Influence of Plasticizers Concentration on Thermal, Mechanical, and Physicochemical Properties on Starch Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Film Preparation
2.2. Physical Characteristics
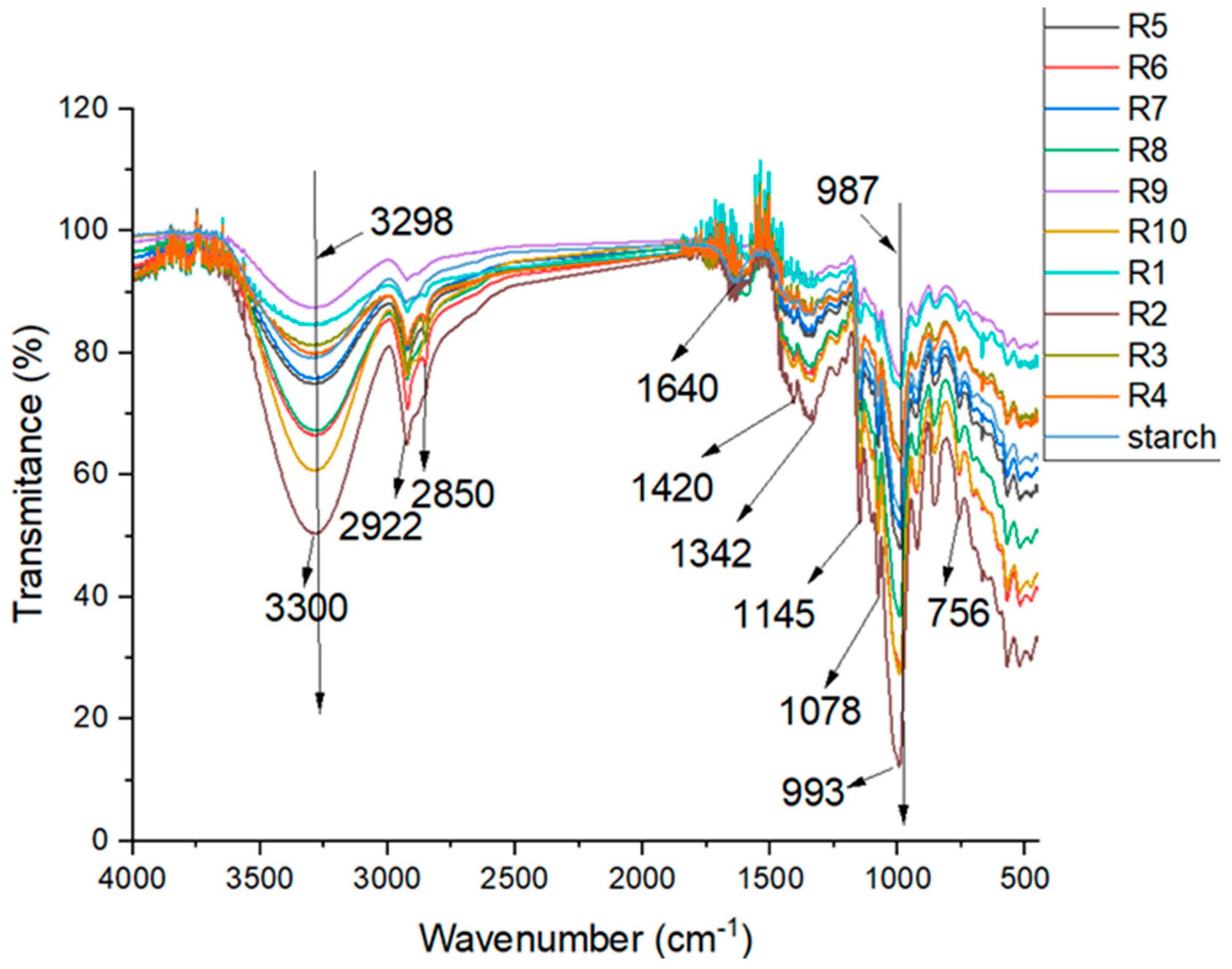
2.2.1. FTIR Analysis
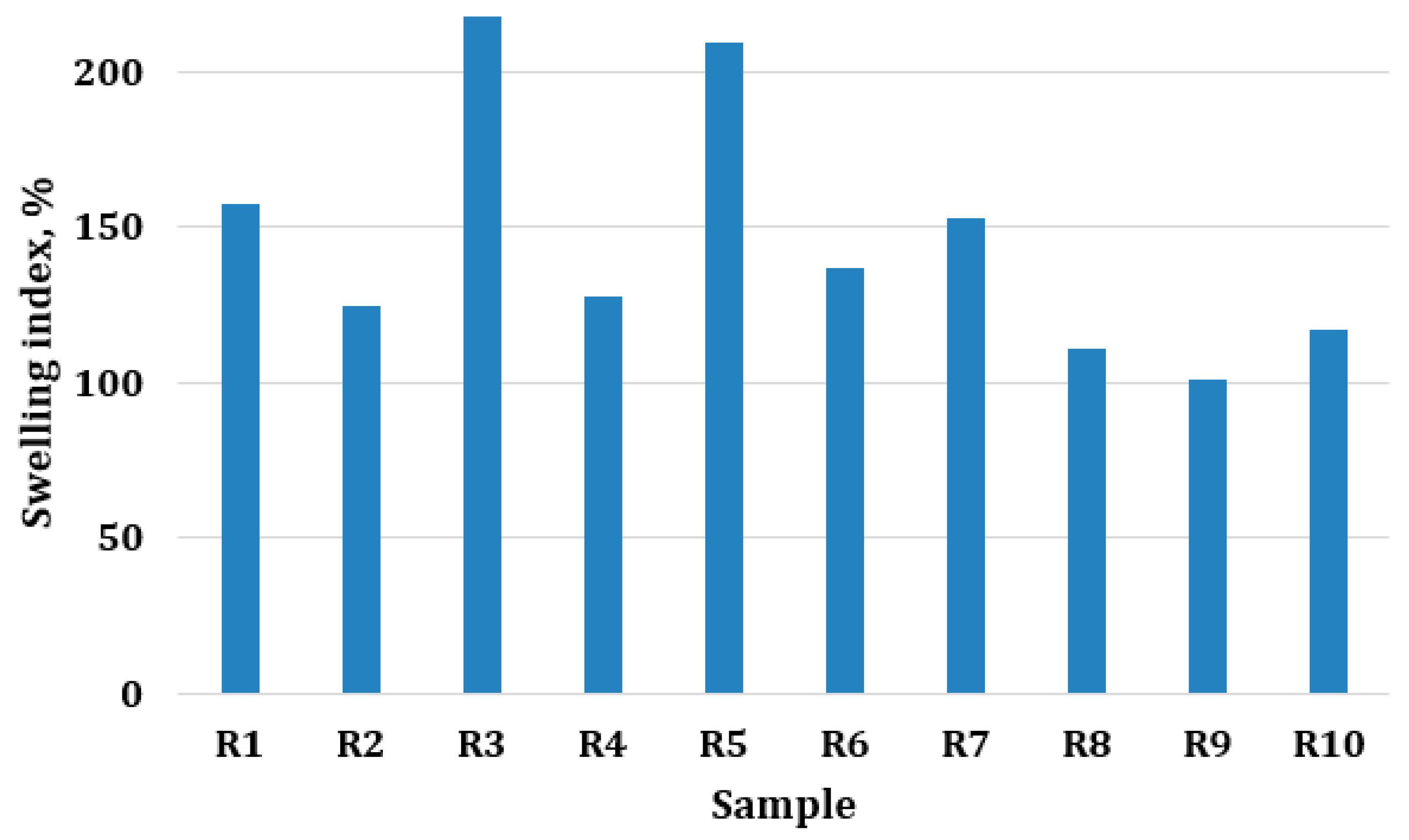
2.2.2. Film Swelling Degree
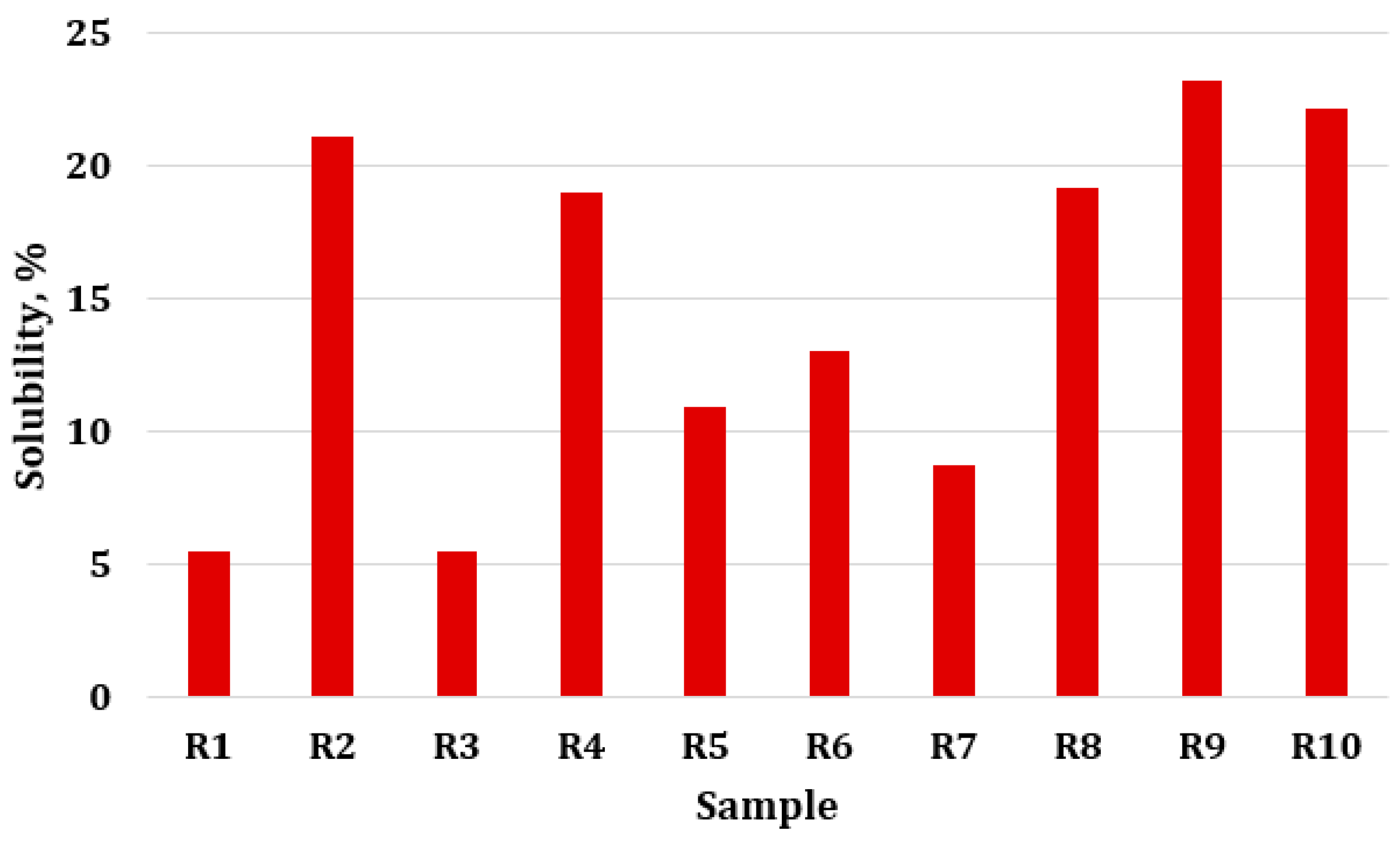
2.2.3. Film Solubility in Water
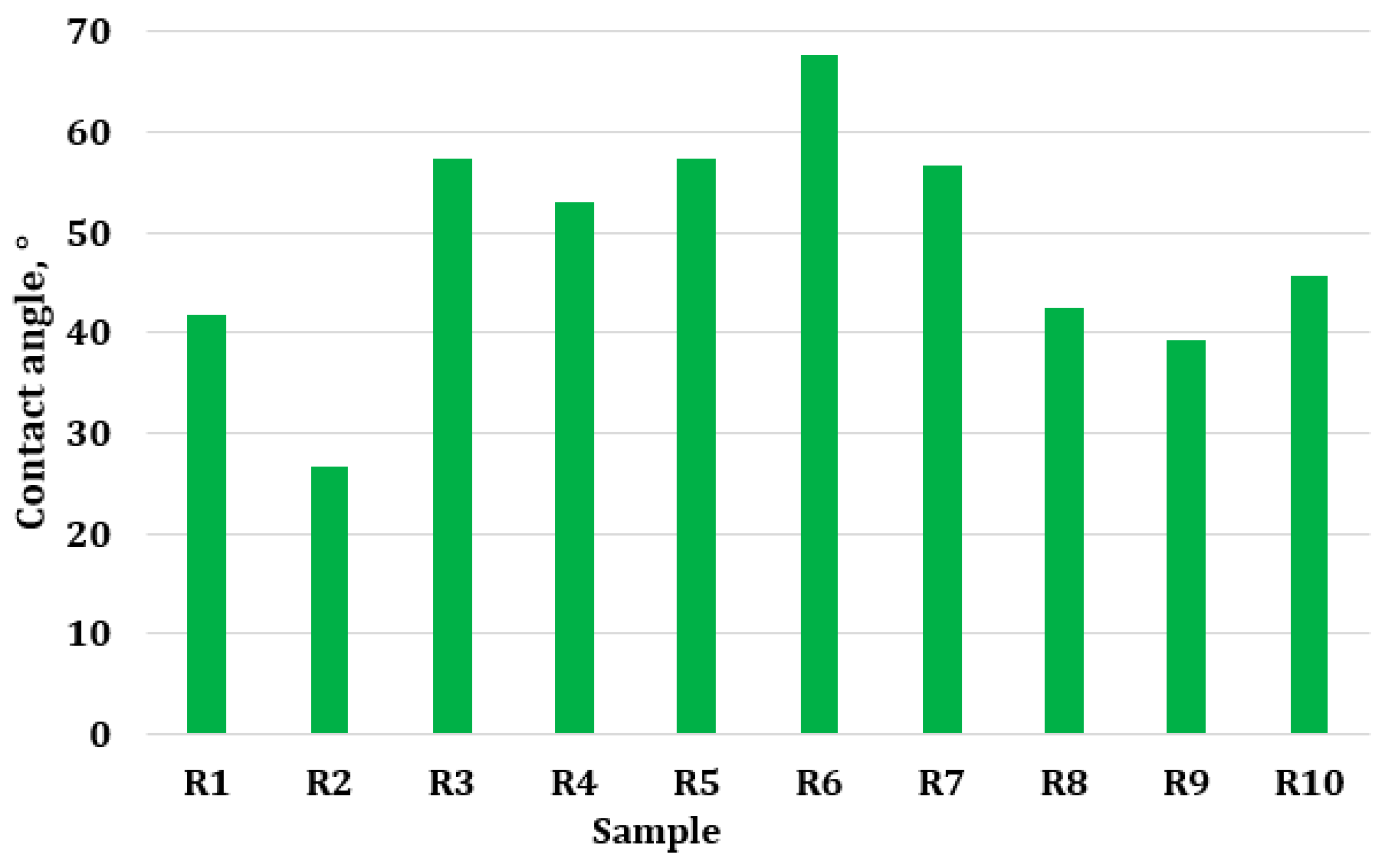
2.2.4. The Contact Angle
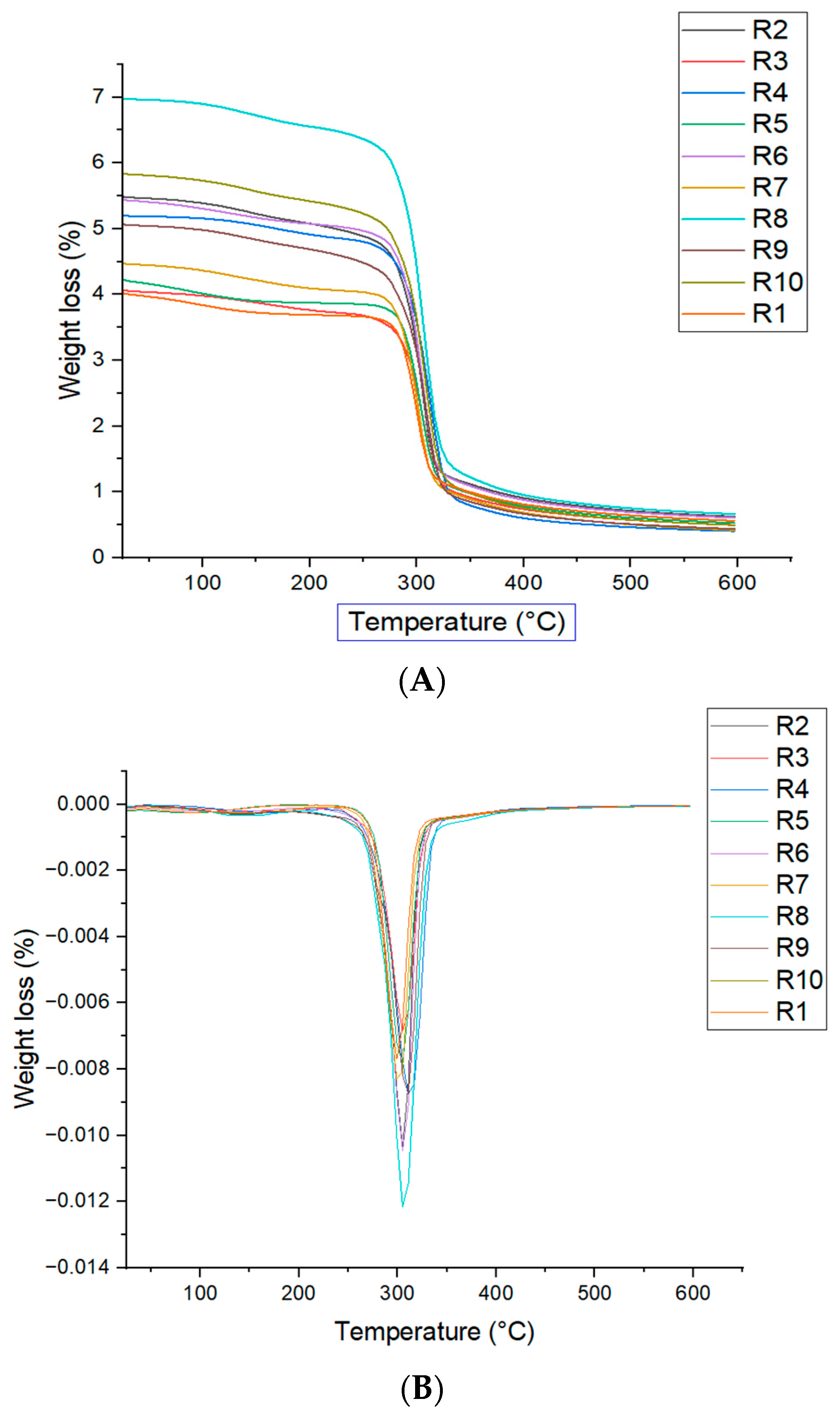
2.2.5. Thermogravimetric Analysis (TGA)
2.2.6. Thickness and Density
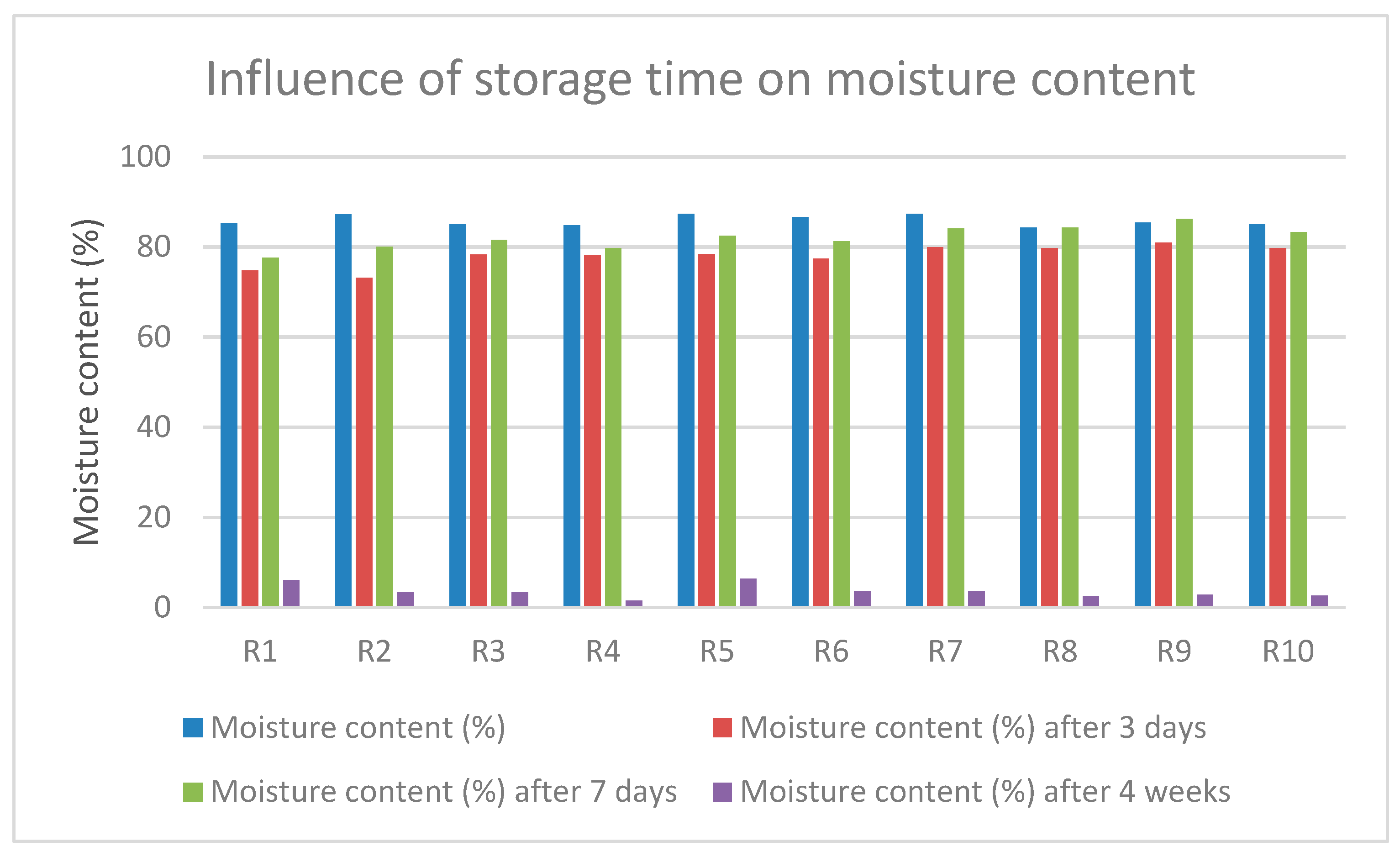
2.2.7. Moisture Content (MC)
2.2.8. Mechanical Properties
3. Results and Discussion
3.1. FTIR Analysis
3.2. Film Swelling Degree
3.3. Film Solubility in Water
3.4. Thermogravimetric Analysis (TGA)
3.5. Water Contact Angle
3.6. Thickness and Density
3.7. Moisture Content (MC)
3.8. Mechanical Testing
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Geiselman, G.M.; Kirby, J.; Landera, A.; Otoupal, P.; Papa, G.; Barcelos, C.; Sundstrom, E.R.; Das, L.; Magurudeniya, H.D.; Wehrs, M.; et al. Conversion of Poplar Biomass into High-Energy Density Tricyclic Sesquiterpene Jet Fuel Blendstocks. Microb. Cell Factories 2020, 19, 208. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Xu, X.; Fang, Y.; Yan, S.; Cui, B.; Abd El-Aty, A.M. Effect of Different Ratios of Glycerol and Erythritol on Properties of Corn Starch-Based Films. Front. Nutr. 2022, 9, 882682. [Google Scholar] [CrossRef]
- Easdani, M.; Ahammed, S.; Saqib, M.N.; Liu, F.; Zhong, F. Engineering Biodegradable Controlled Gelatin-Zein Bilayer Film with Improved Mechanical Strength and Flexibility. Food Hydrocoll. 2024, 148, 109430. [Google Scholar] [CrossRef]
- Dirpan, A.; Ainani, A.F.; Djalal, M. A Review on Biopolymer-Based Biodegradable Film for Food Packaging: Trends over the Last Decade and Future Research. Polymers 2023, 15, 2781. [Google Scholar] [CrossRef]
- Pivnenko, K.; Eriksen, M.K.; Martín-Fernández, J.A.; Eriksson, E.; Astrup, T.F. Recycling of Plastic Waste: Presence of Phthalates in Plastics from Households and Industry. Waste Manag. 2016, 54, 44–52. [Google Scholar] [CrossRef]
- Rochester, J.R. Bisphenol A and Human Health: A Review of the Literature. Reprod. Toxicol. 2013, 42, 132–155. [Google Scholar] [CrossRef] [PubMed]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An Overview of Chemical Additives Present in Plastics: Migration, Release, Fate and Environmental Impact during Their Use, Disposal and Recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef]
- Thompson, R.C.; Moore, C.J.; Vom Saal, F.S.; Swan, S.H. Plastics, the Environment and Human Health: Current Consensus and Future Trends. Phil. Trans. R. Soc. B 2009, 364, 2153–2166. [Google Scholar] [CrossRef] [PubMed]
- Hopewell, J.; Dvorak, R.; Kosior, E. Plastics Recycling: Challenges and Opportunities. Phil. Trans. R. Soc. B 2009, 364, 2115–2126. [Google Scholar] [CrossRef]
- Sanyang, M.L.; Sapuan, S.M.; Jawaid, M.; Ishak, M.R.; Sahari, J. Effect of Plasticizer Type and Concentration on Physical Properties of Biodegradable Films Based on Sugar Palm (Arenga pinnata) Starch for Food Packaging. J. Food Sci. Technol. 2016, 53, 326–336. [Google Scholar] [CrossRef]
- Talja, R.A.; Helén, H.; Roos, Y.H.; Jouppila, K. Effect of Type and Content of Binary Polyol Mixtures on Physical and Mechanical Properties of Starch-Based Edible Films. Carbohydr. Polym. 2008, 71, 269–276. [Google Scholar] [CrossRef]
- Aghazadeh, M.; Karim, R.; Rahman, R.A.; Sultan, M.T.; Johnson, S.K.; Paykary, M. Effect of Glycerol on the Physicochemical Properties of Cereal Starch Films. Czech J. Food Sci. 2018, 36, 403–409. [Google Scholar] [CrossRef]
- Basiak, E.; Lenart, A.; Debeaufort, F. How Glycerol and Water Contents Affect the Structural and Functional Properties of Starch-Based Edible Films. Polymers 2018, 10, 412. [Google Scholar] [CrossRef]
- Tarique, J.; Sapuan, S.M.; Khalina, A. Effect of Glycerol Plasticizer Loading on the Physical, Mechanical, Thermal, and Barrier Properties of Arrowroot (Maranta azrundinacea) Starch Biopolymers. Sci. Rep. 2021, 11, 13900. [Google Scholar] [CrossRef]
- González-Torres, B.; Robles-García, M.Á.; Gutiérrez-Lomelí, M.; Padilla-Frausto, J.J.; Navarro-Villarruel, C.L.; Del-Toro-Sánchez, C.L.; Rodríguez-Félix, F.; Barrera-Rodríguez, A.; Reyna-Villela, M.Z.; Avila-Novoa, M.G.; et al. Combination of Sorbitol and Glycerol, as Plasticizers, and Oxidized Starch Improves the Physicochemical Characteristics of Films for Food Preservation. Polymers 2021, 13, 3356. [Google Scholar] [CrossRef]
- Li, H.; Huneault, M.A. Comparison of Sorbitol and Glycerol as Plasticizers for Thermoplastic Starch in TPS/PLA Blends. J. Appl. Polym. Sci. 2011, 119, 2439–2448. [Google Scholar] [CrossRef]
- Domene-López, D.; Guillén, M.M.; Martin-Gullon, I.; García-Quesada, J.C.; Montalbán, M.G. Study of the Behavior of Biodegradable Starch/Polyvinyl Alcohol/Rosin Blends. Carbohydr. Polym. 2018, 202, 299–305. [Google Scholar] [CrossRef]
- Torres, F.G.; Troncoso, O.P.; Torres, C.; Díaz, D.A.; Amaya, E. Biodegradability and Mechanical Properties of Starch Films from Andean Crops. Int. J. Biol. Macromol. 2011, 48, 603–606. [Google Scholar] [CrossRef]
- Luchese, C.L.; Spada, J.C.; Tessaro, I.C. Starch Content Affects Physicochemical Properties of Corn and Cassava Starch-Based Films. Ind. Crop. Prod. 2017, 109, 619–626. [Google Scholar] [CrossRef]
- Luchese, C.L.; Benelli, P.; Spada, J.C.; Tessaro, I.C. Impact of the Starch Source on the Physicochemical Properties and Biodegradability of Different Starch-Based Films. J. Appl. Polym. Sci. 2018, 135, 46564. [Google Scholar] [CrossRef]
- Nordin, N.; Othman, S.H.; Kadir Basha, R.; Abdul Rashid, S. Mechanical and Thermal Properties of Starch Films Reinforced with Microcellulose Fibres. Food Res. 2018, 2, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Thakur, R.; Pristijono, P.; Scarlett, C.J.; Bowyer, M.; Singh, S.P.; Vuong, Q.V. Starch-Based Films: Major Factors Affecting Their Properties. Int. J. Biol. Macromol. 2019, 132, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Malmir, S.; Montero, B.; Rico, M.; Barral, L.; Bouza, R.; Farrag, Y. Effects of Poly (3-Hydroxybutyrate-Co-3-Hydroxyvalerate) Microparticles on Morphological, Mechanical, Thermal, and Barrier Properties in Thermoplastic Potato Starch Films. Carbohydr. Polym. 2018, 194, 357–364. [Google Scholar] [CrossRef]
- Li, M.; Xie, F.; Hasjim, J.; Witt, T.; Halley, P.J.; Gilbert, R.G. Establishing Whether the Structural Feature Controlling the Mechanical Properties of Starch Films Is Molecular or Crystalline. Carbohydr. Polym. 2015, 117, 262–270. [Google Scholar] [CrossRef]
- Domene-López, D.; García-Quesada, J.C.; Martin-Gullon, I.; Montalbán, M.G. Influence of Starch Composition and Molecular Weight on Physicochemical Properties of Biodegradable Films. Polymers 2019, 11, 1084. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, L.; Li, H.; Chi, Y.; Zhang, H.; Xia, N.; Ma, Y.; Jiang, L.; Zhang, X. Changes in Properties of Soy Protein Isolate Edible Films Stored at Different Temperatures: Studies on Water and Glycerol Migration. Foods 2021, 10, 1797. [Google Scholar] [CrossRef]
- Neira, L.M.; Martucci, J.F.; Stejskal, N.; Ruseckaite, R.A. Time-Dependent Evolution of Properties of Fish Gelatin Edible Films Enriched with Carvacrol during Storage. Food Hydrocoll. 2019, 94, 304–310. [Google Scholar] [CrossRef]
- Chen, C.; Du, Y.; Zuo, G.; Chen, F.; Liu, K.; Zhang, L. Effect of Storage Condition on the Physico-Chemical Properties of Corn–Wheat Starch/Zein Edible Bilayer Films. R. Soc. Open Sci. 2020, 7, 191777. [Google Scholar] [CrossRef] [PubMed]
- Cano, A.; Fortunati, E.; Cháfer, M.; Kenny, J.M.; Chiralt, A.; González-Martínez, C. Properties and Ageing Behaviour of Pea Starch Films as Affected by Blend with Poly(Vinyl Alcohol). Food Hydrocoll. 2015, 48, 84–93. [Google Scholar] [CrossRef]
- Mali, S.; Grossmann, M.V.E.; García, M.A.; Martino, M.N.; Zaritzky, N.E. Effects of Controlled Storage on Thermal, Mechanical and Barrier Properties of Plasticized Films from Different Starch Sources. J. Food Eng. 2006, 75, 453–460. [Google Scholar] [CrossRef]
- Usman, N.; Hassan, L.G.; Almustapha, M.N.; Achor, M.; Agwamba, E.C. Preparation and Characterization of Thermoplastic Cassava and Sweet Potato Starches. Niger. J. Basic Appl. Sci. 2023, 30, 118–125. [Google Scholar] [CrossRef]
- Muhammad, A.; Roslan, A.; Sanusi, S.N.A.; Shahimi, M.Q.; Nazari, N.Z. Mechanical Properties of Bioplastic Form Cellulose Nanocrystal (CNC) Mangosteen Peel Using Glycerol as Plasticizer. J. Phys. Conf. Ser. 2019, 1349, 012099. [Google Scholar] [CrossRef]
- Nurhajati, D.W.; Pidhatika, B.; Harjanto, S. Biodegradable Plastics from Linier Low-Density Polyethylene and Polysaccharide: The Influence of Polysaccharide and Acetic Acid. Maj. Kulit Karet Plast. 2019, 35, 33. [Google Scholar] [CrossRef]
- Mojibayo, I.; Samson, A.O. A Preliminary Investigation of Cassava Starch Potentials As Natural Polymer In Bioplastic Production. Am. J. Interdiscip. Innov. Res. 2020, 02, 31–39. [Google Scholar] [CrossRef]
- Jia, R.; Cui, C.; Gao, L.; Qin, Y.; Ji, N.; Dai, L.; Wang, Y.; Xiong, L.; Shi, R.; Sun, Q. A Review of Starch Swelling Behavior: Its Mechanism, Determination Methods, Influencing Factors, and Influence on Food Quality. Carbohydr. Polym. 2023, 321, 121260. [Google Scholar] [CrossRef]
- Ibrahim, M.I.J.; Sapuan, S.M.; Zainudin, E.S.; Zuhri, M.Y.M. Physical, Thermal, Morphological, and Tensile Properties of Cornstarch-Based Films as Affected by Different Plasticizers. Int. J. Food Prop. 2019, 22, 925–941. [Google Scholar] [CrossRef]
- ASTM D882-18; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM International: West Conshohocken, PA, USA, 2018.
- ASTM International 638-14; Standard Test Method for Tensile Properties of Plastics (Metric); Annual Book of ASTM Standards, Part 08.01, Plastics-General Test Method; American Society for Testing and Materials. ASTM International: West Conshohocken, PA, USA, 2014.
- ISO 527-1; Plastics–Determination of Tensile Properties, Part. 1 General Principles. International Organization for Standardization: Geneva, Switzerland, 2019.
- Santha, N.; Sudha, K.G.; Vijayakumari, K.P.; Nayar, V.U.; Moorthy, S.N. Raman and Infrared Spectra of Starch Samples of Sweet Potato and Cassava. J. Chem. Sci. 1990, 102, 705–712. [Google Scholar] [CrossRef]
- Ma, C.; Tao, H.; Tan, C.; Gao, S.; Wu, Z.; Guo, L.; Cui, B.; Yuan, F.; Zou, F.; Liu, P.; et al. Effects of Polyols with Different Hydroxyl Numbers on the Structure and Properties of Starch Straws. Carbohydr. Polym. 2023, 321, 121297. [Google Scholar] [CrossRef]
- Awol, A.; Waghray, K.; Prabhakara Rao, P.G.; Math, R. Preparation and Characterization of Enset Starch ES) Based Films: Plasticized by Glycerol and Sorbitol. Iran. J. Chem. Chem. Eng. 2021, 41, 446–454. [Google Scholar] [CrossRef]
- Arnold, J.C. Environmental Effects on Crack Growth in Polymers. In Comprehensive Structural Integrity; Elsevier: Amsterdam, The Netherlands, 2003; pp. 281–319. ISBN 978-0-08-043749-1. [Google Scholar]
- Guo, S.; Fu, Z.; Sun, Y.; Wang, X.; Wu, M. Effect of Plasticizers on the Properties of Potato Flour Films. Starch Stärke 2022, 74, 2100179. [Google Scholar] [CrossRef]
- Ballesteros-Mártinez, L.; Pérez-Cervera, C.; Andrade-Pizarro, R. Effect of Glycerol and Sorbitol Concentrations on Mechanical, Optical, and Barrier Properties of Sweet Potato Starch Film. NFS J. 2020, 20, 1–9. [Google Scholar] [CrossRef]
- Nordin, N.; Othman, S.H.; Rashid, S.A.; Basha, R.K. Effects of Glycerol and Thymol on Physical, Mechanical, and Thermal Properties of Corn Starch Films. Food Hydrocoll. 2020, 106, 105884. [Google Scholar] [CrossRef]
- Aydın, A.A.; Ilberg, V. Effect of Different Polyol-Based Plasticizers on Thermal Properties of Polyvinyl Alcohol:Starch Blends. Carbohydr. Polym. 2016, 136, 441–448. [Google Scholar] [CrossRef] [PubMed]
- González, A.; Alvarez Igarzabal, C.I. Soy Protein—Poly (Lactic Acid) Bilayer Films as Biodegradable Material for Active Food Packaging. Food Hydrocoll. 2013, 33, 289–296. [Google Scholar] [CrossRef]
- Gao, W.; Zhu, J.; Kang, X.; Wang, B.; Liu, P.; Cui, B.; Abd El-Aty, A.M. Development and Characterization of Starch Films Prepared by Extrusion Blowing: The Synergistic Plasticizing Effect of Water and Glycerol. LWT 2021, 148, 111820. [Google Scholar] [CrossRef]
- Sothornvit, R.; Krochta, J.M. Plasticizer Effect on Mechanical Properties of β-Lactoglobulin Films. J. Food Eng. 2001, 50, 149–155. [Google Scholar] [CrossRef]
- Farahnaky, A.; Saberi, B.; Majzoobi, M. Effect of Glycerol on Physical and Mechanical Properties of Wheat Starch Edible Films. J. Texture Stud. 2013, 44, 176–186. [Google Scholar] [CrossRef]
- Mohammed, A.A.B.A.; Hasan, Z.; Omran, A.A.B.; Elfaghi, A.M.; Khattak, M.A.; Ilyas, R.A.; Sapuan, S.M. Effect of Various Plasticizers in Different Concentrations on Physical, Thermal, Mechanical, and Structural Properties of Wheat Starch-Based Films. Polymers 2022, 15, 63. [Google Scholar] [CrossRef]
- Prasetyo, D.J.; Apriyana, W.; Jatmiko, T.H.; Hernawan; Hayati, S.N.; Rosyida, V.T.; Pranoto, Y.; Poeloengasih, C.D. Physicochemical Properties of Sugar Palm Starch Film: Effect of Concentration and Plasticizer Type. IOP Conf. Ser. Mater. Sci. Eng. 2017, 223, 012049. [Google Scholar] [CrossRef]
- Song, X. Effect of Storage Conditions on the Physicochemical Characteristics of Bilayer Edible Films Based on Iron Yam–Pea Starch Blend and Corn Zein. Coatings 2022, 12, 1524. [Google Scholar] [CrossRef]
- Lim, W.S.; Ock, S.Y.; Park, G.D.; Lee, I.W.; Lee, M.H.; Park, H.J. Heat-Sealing Property of Cassava Starch Film Plasticized with Glycerol and Sorbitol. Food Packag. Shelf Life 2020, 26, 100556. [Google Scholar] [CrossRef]
- Hazrol, M.D.; Sapuan, S.M.; Zainudin, E.S.; Zuhri, M.Y.M.; Abdul Wahab, N.I. Corn Starch (Zea Mays) Biopolymer Plastic Reaction in Combination with Sorbitol and Glycerol. Polymers 2021, 13, 242. [Google Scholar] [CrossRef] [PubMed]
- Laohakunjit, N.; Noomhorm, A. Effect of Plasticizers on Mechanical and Barrier Properties of Rice Starch Film. Starch Stärke 2004, 56, 348–356. [Google Scholar] [CrossRef]

| Abbreviation | Glycerol (%) | Sorbitol (%) |
|---|---|---|
| R1 | 5 | 0 |
| R2 | 30 | 0 |
| R3 | 0 | 5 |
| R4 | 0 | 30 |
| R5 | 2.5 | 2.5 |
| R6 | 1.67 | 3.33 |
| R7 | 3.33 | 1.67 |
| R8 | 10 | 20 |
| R9 | 20 | 10 |
| R10 | 15 | 15 |
| Abbreviation | First Stage of Decomposition (%) | Second Stage of Decomposition (%) | Mass Residue (%) | Td (°C) | T10 (°C) | T50 (°C) | T85 (°C) |
|---|---|---|---|---|---|---|---|
| R1 | 6.06 | 72.41 | 3.75 | 300 | 200 | 299 | 526 |
| R2 | 3.36 | 77.61 | 2.94 | 305.67 | 229 | 299 | 428 |
| R3 | 3.39 | 76.48 | 3.60 | 306.17 | 246 | 305 | 462 |
| R4 | 1.47 | 84.41 | 2.30 | 312.67 | 264 | 310 | 351 |
| R5 | 6.37 | 74.21 | 3.70 | 303.92 | 235 | 305 | 468 |
| R6 | 3.65 | 78.86 | 3.02 | 306 | 258 | 305 | 421 |
| R7 | 3.57 | 77.45 | 3.61 | 301.67 | 205 | 299 | 398 |
| R8 | 2.56 | 81.59 | 2.52 | 306.25 | 252 | 305 | 375 |
| R9 | 2.82 | 81.83 | 2.87 | 309.5 | 235 | 305 | 369 |
| R10 | 2.59 | 82.08 | 2.71 | 307.92 | 246 | 305 | 369 |
| Abbreviation | Thickness (cm) | Density (g/cm3) |
|---|---|---|
| R1 | 0.091 | 1.195 |
| R2 | 0.100 | 1.360 |
| R3 | 0.103 | 1.389 |
| R4 | 0.090 | 1.530 |
| R5 | 0.096 | 1.425 |
| R6 | 0.084 | 1.519 |
| R7 | 0.103 | 1.533 |
| R8 | 0.145 | 1.316 |
| R9 | 0.206 | 1.198 |
| R10 | 0.248 | 0.925 |
| Probe Number | R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 | R9 | R10 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Max. force | 1 day | * | 43.5 N | * | * | * | 49.1 N | 28.7 N | 68.3 N | 58.4 N | 88.5 N |
| / | / | / | / | / | / | ||||||
| 4.43 kg | 5 kg | 6.96 kg | 6.96 | 5.89 kg | 9.02 kg | ||||||
| 14 days | * | 45.8 N | * | * | * | * | 65.8 N | 58.9 N | 73.1 N | 76.5 N | |
| / | / | / | / | / | |||||||
| 4.67 kg | 6.70 kg | 6.00 kg | 7.45 kg | 7.80 kg | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sirbu, E.-E.; Dinita, A.; Tănase, M.; Portoacă, A.-I.; Bondarev, A.; Enascuta, C.-E.; Calin, C. Influence of Plasticizers Concentration on Thermal, Mechanical, and Physicochemical Properties on Starch Films. Processes 2024, 12, 2021. https://doi.org/10.3390/pr12092021
Sirbu E-E, Dinita A, Tănase M, Portoacă A-I, Bondarev A, Enascuta C-E, Calin C. Influence of Plasticizers Concentration on Thermal, Mechanical, and Physicochemical Properties on Starch Films. Processes. 2024; 12(9):2021. https://doi.org/10.3390/pr12092021
Chicago/Turabian StyleSirbu, Elena-Emilia, Alin Dinita, Maria Tănase, Alexandra-Ileana Portoacă, Andreea Bondarev, Cristina-Emanuela Enascuta, and Catalina Calin. 2024. "Influence of Plasticizers Concentration on Thermal, Mechanical, and Physicochemical Properties on Starch Films" Processes 12, no. 9: 2021. https://doi.org/10.3390/pr12092021
APA StyleSirbu, E.-E., Dinita, A., Tănase, M., Portoacă, A.-I., Bondarev, A., Enascuta, C.-E., & Calin, C. (2024). Influence of Plasticizers Concentration on Thermal, Mechanical, and Physicochemical Properties on Starch Films. Processes, 12(9), 2021. https://doi.org/10.3390/pr12092021









