Preparation of Bioaerogel from Iron-Rich Microalgae for the Removal of Water Pollutants
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
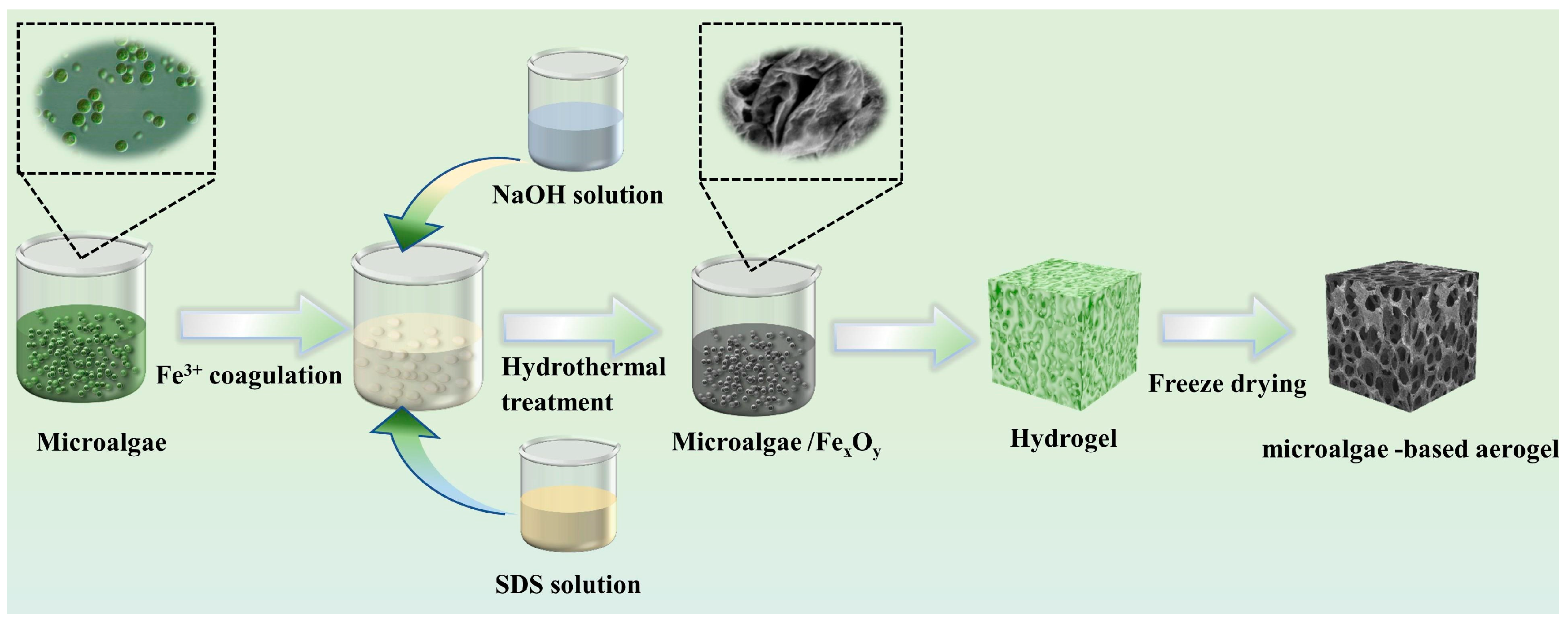
2.2. Preparation of the Fe-CP Aerogels
2.3. Characterization
2.4. Adsorption Experiments
2.5. Separation Performance of Fe-CP Aerogel for Oil/Water and Dye Solution
2.6. Photo-Fenton Catalytic Performance of the Fe-CP Aerogel
3. Results and Discussion
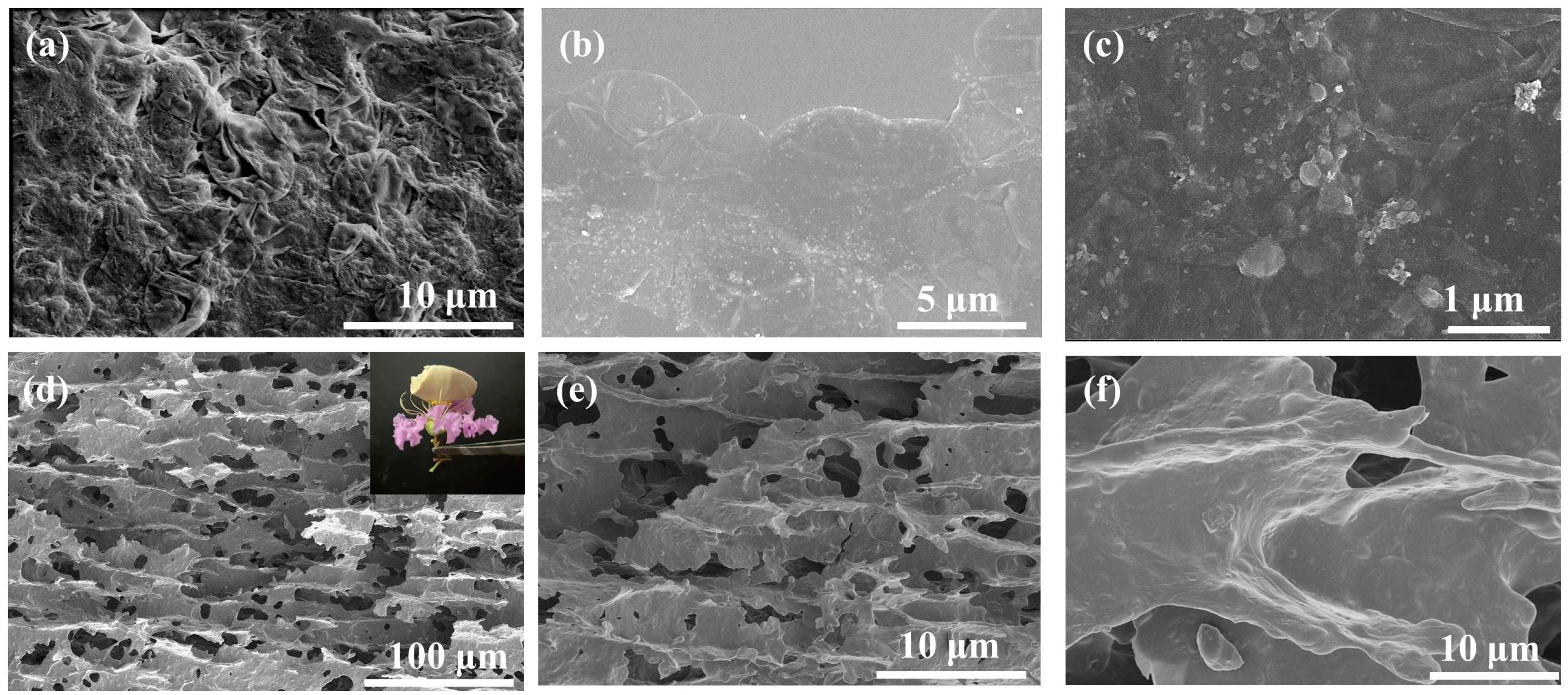
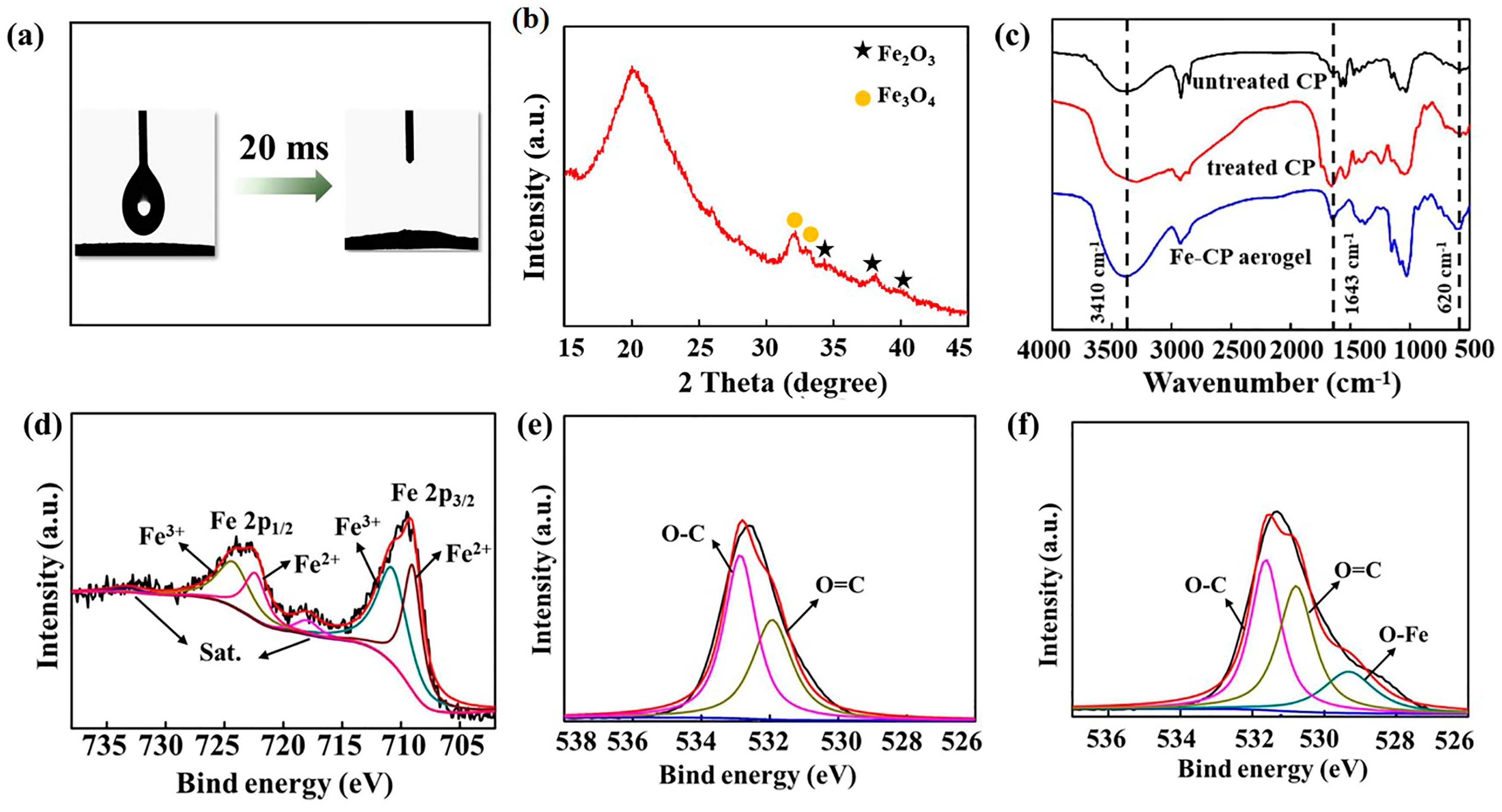
3.1. Characterization of the Microalgae Aerogel
3.2. Adsorption Performance of Microalgae Aerogel for Heavy Metal Ion
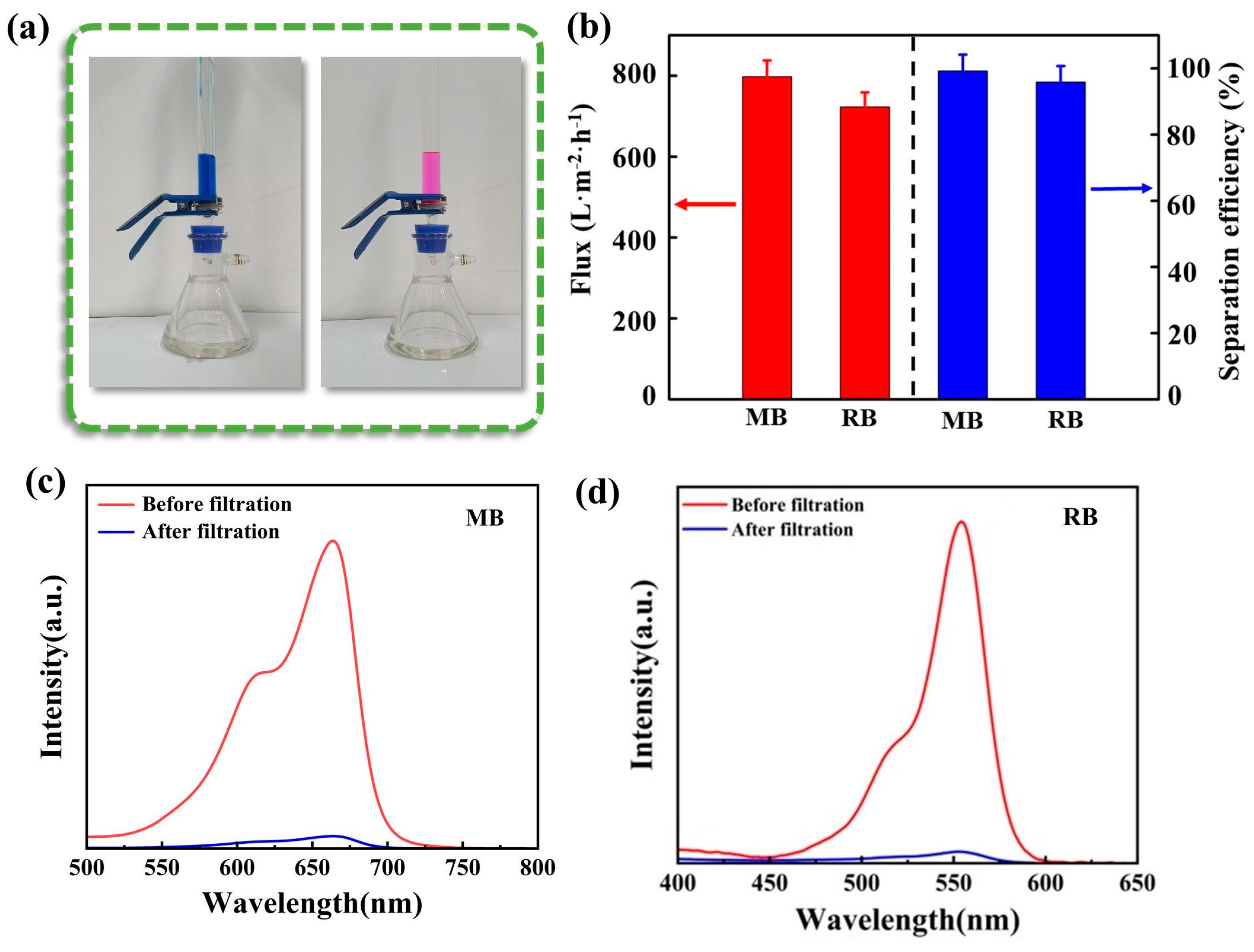
3.3. Oil/Water Emulsion and Dye Wastewater Separation Capacity of Fe-CP Aerogel
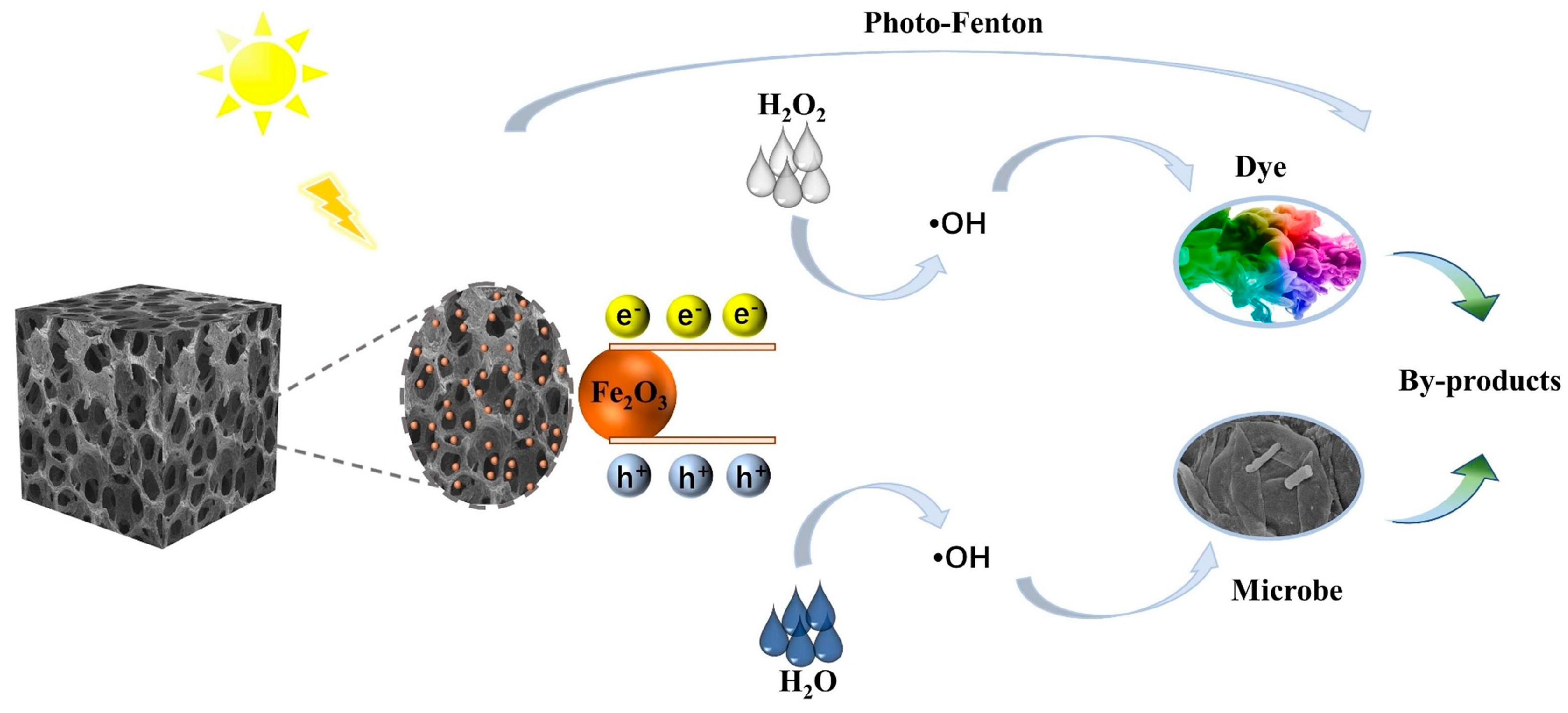
3.4. Photocatalytic Capacity of Fe-CP Aerogel
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, X.; Liu, Y.; Zhang, C.L.; Wen, T.; Zhuang, L.; Wang, X.X.; Song, G.; Chen, D.Y.; Ai, Y.J.; Hayat, T.; et al. Porous Fe2O3 microcubes derived from metal organic frameworks for efficient elimination of organic pollutants and heavy metal ions. Chem. Eng. J. 2018, 336, 241–252. [Google Scholar] [CrossRef]
- Le, V.G.; Nguyen, M.N.; Nguyen, H.L.; Thai, V.A.; Le, V.R.; Manh, V.Q.; Asaithambi, P.; Woong, C.S.; Nguyen, D.D. Ecotoxicological response of algae to contaminants in aquatic environments: A review. Environ. Chem. Lett. 2024, 22, 919–939. [Google Scholar] [CrossRef]
- Liu, X.G.; Shan, Y.Y.; Zhang, S.T.; Kong, Q.Q.; Pang, H. Application of metal organic framework in wastewater treatment. Green Energy Environ. 2023, 8, 698–721. [Google Scholar] [CrossRef]
- Wei, Z.M.; Su, Q.; Lin, Q.Y.; Wang, X.J.; Long, S.R.; Zhang, G.; Yang, J. Multifunctional oxidized poly (arylene sulfide sulfone)/UiO-66 nanofibrous membrane with efficient adsorption/separation ability in harsh environment. Chem. Eng. J. 2022, 430, 133021. [Google Scholar] [CrossRef]
- Fajal, S.; Dutta, S.; Ghosh, S.K. Porous organic polymers (POPs) for environmental remediation. Mater. Horiz. 2023, 10, 4083–4138. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Zhuang, S.T. Removal of cesium ions from aqueous solutions using various separation technologies. Rev. Environ. Sci. Bio/Technol. 2019, 18, 231–269. [Google Scholar] [CrossRef]
- Vinothkumar, K.; Jyothi, M.S.; Lavanya, C.; Sakar, M.; Valiyaveettil, S.; Balakrishna, R.G. Strongly co-ordinated MOF-PSF matrix for selective adsorption, separation and photodegradation of dyes. Chem. Eng. J. 2022, 428, 132561. [Google Scholar] [CrossRef]
- Zhang, L.P.; Liu, Z.; Zhou, X.L.; Zhang, C.; Cai, Q.W.; Xie, R.; Ju, X.J.; Wang, W.; Faraj, Y.; Chu, L.Y. Novel composite membranes for simultaneous catalytic degradation of organic contaminants and adsorption of heavy metal ions. Sep. Purif. Technol. 2019, 237, 116364. [Google Scholar] [CrossRef]
- Chen, X.; Cui, J.; Xu, X.R.; Sun, B.J.; Zhang, L.; Dong, W.; Chen, C.T.; Sun, D.P. Bacterial cellulose/attapulgite magnetic composites as an efficient adsorbent for heavy metal ions and dye treatment. Carbohydr. Polym. 2020, 229, 115512. [Google Scholar] [CrossRef] [PubMed]
- Elattar, R.H.; El-Deen, A.K. Porous material-based quechers: Exploring new horizons in sample preparation. Trends Anal. Chem. 2024, 172, 117571. [Google Scholar] [CrossRef]
- Liu, Y.L.; Jin, C.; Yang, Z.Z.; Wu, G.M.; Liu, G.F.; Kong, Z.W. Recent advances in lignin-based porous materials for pollutants removal from wastewater. Int. J. Biol. Macromol. 2021, 187, 880–891. [Google Scholar] [CrossRef]
- Lim, Y.J.; Goh, K.; Goto, A.; Zhao, Y.L.; Wang, R. Uranium and lithium extraction from seawater: Challenges and opportunities for a sustainable energy future. J. Mater. Chem. A 2023, 11, 22551–22589. [Google Scholar] [CrossRef]
- Liu, H.W.; Li, X.Y.; Zhang, X.X.; Coulon, F.; Wang, C.Q. Harnessing the power of natural minerals: A comprehensive review of their application as heterogeneous catalysts in advanced oxidation processes for organic pollutant degradation. Chemosphere 2023, 337, 139404. [Google Scholar] [CrossRef]
- He, W.; Cao, J.Z.; Guo, F.Y.; Guo, Z.H.; Zhou, P.G.; Wang, R.; Liang, S.; Pang, Q.Y.; Wei, B.R.; Jiao, Y.; et al. Nanostructured carboxylated-wood aerogel membrane for high-efficiency removal of Cu (II) ions from wastewater. Chem. Eng. J. 2023, 468, 143747. [Google Scholar] [CrossRef]
- Hu, X.D.; Zhang, T.Y.; Yang, B.; Hao, M.; Chen, Z.J.; Wei, Y.; Liu, Y.B.; Wang, X.X.; Yao, J.B. Water-resistant nanocellulose/gelatin biomass aerogel for anionic/cationic dye adsorption. Sep. Purif. Technol. 2024, 330, 125367. [Google Scholar] [CrossRef]
- Chew, K.W.; Khoo, K.S.; Foo, H.T.; Chia, S.R.; Walvekar, R.; Lim, S.S. Algae utilization and its role in the development of green cities. Chemosphere 2021, 268, 129332. [Google Scholar] [CrossRef]
- Xu, Y.A.; Hellier, P.; Purton, S.; Baganz, F.; Ladommatos, N. Algal biomass and diesel emulsions: An alternative approach for utilizing the energy content of microalgal biomass in diesel engines. Appl. Energy 2016, 172, 80–95. [Google Scholar] [CrossRef]
- Daneshvar, E.; Zarrinmehr, M.J.; Kousha, M.; Hashtjin, A.M.; Saratale, G.D.; Maiti, A.; Vithanage, M.; Bhatnagar, A. Hexavalent chromium removal from water by microalgal-based materials: Adsorption, desorption and recovery studies. Bioresour. Technol. 2019, 293, 122064. [Google Scholar] [CrossRef]
- Francavilla, M.; Pineda, A.; Lin, C.S.; Franchi, M.; Trotta, P.; Romero, A.A.; Luque, R. Natural porous agar materials from macroalgae. Carbohydr. Polym. 2013, 92, 1555–1560. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, W.A.; Chakraborty, S.; Islam, R.U.; Ghfa, A.A.; Naushad, M.; Bundschuh, J.; Maity, J.P.; Mondal, N.K. Fabrication of biochar-based hybrid Ag nanocomposite from algal biomass waste for toxic dye-laden wastewater treatment. Chemosphere 2022, 289, 133243. [Google Scholar] [CrossRef]
- Hu, X.; Liu, W.J.; Ma, L.L.; Yu, H.Q. Sustainable Conversion of Harmful Algae Biomass into a CO2 Reduction Electrocatalyst for Two-Fold Carbon Utilization. Environ. Sci. Technol. 2023, 57, 1157–1166. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lu, W.M.; Esakkimuthu, S.; Chen, H.; Yang, J.W.; Mu, M.; Gong, X. Life cycle assessment of carbon-based adsorbent preparation from algal biomass. J. Clean. Prod. 2023, 427, 139269. [Google Scholar] [CrossRef]
- Ji, X.Y.; Luo, X.; Zhang, J.B.; Huang, D.Y. Effects of exogenous vitamin B12 on nutrient removal and protein expression of algal-bacterial consortium. Environ. Sci. Pollut. Res. 2021, 28, 15954–15965. [Google Scholar] [CrossRef] [PubMed]
- Baskar, A.V.; Bolan, N.; Hoang, S.A.; Sooriyakumar, P.; Kumar, M.; Singh, L.; Jasemizad, T.; Padhye, L.P.; Singh, G.; Vinu, A.; et al. Recovery, regeneration and sustainable management of spent adsorbents from wastewater treatment streams: A review. Sci. Total Environ. 2022, 822, 153555. [Google Scholar] [CrossRef]
- Zhu, S.Y.; Xu, J.; Wang, B.; Xie, J.X.; Ying, G.D.; Li, J.P.; Cheng, Z.; Li, J.; Chen, K.F. Highly efficient and rapid purification of organic dye wastewater using lignin-derived hierarchical porous carbon. J. Colloid Interface Sci. 2022, 625, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.Y.; Wang, H.Q.; Hu, E.; Lei, Z.W.; Fan, B.L.; Wang, Q.L. Efficient adsorption of uranium from aqueous solutions by microalgae based aerogel. Microporous Mesoporous Mater. 2020, 305, 110383. [Google Scholar] [CrossRef]
- Tee, G.T.; Gok, X.Y.; Yong, W.F. Adsorption of pollutants in wastewater via biosorbents, nanoparticles and magnetic biosorbents: A review. Environ. Res. 2022, 212, 113248. [Google Scholar] [CrossRef] [PubMed]
- Prasad, C.; Jeong, S.G.; Won, J.S.; Ramanjaneyulu, S.; Sangaraju, S.; Kerru, N.; Choi, H.Y. Review on recent advances in cellulose nanofibril based hybrid aerogels: Synthesis, properties and their applications. Int. J. Biol. Macromol. 2024, 261, 129460. [Google Scholar] [CrossRef]
- Bi, L.; Luan, X.; Geng, F.L.; Xu, X.L.; Chen, Y.P.; Zhang, F. Microwave-assisted synthesis of hollow microspheres with multicomponent nanocores for heavy-metal removal and magnetic sensing. ACS Appl. Mater. Interfaces 2020, 12, 46779–46787. [Google Scholar] [CrossRef]
- Liu, X.; Wu, Z.C.; Han, Y.H.; Han, L.J. Characteristic modification of alkalized corn stalk and contribution to the bonding mechanism of fuel briquette. Energy 2017, 133, 299–305. [Google Scholar] [CrossRef]
- Tu, F.; Yu, Y.X.; Wang, Y.; Huang, L.Y.; Ye, D.H.; Fu, Z.Y. Preparation of SiO2/Fe2O3 composite aerogels for thermal insulation enhancement. Ceram. Int. 2024, 50, 2976–2986. [Google Scholar] [CrossRef]
- Han, X.H.; Ding, S.Q.; Zhu, L.J.; Wang, S.R. Preparation and characterization of flame-retardant and thermal insulating bio-based composite aerogels. Energy Build. 2023, 278, 112656. [Google Scholar] [CrossRef]
- Abdullah, J.A.; Jiménez-Rosado, M.; Perez-Puyana, V.; Guerrero, A.; Romero, A. Green synthesis of FexOy nanoparticles with potential antioxidant properties. Nanomaterials 2022, 12, 2449. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Kobe, B.A.; Biesinger, M.C.; McIntyre, N.S. Investigation of multiplet splitting of Fe 2p XPS spectra and bonding in iron compounds. Surf. Interface Anal. 2004, 36, 1564–1574. [Google Scholar] [CrossRef]
- Ateia, E.E.; Saeid, Y.A.; Abdelmaksoud, M.K. Synthesis and characterization of CaFe1.925Gd0.025Sm0.05O4/PEG core–shell nanoparticles for diverse applications. J. Supercond. Nov. Magn. 2023, 36, 1011–1024. [Google Scholar] [CrossRef]
- Wang, T.N.; Jiao, Y.H.; He, M.C.; Peng, X.J.; Liu, P.; Ouyang, W.; Lin, C.Y.; Liu, X.T.; Xie, H.J. Efficient removal of antimony by a facile liquid-controlled strategy reinforced hematite-spinel (Fe2O3-MnFe2O4) composite: Construction, simulation and practical evaluation. Chem. Eng. J. 2023, 451, 138974. [Google Scholar] [CrossRef]
- Huo, J.B.; Yu, G.C. Hexacyanoferrate-modified polyvinyl alcohol/graphene oxide aerogel as an efficient and retrievable adsorbent for cesium. J. Mater. Sci. 2022, 57, 351–365. [Google Scholar] [CrossRef]
- Li, Q.M.; Fu, L.C.; Wang, Z.; Li, A.; Shuang, C.D.; Gao, C.Z. Synthesis and characterization of a novel magnetic cation exchange resin and its application for efficient removal of Cu2+ and Ni2+ from aqueous solutions. J. Clean. Prod. 2017, 165, 801–810. [Google Scholar] [CrossRef]
- Meng, J.W.; Guan, H.; Dai, X.J.; Wang, X.Q. Amino-functionalized wood aerogel for efficient removal of copper ions from water. Int. J. Polym. Sci. 2021, 8, 4913226. [Google Scholar]
- Li, D.W.; Tian, X.J.; Wang, Z.Q.; Guan, Z.; Li, X.Q.; Qiao, H.; Ke, H.Z.; Wei, Q. Multifunctional adsorbent based on metal-organic framework modified bacterial cellulose/chitosan composite aerogel for high efficient removal of heavy metal ion and organic pollutant. Chem. Eng. J. 2020, 383, 123127. [Google Scholar] [CrossRef]
- Ye, X.; Shang, S.S.; Zhao, Y.F.; Cui, S.; Zhong, Y.; Huang, L.J. Ultra-efficient adsorption of copper ions in chitosan–montmorillonite composite aerogel at wastewater treatment. Cellulose 2021, 28, 7201–7212. [Google Scholar] [CrossRef]
- Wang, M.; Yang, Q.; Zhao, X.Q.; Wang, Z.Q. Highly efficient removal of copper ions from water by using a novel alginate-polyethyleneimine hybrid aerogel. Int. J. Biol. Macromol. 2019, 138, 1079–1086. [Google Scholar] [CrossRef]
- Tang, C.X.; Brodie, P.; Li, Y.Z.; Grishkewich, N.J.; Brunsting, M.; Tam, K.C. Shape recoverable and mechanically robust cellulose aerogel beads for efficient removal of copper ions. Chem. Eng. J. 2020, 392, 124821. [Google Scholar] [CrossRef]
- Wang, Q.Z.; Li, Y. Facile and green fabrication of porous chitosan aerogels for highly efficient oil/water separation and metal ions removal from water. J. Environ. Chem. Eng. 2023, 11, 109689. [Google Scholar] [CrossRef]
- Wang, Q.Y.; Li, L.P.; Kong, L.C.; Cai, G.Y.; Wang, P.; Zhang, J.; Zuo, W.; Tian, Y. Compressible amino-modified carboxymethyl chitosan aerogel for efficient Cu(II) adsorption from wastewater. Sep. Purif. Technol. 2022, 293, 121146. [Google Scholar] [CrossRef]
- Li, J.; Zuo, K.; Wu, W.B.; Xu, Z.Y.; Yi, Y.G.; Jing, Y.; Dai, H.Q.; Fang, G.G. Shape memory aerogels from nanocellulose and polyethyleneimine as a novel adsorbent for removal of Cu(II) and Pb(II). Carbohydr. Polym. 2018, 196, 376–384. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, Z.; Jiang, W.; Fang, X.R.; Xia, Z.G.; Niu, H.T.; Zhou, H. Multifunctional amphibious superhydrophilic-oleophobic cellulose nanofiber aerogels for oil and water purification. Carbohydr. Polym. 2024, 330, 121774. [Google Scholar] [CrossRef]
- He, X.H.; Wang, L.L.; Sun, S.M.; Yang, X.H.; Tian, H.Y.; Xia, Z.J.; Li, X.L.; Yan, X.L.; Pu, X.J.; Jiao, Z. Self-assembled synthesis of recyclable g-C3N4/NH2-MIL-53(Fe) aerogel for enhanced photocatalytic degradation of organic pollutants. J. Alloys Compd. 2023, 964, 169391. [Google Scholar] [CrossRef]
- Mei, J.; Yuan, G.J.; Ma, Y.S.; Chen, X.; Ren, L.L. Fe-Doped alumina aerogels as a green heterogeneous catalyst for efficient degradation of organic pollutants. Catal. Lett. 2019, 149, 1874–1887. [Google Scholar] [CrossRef]
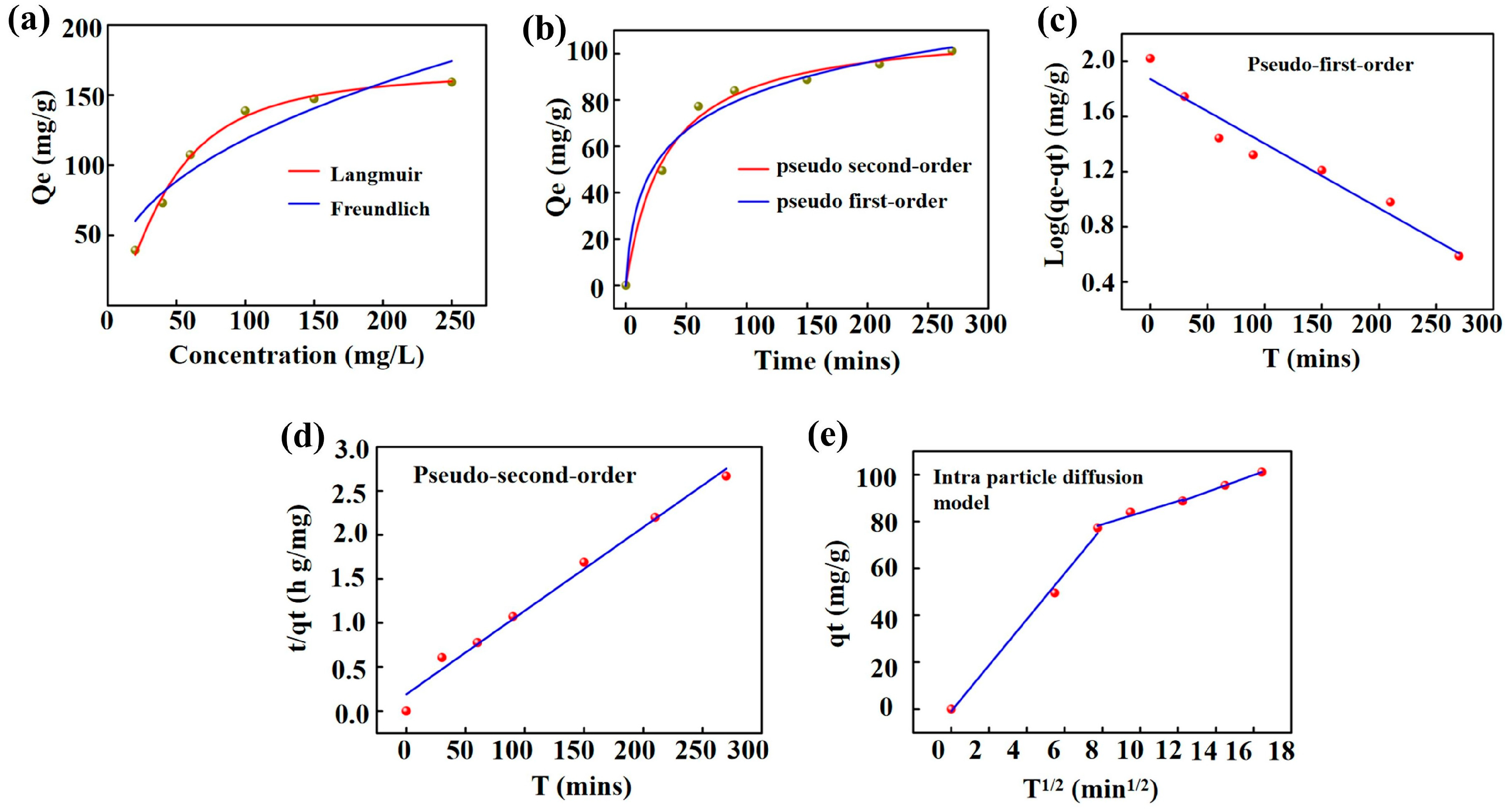


| Kinetics Models | Parameters | |
|---|---|---|
| Langmuir | Qm (mg/g) | 208.3 |
| KL (L/mg) | 0.015 | |
| R2 | 0.975 | |
| Freundlich | 1/n | 0.554 |
| Kf (L/mg) | 9.124 | |
| R2 | 0.897 | |
| Kinetics Models | Parameters | ||
|---|---|---|---|
| Pseudo-first-order | Qe (mg/g) | 105.55 | |
| K1 (min−1) | 0.011 | ||
| R2 | 0.948 | ||
| Pseudo-second-order | Qe (mg/g) | 105.26 | |
| K2 (g.mg−1.min−1) | 0.00047 | ||
| R2 | 0.987 | ||
| Intra-particle diffusion | Stage 1 | K3 | 9.799 |
| C | −0.949 | ||
| R2 | 0.989 | ||
| Stage 2 | K3 | 2.486 | |
| C | 58.902 | ||
| R2 | 0.899 | ||
| Stage 3 | K3 | 2.951 | |
| C | 52.657 | ||
| R2 | 0.999 | ||
| Aerogel Samples | Initial Concentration | pH | Adsorbent Dose | Qmax (mg.g−1) | Ref. |
|---|---|---|---|---|---|
| PEI@wood aerogel | 150 mg/L | 5 | 1000 mg/L | 59.80 | [39] |
| ZIF-67/BC/CH | N/A | 6 | 1000 mg/L | 200.06 | [40] |
| CS-MMT2 | 10 mg/L | 6 | 1000 mg/L | 86.95 | [41] |
| Alg-PEI | 0.1 mM | 1–7 | 5000 mg/L | 214.40 | [42] |
| CGP3 | 100 mg/L | 3–6.2 | 2000 mg/L | 163.40 | [43] |
| Chitosan aerogels | 100 mg/L | N/A | 1500 mg/L | 116.70 | [44] |
| PEI modified CCS aerogel | 50 mg/L | 6 | 400 mg/L | 175.56 | [45] |
| NFC/PEI | 20 mg/L | 5 | 300 mg/L | 175.44 | [46] |
| Fe-CP aerogel | 50 mg/L | 6 | 1000 mg/L | 208.30 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, X.; Si, J.; Chen, B.; Wang, Q.; Zeng, S.; Cui, Z. Preparation of Bioaerogel from Iron-Rich Microalgae for the Removal of Water Pollutants. Processes 2024, 12, 1313. https://doi.org/10.3390/pr12071313
Niu X, Si J, Chen B, Wang Q, Zeng S, Cui Z. Preparation of Bioaerogel from Iron-Rich Microalgae for the Removal of Water Pollutants. Processes. 2024; 12(7):1313. https://doi.org/10.3390/pr12071313
Chicago/Turabian StyleNiu, Xinqi, Junhui Si, Binyi Chen, Qianting Wang, Sen Zeng, and Zhixiang Cui. 2024. "Preparation of Bioaerogel from Iron-Rich Microalgae for the Removal of Water Pollutants" Processes 12, no. 7: 1313. https://doi.org/10.3390/pr12071313





