Nano-Hydroxyapatite Modified Tobacco Stalk-Based Biochar for Immobilizing Cd(II): Interfacial Adsorption Behavior and Mechanisms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Tobacco Stalk-Derived Biochar and Its Modified Materials Preparation
2.3. Batch Adsorption Experiment
2.4. Analytical Methods
2.5. Characterization of Experimental Samples
3. Results and Discussions
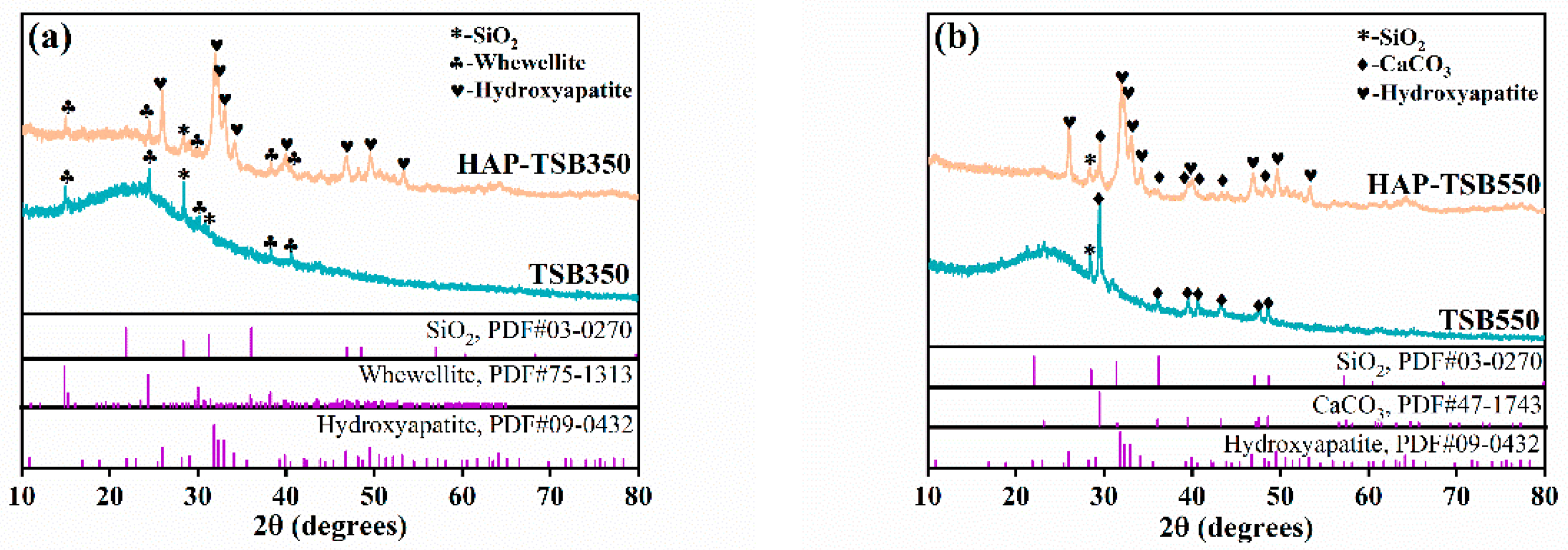
3.1. Characterization of the Biochar with Modification
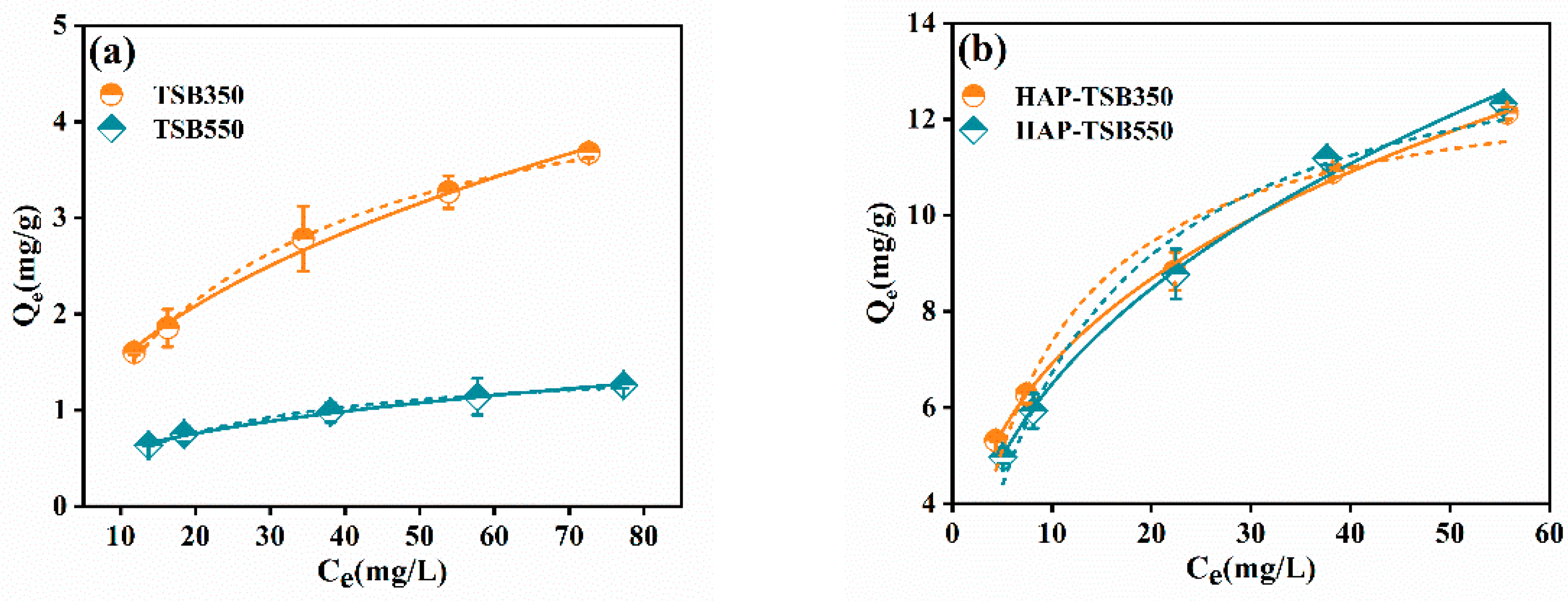
3.2. Adsorption Isotherm
3.3. Adsorption Kinetics
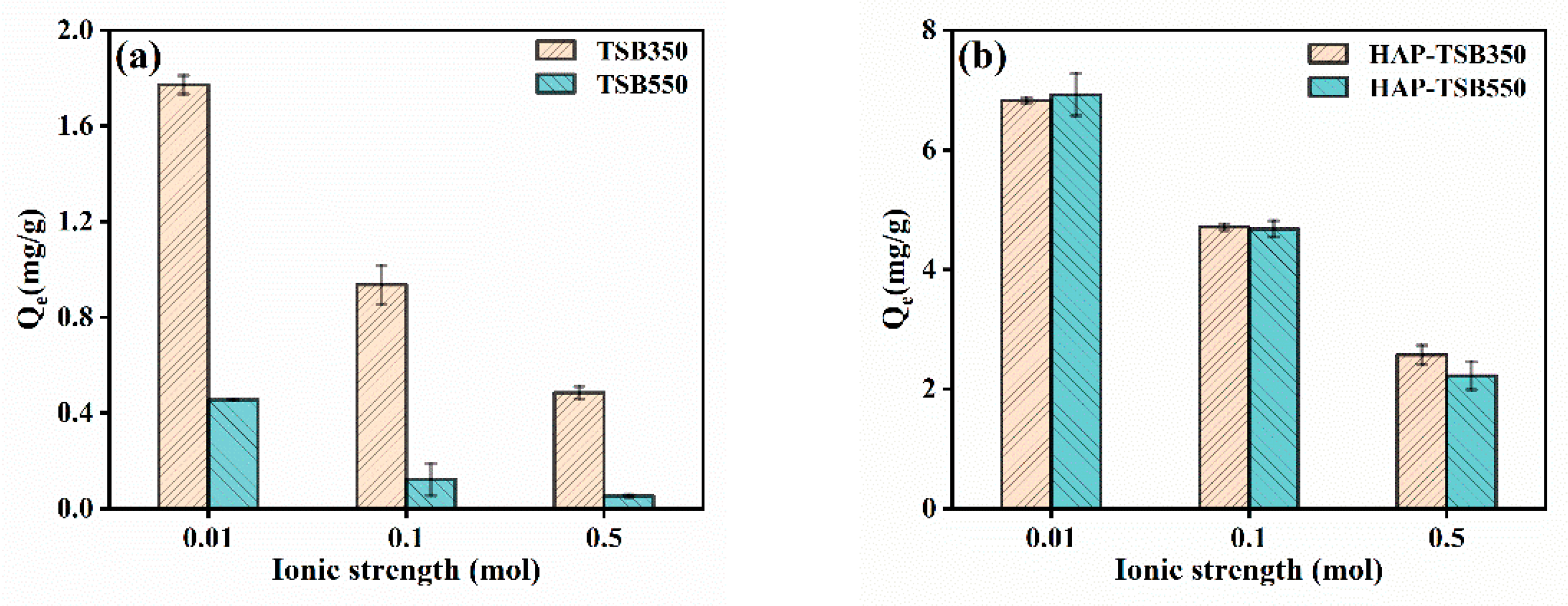
3.4. Effects of pH and Ionic Strength
3.5. X-ray Photoelectron Spectroscopy Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Oh, S.Y.; Yoon, M.K. Biochar for Treating Acid Mine Drainage. Environ. Eng. Sci. 2013, 30, 589–593. [Google Scholar] [CrossRef]
- Xiang, M.T.; Li, Y.; Yang, J.Y.; Lei, K.G.; Li, Y.; Li, F.; Zheng, D.F.; Fang, X.Q.; Cao, Y. Heavy metal contamination risk assessment and correlation analysis of heavy metal contents in soil and crops. Environ. Pollut. 2021, 278, 116911. [Google Scholar] [CrossRef] [PubMed]
- He, L.Z.; Xu, Y.; Zhang, M.E.; Gul, S.; Zhang, X.K.; Zhong, H.; Tang, Y.X.; Dong, D.B.; Xu, Y.; Liu, D.; et al. Effect of remediation technologies on soil fertility in heavy metal(loid)-contaminated soils: A critical review. Crit. Rev. Environ. Sci. Technol. 2024, 54, 1417–1435. [Google Scholar] [CrossRef]
- Liu, T.; Yuan, X.M.; Luo, K.; Xie, C.; Zhou, L.Y. Molecular engineering of a new method for effective removal of cadmium from water. Water. Res. 2024, 253, 121326. [Google Scholar] [CrossRef]
- Lange, K.; Viklander, M.; Blecken, G.T. Investigation of intra-event variations of total, dissolved and truly dissolved metal concentrations in highway runoff and a gross pollutant trap—Bioretention stormwater treatment train. Water. Res. 2022, 216, 118284. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.R.; Chen, H.B.; Fang, Z.; Niazi, N.K.; Adusei-Fosu, K.; Li, J.H.; Yang, X.; Liu, Z.Z.; Bolan, N.S.; Gao, B.; et al. Coupled sorptive and oxidative antimony(III) removal by iron-modified biochar: Mechanisms of electron-donating capacity and reactive Fe species. Environ. Pollut. 2023, 337, 122637. [Google Scholar] [CrossRef]
- Inyang, M.I.; Gao, B.; Yao, Y.; Xue, Y.W.; Zimmerman, A.; Mosa, A.; Pullammanappallil, P.; Ok, Y.S.; Cao, X.D. A review of biochar as a low-cost adsorbent for aqueous heavy metal removal. Crit. Rev. Environ. Sci. Technol. 2016, 46, 406–433. [Google Scholar] [CrossRef]
- Tran, H.T.; Bolan, N.S.; Lin, C.; Binh, Q.A.; Nguyen, M.K.; Luu, T.A.; Le, V.G.; Pham, C.Q.; Hoang, H.G.; Vo, D.V.N. Succession of biochar addition for soil amendment and contaminants remediation during co-composting: A state of art review. J. Environ. Manag. 2023, 342, 118191. [Google Scholar] [CrossRef]
- Biswal, B.K.; Balasubramanian, R. Use of biochar as a low-cost adsorbent for removal of heavy metals from water and wastewater: A review. J. Environ. Chem. Eng. 2023, 11, 110986. [Google Scholar] [CrossRef]
- Fakayode, O.A.; Aboagarib, E.A.A.; Zhou, C.S.; Ma, H.L. Co-pyrolysis of lignocellulosic and macroalgae biomasses for the production of biochar—A review. Bioresour. Technol. 2020, 297, 122408. [Google Scholar] [CrossRef]
- Yang, X.; Lu, K.; McGrouther, K.; Che, L.; Hu, G.; Wang, Q.; Liu, X.; Shen, L.; Huang, H.; Ye, Z.; et al. Bioavailability of Cd and Zn in soils treated with biochars derived from tobacco stalk and dead pigs. J. Soils Sediments 2017, 17, 751–762. [Google Scholar] [CrossRef]
- Zhang, W.; Li, C.; Li, G.; Lin, Q.; Zhao, X.; He, Y.; Liu, Y.; Luo, Z. Biochar alters inorganic phosphorus fractions in tobacco-growing soil. J. Soil Sci. Plant Nut. 2021, 21, 1689–1699. [Google Scholar] [CrossRef]
- Zheng, X.; Song, W.; Guan, E.; Wang, Y.; Hu, X.; Liang, H.; Dong, J. Response in physicochemical properties of tobacco-growing soils and N/P/K accumulation in tobacco plant to tobacco straw biochar. J. Soil Sci. Plant Nutr. 2020, 20, 293–305. [Google Scholar] [CrossRef]
- Yu, X.N.; Zhou, H.J.; Ye, X.F.; Wang, H.L. From hazardous agriculture waste to hazardous metal scavenger: Tobacco stalk biochar-mediated sequestration of Cd leads to enhanced tobacco productivity. J. Hazard. Mater. 2021, 413, 125303. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, M.K. Engineered biochar: A multifunctional material for energy and environment. Environ. Pollut. 2022, 298, 118831. [Google Scholar] [CrossRef]
- Chen, H.B.; Gao, Y.R.; Li, J.H.; Fang, Z.; Bolan, N.; Bhatnagar, A.; Gao, B.; Hou, D.Y.; Wang, S.S.; Song, H.; et al. Engineered biochar for environmental decontamination in aquatic and soil systems: A review. Carbon Res. 2022, 1, 4. [Google Scholar] [CrossRef]
- Xiao, J.; Hu, R.; Chen, G.C. Micro-nano-engineered nitrogenous bone biochar developed with a ball-milling technique for high-efficiency removal of aquatic Cd(II), Cu(II) and Pb(II). J. Hazard. Mater. 2020, 387, 121980. [Google Scholar] [CrossRef]
- Duan, W.Y.; Oleszczuk, P.; Pan, B.; Xing, B.S. Environmental behavior of engineered biochars and their aging processes in soil. Biochar 2019, 1, 339–351. [Google Scholar] [CrossRef]
- Medeiros, D.; Nzediegwu, C.; Benally, C.; Messele, S.A.; Kwak, J.H.; Naeth, M.A.; Ok, Y.S.; Chang, S.X.; El-Din, M.G. Pristine and engineered biochar for the removal of contaminants co-existing in several types of industrial wastewaters: A critical review. Sci. Total Environ. 2022, 809, 151120. [Google Scholar] [CrossRef]
- Li, M.P.; Dong, C.; Guo, C.X.; Yu, L.G. Magnetic activated biochar Fe3O4-MOS made from moringa seed shells for the adsorption of methylene blue. Processes 2022, 10, 2720. [Google Scholar] [CrossRef]
- Lan, G.X.; Yan, X.X.; Deng, P.Y.; Li, T.Z.; Xia, Y.P.; Zhu, Z.H.; Wu, Y.; Fu, C.A. Preparation of iron salt-modified sludge biochar and its uptake behavior for phosphate. Processes 2022, 10, 2122. [Google Scholar] [CrossRef]
- Chen, H.B.; Gao, Y.R.; Fang, Z.; Li, J.Y.; Pillai, S.C.; Song, H.C.; Sun, C.H.; Bolan, N.; Yang, X.; Vithanage, M.; et al. Investigating the electron-scale adsorption mechanisms using DFT calculations and experimental studies in self-assembly magnetic biochar gel incorporated with graphene nanosheets for enhanced Sb(III) removal. Chem. Eng. J. 2024, 487, 150740. [Google Scholar] [CrossRef]
- Ban, S.E.; Lee, E.J.; Lim, D.J.; Kim, I.S.; Lee, J.W. Evaluation of sulfuric acid-pretreated biomass-derived biochar characteristics and its diazinon adsorption mechanism. Bioresour. Technol. 2022, 348, 126828. [Google Scholar] [CrossRef]
- Bolan, S.; Hou, D.Y.; Wang, L.W.; Hale, L.; Egamberdieva, D.; Tammeorg, P.; Li, R.; Wang, B.; Xu, J.P.; Wang, T.; et al. The potential of biochar as a microbial carrier for agricultural and environmental applications. Sci. Total Environ. 2023, 886, 163968. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh, E.; Mishra, R.; Kumar, S. Biochar as environmental armour and its diverse role towards protecting soil, water and air. Sci. Total Environ. 2022, 806, 150444. [Google Scholar] [CrossRef]
- Chen, Y.N.; Li, M.L.; Li, Y.P.; Liu, Y.H.; Chen, Y.R.; Li, H.; Li, L.S.Z.; Xu, F.T.; Jiang, H.J.; Chen, L. Hydroxyapatite modified sludge-based biochar for the adsorption of Cu2+ and Cd2+: Adsorption behavior and mechanisms. Bioresour. Technol. 2021, 321, 124413. [Google Scholar] [CrossRef]
- Luo, Y.; Li, Z.P.; Xu, H.C.; Xu, X.Y.; Qiu, H.; Cao, X.D.; Zhao, L. Development of phosphorus composite biochar for simultaneous enhanced carbon sink and heavy metal immobilization in soil. Sci. Total Environ. 2022, 831, 154845. [Google Scholar] [CrossRef]
- Zhu, Z.Q.; Yang, Y.H.; Fan, Y.M.; Zhang, L.H.; Tang, S.; Zhu, Y.N.; Zhou, X.B. Strontium-doped hydroxyapatite as an efficient adsorbent for Cd(II) removal from wastewater: Performance, kinetics, and mechanism. Environ. Technol. Innov. 2022, 28, 102575. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Liu, Y.X.; Lu, H.H.; Yang, R.Q.; Yang, S.M. Competitive adsorption of Pb(II), Cu(II), and Zn(II) ions onto hydroxyapatite-biochar nanocomposite in aqueous solutions. J. Solid State Chem. 2018, 261, 53–61. [Google Scholar] [CrossRef]
- Sun, L.M.; Wu, J.S.; Wang, J.S.; Xu, M.; Zhou, W.Y.; Du, Y.C.; Li, Y.L.; Li, H.Y. Fabricating hydroxyapatite functionalized biochar composite using steel slag and Hami melon peel for Pb(II) and Cd(II) removal. Colloids Surf. Physicochem. Eng. Aspects. 2023, 666, 131310. [Google Scholar] [CrossRef]
- Gao, R.; Fu, Q.; Hu, H.; Wang, Q.; Liu, Y.; Zhu, J. Highly-effective removal of Pb by co-pyrolysis biochar derived from rape straw and orthophosphate. J. Hazard. Mater. 2019, 371, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Langmuir, I. The Adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Über die adsorption in lösungen Z. Phys. Chem. 1906, 57, 385–470. [Google Scholar] [CrossRef]
- Liao, J.; He, X.S.; Zhang, Y.; Zhang, L.; He, Z.B. The construction of magnetic hydroxyapatite-functionalized pig manure-derived biochar for the efficient uranium separation. Chem. Eng. J. 2023, 457, 141367. [Google Scholar] [CrossRef]
- Li, J.Y.; Gao, Y.R.; Li, C.B.; Wang, F.L.; Chen, H.B.; Yang, X.; Jeyakumar, P.; Sarkar, B.; Luo, Z.B.; Bolan, N.; et al. Pristine and Fe-functionalized biochar for the simultaneous immobilization of arsenic and antimony in a contaminated mining soil. J. Hazard. Mater. 2024, 469, 133937. [Google Scholar] [CrossRef]
- Qian, L.B.; Zhang, W.Y.; Yan, J.C.; Han, L.; Chen, Y.; Ouyang, D.; Chen, M.F. Nanoscale zero-valent iron supported by biochars produced at different temperatures: Synthesis mechanism and effect on Cr(VI) removal. Environ. Pollut. 2017, 223, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Ghosh, G.K.; Avasthe, R.K.; Sinha, K. Compositional heterogeneity of different biochar: Effect of pyrolysis temperature and feedstocks. J. Environ. Manag. 2021, 278, 111501. [Google Scholar] [CrossRef]
- Yang, F.; Jiang, Y.T.; Dai, M.; Hou, X.T.; Peng, C.S. Active biochar-supported iron oxides for Cr(VI) removal from groundwater: Kinetics, stability and the key role of FeO in electron-transfer mechanism. J. Hazard. Mater. 2022, 424, 127542. [Google Scholar] [CrossRef]
- Chia, C.H.; Gong, B.; Joseph, S.D.; Marjo, C.E.; Munroe, P.; Rich, A.M. Imaging of mineral-enriched biochar by FTIR, Raman and SEM-EDX. Vib. Spectrosc. 2012, 62, 248–257. [Google Scholar] [CrossRef]
- Sun, Y.Q.; Xiong, X.N.; He, M.J.; Xu, Z.B.; Hou, D.Y.; Zhang, W.H.; Ok, Y.S.; Rinklebe, J.; Wang, L.L.; Tsang, D.C.W. Roles of biochar-derived dissolved organic matter in soil amendment and environmental remediation: A critical review. Chem. Eng. J. 2021, 424, 130387. [Google Scholar] [CrossRef]
- Zhu, S.H.; Irshad, M.K.; Ibrahim, M.; Chen, Q.; Shang, J.Y.; Zhang, Q.R. The distinctive role of nano-hydroxyapatite modified biochar for alleviation of cadmium and arsenic toxicity in aqueous system. J. Water Process. Eng. 2022, 49, 103054. [Google Scholar] [CrossRef]
- Li, X.F.; Guo, C.L.; Jin, X.H.; Yao, Q.; Liu, Q.Q.; Zhang, L.J.; Lu, G.N.; Reinfelder, J.R.; Huang, W.L.; Dang, Z. Molecular-scale study of Cr(vi) adsorption onto lepidocrocite facets by EXAFS, in situ ATR-FTIR, theoretical frequency calculations and DFT+ U techniques. Environ. Sci. Nano 2022, 9, 568–581. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Adi, V.S.K.; Huang, H.L.; Lin, H.P.; Huang, Z.H. Adsorption of metal ions with biochars derived from biomass wastes in a fixed column: Adsorption isotherm and process simulation. J. Ind. Eng. Chem. 2019, 76, 240–244. [Google Scholar] [CrossRef]
- Li, Z.; Li, M.; Che, Q.; Li, Y.D.; Liu, X. Synergistic removal of tylosin/sulfamethoxazole and copper by nanohydroxyapatite modified biochar. Bioresour. Technol. 2019, 294, 122163. [Google Scholar] [CrossRef]
- Liu, N.; Wang, M.X.; Liu, M.M.; Liu, F.; Weng, L.; Koopal, L.K.; Tan, W.F. Sorption of tetracycline on organo-montmorillonites. J. Hazard. Mater. 2012, 225–226, 28–35. [Google Scholar] [CrossRef]
- Liu, C.W.; Ye, J.; Lin, Y.; Wu, J.; Price, G.W.; Burton, D.; Wang, Y.X. Removal of Cadmium (II) using water hyacinth (Eichhornia crassipes) biochar alginate beads in aqueous solutions. Environ. Pollut. 2020, 264, 114785. [Google Scholar] [CrossRef]
- Zhou, Z.; Xu, Z.; Feng, Q.; Yao, D.; Yu, J.; Wang, D.; Lv, S.; Liu, Y.; Zhou, N.; Zhong, M.-E. Effect of pyrolysis condition on the adsorption mechanism of lead, cadmium and copper on tobacco stem biochar. J. Clean. Prod. 2018, 187, 996–1005. [Google Scholar] [CrossRef]
- Li, Z.W.; Niu, R.Y.; Yu, J.H.; Yu, L.Y.; Cao, D. Removal of cadmium from aqueous solution by magnetic biochar: Adsorption characteristics and mechanism. Environ. Sci. Pollut. Res. 2024, 31, 6543–6557. [Google Scholar] [CrossRef]
- Wu, W.L.; Liu, Z.H.; Azeem, M.; Guo, Z.Q.; Li, R.H.; Li, Y.G.; Peng, Y.R.; Ali, E.F.; Wang, H.L.; Wang, S.S.; et al. Hydroxyapatite tailored hierarchical porous biochar composite immobilized Cd(II) and Pb(II) and mitigated their hazardous effects in contaminated water and soil. J. Hazard. Mater. 2022, 437, 129330. [Google Scholar] [CrossRef]
- Li, X.F.; Guo, C.L.; Jin, X.H.; He, C.C.; Dang, Z. Mechanisms of Cr(VI) adsorption on schwertmannite under environmental disturbance: Changes in surface complex structures. J. Hazard. Mater. 2021, 416, 125781. [Google Scholar] [CrossRef]
- Wang, H.Y.; Gao, B.; Wang, S.S.; Fang, J.; Xue, Y.W.; Yang, K. Removal of Pb(II), Cu(II), and Cd(II) from aqueous solutions by biochar derived from KMnO4 treated hickory wood. Bioresour. Technol. 2015, 197, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Hu, J.; Yu, Y.; Ma, D.; Gong, W.; Qiu, H.; Hu, Z.; Gao, H.W. Facile preparation of sulfonated biochar for highly efficient removal of toxic Pb(II) and Cd(II) from wastewater. Sci. Total Environ. 2021, 750, 141545. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Liu, X.; Zhang, J.; Tie, B.; Lei, M.; Wei, X.; Peng, O.; Du, H. Silicate-modified oiltea camellia shell-derived biochar: A novel and cost-effective sorbent for cadmium removal. J. Clean. Prod. 2021, 281, 125390. [Google Scholar] [CrossRef]





| Parameters | TSB350 | TSB550 | HAP–TSB350 | HAP–TSB550 |
|---|---|---|---|---|
| Langmuir | ||||
| Qmax | 4.92 | 1.57 | 13.17 | 14.50 |
| KL | 0.038 | 0.048 | 0.127 | 0.086 |
| R2 | 0.995 | 0.988 | 0.939 | 0.966 |
| Freundlich | ||||
| 1/n | 0.45 | 0.38 | 0.33 | 0.39 |
| KF | 0.54 | 0.24 | 3.23 | 2.66 |
| R2 | 0.991 | 0.995 | 0.998 | 0.993 |
| Pseudo-First-Order Model | Pseudo-Second-Order Model | |||||
|---|---|---|---|---|---|---|
| Qe | K1 | R2 | Qe | K2 | R2 | |
| TSB350 | 2.08 | 0.89 | 0.383 | 2.26 | 0.24 | 0.997 |
| TSB550 | 1.09 | 1.85 | 0.819 | 1.09 | 3.94 | 0.999 |
| HAP–TSB350 | 5.37 | 0.91 | 0.424 | 6.04 | 0.07 | 0.999 |
| HAP–TSB550 | 1.13 | 6.21 | 0.393 | 6.76 | 0.11 | 0.999 |
| C Speciation Proportion (%) | O Speciation Proportion (%) | |||||
|---|---|---|---|---|---|---|
| C–C | C–O | C=O | O–C=O | C=O | O–H | |
| TSB350 | 65.59 | 31.57 | 2.84 | 21.18 | 47.28 | 31.54 |
| Cd–TSB350 | 50.62 | 28.25 | 21.13 | 23.30 | 55.87 | 20.83 |
| HAP–TSB350 | 68.84 | 19.33 | 11.83 | 8.49 | 49.55 | 41.96 |
| Cd–HAP–TSB350 | 61.52 | 18.08 | 20.40 | 26.26 | 67.55 | 6.19 |
| TSB550 | 85.06 | 10.96 | 3.98 | 27.17 | 42.60 | 30.23 |
| Cd–TSB550 | 77.33 | 10.76 | 11.91 | 28.55 | 43.63 | 27.82 |
| HAP–TSB550 | 63.83 | 33.02 | 3.15 | 12.18 | 64.93 | 22.89 |
| Cd–HAP–TSB550 | 69.79 | 20.08 | 10.13 | 19.59 | 76.24 | 4.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Li, X.; Shen, C.; Chen, D.; Li, F.; Xu, W.; Wu, X.; Bao, Y. Nano-Hydroxyapatite Modified Tobacco Stalk-Based Biochar for Immobilizing Cd(II): Interfacial Adsorption Behavior and Mechanisms. Processes 2024, 12, 1924. https://doi.org/10.3390/pr12091924
Li T, Li X, Shen C, Chen D, Li F, Xu W, Wu X, Bao Y. Nano-Hydroxyapatite Modified Tobacco Stalk-Based Biochar for Immobilizing Cd(II): Interfacial Adsorption Behavior and Mechanisms. Processes. 2024; 12(9):1924. https://doi.org/10.3390/pr12091924
Chicago/Turabian StyleLi, Tianfu, Xiaofei Li, Chaoran Shen, Dian Chen, Fuhua Li, Weicheng Xu, Xiaolian Wu, and Yanping Bao. 2024. "Nano-Hydroxyapatite Modified Tobacco Stalk-Based Biochar for Immobilizing Cd(II): Interfacial Adsorption Behavior and Mechanisms" Processes 12, no. 9: 1924. https://doi.org/10.3390/pr12091924







