Molecular Insight into the Occurrence Characteristics of Deep Oil with Associated Gas Methane and the Displacement Resistance in Water Flooding in Nano-Pore Throat
Abstract
:1. Introduction
2. Simulation Model and Method
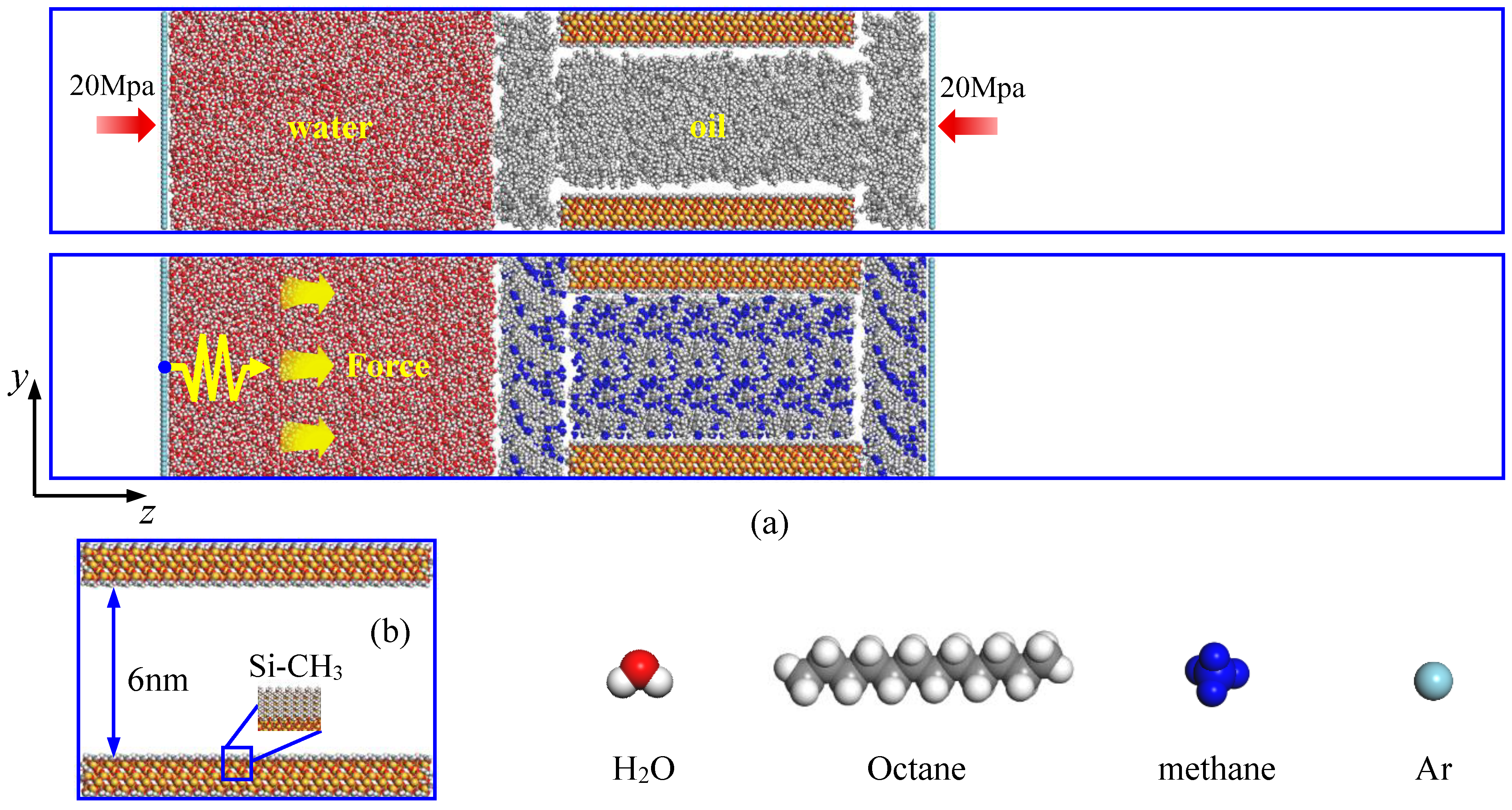
2.1. Simulation Models
2.2. Force Fields
2.3. Equilibrium Molecular Dynamics (EMD)
2.4. Steered Molecular Dynamics (SMD)
3. Results and Discussion
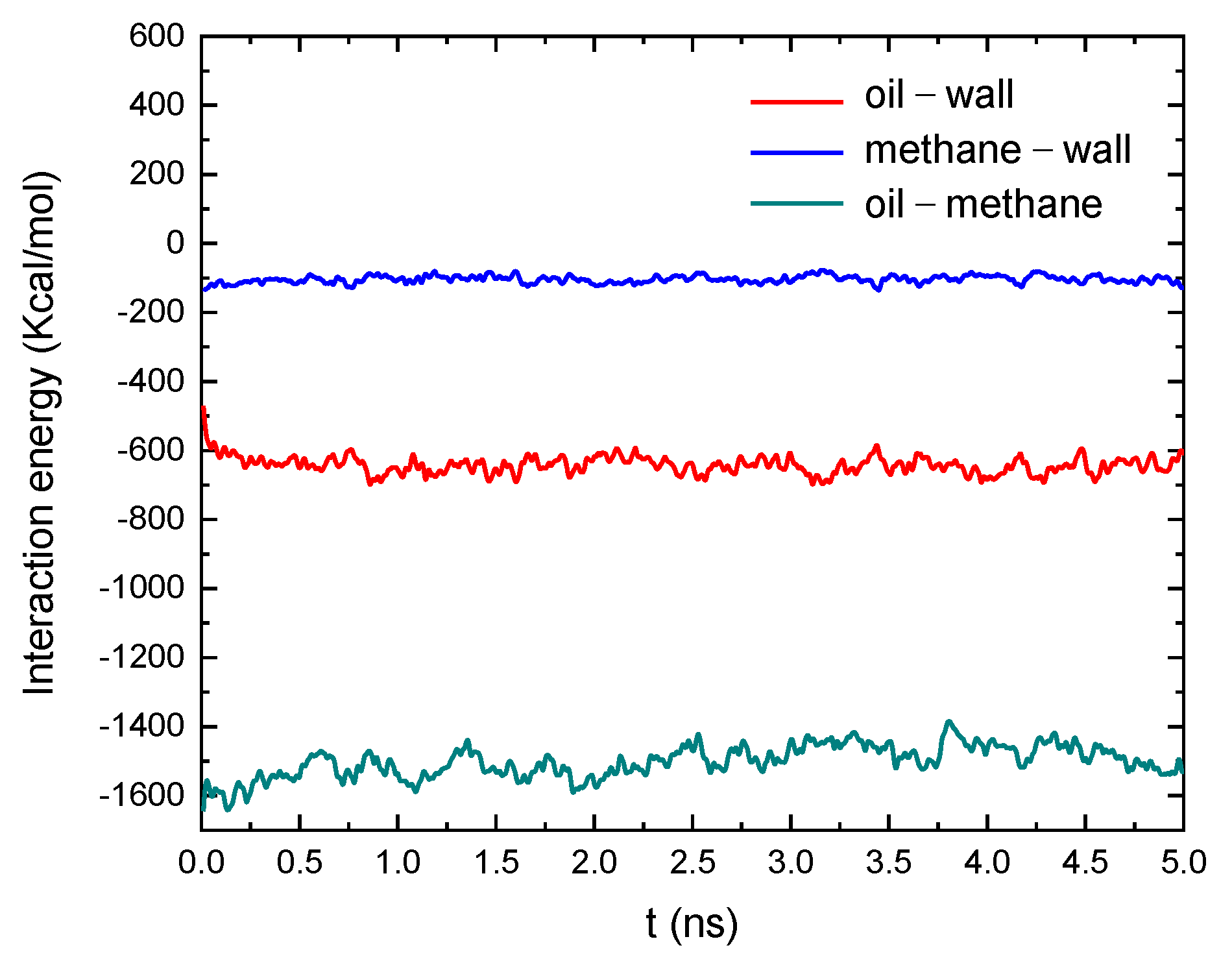
3.1. Microstates and Distribution of the Oil and Methane in the Quartz Slits
3.2. Water Displacement Oil and Gas Process in Nano-Pore Throat
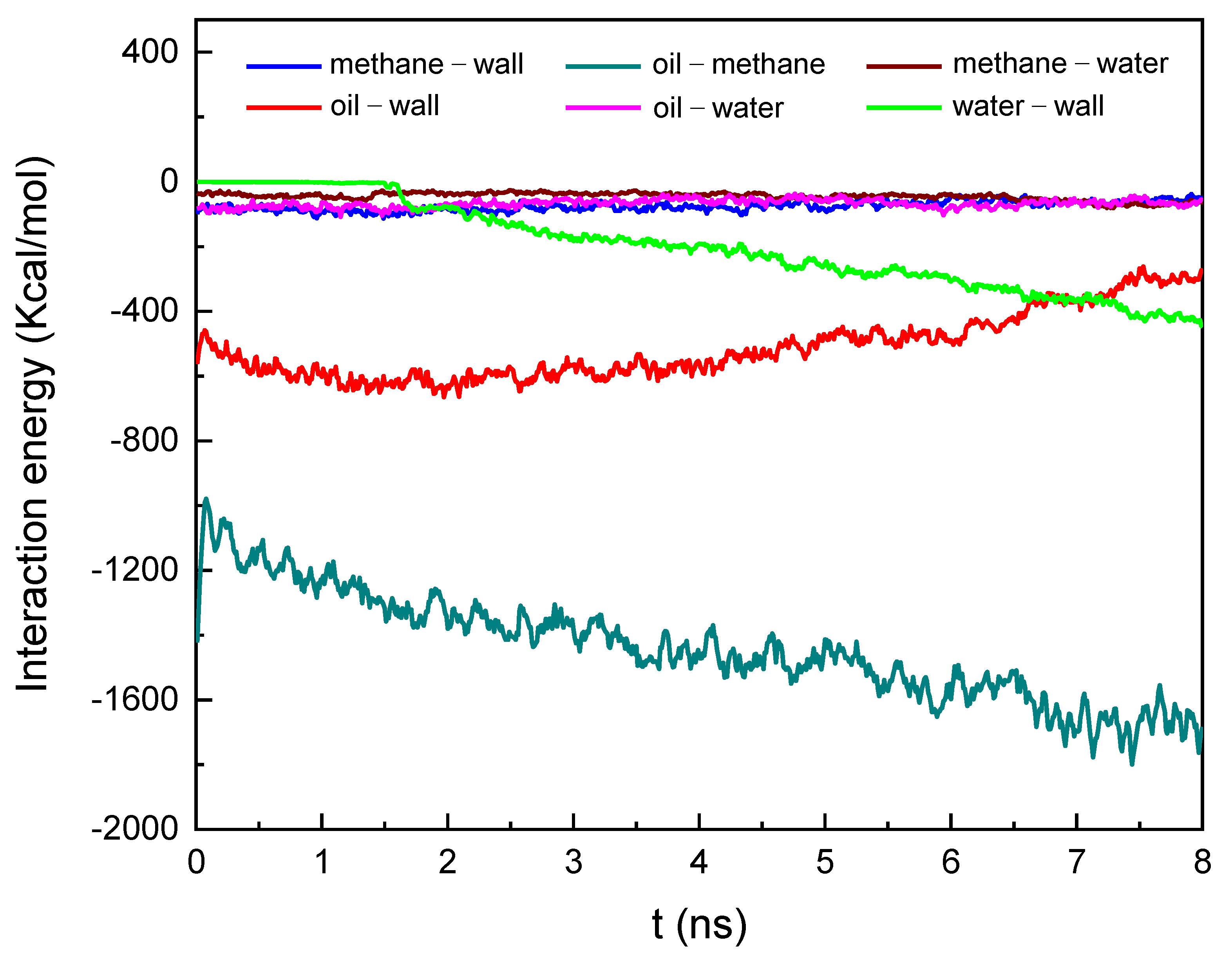
3.2.1. Water Displacement Oil and Gas Process
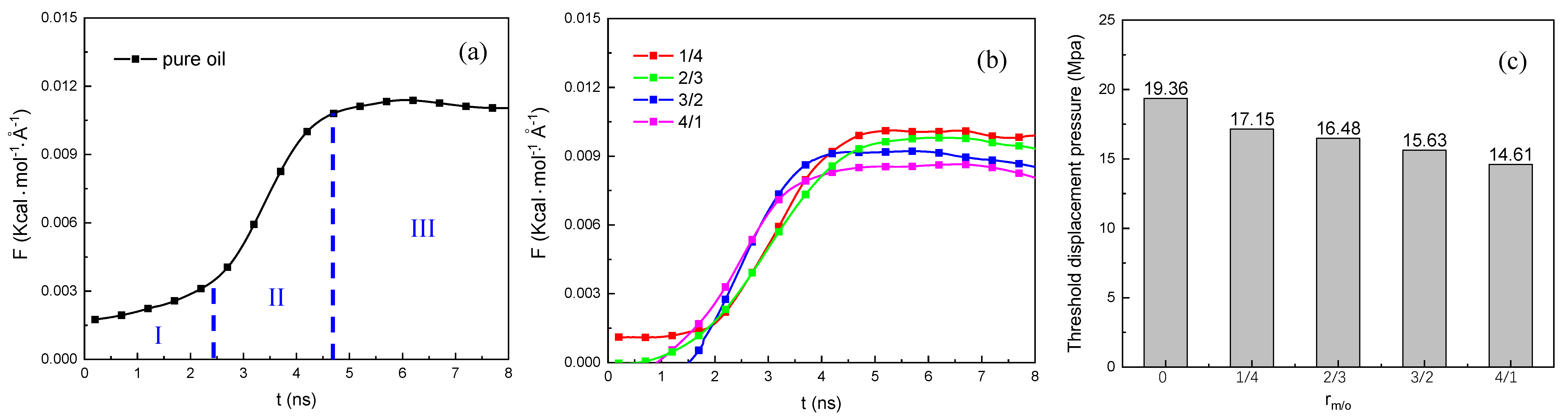
3.2.2. The Threshold Displacement Pressure
3.2.3. The Displacement Rate of Oil and Gas
4. Conclusions
- (1).
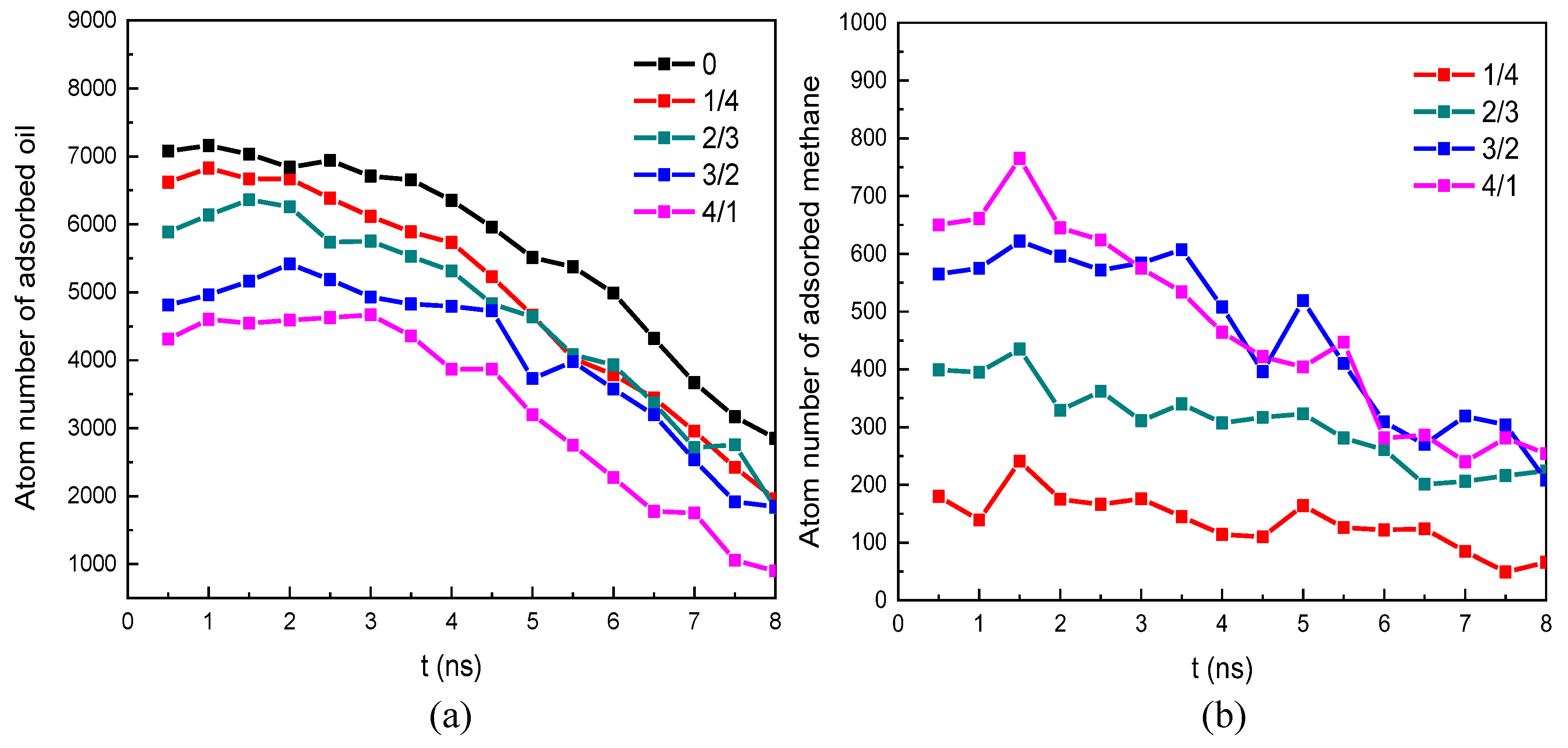
- The occurrence characteristics of oil with associated gas methane in the nano-pore throat indicated that there were two occurrence statuses of oil and methane: free status located within the nano-pore throat and adsorption status on the rock surface. We found methane molecules tend to cluster together and form a free status. Compared to methane, oil molecules were more likely to adsorb on the rock surface. We also found that the adsorption-status oil molecules gradually decrease and the adsorption-status methane molecules gradually increase as the methane content increases, which would be beneficial to improving oil and methane displacement efficiency in the water flooding process.
- (2).
- In the water displacement oil and gas process, the trajectory of the displacement process and the atom number of water in the nano-pore throat showed that the oil and methane displacement efficiency was enhanced as the methane content increased, which could be ascribed to a decreasing amount of adsorbed oil and an increasing amount of adsorbed methane as the methane content increased. In addition, we found that the threshold displacement pressure in the displacement process gradually decreased as the methane content increased as follows: Prm/o=0 > Prm/o=1/4 > Prm/o=2/3 > Prm/o=3/2 > Prm/o=4/1, which could be ascribed to the decrease of the oil/water IFT and the oil viscosity as the methane content increases.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jiao, F.Z. Re-recognition of “unconventional” in unconventional oil and gas. Petrol. Explor. Dev. 2019, 46, 847–855. [Google Scholar] [CrossRef]
- Tian, Y.X.; Qi, Y.M.; Chen, S.J.; Han, H.J.; Wang, H.Y.; Gong, X.Z.; Zhang, M.; Jiang, X.D.; Chen, Y.G. Recombination of hydrogen bonds clipping interfacial film effectively for dehydrated tight oil. Sep. Purif. Technol. 2023, 319, 124093. [Google Scholar] [CrossRef]
- Lior, N. Exergy, Energy, and Gas Flow Analysis of Hydrofractured Shale Gas Extraction. J. Energy Resour. Technol. 2016, 138, 061601. [Google Scholar] [CrossRef]
- Liu, C.Y.; Huang, L.; Zhang, D.D.; Zhao, J.F.; Deng, Y.; Guo, P.; Huang, Y.J.; Wang, J.Q. Genetic causes of oil-rich and oil-poor reservoirs: Implications from two Cenozoic basins in the eastern North China Craton. Sci. China Earth Sci. 2018, 61, 1910–1931. [Google Scholar] [CrossRef]
- You, X.L.; Wu, S.N.; Xu, F. The characteristics and main control factors of hydrocarbon accumulation of ultra-deep marine carbonates in the Tarim Basin, Nw China—A review. Carpath. J. Earth Environ. 2018, 13, 135–146. [Google Scholar] [CrossRef]
- Nurbekova, R.; Smirnova, N.; Goncharev, I.; Sachsenhofer, R.F.; Hazlett, R.; Smirnov, G.; Yensepbayev, T.; Mametov, S.; Fustic, M. High-quality source rocks in an underexplored basin: The upper Carboniferous-Permian succession in the Zaysan Basin (Kazakhstan). Int. J. Coal. Geol. 2023, 272, 104254. [Google Scholar] [CrossRef]
- Zhu, G.Y.; Zou, C.N.; Yang, H.J.; Wang, K.; Zheng, D.M.; Zhu, Y.F.; Wang, Y. Hydrocarbon accumulation mechanisms and industrial exploration depth of large-area fracture-cavity carbonates in the Tarim Basin, western China. J. Petrol. Sci. Eng. 2015, 133, 889–907. [Google Scholar] [CrossRef]
- Sutton, R.P. Fundamental PVT Calculations for Associated and Gas/Condensate Natural-Gas Systems. SPE Res. Eval. Eng. 2007, 10, 270–284. [Google Scholar] [CrossRef]
- Tao, K.; Cao, J.; Chen, X.; Nueraili, Z.; Hu, W. Deep hydrocarbons in the northwestern Junggar Basin (NW China): Geochemistry, origin, and implications for the oil vs gas generation potential of post-mature saline lacustrine source rocks. Mar. Petrol. Geol. 2019, 109, 623–640. [Google Scholar] [CrossRef]
- Su, Y.L.; Zhang, X.; Li, L.; Hao, Y.M.; Zhan, S.Y.; Wang, W.D.; Wu, Z.Y.; Zhang, W.J. Experimental study on microscopic mechanisms and displacement efficiency of N-2 flooding in deep-buried clastic reservoirs. J. Petrol. Sci. Eng. 2022, 208, 109789. [Google Scholar] [CrossRef]
- Zhu, G.Y.; Zhang, Z.Y.; Zhou, X.X.; Yan, L.; Sun, C.H.; Zhao, B. Preservation of Ultradeep Liquid Oil and Its Exploration Limit. Energy Fuels 2018, 32, 11165–11176. [Google Scholar] [CrossRef]
- Zhang, P.F.; Lu, S.F.; Li, J.Q.; Chang, X.C.; Lin, Z.Z.; Chen, G.; Li, J.J.; Liu, J.Z.; Tian, S.S. Evaluating microdistribution of adsorbed and free oil in a lacustrine shale using nuclear magnetic resonance: A theoretical and experimental study. J. Petrol. Sci. Eng. 2022, 212, 110208. [Google Scholar] [CrossRef]
- Li, J.; Wu, Q.Z.; Jin, W.J.; Lu, J.; Nan, Z.Y. Logging evaluation of free-gas saturation and volume content in Wufeng-Longmaxi organic-rich shales in the Upper Yangtze Platform, China. Mar. Petrol. Geol. 2019, 100, 530–539. [Google Scholar] [CrossRef]
- Zhao, H.J.; Lai, Z.P.; Firoozabadi, A. Sorption Hysteresis of Light Hydrocarbons and Carbon Dioxide in Shale and Kerogen. Sci. Rep. 2017, 7, 16209. [Google Scholar] [CrossRef] [PubMed]
- Rezlerová, E.; Jain, S.K.; Lísal, M. Adsorption, Diffusion, and Transport of C1 to C3 Alkanes and Carbon Dioxide in Dual-Porosity Kerogens: Insights from Molecular Simulations. Energy Fuels 2023, 37, 492–508. [Google Scholar] [CrossRef]
- Wu, S.; Luo, W.N.; Fang, S.D.; Sun, Z.; Yan, S.H.; Li, Y.H. Wettability Impact on Nanoconfined Methane Adsorption Behavior: A Simplified Local Density Investigation. Energy Fuels 2022, 36, 14204–14219. [Google Scholar] [CrossRef]
- Devegowda, D.; Sapmanee, K.; Civan, F.; Sigal, R. Phase Behavior of Gas Condensates in Shales Due to Pore Proximity Effects: Implications for Transport, Reserves and Well Productivity. In Proceedings of the SPE Annual Technical Conference and Exhibition, San Antonio, TX, USA, 8–10 October 2012. [Google Scholar] [CrossRef]
- Beal, C. The viscosity of air, water, natural gas, crude oil and its associated gases at oil field temperatures and pressures. Trans. AIME 1946, 165, 94–115. [Google Scholar] [CrossRef]
- Jennings, H.Y., Jr.; Newman, G.H. The effect of temperature and pressure on the interfacial tension of water against methanenormal decane mixtures. SPEJ Soc. Pet. Eng. J. 1971, 11, 171–175. [Google Scholar] [CrossRef]
- Moh, D.Y.; Zhang, H.W.; Sun, S.Y.; Qiao, R. Molecular anatomy and macroscopic behavior of oil extraction from nanopores by CO2 and CH4. Fuel 2022, 324, 124662. [Google Scholar] [CrossRef]
- Fang, T.M.; Li, S.J.; Zhang, Y.N.; Su, Y.L.; Yan, Y.G.; Zhang, J. How the oil recovery in deep oil reservoirs is affected by injected gas types: A molecular dynamics simulation study. Chem. Eng. Sci. 2021, 231, 116286. [Google Scholar] [CrossRef]
- Zhang, J.; Dong, Z.H.; Wang, M.H.; Yan, Y.G. Effects of the Methane Content on the Water−Oil Interface: Insights from the Molecular Level. Energy Fuels 2017, 31, 7026–7032. [Google Scholar] [CrossRef]
- Wu, K.; Chen, Z.; Li, J.; Li, X.; Xu, J.; Dong, X. Wettability effect on nanoconfined water flow. Proc. Natl. Acad. Sci. USA 2017, 114, 3358–3363. [Google Scholar] [CrossRef]
- Akkutlu, I.Y.; Fathi, E. Multiscale gas transport in shales with local kerogen heterogeneities. SPE J. 2012, 17, 1002–1011. [Google Scholar] [CrossRef]
- Yang, T.; Li, X.; Zhang, D. Quantitative dynamic analysis of gas desorption contribution to production in shale gas reservoirs. J. Unconv. Oil Gas Resour. 2015, 9, 18–30. [Google Scholar] [CrossRef]
- Wang, S.; Feng, Q.H.; Javadpour, F.; Yang, Y.B. Breakdown of Fast Mass Transport of Methane through Calcite Nanopores. J. Phys. Chem. C 2016, 120, 14260–14269. [Google Scholar] [CrossRef]
- Xiong, C.M.; Li, S.J.; Ding, B.; Geng, X.F.; Zhang, J.; Yan, Y.G. Molecular insight into the oil displacement mechanism of gas flooding in deep oil reservoir. Chem. Phys. Lett. 2021, 783, 139044. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Fang, T.M.; Ding, B.; Wang, W.D.; Yan, Y.G.; Li, Z.; Guo, W.Y.; Zhang, J. Migration of oil/methane mixture in shale inorganic nano-pore throat: A molecular dynamics simulation study. J. Petrol. Sci. Eng. 2020, 187, 106784. [Google Scholar] [CrossRef]
- Wang, S.; Javadpour, F.; Feng, Q.H. Molecular dynamics simulations of oil transport through inorganic nanopores in shale. Fuel 2016, 171, 74–86. [Google Scholar] [CrossRef]
- Liang, S.X.; Fang, T.M.; Xiong, W.; Ding, B.; Yan, Y.G.; Zhang, J. Oil detachment by modified nanoparticles: A molecular dynamics simulation study. Comput. Mater. Sci. 2019, 170, 109177. [Google Scholar] [CrossRef]
- Qin, Y.; Yang, X.N.; Zhu, Y.F.; Ping, J.L. Molecular Dynamics Simulation of Interaction between Supercritical CO2 Fluid and Modified Silica Surfaces. J. Phys. Chem. C 2008, 112, 12815–12824. [Google Scholar] [CrossRef]
- Lavoie, R.G.; Ouellet, M.; Hamelin, J.; Benard, P. Numerical Implementation of the Multicomponent Potential Theory of Adsorption in Python Using the NIST Refprop Database. Commun. Comput. Phys. 2018, 23, 1602–1625. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Grigera, J.R.; Straatsma, T.P. The missing term in effective pair potentials. J. Phys. Chem. 1987, 91, 6269–6271. [Google Scholar] [CrossRef]
- Dauber-Osguthorpe, P.; Roberts, V.A.; Osguthorpe, D.J.; Wolff, J.; Genest, M.; Hagler, A.T. Structure and energetics of ligand binding to proteins: Escherichia coli dihydrofolate reductase-trimethoprim, a drug-receptor system. Proteins Struct. Funct. Genet. 1988, 4, 31–47. [Google Scholar] [CrossRef] [PubMed]
- Forte, G.; Grassi, A.; Marletta, G. Molecular Modeling of Oligopeptide Adsorption onto Functionalized Quartz Surfaces. J. Phys. Chem. B 2007, 111, 11237–11243. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Maxwell, D.S.; Tirado-Rives, J. Development and testing of the OPLS all-atom force field on conformational energetics and properties of organic liquids. J. Am. Chem. Soc. 1996, 118, 11225–11236. [Google Scholar] [CrossRef]
- Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Nosé, S. A molecular dynamics method for simulations in the canonical ensemble. Mol. Phys. 1984, 52, 255–268. [Google Scholar] [CrossRef]
- Hockney, R.W.; Eastwood, J.W. Computer Simulation Using Particles; CRC Press: Boca Raton, FL, USA, 1988. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. Model. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Izrailev, S.; Stepaniants, S.; Isralewitz, B.; Kosztin, D.; Lu, H.; Molnar, F.; Wriggers, W.; Schulten, K. Steered Molecular Dynamics. Computational Molecular Dynamics: Challenges, Methods, Ideas; Springer: Berlin/Heidelberg, Germany, 1999; pp. 39–65. [Google Scholar]
- Chen, W.; Foster, A.S.; Alava, M.J.; Laurson, L. Stick-slip control in nanoscale boundary lubrication by surface wettability. Phys. Rev. Lett. 2015, 114, 095502. [Google Scholar] [CrossRef]
- Castro, M.A.; Clarke, S.M.; Inaba, A.; Arnold, T.; Thomas, R.K. Competitive adsorption of simple linear alkane mixtures onto graphite. J. Phys. Chem. B 1998, 102, 10528–10534. [Google Scholar] [CrossRef]
- Harrison, A.; Cracknell, R.F.; Krueger-Venus, J.; Sarkisov, L. Branched versus linear alkane adsorption in carbonaceous slit pores. Adsorption 2014, 20, 427–437. [Google Scholar] [CrossRef]
- Dong, H.; Zhu, Q.Y.; Wang, L.; Yue, X.K.; Fang, H.X.; Wang, Z.J.; Liu, S.Y.; Wei, S.X.; Lu, X.Q. Effects of Shale Pore Size and Connectivity on scCO2 Enhanced Oil Recovery: A Molecular Dynamics Simulation Investigation. Langmuir 2023, 39, 6287–6299. [Google Scholar] [CrossRef] [PubMed]
- Sui, H.G.; Zhang, F.Y.; Wang, Z.Q.; Wang, D.S.; Wang, Y.D. Molecular simulations of oil adsorption and transport behavior in inorganic shale. J. Mol. Liq. 2020, 305, 112745. [Google Scholar] [CrossRef]
- Hao, X.S.; Jia, Z.H.; Zhang, L.L.; Wang, X.; Zhang, J.; Zhou, L.X.; Yan, Y.G. Molecular Insight into the Methane Occurrence inside a Shale Nanochannel with Formation Water. Energy Fuel 2023, 37, 1207–1215. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.; Zhang, J.; He, J. Insight into the pressure-induced displacement mechanism for selecting efficient nanofluids in various capillaries. Environ. Sci. Nano 2020, 7, 2785. [Google Scholar] [CrossRef]
- Bosanquet, C.H. On the flow of liquids into capillary tubes. Philos. Mag. Ser. 6 1923, 45, 525–531. [Google Scholar] [CrossRef]
- Oyarzua, E.; Walther, J.H.; Mejia, A.; Zambrano, H.A. Early regimes of water capillary flow in slit silica nanochannels. Phys. Chem. Chem. Phys. 2015, 17, 14731–14739. [Google Scholar] [CrossRef]
- Smith, W.O.; Crane, M.D. The Jamin effect in cylindrical tubes. J. Am. Chem. Soc. 1930, 52, 1345–1349. [Google Scholar] [CrossRef]
- Liang, M.; Yang, S.; Miao, T.; Yu, B. Minimum applied pressure for a drop through an abruptly constricted capillary. Microfluid. Nanofluidics 2015, 19, 1–8. [Google Scholar] [CrossRef]
- Jang, S.S.; Lin, S.T.; Maiti, P.K.; Blanco, M.; Goddard, W.A.; Shuler, P.; Tang, Y.C. Molecular Dynamics Study of a Surfactant-Mediated Decane-Water Interface: Effect of Molecular Architecture of Alkyl Benzene Sulfonate. J. Phys. Chem. B 2004, 108, 12130–12140. [Google Scholar] [CrossRef]
- Ndao, M.; Goujon, F.; Ghoufi, A.; Patrice, M. Coarse-grained modeling of the oil-water-surfactant interface through the local definition of the pressure tensor and interfacial tension. Theor. Chem. Acc. 2017, 136, 21. [Google Scholar] [CrossRef]
- Kunieda, M.; Nakaoka, K.; Liang, Y.; Miranda, C.; Ueda, A.; Takahashi, S.; Okabe, H.; Matsuoka, T. Self-Accumulation of Aromatics at the Oil-Water Interface through Weak Hydrogen Bonding. J. Am. Chem. Soc. 2010, 132, 18281–18286. [Google Scholar] [CrossRef] [PubMed]






| Molar Ratio of Methane to Oil | Density a (g/cm3) | Octane Number | Methane Number |
|---|---|---|---|
| 0 | 0.676 | 788 | 0 |
| 1/4 | 0.624 | 768 | 192 |
| 2/3 | 0.581 | 690 | 460 |
| 3/2 | 0.520 | 600 | 900 |
| 4/1 | 0.519 | 461 | 1844 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, L.; Wang, W.; Yan, Y.; Zhao, C.; Zhong, J.; Liu, Y. Molecular Insight into the Occurrence Characteristics of Deep Oil with Associated Gas Methane and the Displacement Resistance in Water Flooding in Nano-Pore Throat. Processes 2023, 11, 2529. https://doi.org/10.3390/pr11092529
Zhou L, Wang W, Yan Y, Zhao C, Zhong J, Liu Y. Molecular Insight into the Occurrence Characteristics of Deep Oil with Associated Gas Methane and the Displacement Resistance in Water Flooding in Nano-Pore Throat. Processes. 2023; 11(9):2529. https://doi.org/10.3390/pr11092529
Chicago/Turabian StyleZhou, Lixia, Weiming Wang, Youguo Yan, Chengen Zhao, Jiahao Zhong, and Yuqi Liu. 2023. "Molecular Insight into the Occurrence Characteristics of Deep Oil with Associated Gas Methane and the Displacement Resistance in Water Flooding in Nano-Pore Throat" Processes 11, no. 9: 2529. https://doi.org/10.3390/pr11092529
APA StyleZhou, L., Wang, W., Yan, Y., Zhao, C., Zhong, J., & Liu, Y. (2023). Molecular Insight into the Occurrence Characteristics of Deep Oil with Associated Gas Methane and the Displacement Resistance in Water Flooding in Nano-Pore Throat. Processes, 11(9), 2529. https://doi.org/10.3390/pr11092529












