Scaling Fed-Batch and Perfusion Antibody Production Processes in Geometrically Dissimilar Stirred Bioreactors
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Line and Medium
2.2. Inoculum Production
2.3. Fed-Batch Cultivations
- Shake flasks, 250 mL (Corning).
- An Ambr 250 modular (Sartorius).
- A 3 L HyPerforma Glass bioreactor (Thermo Scientific); controlled by a HyPerforma G3Lab Bioprocess Controller.
- A 50 L HyPerforma DynaDrive S.U.B. (Thermo Scientific); controlled by a HyPerforma G3Pro Bioprocess Controller.
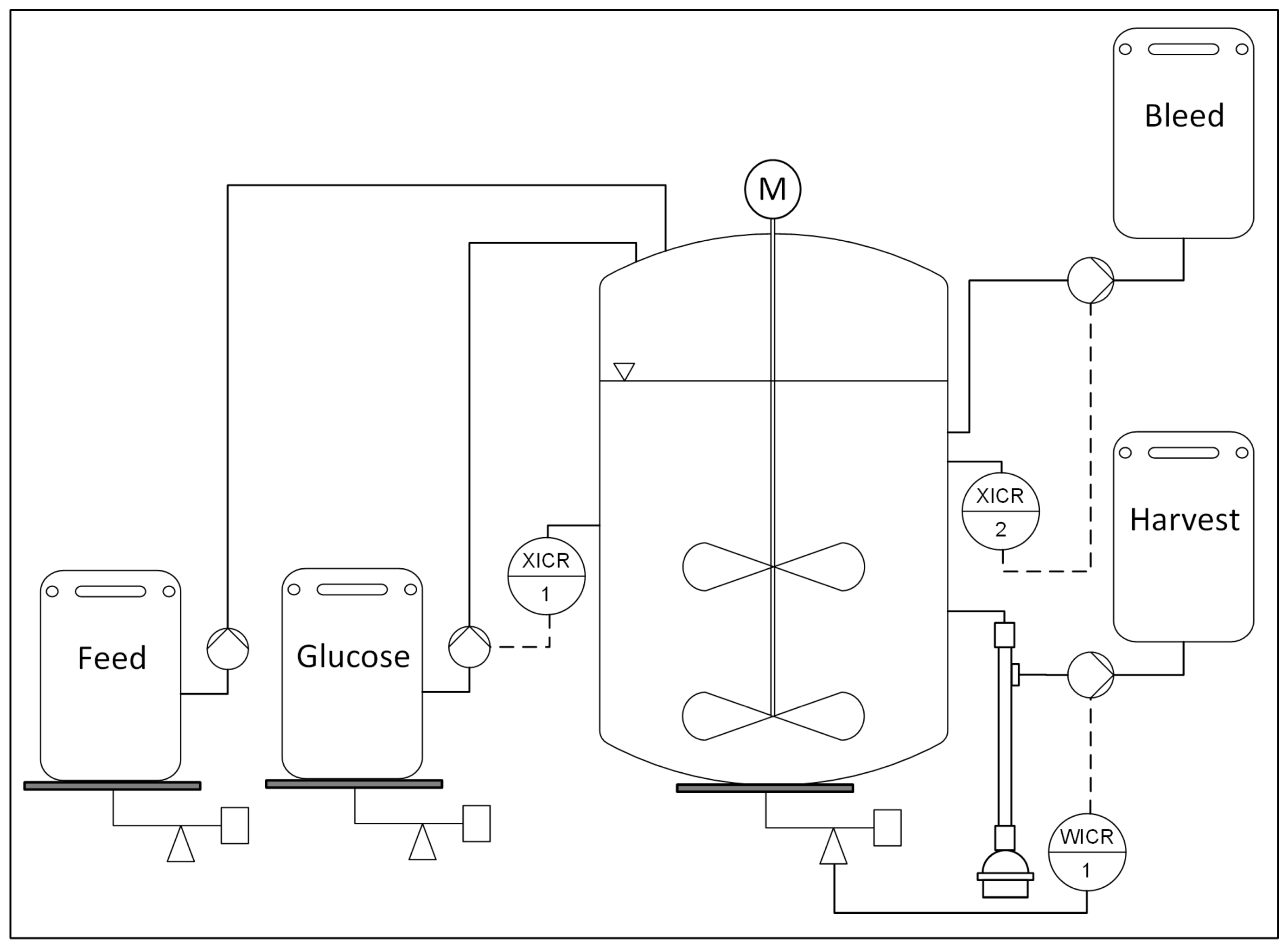
2.4. Perfusion Cultivations at 2 L and 50 L Scale
2.5. Analytics
3. Results
3.1. Fed Batch
3.1.1. Scaling Considerations
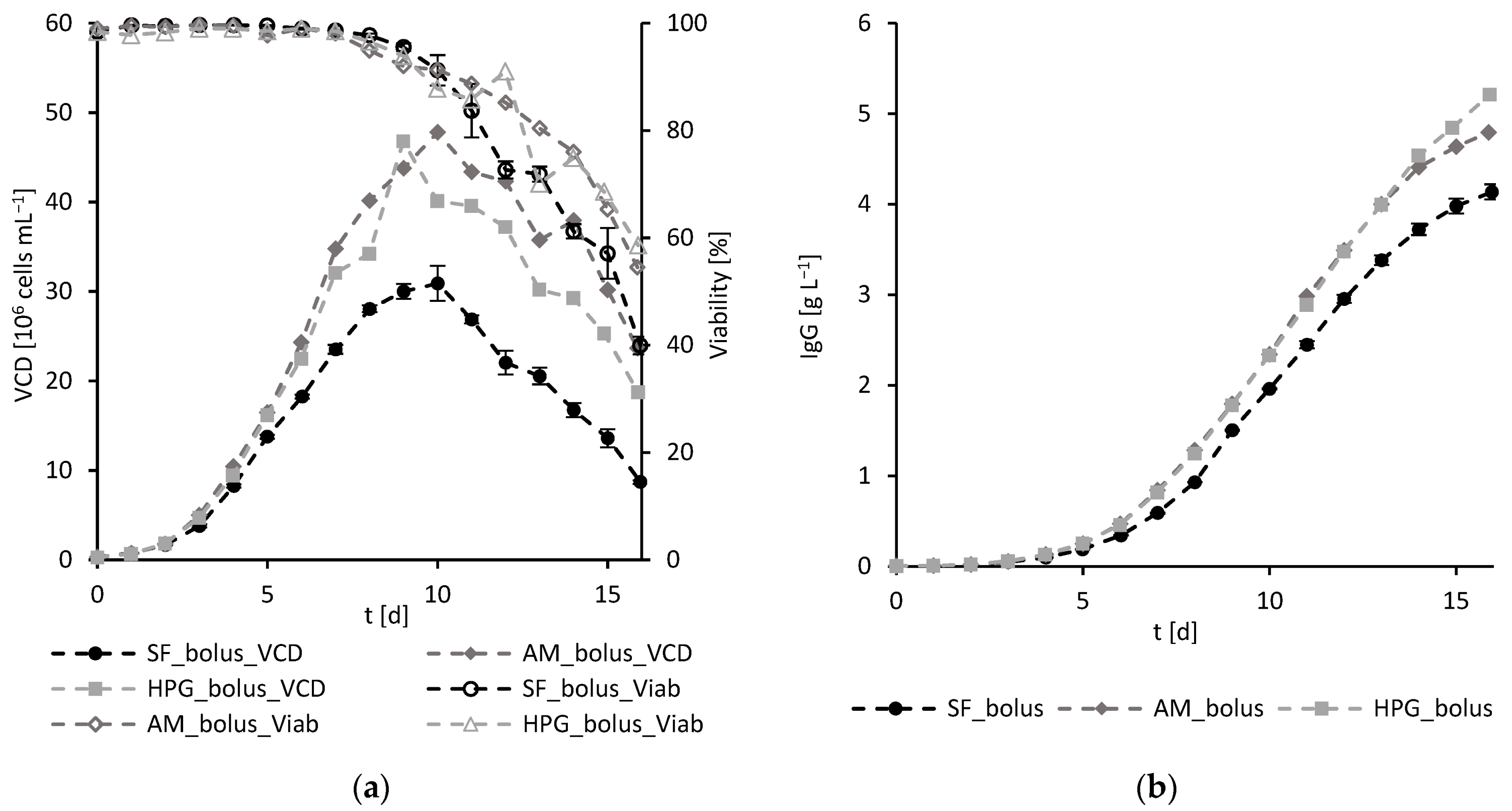
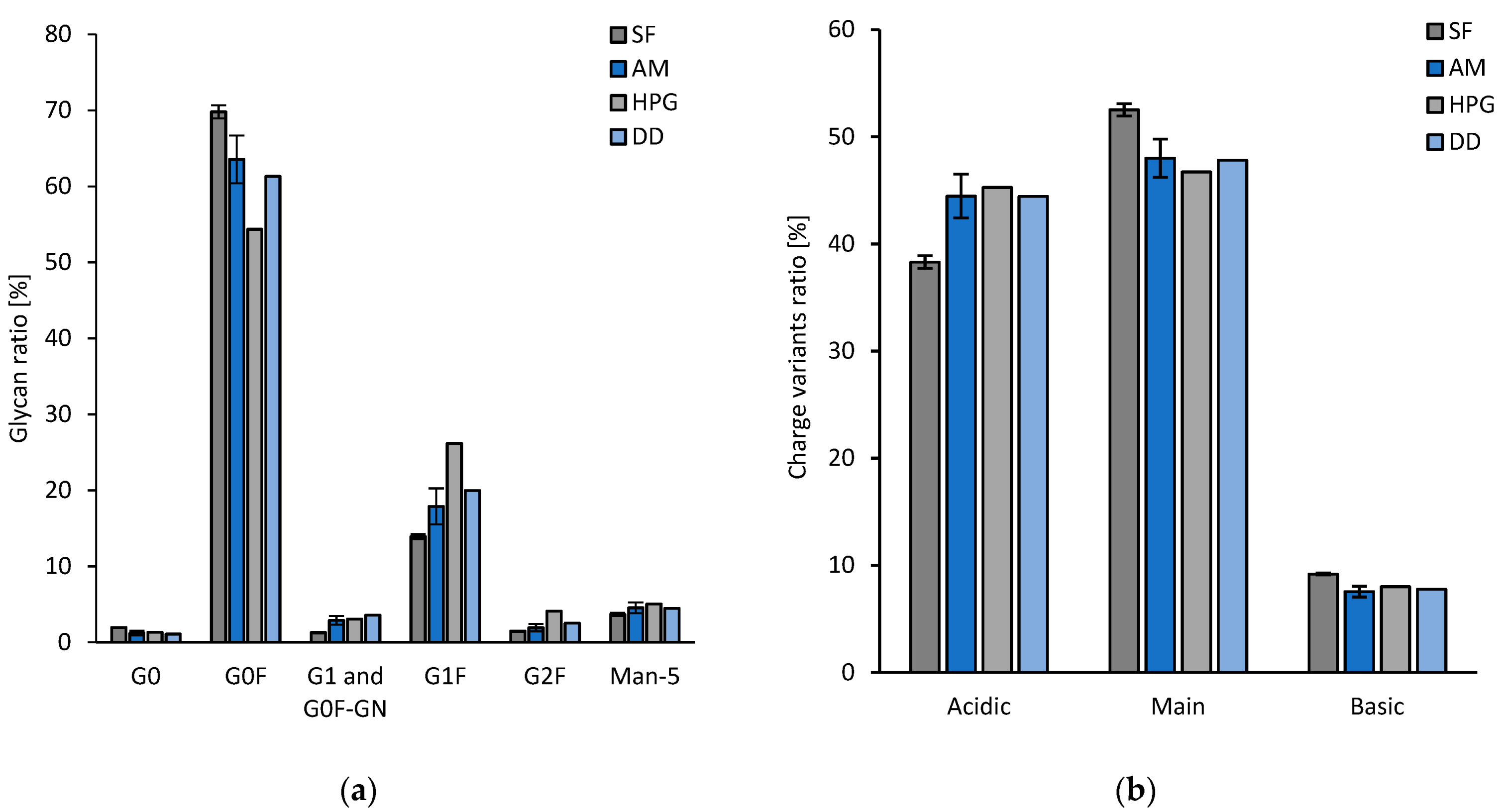
3.1.2. Experimental Results
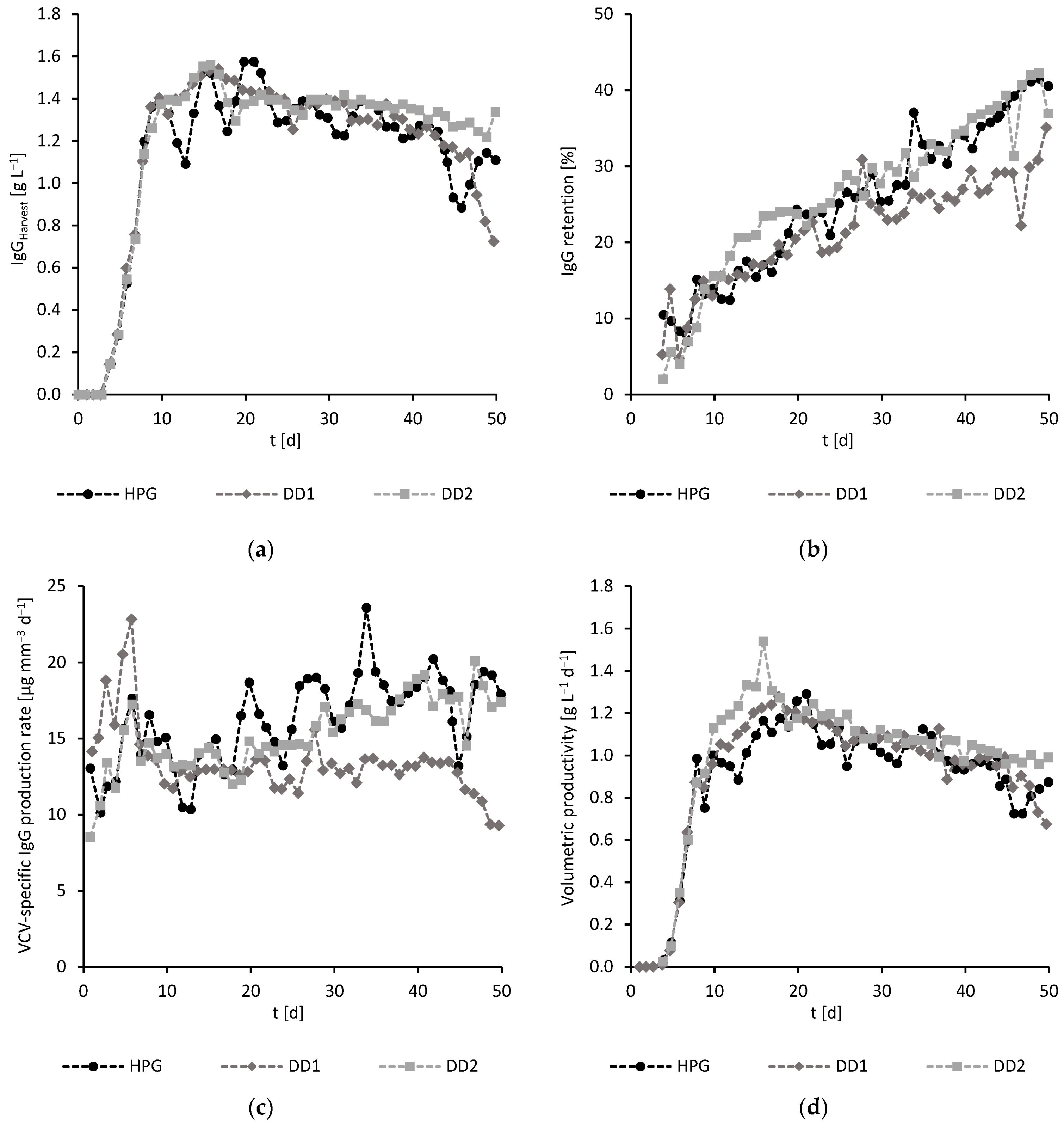
3.2. Perfusion
3.2.1. Scaling Considerations
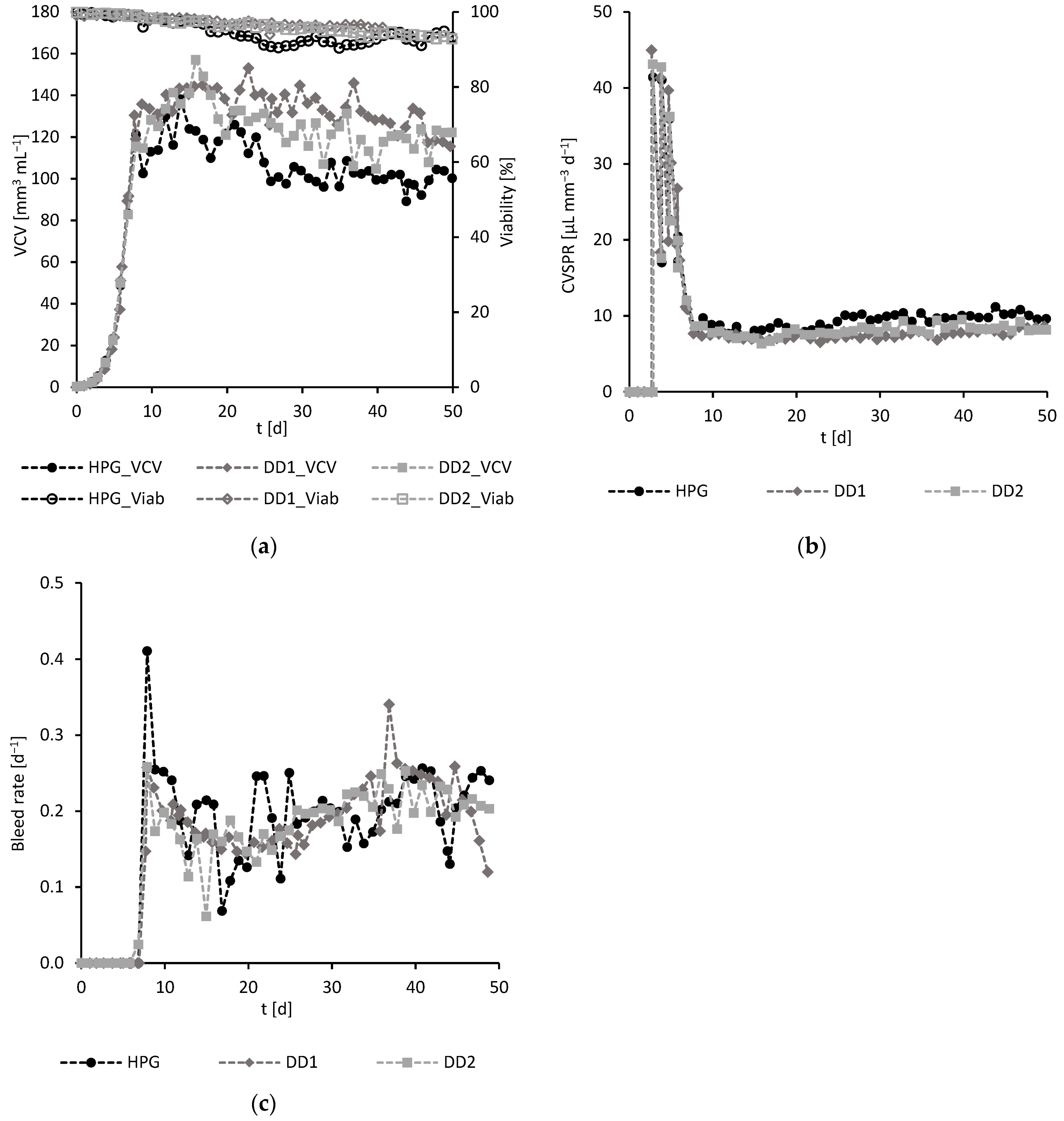
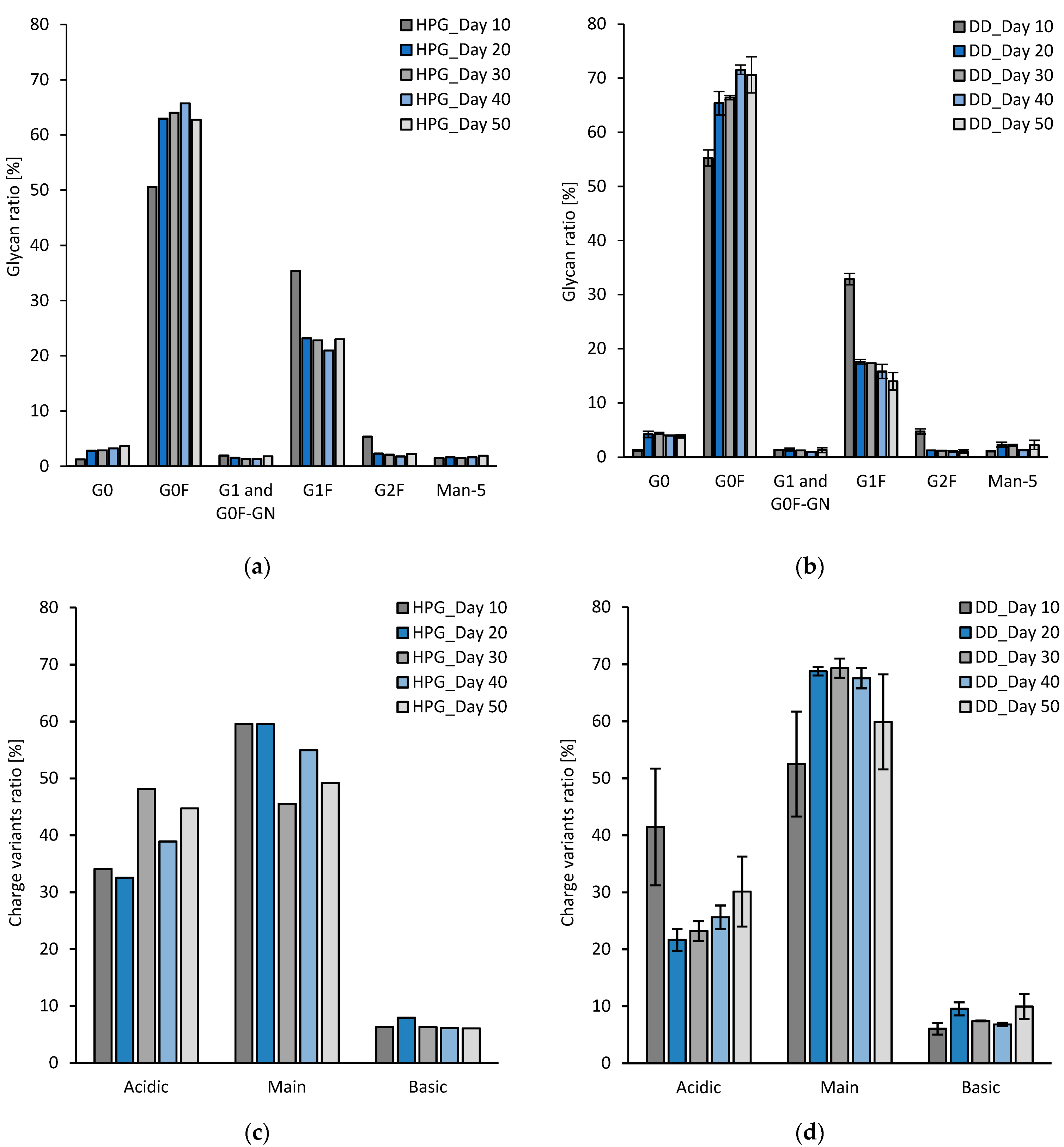
3.2.2. Experimental Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walsh, G.; Walsh, E. Biopharmaceutical Benchmarks 2022. Nat. Biotechnol. 2022, 40, 1722–1760. [Google Scholar] [CrossRef]
- MacDonald, M.A.; Nöbel, M.; Roche Recinos, D.; Martínez, V.S.; Schulz, B.L.; Howard, C.B.; Baker, K.; Shave, E.; Lee, Y.Y.; Marcellin, E.; et al. Perfusion Culture of Chinese Hamster Ovary Cells for Bioprocessing Applications. Crit. Rev. Biotechnol. 2022, 42, 1099–1115. [Google Scholar] [CrossRef]
- Wong, H.E.; Chen, C.; Le, H.; Goudar, C.T. From Chemostats to High-Density Perfusion: The Progression of Continuous Mammalian Cell Cultivation. J. Chem. Technol. Biotechnol. 2022, 97, 2297–2304. [Google Scholar] [CrossRef]
- Matanguihan, C.; Wu, P. Upstream Continuous Processing: Recent Advances in Production of Biopharmaceuticals and Challenges in Manufacturing. Curr. Opin. Biotechnol. 2022, 78, 102828. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Teale, M.; Steiner, S.; Junne, S.; Neubauer, P.; Eibl, D.; Eibl, R. Intensified and Continuous MAb Production with Single-Use Systems. In Cell Culture Engineering and Technology; Pörtner, R., Ed.; Springer: Cham, Switzerland, 2021; pp. 401–429. ISBN 978-3-030-79871-0. [Google Scholar]
- Lavado-García, J.; Pérez-Rubio, P.; Cervera, L.; Gòdia, F. The Cell Density Effect in Animal Cell-Based Bioprocessing: Questions, Insights and Perspectives. Biotechnol. Adv. 2022, 60, 108017. [Google Scholar] [CrossRef]
- Schwarz, H.; Mäkinen, M.E.; Castan, A.; Chotteau, V. Monitoring of Amino Acids and Antibody N-Glycosylation in High Cell Density Perfusion Culture Based on Raman Spectroscopy. Biochem. Eng. J. 2022, 182, 108426. [Google Scholar] [CrossRef]
- Romann, P.; Kolar, J.; Chappuis, L.; Herwig, C.; Villiger, T.K.; Bielser, J.M. Maximizing Yield of Perfusion Cell Culture Processes: Evaluation and Scale-up of Continuous Bleed Recycling. Biochem. Eng. J. 2023, 193, 108873. [Google Scholar] [CrossRef]
- Bausch, M.; Schultheiss, C.; Sieck, J.B. Recommendations for Comparison of Productivity Between Fed-Batch and Perfusion Processes. Biotechnol. J. 2019, 14, 1700721. [Google Scholar] [CrossRef] [PubMed]
- Gomez, N.; Lull, J.; Yang, X.; Wang, Y.; Zhang, X.; Wieczorek, A.; Harrahy, J.; Pritchard, M.; Cano, D.M.; Shearer, M.; et al. Improving Product Quality and Productivity of Bispecific Molecules through the Application of Continuous Perfusion Principles. Biotechnol. Prog. 2020, 36, e2973. [Google Scholar] [CrossRef]
- Walther, J.; Lu, J.; Hollenbach, M.; Yu, M.; Hwang, C.; McLarty, J.; Brower, K. Perfusion Cell Culture Decreases Process and Product Heterogeneity in a Head-to-Head Comparison with Fed-Batch. Biotechnol. J. 2019, 14, 1700733. [Google Scholar] [CrossRef]
- Bielser, J.-M.; Chappuis, L.; Xiao, Y.; Souquet, J.; Broly, H.; Morbidelli, M. Perfusion Cell Culture for the Production of Conjugated Recombinant Fusion Proteins Reduces Clipping and Quality Heterogeneity Compared to Batch-Mode Processes. J. Biotechnol. 2019, 302, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Arnold, L.; Lee, K.; Rucker-Pezzini, J.; Lee, J.H. Implementation of Fully Integrated Continuous Antibody Processing: Effects on Productivity and COGm. Biotechnol. J. 2019, 14, 1800061. [Google Scholar] [CrossRef] [PubMed]
- Mahal, H.; Branton, H.; Farid, S.S. End-to-End Continuous Bioprocessing: Impact on Facility Design, Cost of Goods, and Cost of Development for Monoclonal Antibodies. Biotechnol. Bioeng. 2021, 118, 3468–3485. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, H.; Sponchioni, M.; Morbidelli, M. Integration and Digitalization in the Manufacturing of Therapeutic Proteins. Chem. Eng. Sci. 2022, 248, 117159. [Google Scholar] [CrossRef]
- Dreher, T.; Husemann, U.; Adams, T.; de Wilde, D.; Greller, G. Design Space Definition for a Stirred Single-Use Bioreactor Family from 50 to 2000 L Scale. Eng. Life Sci. 2014, 14, 304–310. [Google Scholar] [CrossRef]
- Tregidgo, M.; Dorn, M.; Lucas, C.; Micheletti, M. Design and Characterization of a Novel Perfusion Reactor for Biopharmaceuticals Production. Chem. Eng. Res. Des. 2023, 194, 344–357. [Google Scholar] [CrossRef]
- Nienow, A.W.; Scott, W.H.; Hewitt, C.J.; Thomas, C.R.; Lewis, G.; Amanullah, A.; Kiss, R.; Meier, S.J. Scale-down Studies for Assessing the Impact of Different Stress Parameters on Growth and Product Quality during Animal Cell Culture. Chem. Eng. Res. Des. 2013, 91, 2265–2274. [Google Scholar] [CrossRef]
- Sieck, J.B.; Cordes, T.; Budach, W.E.; Rhiel, M.H.; Suemeghy, Z.; Leist, C.; Villiger, T.K.; Morbidelli, M.; Soos, M. Development of a Scale-Down Model of Hydrodynamic Stress to Study the Performance of an Industrial CHO Cell Line under Simulated Production Scale Bioreactor Conditions. J. Biotechnol. 2013, 164, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Freiberger, F.; Budde, J.; Ateş, E.; Schlüter, M.; Pörtner, R.; Möller, J. New Insights from Locally Resolved Hydrodynamics in Stirred Cell Culture Reactors. Processes 2022, 10, 107. [Google Scholar] [CrossRef]
- Jossen, V.; Eibl, R.; Eibl, D. Single-Use Bioreactors—An Overview. In Single-Use Technology in Biopharmaceutical Manufacture; Eibl, R., Eibl, D., Eds.; Wiley: Hoboken, NJ, USA, 2019; pp. 37–52. ISBN 9781119477891. [Google Scholar]
- Thermo Fisher Scientific HyPerforma DynaDrive Single-Use Bioreactor Service Guide. Available online: https://assets.thermofisher.com/TFS-Assets/BPD/Reference-Materials/hyperforma-dynadrive-single-use-bioreactor-service-guide-2022.pdf (accessed on 23 November 2023).
- Schirmer, C.; Müller, J.; Steffen, N.; Werner, S.; Eibl, R.; Eibl, D. How to Produce MAbs in a Cube-Shaped Stirred Single-Use Bioreactor at 200 L Scale. In Animal Cell Biotechnology: Methods and Protocols; Pörtner, R., Ed.; Humana Press: New York, NY, USA, 2020; pp. 169–186. ISBN 978-1-0716-0191-4. [Google Scholar]
- Thermo Fisher Scientific Data Sheet—HyPerforma DynaDrive Single-Use Bioreactor. Available online: https://assets.thermofisher.com/TFS-Assets/BPD/Datasheets/50l-dynadrive-sub-data-sheet.pdf (accessed on 23 November 2023).
- Karimi Alavijeh, M.; Baker, I.; Lee, Y.Y.; Gras, S.L. Digitally Enabled Approaches for the Scale up of Mammalian Cell Bioreactors. Digit. Chem. Eng. 2022, 4, 100040. [Google Scholar] [CrossRef]
- Kaiser, S.C. Characterization and Optimization of Single-Use Bioreactors and Biopharmaceutical Production Processes Using Computational Fluid Dynamics. Doctoral Thesis, Technische Universität, Berlin, Germany, 2014. [Google Scholar]
- Junker, B.H. Scale-up Methodologies for Escherichia Coli and Yeast Fermentation Processes. J. Biosci. Bioeng. 2004, 97, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Maschke, R.W.; Seidel, S.; Bley, T.; Eibl, R.; Eibl, D. Determination of Culture Design Spaces in Shaken Disposable Cultivation Systems for CHO Suspension Cell Cultures. Biochem. Eng. J. 2022, 177, 108224. [Google Scholar] [CrossRef]
- Liepe, F.; Sperling, R.; Jembere, S. Rührwerke: Theoretische Grundlagen, Auslegung und Bewertung; Fachhochschule Anhalt: Köthen, Germany, 1998; ISBN 9783000031953. [Google Scholar]
- Zlokarnik, M. Rührtechnik—Theorie und Praxis; Springer: Berlin/Heidelberg, Germany, 1999; ISBN 978-3-642-63675-2. [Google Scholar]
- Nienow, A.W. Re “Development of a Scale-down Model of Hydrodynamic Stress to Study the Performance of an Industrial CHO Cell Line under Simulated Production Scale Bioreactor Conditions” [Sieck, J.B., Cordes, T., Budach, W.E., Rhiel, M.H., Suemeghy, Z., Leist, C., Villiger, T.K., Morbidelli, M., Soos, M., 2013. Journal of Biotechnology 164, 41–49]. J. Biotechnol. 2014, 171, 82–84. [Google Scholar] [CrossRef] [PubMed]
- Seidel, S.; Maschke, R.W.; Werner, S.; Jossen, V.; Eibl, D. Oxygen Mass Transfer in Biopharmaceutical Processes: Numerical and Experimental Approaches. Chem. Ing. Tech. 2021, 93, 42–61. [Google Scholar] [CrossRef]
- Teworte, S.; Malcı, K.; Walls, L.E.; Halim, M.; Rios-Solis, L. Recent Advances in Fed-Batch Microscale Bioreactor Design. Biotechnol. Adv. 2022, 55, 107888. [Google Scholar] [CrossRef]
- Frey, L.J.; Krull, R. Microbioreactors for Process Development and Cell-Based Screening Studies. In Microfluidics in Biotechnology. Advances in Biochemical Engineering/Biotechnology; Bahnemann, J., Grünberger, A., Eds.; Springer: Cham, Switzerland, 2020; Volume 179, pp. 67–100. ISBN 978-3-031-04188-4. [Google Scholar]
- Bielser, J.M.; Chami, J.; Markarian, J. Continuous Bleed Recycling Significantly Increases Recombinant Protein Production Yield in Perfusion Cell Cultures. Biochem. Eng. J. 2021, 169, 107966. [Google Scholar] [CrossRef]
- Jefferis, R. Glycosylation of Recombinant Antibody Therapeutics. Biotechnol. Prog. 2005, 21, 11–16. [Google Scholar] [CrossRef]
- Cameron, D.; Piccart-Gebhart, M.J.; Gelber, R.D.; Procter, M.; Goldhirsch, A.; de Azambuja, E.; Castro, G.; Untch, M.; Smith, I.; Gianni, L.; et al. 11 Years’ Follow-up of Trastuzumab after Adjuvant Chemotherapy in HER2-Positive Early Breast Cancer: Final Analysis of the HERceptin Adjuvant (HERA) Trial. Lancet 2017, 389, 1195–1205. [Google Scholar] [CrossRef]
- European Medicines Agency Summary of Product Characteristics—Herceptin. Available online: https://www.ema.europa.eu/en/documents/product-information/herceptin-epar-product-information_en.pdf (accessed on 29 November 2023).
- Gerstweiler, L.; Bi, J.; Middelberg, A.P.J. Continuous Downstream Bioprocessing for Intensified Manufacture of Biopharmaceuticals and Antibodies. Chem. Eng. Sci. 2021, 231, 116272. [Google Scholar] [CrossRef]

| Parameter | Ambr 250 | 3 L HyPerforma Glass | HyPerforma DynaDrive |
|---|---|---|---|
| Volume | 175–220 mL | 1.45–2.0 L | 35–50 L |
| Temperature | 37 °C | 37 °C | 37 °C |
| Feed addition | Bolus or continuous | Bolus | Continuous |
| Stirrer configuration | 2× pitched blade (3 blades) | 2× pitched blade (3 blades) | Flexible rope ladder with 3× pitched blade (2 blades) 1× sweep impeller (2 blades) |
| Stirrer speed | 522–582 min−1 | 200–250 min−1 | 125–140 min−1 |
| Stirrer tip speed | 0.71–0.79 m s−1 | 0.59–0.73 m s−1 | 0.71–0.79 m s−1 |
| Specific power input | 60–90 W m−3 | 30–63 W m−3 | 40 W m−3 |
| Sparger | Open pipe sparger | Ring sparger | Drilled hole sparger |
| pH | ≤7.15 (CO2 via sparger) | ≤7.15 (CO2 via sparger) | ≤7.15 (CO2 via sparger) |
| DO | ≥40% (O2 and air via sparger) | ≥40% (O2 via sparger) | =40% (N2, O2 and air via sparger) |
| Parameter | 3 L HyPerforma Glass | HyPerforma DynaDrive |
|---|---|---|
| Volume | 2 L | 50 L |
| Temperature | 37 °C | 37 °C |
| Cell retention | XCell ATF 2 single-use | XCell ATF 6 single-use |
| Stirrer configuration | 2× pitched blade (3 blades) | Flexible rope ladder with 3× pitched blade (2 blades) 1× sweep impeller (2 blades) |
| Stirrer speed | 435 min−1 | 136.5 min−1 |
| Stirrer tip speed | 1.28 m s−1 | 0.77 m s−1 |
| Specific power input | 320 W m−3 | 40 W m−3 |
| Sparger | Ring sparger | Drilled hole sparger |
| pH | ≤7.15 (CO2 via sparger) | ≤7.15 (CO2 via sparger) |
| DO | ≥40% (O2 and air via sparger) | =40% (N2, O2 and air via sparger) |
| Fed Batch | Perfusion | |
|---|---|---|
| Process duration [d] | 15 | 50 |
| Yield per 50 L batch [kg] | 0.245 | 2.28–2.44 |
| Space-time yield [g L−1 d−1] | 0.33 | 0.91–0.97 |
| Volumetric productivity * [g L−1 d−1] | - | 1.05–1.12 |
| Annual productivity (50 L bioreactor) [kg] | 6.0 | 16.6–17.7 |
| Increase [%] | - | 179–197 |
| Possible annual therapies ** | 750 | 2089–2227 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ott, V.; Ott, J.; Eibl, D.; Eibl, R. Scaling Fed-Batch and Perfusion Antibody Production Processes in Geometrically Dissimilar Stirred Bioreactors. Processes 2024, 12, 806. https://doi.org/10.3390/pr12040806
Ott V, Ott J, Eibl D, Eibl R. Scaling Fed-Batch and Perfusion Antibody Production Processes in Geometrically Dissimilar Stirred Bioreactors. Processes. 2024; 12(4):806. https://doi.org/10.3390/pr12040806
Chicago/Turabian StyleOtt, Vivian, Jan Ott, Dieter Eibl, and Regine Eibl. 2024. "Scaling Fed-Batch and Perfusion Antibody Production Processes in Geometrically Dissimilar Stirred Bioreactors" Processes 12, no. 4: 806. https://doi.org/10.3390/pr12040806
APA StyleOtt, V., Ott, J., Eibl, D., & Eibl, R. (2024). Scaling Fed-Batch and Perfusion Antibody Production Processes in Geometrically Dissimilar Stirred Bioreactors. Processes, 12(4), 806. https://doi.org/10.3390/pr12040806







