Modeling the Analysis Process of a Lipid-Based, Multi-Compartment Drug Delivery System
Abstract
1. Introduction
2. Types of Solid Lipid Particles
3. Nomenclature
4. Advantages and Routes of Administration of SLMs
- Particles formed from a solid lipid at body temperature can provide not only a prolonged release effect but also protection of the incorporated drug substance or taste masking;
- Due to the size of the particles, compared to SLNs, they provide a higher level of incorporation of the drug substance in the lipid matrix and a more visible prolonged release effect;
- As a multi-compartment carrier, they offer uniform dispersion at the site of administration, which ensures more even absorption and better protection of the applied drug substance;
- Cost-effective production on a large scale;
- Possibility of production using various techniques, depending on the desired size of the microparticles or form (powder or dispersion);
- Due to the composition of the matrix consisting of GRAS (generally recognized as safe) substances, the carrier is well tolerated, considered non-toxic, biocompatible and biodegradable;
- Unlike SLNs, they do not require a high-pressure homogenization process at the production stage;
- SLMs in a powder form can be obtained without the use of organic solvents;
- Possibility of thermal sterilization of dispersion and application of the sterile formulation;
- Possibility of application in both solid and liquid forms;
- Possibility of administration by the most common routes, such as oral or topical.
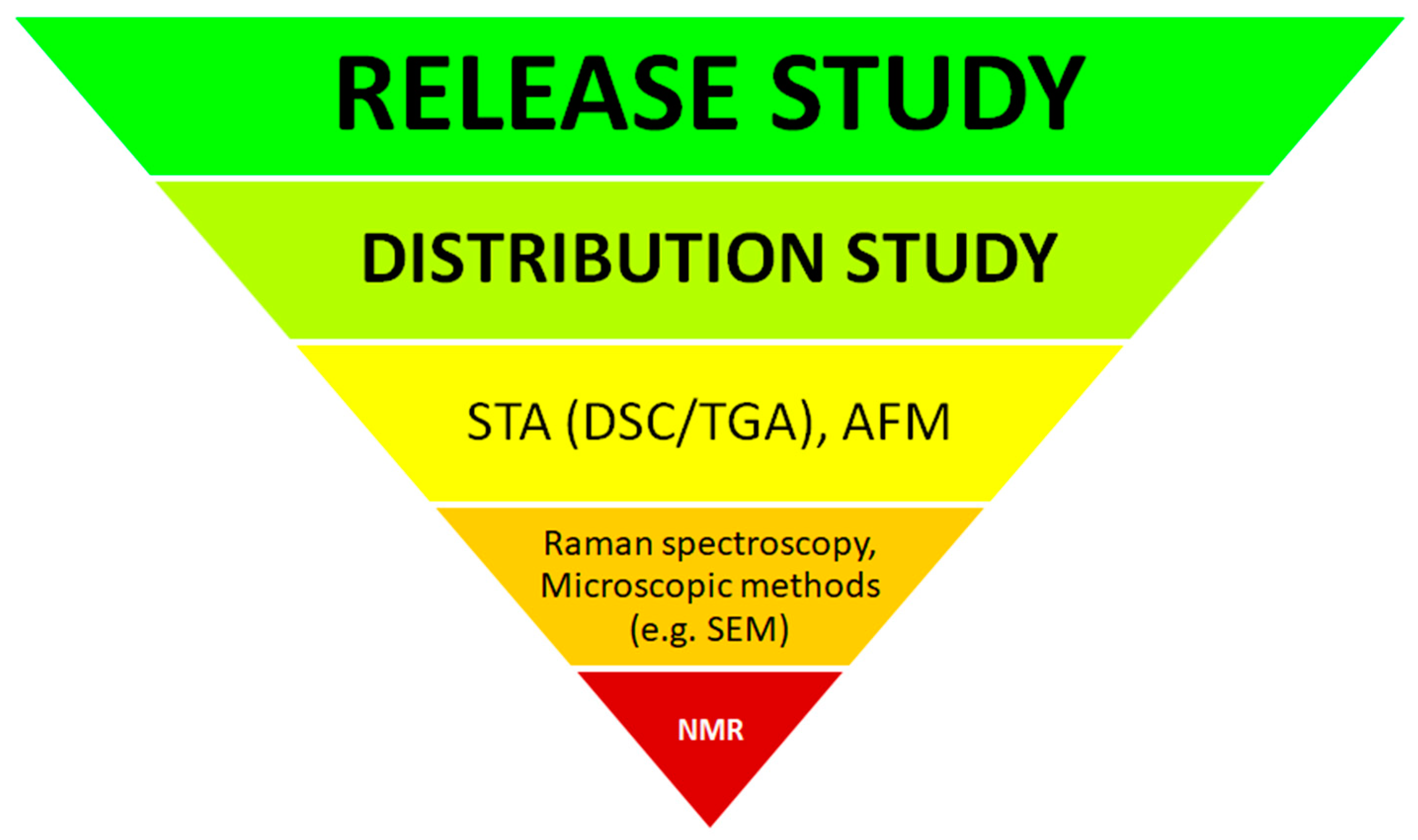
5. Assessment of Drug Substance Distribution in SLMs
5.1. Instrumental Methods for Characterizing API Distribution in SLMs
5.1.1. Simultaneous Thermogravimetric Analysis (STA)
5.1.2. Atomic Force Microscopy (AFM)
5.1.3. Raman Spectroscopy
5.1.4. Nuclear Magnetic Resonance (NMR)
5.2. Quantitative Method for Assessing API Distribution in SLMs
6. Models, Conditions and Limitations in Drug Substance Release Studies from SLMs
7. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kállai-Szabó, N.; Farkas, D.; Lengyel, M.; Basa, B.; Fleck, C.; Antal, I. Microparticles and multi-unit systems for advanced drug delivery. Eur. J. Pharm. Sci. 2024, 194, 106704. [Google Scholar] [CrossRef] [PubMed]
- Koroleva, M. Multicompartment colloid systems with lipid and polymer membranes for biomedical applications. Phys. Chem. Chem. Phys. 2023, 25, 21836–21859. [Google Scholar] [CrossRef]
- Rehman, M.; Tahir, N.; Sohail, M.F.; Qadri, M.U.; Duarte, S.O.D.; Brandão, P.; Esteves, T.; Javed, I.; Fonte, P. Lipid-based nanoformulations for drug delivery: An ongoing perspective. Pharmaceutics 2024, 16, 1376. [Google Scholar] [CrossRef]
- Keerthana, R.; Gayathri, P.S.; Gayathri, K.; Sreeja, C.N. Vesosomes: New prospects in multi compartment vesicular drug delivery system. Int. J. Pharm. Res. 2020, 12, 869–877. [Google Scholar]
- The United States Pharmacopoeia Website. Available online: https://www.usp.org/small-molecules/dissolution (accessed on 25 March 2024).
- Jaspart, S.; Piel, G.; Delattre, L.; Evrard, B. Solid lipid microparticles: Formulation, preparation, characterisation, drug release and applications. Expert Opin. Drug Deliv. 2005, 2, 75–87. [Google Scholar] [CrossRef]
- Scalia, S.; Young, P.M.; Traini, D. Solid lipid microparticles as an approach to drug delivery. Expert Opin. Drug Deliv. 2015, 12, 583–599. [Google Scholar] [CrossRef]
- Wolska, E.; Sznitowska, M.; Chorążewicz, J.; Szerkus, O.; Radwańska, A.; Markuszewski, M.J.; Kaliszan, R.; Raczyńska, K. Ocular irritation and cyclosporine A distribution in the eye tissues after administration of Solid Lipid Microparticles in the rabbit model. Eur. J. Pharm. Sci. 2018, 121, 95–105. [Google Scholar] [CrossRef]
- Abdelaziz, H.M.; Gaber, M.; Abd-Elwakil, M.M.; Mabrouk, M.T.; Elgohary, M.M.; Kamel, N.M.; Kabary, D.M.; Freag, M.S.; Samaha, M.W.; Mortada, S.M.; et al. Inhalable particulate drug delivery systems for lung cancer therapy: Nanoparticles, microparticles, nanocomposites and nanoaggregates. J. Control. Release. 2018, 269, 374–392. [Google Scholar] [CrossRef] [PubMed]
- Prow, T.W.; Grice, J.E.; Lin, L.L.; Faye, R.; Butler, M.; Becker, W.; Wurm, E.M.T.; Yoong, C.; Robertson, T.A.; Soyer, H.P.; et al. Nanoparticles and microparticles for skin drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 470–491. [Google Scholar] [CrossRef]
- Di Sabatino, M.; Albertini, B.; Kett, V.L.; Passerini, N. Spray congealed lipid microparticles with high protein loading: Preparation and solid state characterisation. Eur. J. Pharm. Sci. 2012, 46, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.H.; Radtke, M.; Wissing, S.A. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv. Drug Deliv. Rev. 2002, 54, 131–155. [Google Scholar] [CrossRef] [PubMed]
- Üner, M.; Yener, G. Importance of solid lipid nanoparticles (SLN) in various administration routes and future perspective. Int. J. Nanomed. 2007, 2, 289–300. [Google Scholar]
- Patel, P.; Vyas, N.; Raval, M. Safety and toxicity issues of polymeric nanoparticles. Nanotechnol. Med. 2021, 9, 156–173. [Google Scholar] [CrossRef]
- Borandeh, S.; van Bochove, B.; Teotia, A.; Seppälä, J. Polymeric drug delivery systems by additive manufacturing. Adv. Drug Deliv. Rev. 2021, 173, 349–373. [Google Scholar] [CrossRef]
- Sharma, S.; Parveen, R.; Chatterji, B.P. Toxicology of Nanoparticles in Drug Delivery. Curr. Pathobiol. Rep. 2021, 9, 133–144. [Google Scholar] [CrossRef]
- Lengyel, M.; Kállai-Szabó, N.; Antal, V.; Laki, A.J.; Antal, I. Microparticles, microspheres, and microcapsules for advanced drug delivery. Sci. Pharm. 2019, 87, 20. [Google Scholar] [CrossRef]
- Capretto, L.; Mazzitelli, S.; Nastruzzi, C. Design, production and optimization of solid lipid microparticles (SLM) by a coaxial microfluidic device. J. Control. Release 2012, 160, 409–417. [Google Scholar] [CrossRef]
- da Silva, R.Y.P.; de Menezes, D.L.B.; Oliveira, V.d.S.; Converti, A.; de Lima, Á.A.N. Microparticles in the development and improvement of pharmaceutical formulations: An analysis of in vitro and in vivo studies. Int. J. Mol. Sci. 2023, 24, 5441. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://microspheres.us/what-are-microspheres/ (accessed on 25 March 2024).
- Wong, C.Y.; Al-Salamia, H.; Dassa, C.R. Microparticles, microcapsules and microspheres: A review of recent develop-ments and prospects for oral delivery of insulin. Int. J. Pharm. 2018, 537, 223–244. [Google Scholar] [CrossRef] [PubMed]
- Dudala, T.B.; Yalavarthi, P.R.; Vadlamudi, H.V.; Thanniru, J.; Yaga, G.; Mudumala, N.L.; Pasupati, V.K. A perspective overview on lipospheres as lipid carrier systems. Int. J. Pharm. Investig. 2014, 4, 149–155. [Google Scholar] [CrossRef] [PubMed]
- López-Iglesias, C.; Quílez, C.; Barros, J.; Velasco, D.; Alvarez-Lorenzo, C.; Jorcano, J.L.; Monteiro, F.J.; García-González, C.A. Lidocaine-loaded solid lipid microparticles (SLMPS) produced from gas-saturated solutions for wound applications. Pharmaceutics 2020, 12, 870. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shena, L.; Wanga, T.; Lia, H.; Huang, R.; Zhang, Z.; Wang, Y.; Quana, D. Taste masking of water-soluble drug by solid lipid microspheres: A child-friendly system established by reversed lipid-based nanoparticle technique. J. Pharm. Pharmacol. 2020, 72, 776–786. [Google Scholar] [CrossRef] [PubMed]
- Bertoni, S.; Tedesco, D.; Bartolini, M.; Prata, C.; Passerini, N.; Albertini, B. Solid lipid microparticles for oral delivery of catalase: Focus on the protein structural integrity and gastric protection. Mol. Pharm. 2020, 17, 3609–3621. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Salituro, G.M.; Lee, K.-J.; Bak, A.; Leung, D.H. Modulating drug release and enhancing the oral bioavailability of torcetrapib with solid lipid dispersion formulations. AAPS Pharmscitech 2015, 16, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Luo, X.; Baldursdottir, S.G.; Yang, M.; Sun, X.; Mu, H. In vivo evaluation of solid lipid microparticles and hybrid polymer-lipid microparticles for sustained delivery of leuprolide. Eur. J. Pharm. Biopharm. 2019, 142, 315–321. [Google Scholar] [CrossRef]
- Li, Y.-Z.; Sun, X.; Gong, T.; Liu, J.; Zuo, J.; Zhang, Z.-R. Inhalable microparticles as carriers for pulmonary delivery of thymopentin-loaded solid lipid nanoparticles. Pharm. Res. 2010, 27, 1977–1986. [Google Scholar] [CrossRef] [PubMed]
- Nemati, E.; Mokhtarzadeh, A.; Panahi-Azar, V.; Mohammadi, A.; Hamishehkar, H.; Mesgari-Abbasi, M.; Dolatabadi, J.E.N.; de la Guardia, M. Ethambutol-loaded solid lipid nanoparticles as dry powder inhalable formulation for tuberculosis therapy. AAPS Pharmscitech 2019, 20, 120. [Google Scholar] [CrossRef]
- Kim, J.T.; Barua, S.; Kim, H.; Hong, S.-C.; Yoo, S.-Y.; Jeon, H.; Cho, Y.; Gil, S.; Oh, K.; Lee, J. Absorption study of genistein using solid lipid microparticles and nanoparticles: Control of oral bioavailability by particle sizes. Biomol. Ther. 2017, 25, 452–459. [Google Scholar] [CrossRef]
- Reithmeier, H.; Herrmann, J.; Göpferich, A. Lipid microparticles as a parenteral controlled release device for peptides. J. Control. Release 2001, 73, 339–350. [Google Scholar] [CrossRef]
- Rahimpour, Y.; Javadzadeh, Y.; Hamishehkar, H. Solid lipid microparticles for enhanced dermal delivery of tetracycline HCl. Colloids Surf. B Biointerfaces 2016, 145, 14–20. [Google Scholar] [CrossRef]
- Gavini, E.; Albertini, B.; Rassu, G.; Di Sabatino, M.; Sanna, V.; Giunchedi, P.; Rodriguez, L.; Passerini, N. Evaluation of solid lipid microparticles produced by spray congealing for topical application of econazole nitrate. J. Pharm. Pharmacol. 2009, 61, 559–567. [Google Scholar] [CrossRef]
- Lauterbach, A.; Mueller-Goymann, C.C. Development, formulation, and characterization of an adapalene-loaded solid lipid microparticle dispersion for follicular penetration. Int. J. Pharm. 2014, 466, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Melike, Ü.; Karaman, E.F. Preliminary studies on solid lipid microparticles of loratadine for the treatment of allergic re-actions via the nasal route. Trop. J. Pharm. Res. 2013, 12, 287–293. [Google Scholar]
- Mezzena, M.; Scalia, S.; Young, P.M.; Traini, D. Solid lipid budesonide microparticles for controlled release inhalation therapy. AAPS J. 2009, 11, 771–778. [Google Scholar] [CrossRef]
- Dolci, L.S.; Panzavolta, S.; Albertini, B.; Campisi, B.; Gandolfi, M.; Bigi, A.; Passerini, N. Spray-congealed solid lipid mi-croparticles as a new tool for the controlled release of bisphosphonates from a calcium phosphate bone cement. Eur. J. Pharm. Biopharm. 2018, 122, 6–16. [Google Scholar] [CrossRef]
- Scalia, S.; Haghi, M.; Losi, V.; Trotta, V.; Young, P.M.; Traini, D. Quercetin solid lipid microparticles: A flavonoid for inhalation lung delivery. Eur. J. Pharm. Sci. 2013, 49, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.E.; Lamprecht, A. Spray freeze drying as an alternative technique for lyophilization of polymeric and lipid-based nanoparticles. Int. J. Pharm. 2017, 516, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Freitas, C.; Müller, R.H. Spray-drying of solid lipid nanoparticles (SLN). Eur. J. Pharm. Biopharm. 1998, 46, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Freitas, C.; Müller, R. Correlation between long-term stability of solid lipid nanoparticles (SLN) and crystallinity of the lipid phase. Eur. J. Pharm. Biopharm. 1999, 47, 125–132. [Google Scholar] [CrossRef]
- Martins, S.; Tho, I.; Ferreira, D.C.; Souto, E.B.; Brandl, M. Physicochemical properties of lipid nanoparticles: Effect of lipid and surfactant composition. Drug Dev. Ind. Pharm. 2011, 37, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, L.; Gallarate, M.; Panciani, P.P.; Ugazio, E.; Sapino, S.; Peira, E.; Chirio, D. Techniques for the preparation of solid lipid nano and microparticles. Appl. Nanotechnol. Drug Deliv. 2014, 2, 51–75. [Google Scholar] [CrossRef]
- Albertini, B.; Passerini, N.; González-Rodríguez, M.L.; Perissutti, B.; Rodriguez, L. Effect of Aerosil on the properties of lipid controlled release microparticles. J. Control. Release 2004, 100, 233–246. [Google Scholar] [CrossRef]
- Wolska, E. Fine powder of lipid microparticles—Spray drying process development and optimization. J. Drug Deliv. Sci. Technol. 2021, 64, 102640. [Google Scholar] [CrossRef]
- Santos, D.; Maurício, A.C.; Sencadas, V.; Santos, J.D.; Fernandes, M.H.; Gomes, P.S. Spray Drying: An Overview. In Biomaterials-Physics and Chemistry-New Edition; InTech: London, UK, 2018; Chapter 2; pp. 9–35. [Google Scholar] [CrossRef]
- Gugu, T.H.; Chime, S.A.; Attama, A.A. Solid lipid microparticles: An approach for improving oral bioavailability of aspirin. Asian J. Pharm. Sci. 2015, 10, 425–432. [Google Scholar] [CrossRef]
- Andonova, V.; Peneva, P. Characterization methods for solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC). Curr. Pharm. Des. 2018, 23, 6630–6642. [Google Scholar] [CrossRef]
- Gordillo-Galeano, A.; Mora-Huertas, C.E. Solid lipid nanoparticles and nanostructured lipid carriers: A review empha-sizing on particle structure and drug release. Eur. J. Pharm. Biopharm. 2018, 133, 285–308. [Google Scholar] [CrossRef]
- Liu, P.C.; Christophersen, J.; Yang, M.; Nielsen, H.M.; Mu, H. The impact of particle preparation methods and polymorphic stability of lipid excipients on protein distribution in microparticles. Drug Dev. Ind. Pharm. 2017, 43, 2032–2042. [Google Scholar] [CrossRef] [PubMed]
- Boreham, A.; Volz, P.; Peters, D.; Keck, C.M.; Alexiev, U. Determination of nanostructures and drug distribution in lipid nanoparticles by single molecule microscopy. Eur. J. Pharm. Biopharm. 2017, 110, 31–38. [Google Scholar] [CrossRef]
- Available online: https://hha.hitachi-hightech.com/en/blogs-events/blogs/2022/08/03/what-is-simultaneous-hermogravimetric-analysis-sta/ (accessed on 25 March 2024).
- Wolska, E.; Regdon, G., Jr. Thermal analysis in the evaluation of Solid Lipid Microparticles in the form of aqueous dispersion and fine powder. Appl. Sci. 2023, 13, 13282. [Google Scholar] [CrossRef]
- Jiang, X.; Zhao, Y.; Guan, Q.; Xiao, S.; Dong, W.; Lian, S.; Zhang, H.; Liu, M.; Wang, Z.; Han, J. Amorphous solid dispersions of cyclosporine A with improved bioavailability prepared via hot melt extrusion: Formulation, physicochemical characterization, and in vivo evaluation. Eur. J. Pharm. Sci. 2022, 168, 106036. [Google Scholar] [CrossRef]
- Silveira, E.F.; Rannier, L.; Nalone, L.; da Silva, C.F.; Chaud, M.V.; de MBarbosa, R.; Junior, R.L.; da Costa, L.P.; Souto, E.B.; Severino, P. Loading of 5-aminosalicylic in solid lipid microparticles (SLM): Solubility screening of lipid excipients and physicochemical characterization. J. Therm. Anal. Calorim. 2020, 139, 1151–1159. [Google Scholar] [CrossRef]
- Bunjes, H.; Unruh, T. Characterization of lipid nanoparticles by differential scanning calorimetry, X-ray and neutron scattering. Adv. Drug Deliv. Rev. 2007, 59, 379–402. [Google Scholar] [CrossRef]
- Wong, P.C.H.; Heng, P.W.S.; Chan, L.W. A study on the solid state characteristics of spray-congealed glyceryl dibehenate solid lipid microparticles containing ibuprofen. Drug Dev. Ind. Pharm. 2016, 42, 364–377. [Google Scholar] [CrossRef] [PubMed]
- Passerini, N.; Qi, S.; Albertini, B.; Grassi, M.; Rodriguez, L.; Craig, D.Q. Solid lipid microparticles produced by spray congealing: Influence of the atomizer on microparticle characteristics and mathematical modeling of the drug release. J. Pharm. Sci. 2010, 99, 916–931. [Google Scholar] [CrossRef]
- Onoue, S.; Sato, H.; Ogawa, K.; Kawabata, Y.; Mizumoto, T.; Yuminoki, K.; Hashimoto, N.; Yamada, S. Improved dis-solution and pharmacokinetic behavior of cyclosporine A using high-energy amorphous solid dispersion approach. Int. J. Pharm. 2010, 399, 94–101. [Google Scholar] [CrossRef]
- Wolska, E.; Sznitowska, M. Technology of stable, prolonged-release eye-drops containing Cyclosporine A, distributed between lipid matrix and surface of the solid lipid microspheres (SLM). Int. J. Pharm. 2013, 441, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, E.; Souto, E.B.; Müller, R.H. Physicochemical investigations on the structure of drug-free and drug-loaded solid lipid nanoparticles (SLNTM) by means of DSC and 1H NMR. Pharmazie 2005, 60, 508–513. [Google Scholar]
- Jayawardena, H.S.N.; Liyanage, S.H.; Rathnayake, K.; Patel, U.; Yan, M. Analytical Methods for Characterization of Nanomaterial Surfaces. Anal. Chem. 2021, 93, 1889–1911. [Google Scholar] [CrossRef]
- Wolska, E.; Sznitowska, M.; Krzemińska, K.; Ferreira Monteiro, M. Analytical techniques for the assessment of drug-lipid interactions and the active substance distribution in liquid dispersions of Solid Lipid Microparticles (SLM) produced de novo and reconstituted from spray-dried powders. Pharmaceutics 2020, 12, 664. [Google Scholar] [CrossRef]
- Ali, H.; El-Sayed, K.; Sylvester, P.W.; Nazzal, S. Molecular interaction and localization of tocotrienol-rich fraction (TRF) within the matrices of lipid nanoparticles: Evidence studies by Differential Scanning Calorimetry (DSC) and Proton Nu-clear Magnetic Resonance spectroscopy (1H NMR). Colloids Surf. B Biointerfaces 2010, 77, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Jenning, V.; Thünemann, A.F.; Gohla, S.H. Characterisation of a novel solid lipid nanoparticle carrier system based on binary mixtures of liquid and solid lipids. Int. J. Pharm. 2000, 199, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Sahu, I.D.; Liu, L.; Osatuke, A.; Comer, R.G.; Dabney-Smith, C.; Lorigan, G.A. Characterizing the structure of lipodisq nanoparticles for membrane protein spectroscopic studies. Biochim. Biophys. Acta (BBA) Biomembr. 2015, 1848, 329–333. [Google Scholar] [CrossRef]
- Noack, A.; Hause, G.; Mäder, K. Physicochemical characterization of curcuminoid-loaded solid lipid nanoparticles. Int. J. Pharm. 2012, 423, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, P.; Khatik, R.; Khandelwal, K.; Taneja, I.; Raju, K.S.R.; Wahajuddin; Paliwal, S.K.; Dwivedi, A.K.; Mishra, P.R. Pharmacokinetics study of arteether loaded solid lipid nanoparticles: An improved oral bioavailability in rats. Int. J. Pharm. 2014, 466, 321–327. [Google Scholar] [CrossRef]
- Zhang, R.; Sahu, I.D.; Bali, A.P.; Dabney-Smith, C.; Lorigan, G.A. Characterization of the structure of lipodisq nanopar-ticles in the presence of KCNE1 by dynamic light scattering and transmission electron microscopy. Chem. Phys. Lipids 2017, 203, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Rakotoarisoa, M.; Angelov, B.; Drechsler, M.; Nicolas, V.; Bizien, T.; Gorshkova, Y.E.; Deng, Y.; Angelova, A. Liquid crystalline lipid nanoparticles for combined delivery of curcumin, fish oil and BDNF: In vitro neuroprotective potential in a cellular model of tunicamycin-induced endoplasmic reticulum stress. Smart Mater. Med. 2022, 3, 274–288. [Google Scholar] [CrossRef]
- Wei, L.; Yang, Y.; Shi, K.; Wu, J.; Zhao, W.; Mo, J. Preparation and characterization of loperamide-loaded Dynasan solid lipid nanoparticles for increased oral absorption in the treatment of diarrhea. Front. Pharmacol. 2016, 7, 332. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.N.; Kummer, K.M.; Dyondi, D.; Webster, T.J.; Bannerjee, R. Multi-scale strategy to eradicate Pseudomonas aeruginosa on surfaces using solid lipid nanoparticles loaded with free fatty acids. Nanoscale 2014, 6, 825–832. [Google Scholar] [CrossRef]
- Lauterbach, A.; Müller-Goymann, C.C. Design of lipid microparticle dispersions based on the physicochemical properties of the lipid and aqueous phase. Int. J. Pharm. 2015, 494, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Chinaeke, E.E.; Chime, S.A.; Onyishi, V.I.; Attama, A.A.; Okore, V.C. Formulation development and evaluation of the anti-malaria properties of sustained release artesunate-loaded solid lipid microparticles based on phytolipids. Drug Deliv. 2013, 22, 652–665. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gavini, E.; Sanna, V.; Sharma, R.; Juliano, C.; Usai, M.; Marchetti, M.; Karlsen, J.; Giunchedi, P. Solid lipid microparticles (SLM) containing juniper oil as anti-acne topical carriers: Preliminary studies. Pharm. Dev. Technol. 2005, 10, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Islam, N.; Irfan, M.; Zahoor, A.F.; Syed, H.K.; Khan, I.U.; Iqbal, M.S.; Rasul, A.; Abbas, N.; Hussain, A.; Mohsin, N.U.A.; et al. Evaluation of solubility and dissolution of lamotrigine using lipid based micro-particulate carriers: An in-vitro analysis. Pak. J. Pharm. Sci. 2020, 33, 299–306. [Google Scholar] [PubMed]
- Wolska, E.; Brach, M. Distribution of drug substances in Solid Lipid Microparticles (SLM)—Methods of analysis and interpretation. Pharmaceutics 2022, 14, 335. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.intertek.com/pharmaceutical/gmp-cmclaboratory/pharmaceutical-dissolution-testing/ (accessed on 25 March 2024).
- Zolnik, B.S.; Raton, J.-L.; Burgess, D.J. Application of USP apparatus 4 and in situ fiber optic analysis to microsphere release testing. Dissolut. Technol. 2005, 12, 11–14. [Google Scholar] [CrossRef]
- Shen, J.; Burgess, D.J. Accelerated in-vitro release testing methods for extended-release parenteral dosage forms. J. Pharm. Pharmacol. 2012, 64, 986–996. [Google Scholar] [CrossRef] [PubMed]
- Wolska, E.; Szymańska, M. Comparison of the in vitro drug release methods for the selection of test conditions to char-acterize Solid Lipid Microparticles. Pharmaceutics 2023, 15, 511. [Google Scholar] [CrossRef]
- Albertini, B.; Bertoni, S.; Perissutti, B.; Passerini, N. An investigation into the release behavior of solid lipid microparticles in different simulated gastrointestinal fluids. Colloids Surf. B Biointerfaces 2018, 173, 276–285. [Google Scholar] [CrossRef]
- Agubata, C.O.; Nzekwe, I.T.; Attama, A.A.; Mueller-Goymann, C.C.; Onunkwo, G.C. Formulation, characterization and anti-malarial activity of homolipid-based artemether microparticles. Int. J. Pharm. 2015, 478, 202–222. [Google Scholar] [CrossRef]
- Souto, E.B.; Wissing, S.A.; Barbosa, C.M.; Müller, R.H. Development of a controlled release formulation based on SLN and NLC for topical clotrimazole delivery. Int. J. Pharm. 2004, 278, 71–77. [Google Scholar] [CrossRef]
- Available online: https://www.sigmaaldrich.com/PL/pl/technical-documents/technicalarticle/protein-biology/pro-tein-concentration-and-buffer-exchange/dialysis-tubing (accessed on 25 March 2024).
- Xu, X.; Khan, M.A.; Burgess, D.J. A two-stage reverse dialysis in vitro dissolution testing method for passive targeted liposomes. Int. J. Pharm. 2012, 426, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.; Amaral, M.H.; Gonzalez, E.; Santos, D.; Ferreira, D. Solid lipid nanoparticles (SLN)-based hydrogels as potential carriers for oral transmucosal delivery of risperidone: Preparation and characterization studies. Colloids Surf. B Biointerfaces 2012, 93, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, R.; Caputo, O.; Gasco, M.R. Preparation and characterization of solid lipid nanospheres containing paclitaxel. Eur. J. Pharm. Sci. 2000, 10, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Hengst, L.; Hunt, R.; Feng, X.; Kozak, D.; Choi, S.; Ashraf, M.; Xu, X. Evaluating drug distribution and release in ophthalmic emulsions: Impact of release conditions. J. Control. Release 2020, 327, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Ammar, H.O.; Salama, H.A.; Ghorab, M.; Mahmoud, A.A. Nanoemulsion as a potential ophthalmic delivery system for dorzolamide hydrochloride. AAPS Pharmscitech 2009, 10, 808–819. [Google Scholar] [CrossRef] [PubMed]
- Wolska, E.; Sadowska, K. Drug release from lipid microparticles—Insights into drug incorporation and the influence of physiological factors. Pharmaceutics 2024, 16, 545. [Google Scholar] [CrossRef] [PubMed]
- Satyanarayana, S.D.; Abu Lila, A.S.; Moin, A.; Moglad, E.H.; Khafagy, E.-S.; Alotaibi, H.F.; Obaidullah, A.J.; Charyulu, R.N. Ocular delivery of bimatoprost-loaded solid lipid nanoparticles for effective management of glaucoma. Pharmaceuticals 2023, 16, 1001. [Google Scholar] [CrossRef] [PubMed]
- Eid, H.M.; Elkomy, M.H.; El Menshawe, S.F.; Salem, H.F. Development, optimization, and in vitro/in vivo characterization of enhanced lipid nanoparticles for ocular delivery of ofloxacin: The influence of pegylation and chitosan coating. AAPS Pharmscitech 2019, 20, 183. [Google Scholar] [CrossRef]
- Khames, A.; Khaleel, M.; El-Badawy, M.; El-Nezhawy, A. Natamycin solid lipid nanoparticles—Sustained ocular delivery system of higher corneal penetration against deep fungal keratitis: Preparation and optimization. Int. J. Nanomed. 2019, 14, 2515–2531. [Google Scholar] [CrossRef]
- Khare, A.; Singh, I.; Pawar, P.; Grover, K. Design and evaluation of voriconazole loaded solid lipid nanoparticles for ophthalmic application. J. Drug Deliv. 2016, 2016, 6590361. [Google Scholar] [CrossRef]
- Christophersen, P.; Zhang, L.; Yang, M.; Nielsen, H.; Müllertz, A.; Mu, H. Solid lipid particles for oral delivery of peptide and protein drugs I—Elucidating the release mechanism of lysozyme during lipolysis. Eur. J. Pharm. Biopharm. 2013, 85, 473–480. [Google Scholar] [CrossRef]
- Tseng, R.C.; Chen, C.-C.; Hsu, S.-M.; Chuang, H.-S. Contact-Lens Biosensors. Sensors 2018, 18, 2651. [Google Scholar] [CrossRef]
- Villatoro, A.J.; Fernández, V.; Claros, S.; Alcoholado, C.; Cifuentes, M.; Merayo-Lloves, J.; Andrades, J.A.; Becerra, J. Regenerative therapies in dry eye disease: From growth factors to cell therapy. Int. J. Mol. Sci. 2017, 18, 2264. [Google Scholar] [CrossRef]
- Alexeev, V.L.; Das, S.; Finegold, D.N.; A Asher, S. Photonic crystal glucose-sensing material for noninvasive monitoring of glucose in tear fluid. Clin. Chem. 2005, 50, 2353–2360. [Google Scholar] [CrossRef]
- El-Sabbagh, H.; Ghanem, A.H.; Abdel-Alim, H.M. Solubilization of indometacin. Die Pharm. 1978, 33, 529–531. [Google Scholar]
- Tres, F.; Treacher, K.; Booth, J.; Hughes, L.P.; Wren, S.A.C.; Aylott, J.W.; Burley, J.C. Indomethacin-Kollidon VA64 ex-trudates: A mechanistic study of pH-dependent controlled release. Mol. Pharm. 2016, 13, 1166–1175. [Google Scholar] [CrossRef] [PubMed]
- Committee for Medicinal Products for Human Use (CHMP); Committee for Medicinal Products for Veterinary Use (CVMP); Quality Working Party. Reflection Paper on the Dissolution Specification for Generic Solid Oral Immediate Release Products with Systemic; European Medicines Agency: Amsterdam, The Netherlands, 2017; p. 44. [Google Scholar]
- Siepmann, J.; Siepmann, F. Sink conditions do not guarantee the absence of saturation effects. Int. J. Pharm. 2020, 577, 119009. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Glennon, B.; He, Y.; Donnellan, P. Dissolution kinetics of a BCS class II active pharmaceutical ingredient: Diffusion-based model validation and prediction. ACS Omega 2021, 6, 8056–8067. [Google Scholar] [CrossRef]
- Torrent-Burgués, J. Lysozyme Influence on Monolayers of Individual and Mixed Lipids. Colloids Interfaces 2022, 6, 15. [Google Scholar] [CrossRef]
- Sigma-Aldrich. Product Information, Lysozyme from Chicken Egg White, Catalog Number L7651. Available online: https://www.sigmaaldrich.com (accessed on 25 March 2024).


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolska, E.; Sznitowska, M. Modeling the Analysis Process of a Lipid-Based, Multi-Compartment Drug Delivery System. Processes 2025, 13, 460. https://doi.org/10.3390/pr13020460
Wolska E, Sznitowska M. Modeling the Analysis Process of a Lipid-Based, Multi-Compartment Drug Delivery System. Processes. 2025; 13(2):460. https://doi.org/10.3390/pr13020460
Chicago/Turabian StyleWolska, Eliza, and Małgorzata Sznitowska. 2025. "Modeling the Analysis Process of a Lipid-Based, Multi-Compartment Drug Delivery System" Processes 13, no. 2: 460. https://doi.org/10.3390/pr13020460
APA StyleWolska, E., & Sznitowska, M. (2025). Modeling the Analysis Process of a Lipid-Based, Multi-Compartment Drug Delivery System. Processes, 13(2), 460. https://doi.org/10.3390/pr13020460





