± 4 c | 0.259 ± 0.008 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, P.D.d.F.; Siqueira, L.d.V.; Tadini, C.C.; Favaro-Trindade, C.S. Characterization of Cassava Starch Extruded Sheets Incorporated with Tucumã Oil Microparticles. Processes 2023, 11, 876. https://doi.org/10.3390/pr11030876
Santos PDdF, Siqueira LdV, Tadini CC, Favaro-Trindade CS. Characterization of Cassava Starch Extruded Sheets Incorporated with Tucumã Oil Microparticles. Processes. 2023; 11(3):876. https://doi.org/10.3390/pr11030876
Chicago/Turabian StyleSantos, Priscila Dayane de Freitas, Larissa do Val Siqueira, Carmen Cecilia Tadini, and Carmen Sílvia Favaro-Trindade. 2023. "Characterization of Cassava Starch Extruded Sheets Incorporated with Tucumã Oil Microparticles" Processes 11, no. 3: 876. https://doi.org/10.3390/pr11030876
APA StyleSantos, P. D. d. F., Siqueira, L. d. V., Tadini, C. C., & Favaro-Trindade, C. S. (2023). Characterization of Cassava Starch Extruded Sheets Incorporated with Tucumã Oil Microparticles. Processes, 11(3), 876. https://doi.org/10.3390/pr11030876











