Main Controlling Factors Affecting the Viscosity of Polymer Solution due to the Influence of Polymerized Cations in High-Salt Oilfield Wastewater
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.2. Experimental Methods
2.2.1. Polyacrylamide Mother Liquor Preparation
2.2.2. Effects of Different Ions on the Viscosity of Polymer Solutions
3. Results and Discussion
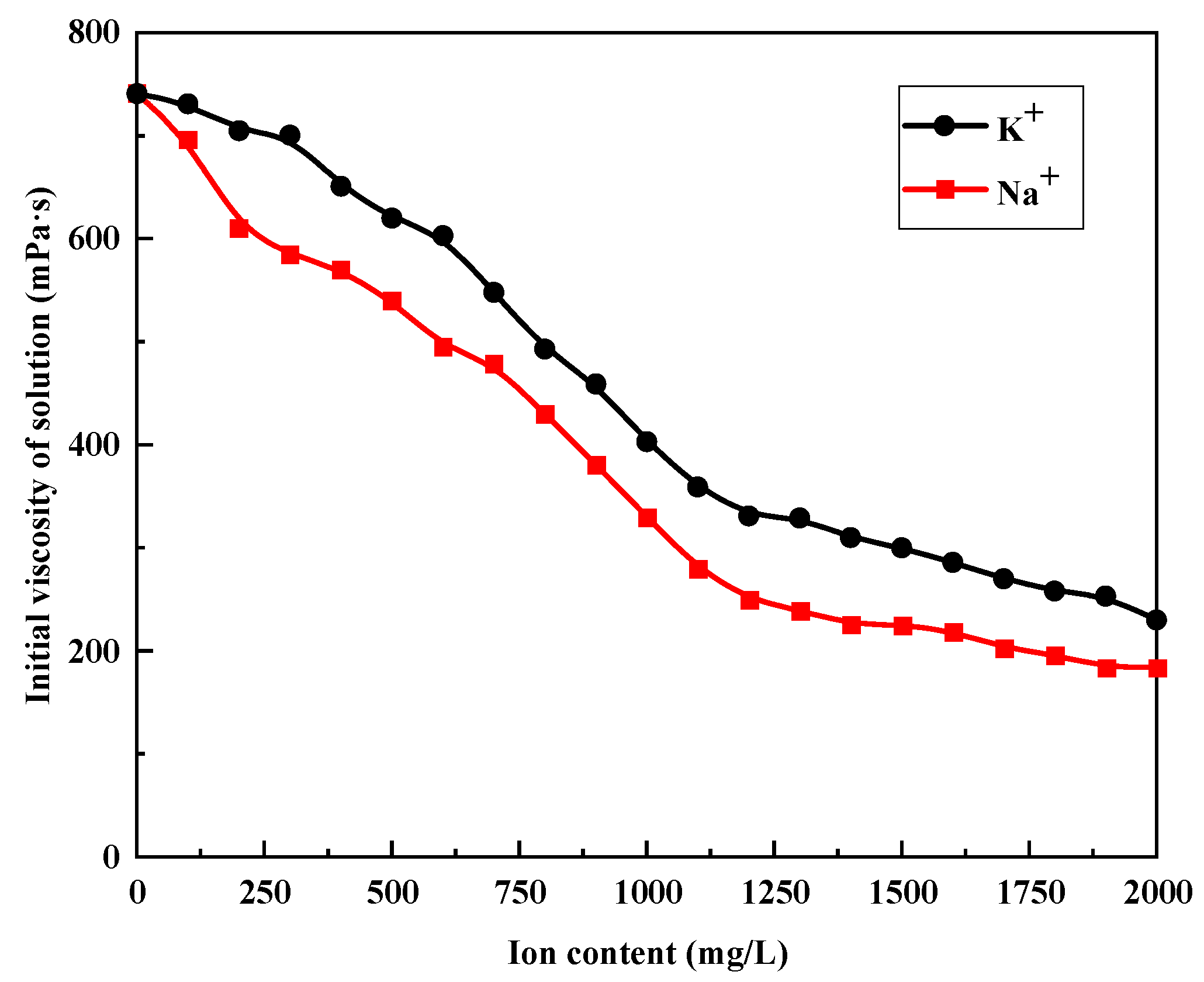
3.1. Effect of K+ and Na+ on the Viscosity of Polymer Solution
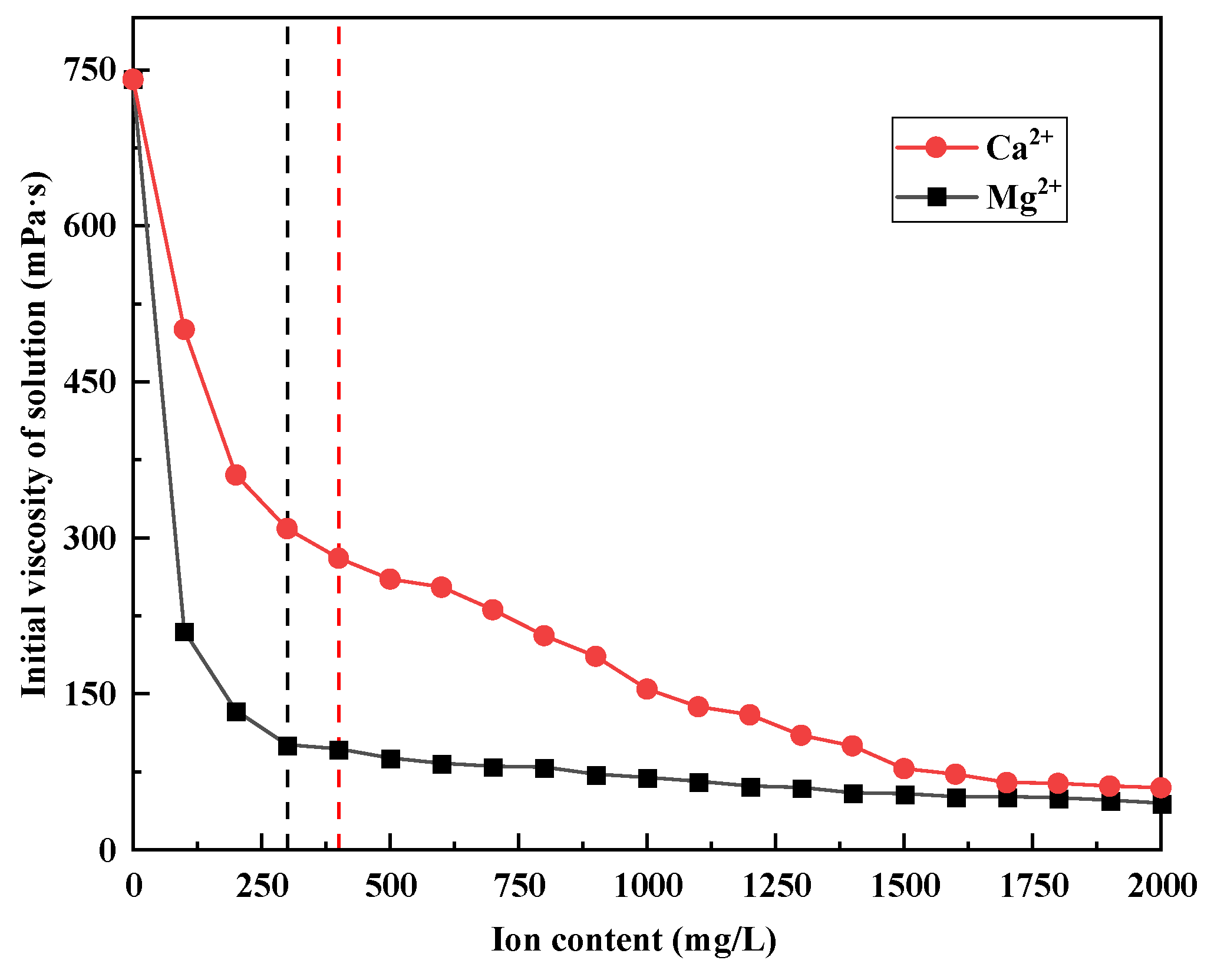
3.2. Effects of Ca2+ and Mg2+ on the Viscosity of Polymer Solutions
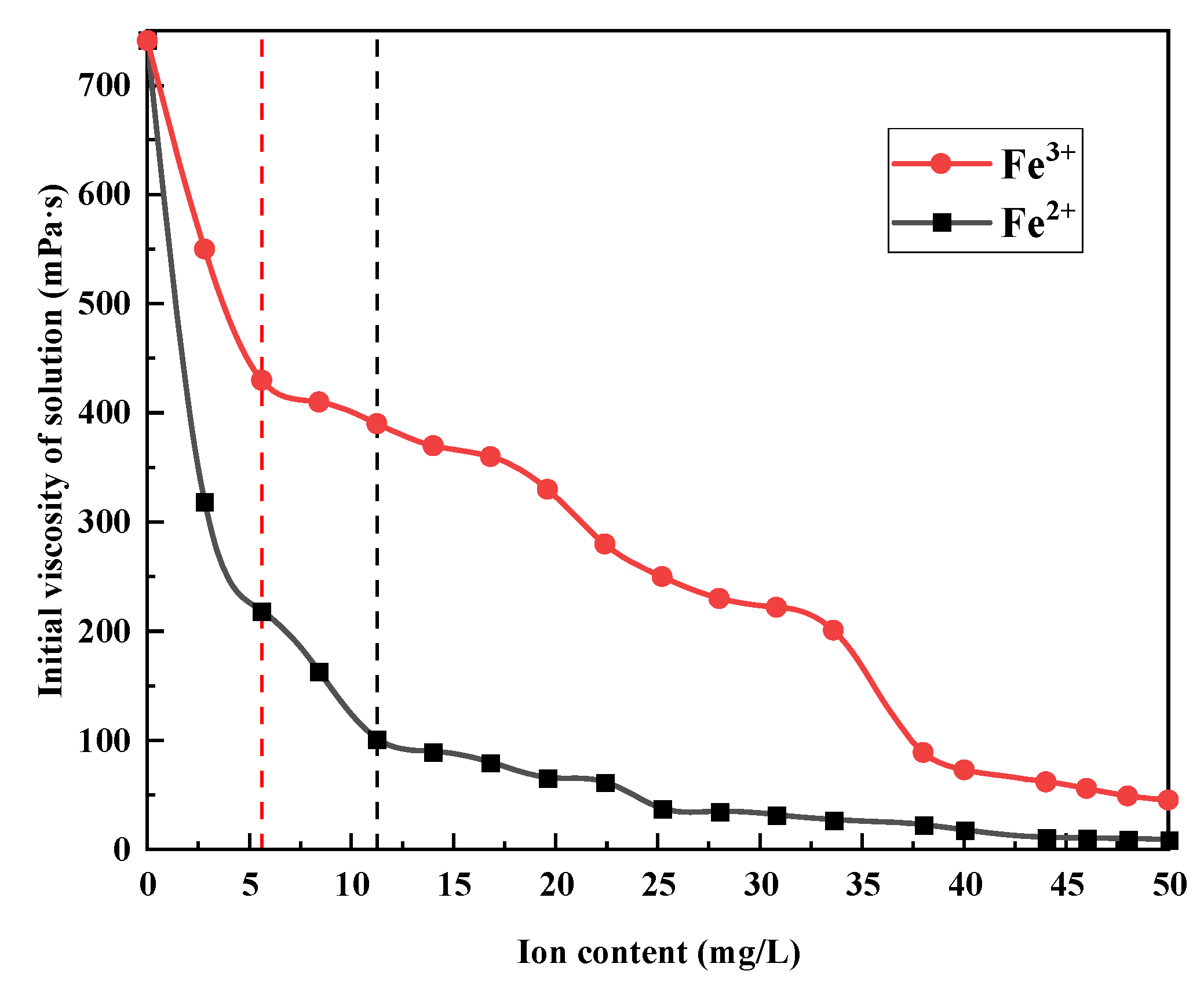
3.3. Effect of Fe2+ and Fe3+ on the Viscosity of Polymer Solution
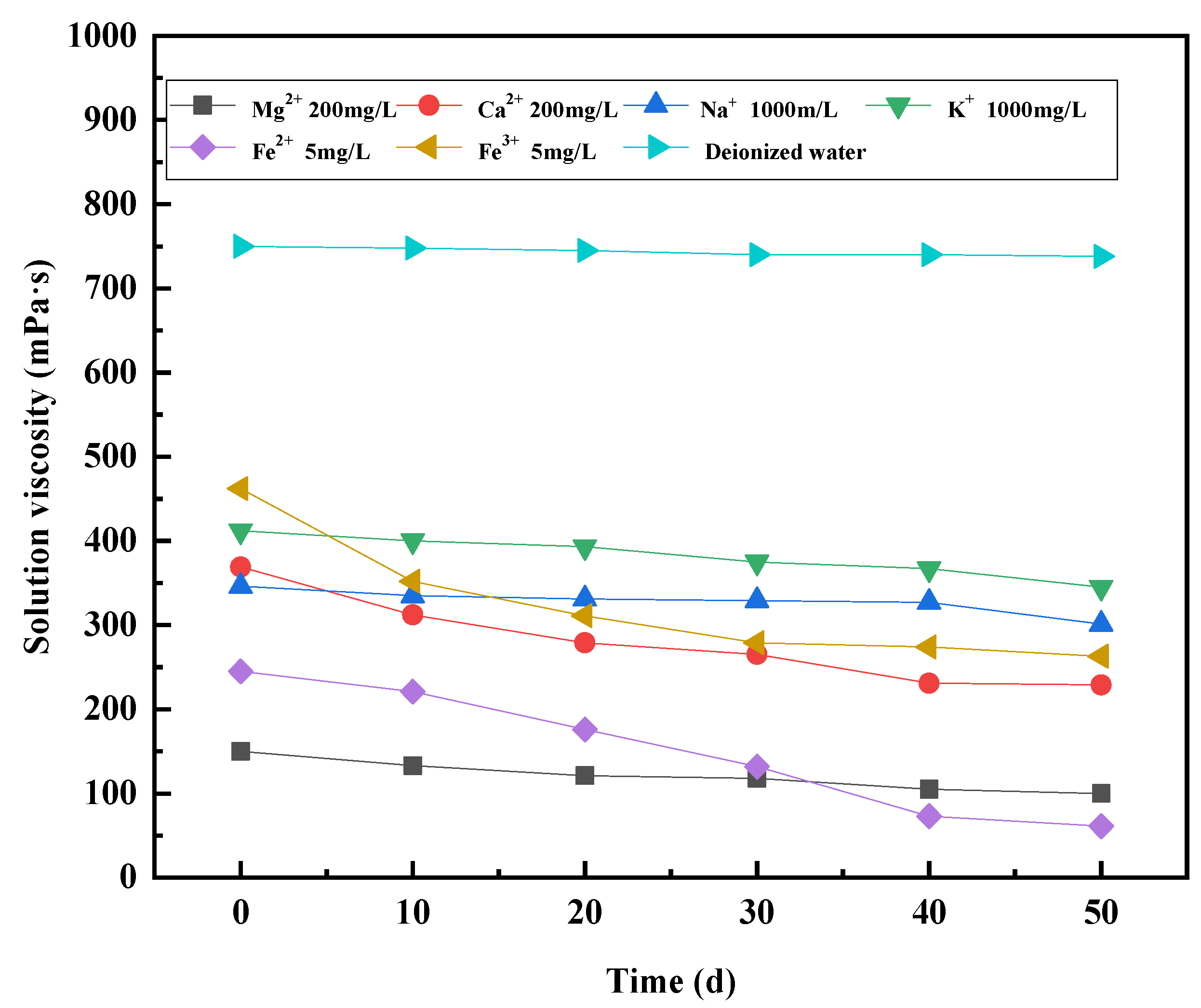
3.4. Effect of Cations on Viscosity Stability of Polymer Solutions
- —viscosity loss intensity, mPa·s/(mg·L−1·d−1);
- —polymer solution viscosity, mPa·s;
- —viscosity of polymer solution after standing, mPa·s;
- _dio—viscosity of polymer solution prepared in deionized water, mPa·s;
- t—viscosity stability test time, d;
- —Cation content in the polymer solution mg·L−1.
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Du, C.; Wang, W.; Wang, Z. The influence mechanism of cations on viscosity loss of polymer solution. J. China Univ. Pet. 2020, 44, 2, 164–168. [Google Scholar]
- Alias, N.H.; Jaafar, J.; Samitsu, S.; Ismail, A.; Othman, M.; Rahman, M.A.; Othman, N.H.; Yusof, N.; Aziz, F.; Mohd, T. Efficient removal of partially hydrolysed polyacrylamide in polymer-flooding produced water using photocatalytic graphitic carbon nitride nanofibres. Arab. J. Chem. 2020, 13, 4341–4349. [Google Scholar] [CrossRef]
- Ding, P.; Dang, W.; Wang, L. Current status and prospects of oilfield produced water reinjection treatment technology. Mod. Chem. 2019, 39, 21–25. [Google Scholar]
- Wang, Z. Situation and countermeasure analysis of upgrading oily sewage quality in Xingbei oilfield. Oil Gas Field Ground Eng. 2020, 39, 47–52. [Google Scholar]
- Wang, H.; Dai, L.; Lu, K. Influence factors of apparent viscosity and molecular chain failure mechanism of polyacrylamide solution used in drilling and production. J. China Univ. Pet. 2022, 46, 115–125. [Google Scholar]
- Zhang, C. Exploration of measures to improve the water quality of oilfield wastewater. China Pet. Chem. Stand. Qual. 2022, 42, 39–41. [Google Scholar]
- Chen, Y. Analysis of factors influencing the treatment effect of extracted water from ternary composite drive in a block and research on treatment measures. Energy Conserv. Pet. Petrochem. Ind. 2019, 9, 24–27+9–10. [Google Scholar]
- Du, Y.; Zhu, Y.; Ji, Y. Effect of salt-resistant mono-mers on viscosity of modified polymers based on the hy-drolyzed poly-acrylamide (HPAM): A molecular dynamics study. J. Mol. Liq. 2021, 325, 115161. [Google Scholar] [CrossRef]
- Sun, L.; Pu, W.; Xin, J. Study on viscosity-affecting factors for alkali/polymer flooding system. Adv. Fine Petrochem. 2007, 25, 11–13+16. [Google Scholar]
- Ren, J.; Zhou, X.; Zhang, D. Analysis on influence factors of formulating polymer solution with sewage and thickening effect. Contemp. Chem. Ind. 2016, 45, 272–275. [Google Scholar]
- Kumar, S.; Panigerhi, P.; Saw, R.K. Interfacial interaction of cationic surfactants and itseffect on wettability alteration of oil-wet carbonate rock. Energy Fuels 2016, 30, 2846–2857. [Google Scholar] [CrossRef]
- Chen, L.; Qian, Z.; Li, L.; Fu, M.; Zhao, H.; Fu, L.; Li, G. Synergism of polyvinyl alcohol fiber to hydrogel for profile modification. Colloids Surf. A Physicochem. Eng. Asp. 2019, 578, 123609. [Google Scholar] [CrossRef]
- Chen, L.; Li, G.; Chen, Y.; Zeng, H.; Mao, Z.; Liu, L.; Wang, X.; Xu, S. Thixotropy research of laponite-hydrogel composites for water shutoff in horizontal wells. J. Pet. Sci. Eng. 2021, 208, 109600. [Google Scholar] [CrossRef]
- Fang, H. Effect of retained polymer in oil produced wastewater on the viscosity of polymer solutions prepared by wastewater. Oilfield Chem. 2021, 38, 173–178. [Google Scholar]
- Wen, F.; Cheng, R. Study and management of polymer solution viscosity instability. J. Shengli Oilfield Staff. Univ. 2006, 20, 47–49. [Google Scholar]
- Fu, M.; Zhou, K.; Zhao, L. Influence of dissolved oxygen in polymer repellent solution on polymer stability. J. Southwest Pet. Inst. 1999, 2, 71–73. [Google Scholar]
- He, J.; Yang, J.; Tang, S.; Yuan, L.; Meng, J. Effects of Fe2+ and S2- on performance of polymer solution. Oilfield Chem. 2015, 32, 370–375. [Google Scholar]
- Zhang, K.; Jiang, W.; Lu, X. A laboratory study on impact of dissolved oxygen to viscosity of polymer solutions prepared in oilfield produced water. Oilfield Chem. 2006, 25, 239–242. [Google Scholar]
- Zhan, Y.; Guo, S.; Yan, G. Study on degradation of partially hydrolysed polyacrylamide. Polym. Bull. 2004, 35, 70–73. [Google Scholar]
- Wu, C.; Su, G.; Chen, S.; Yang, H.; Yu, X. Synthesis and properties evaluation of ultra-high molecular mass salt-resistant polymer. Fine Chem. 2021, 38, 1243–1249+1263. [Google Scholar]
- Guan, S.; Fan, H.; Zhu, J. Examination of viscosity stability of polyacrylamide. J. Northeast. Pet. Univ. 2007, 31, 113–114+121+131–132. [Google Scholar]
- Hu, G. Exploration of viscosity stabilisation technology for polymer deep fluids. Test Min. Technol. 2001, 22, 36–38. [Google Scholar]
- Levitt, D.B.; Slaughter, W.; Pope, G.A.; Jouenne, S. The Effect of Redox Potential and Metal Solubility on Oxidative Polymer Degradation. SPE Reserv. Eval. Eng. 2011, 14, 287–298. [Google Scholar] [CrossRef]
- Chen, L.; Wang, J.; Yu, L.; Zhang, Q.; Fu, M.; Zhao, Z.; Zuo, J. Experimental Investigation on the Nanosilica-Reinforcing Polyacrylamide/Polyethylenimine Hydrogel for Water Shutoff Treatment. Energy Fuels 2018, 32, 6650–6656. [Google Scholar] [CrossRef]
- Tian, J.; Ao, W.; Wu, X. Experimental study on the effect of viscosity of effluent dilution polymer solutions formulated with sewage water. Sci. Technol. Eng. 2016, 16, 166–169. [Google Scholar]
- You, G.; Li, J. Chemical methods for treating produced water from polymer injection oil fields into polymer water. Ind. Water Treat. 2010, 30, 59–61. [Google Scholar]
- Ma, T.; Zhang, X.; Jing, L. Analysis of the main influencing factors of polymer solution viscosity. Chem. Eng. Abstr. 2004, 3, 11–15. [Google Scholar]
- Hu, G.; Xu, J.; Xia, Z. Discussion on viscosity increasing and stabilizing technology of polymer solution. Trial Min. Technol. 2004, 22, 21–22. [Google Scholar]
- Bala, T.; Prasad, B.L.V.; Sastry, M.; Kahaly, M.U.; Waghmare, U.V. Interaction of Different Metal Ions with Carboxylic Acid Group: A Quantitative Study. J. Phys. Chem. A 2007, 111, 6183–6190. [Google Scholar] [CrossRef]
- Li, D.; Zheng, T.; Yu, J.; He, H.; Shi, W.; Ma, J. Enhancement of the electro-Fenton degradation of organic contaminant by accelerating Fe3+/Fe2+ cycle using hydroxylamine. J. Ind. Eng. Chem. 2022, 105, 405–413. [Google Scholar] [CrossRef]
- Quadrado, R.F.; Fajardo, A.R. Fast decolorization of azo methyl orange via heterogeneous Fenton and Fenton-like reactions using alginate-Fe2+/Fe3+ films as catalysts. Carbohydr. Polym. 2017, 177, 443–450. [Google Scholar] [CrossRef]
- Jiang, C.; Pang, S.; Ouyang, F.; Ma, J.; Jiang, J. A new insight into Fenton and Fenton-like processes for water treatment. J. Hazard. Mater. 2010, 174, 813–817. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Peng, J.; Duan, X.; Yin, H.; Huang, B.; Zhou, C.; Lai, B. Redox-active polymers as robust electron-shuttle co-catalysts for fast Fe3+/Fe2+ circulation and green fenton oxidation. Environ. Sci. Technol. 2023, 57, 3334–3344. [Google Scholar] [CrossRef] [PubMed]
- Ling, X.; Cai, A.; Chen, M.; Sun, H.; Xu, S.; Huang, Z.; Deng, J. A comparison of oxidation and re-flocculation behaviors of Fe2+/PAA and Fe2+/H2O2 treatments for enhancing sludge dewatering: A mechanism study. Sci. Total Environ. 2022, 847, 157690. [Google Scholar] [CrossRef] [PubMed]



| Viscosity (mPa·s) | Equivalent Na+ Mass Concentration (mg/L) | Ca2+ Content (mg/L) | Mg2+ Content (mg/L) |
|---|---|---|---|
| 741 | 0 | 0 | 0 |
| 600 | 330.817 | 61.652 | 25.913 |
| 550 | 432.625 | 78.589 | 43.746 |
| 500 | 556.326 | 89.485 | 70.452 |
| 450 | 641.234 | 97.356 | 80.689 |
| 330 | 964.558 | 259.554 | 85.773 |
| 250 | 1624.822 | 615.325 | 110.519 |
| 130 | 2520.061 | 1354.113 | 190.258 |
| Viscosity (mPa·s) | Equivalent Mg2+ Mass Concentration (mg/L) | Fe2+ Content (mg/L) | Fe3+ Content (mg/L) |
|---|---|---|---|
| 741 | 0 | 0 | 0 |
| 600 | 25.939 | 1.122 | 2.139 |
| 550 | 43.732 | 1.613 | 2.732 |
| 500 | 70.426 | 1.882 | 3.926 |
| 450 | 80.664 | 2.481 | 5.066 |
| 330 | 85.779 | 2.615 | 19.620 |
| 250 | 110.510 | 4.130 | 25.217 |
| 130 | 190.275 | 9.580 | 36.470 |
| Ion Type | (mPa·s/(mg·L−1·d−1)) | Ion Type | (mPa·s/(mg·L−1·d−1)) |
|---|---|---|---|
| Na+ | 1.5~7.6 | Fe2+ | 82.4~152.1 |
| Ca2+ | 19.2~100.8 | Fe3+ | 42.5~60.4 |
| Mg2+ | 27.06~110.5 | K+ | 0.029~8.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.; Fu, M.; Li, M.; Luo, Y.; Ni, S.; Hou, L. Main Controlling Factors Affecting the Viscosity of Polymer Solution due to the Influence of Polymerized Cations in High-Salt Oilfield Wastewater. Processes 2024, 12, 791. https://doi.org/10.3390/pr12040791
Hu J, Fu M, Li M, Luo Y, Ni S, Hou L. Main Controlling Factors Affecting the Viscosity of Polymer Solution due to the Influence of Polymerized Cations in High-Salt Oilfield Wastewater. Processes. 2024; 12(4):791. https://doi.org/10.3390/pr12040791
Chicago/Turabian StyleHu, Jiani, Meilong Fu, Minxuan Li, Yuting Luo, Shuai Ni, and Lijuan Hou. 2024. "Main Controlling Factors Affecting the Viscosity of Polymer Solution due to the Influence of Polymerized Cations in High-Salt Oilfield Wastewater" Processes 12, no. 4: 791. https://doi.org/10.3390/pr12040791
APA StyleHu, J., Fu, M., Li, M., Luo, Y., Ni, S., & Hou, L. (2024). Main Controlling Factors Affecting the Viscosity of Polymer Solution due to the Influence of Polymerized Cations in High-Salt Oilfield Wastewater. Processes, 12(4), 791. https://doi.org/10.3390/pr12040791





