Abstract
In general, there are three methods for extracting oil from various sources: mechanical, solvent, and pre-press-solvent. Each of these methods has its own advantages and disadvantages, with extraction efficiency depending on key factors such as the extraction technique, the properties of the plant component matrix, and the solvent used. Factors like temperature, pressure, and time also play a role. Researchers have consistently sought to replace or complement these methods to reduce residual oil in products. This study introduces new oil extraction methods that have gained attention in recent years, including the microwave, pulsed electric field, ultrasound, supercritical fluid, enzymatic, ohmic, and combined methods to enhance efficiency. The research demonstrates that these methods increase oil extraction efficiency and bioactive compound extraction from plant sources, resulting in improved oil quality. Most methods also reduce extraction time, offering researchers and industrialists a variety of options for their oil extraction needs. However, the study notes contradictions in the results, such as varying acidity levels in the oil, which may be attributed to raw materials and study conditions. In the end, it was determined that ultrasound, pulsed electric field, and enzyme methods can be used industrially to extract oil from olives, while supercritical fluid can be used to extract oil from certain seeds.
1. Introduction
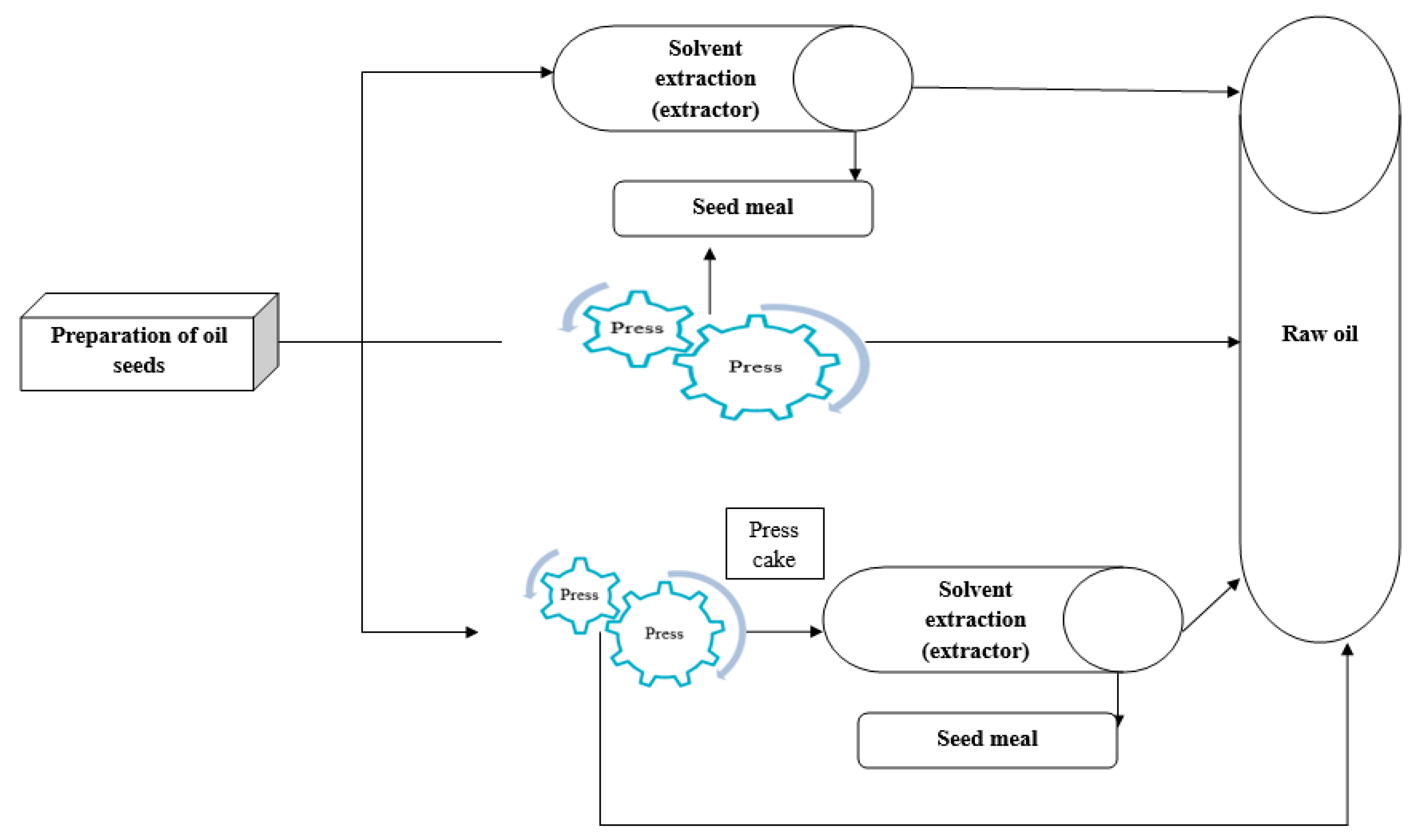
The use of oilseeds in human food consumption, as well as their utilization for animal feed, pharmaceuticals, soap making, and fuel, has piqued farmers’ interest and garnered government support for their cultivation [1]. Oils play a crucial role in enhancing the taste, aroma, color, and texture of food, while also imparting a sense of satiety and deliciousness. Nutritionally, lipids serve as a rich source of calories, containing approximately twice as much energy as carbohydrates or proteins. Additionally, they provide vital nutrients such as linoleic acid, linolenic acid, and fat-soluble vitamins (A, D, E, and K). The primary lipid classes include free fatty acids, triacylglycerols composed of fatty acids esterified to glycerol, and phosphoglycerates containing fatty acids esterified to glycerol, along with phosphoric acid and organic bases [2]. Edible fats and oils supply essential fatty acids crucial for human health. Linoleic acid (18:2) and linolenic acid (18:3) are two essential fatty acids necessary for fetal growth and development. These fatty acids play a vital role in pregnancy, lactation, and capillary strength, as the human body lacks the necessary enzymes to synthesize them and thus must obtain them from dietary sources [3]. Broadly speaking, there are three traditional extraction methods in the oil processing field, both historically and in modern industry, as illustrated in Figure 1.
Figure 1.
Schematic of traditional methods of oil extraction from oilseeds.
- (a)
- Mechanical Extraction (Press)
Mechanical extraction, specifically pressing, is commonly used on a small scale. There are two types of mechanical oil extraction: hot and cold pressing. In the hot pressing process, the seeds’ flakes are cooked due to the heat generated, resulting in the removal of about 75% or more of the oil in the seed. During this method, the oil content in the flour is around 16%. Cold pressing, on the other hand, is not a new concept, as hydraulic presses have been commonly used for centuries. This method does not produce heat during operation, making it ideal for producing high-quality products like olive oil, where maintaining taste is crucial. Additionally, cold pressing prevents the increase in temperature that leads to protein denaturation. It is important to note that the crushing efficiency can vary slightly based on the process, variety, location, and growth environment. While hot pressing has a higher oil extraction efficiency than cold pressing, the resulting oil quality is lower due to the heat involved [4]. Cold pressed oils, however, retain their natural properties better and are chemical-free, leading to increased demand. Various factors, such as press pressure, spiral press rotational speed, grain moisture, and process temperature, affect the efficiency of oil extraction by cold pressing. The absence of chemicals in this method results in oil and flour free of chemical compounds, making them suitable for both human and animal consumption. The low initial and operational investment costs, a wide range of capacities (from small scale to 100 tons per hour), and the ease of the process are other advantages of cold pressing. The main drawback of oil extraction by pressing is the high amount of residual oil in the meal, sometimes reported up to 10–20% in some sources. Strict control of the humidity and temperature of the raw material is essential during this process to prevent damage to flour proteins [5,6,7].
- (b)
- Solvent Extraction
Solvent extraction is a method commonly used for oilseeds containing less than 20% oil, particularly in soybean factories, which play a significant role in the oil industry. The residual oil in the meal after extraction using this method is less than 1%. Establishing initial contact between the solvent and the oil in the seeds is time-consuming, making time an important factor in this extraction method. Hexane is typically the solvent used in this process, as it can easily dissolve vegetable oils. This solvent has a suitable boiling temperature and a relatively low latent heat of evaporation, aiding in energy conservation during distillation. Hexane is non-corrosive to metals, neutral to extracted oil, chemically resistant under process conditions, and readily available at a reasonable price. Despite these benefits, its high flammability necessitates the use of specialized equipment in this extraction method. Due to environmental concerns, low-risk solvents like ethanol, isopropanol, and hydrocarbons are sometimes used in place of hexane. Particle size is a crucial factor in solvent extraction, as reducing it increases solvent penetration into the plant material and improves yield. Drawbacks of using solvents include the lengthy extraction process, high solvent consumption, damage to heat-sensitive compounds, and the production of unwanted materials [8,9,10]. Factors that affect the extraction efficiency of this method include the type and composition of the raw material (such as oil and moisture content), pretreatments that impact cell structure and size, the type of solvent and its temperature, and the ratio of solvent to input grain [11,12]. For instance, Rodrigues et al. (2011) and Toda et al. (2016) noted that raising the temperature results in higher oil extraction efficiency and an increase in free fatty acids. This implies that there is a limit to how much the temperature can be raised [13,14].
- (c)
- Pre-press and solvent method
This extraction method is used for oilseeds with more than 25% oil content (such as sunflower, canola, etc.). In this method, the oil content in the seeds is first reduced to 16–20% using a press. The press cake obtained is then subjected to solvent extraction under specific conditions (temperature of 50 °C for approximately 7 h), where the remaining oil in the press cake is extracted by adding solvent. The amount of oil left in the flour after extraction using this method is less than 0.5% [10,15].
As mentioned, each of the above methods has its own advantages and disadvantages. Generally, the main disadvantage of conventional methods is the lower oil yield with pressing compared to solvent extraction, which can make oil production economically disadvantageous. On the other hand, the use of organic solvents in the other two methods (using solvents and the pre-pressing and solvent method) raises environmental concerns [16]. Therefore, researchers have been working on finding alternative or complementary methods to improve oil extraction efficiency. The aim of this study was to address the lack of a comprehensive analysis of different oil extraction methods, and their advantages and disadvantages, focusing on extraction efficiency and the physicochemical properties of the resulting oil.
2. Using the Microwave Method
Microwaves are non-ionizing electromagnetic waves with a frequency between 300 MHz and 300 GHz. They are located between radio waves and infrared rays in the electromagnetic spectrum, consisting of two oscillating vertical fields: electric and magnetic fields. Normal heating relies on the conduction-displacement phenomenon, resulting in a significant amount of thermal energy being lost to the environment. However, with microwaves, heating occurs in a specific and selective path without heat loss to the environment, similar to heating in a closed system. The principles of heating with microwaves are based on the direct interaction of waves with solvent and polar materials, working through two phenomena: ion transfer and dipole rotation, which typically occur simultaneously [17]. These waves cause water evaporation from the structure of plant material, increasing pressure in the internal environment. This can lead to material decomposition, membrane rupture, improved efficiency of oil extraction through cold pressing, and oil passage through cell membranes [18].The microwave extraction method is a superior alternative to traditional solid–liquid extraction methods for extracting metabolites from plants. Its advantages over traditional methods include (1) significant reduction in extraction time (usually less than 30 min); (2) decreased solvent usage; (3) enhanced extraction efficiency; (4) automatic device adjustment for accuracy; (5) suitability for heat-sensitive components; (6) ability to extract trace components like heavy metals and pesticide residues from small plant samples; and (7) creation of turbulence during extraction to improve mass transfer. One drawback of this method is its low efficiency when target compounds or solvents are non-polar or volatile [19,20]. On the other hand, due to its thermal nature, this method is unsuitable for the extraction of polyunsaturated fatty acids (PUFAs), as PUFAs are susceptible to oxidation and degradation. In addition, the formation of free radicals and heat during this process is also a disadvantage. Some of these drawbacks can be avoided by using temperature-controlled microwave reactors. However, such reactors are expensive for large-scale processing [21]. In a study conducted by Gallego-Schmid et al. (2018) to assess the environmental impact of microwaves and European legislation on energy efficiency and waste management, it was found that this process reduces the carbon footprint by 4–9%. However, there is still limited information on the environmental impact of this method [22]. The variables of this process play a crucial role in optimizing oil yield. The main variables in microwave-assisted extraction include the solid-to-liquid ratio, microwave power and time (W), and the temperature used [23]. Economic studies on oil processing using microwaves are scarce, but studies on the economic viability of this method in other food processing areas exist. For example, Nguyen and Zhang (2020) demonstrated that using microwaves to extract protein from lobster shells resulted in a 2.7-year return on initial investment, indicating the high efficiency and profitability of the process [24].
Microwaves are utilized for industrial, scientific, medical, and communication purposes. In most countries, four frequencies (915, 2450, 5800, and 22,125 MHz) have been allocated for industrial, research, and medical applications. Unlike X-rays and gamma rays, microwaves are unable to break chemical bonds or damage food molecules due to their low frequency [25]. The use of microwaves reduces the oil extraction time and energy consumption. Consequently, numerous studies have explored the use of microwaves in oil extraction, some of which are briefly summarized in Table 1. As shown in this table, the study by Senphan et al. (2024) reported a decrease in extraction efficiency when using microwaves. This decrease is attributed to the significantly shorter processing time of this method [26]. Additionally, when the process time is equal, the extraction efficiency with microwaves is higher. Some researchers have reported that the use of microwaves during oil extraction leads to a decrease in polyunsaturated fatty acids (PUFAs) [27,28]. These researchers stated that the use of microwaves leads to the destruction of PUFAs. On the other hand, some studies indicated that the use of microwaves leads to an increase in tocopherols (vitamin E) due to a decrease in the reaction between these compounds with various polysaccharides, proteins, and peptides present in oilseeds, and the greater penetration of these compounds into the oil [27,29].

Table 1.
Studies conducted in the field of oil extraction using microwaves.
3. Pulsed Electric Field
The pulsed electric field process, as a non-thermal method, has been widely utilized to optimize energy consumption and maintain the quality characteristics of products. In recent years, this method has found applications on an industrial scale in biotechnology, pharmaceuticals, manufacturing stimulating lasers, and cleaning metals and polymer materials, as well as in the preparation and processing of food such as fruit extracts, milk, and liquid eggs. This process involves applying pulses in a short period of time (in the range of microseconds), in a strong electric field, to the food material between two electrodes at room temperature. Alternating electric fields with high intensity causes electrical disintegration of cells and increases their permeability [39,40]. The reason for using this process can be attributed to research conducted in Germany. In 1960, Deon Speck described the equipment and various methods of this process for sausage processing; subsequently, with the help of other German researchers, studies were conducted over many years to further develop this process [41]. By utilizing pulsed electric fields, the efficiency of oil extraction increases due to the increase in cell permeability. The most widely accepted theory of how the PEF method works is consistent with the electromechanical model introduced by Zimmerman et al. in 1974. This model explains that the cell membrane is like a capacitor with a low dielectric constant and contains positive and negative ions in the normal state. The integration of these ions on both sides of the membrane leads to a potential difference of about 10 mV in the cell. If this cell is placed in an external electric field, the ions move along the electric field. The movement of ions increases the potential difference until its value reaches a critical limit, which varies in different cells. This critical value depends on the size and shape of the cell, usually ranging within 1–2 kV/cm for plant cells and 10–14 kV/cm for animal cells. When the potential difference reaches a critical level, the attractive force between charges with opposite poles causes electromechanical forces to dominate, leading to the elastic membrane being damaged. This process results in the formation of pores in the membrane, known as electrical permeability or electroporation [42,43,44]. Many researchers have studied the effect of pulsed electric fields as a pretreatment during mechanical pressing, and their reports have indicated a positive impact on the qualitative and sensory properties of the oil obtained [44,45].
To utilize pulsed electric fields in the food industry, specific equipment is required, such as a high voltage pulse generator, process chamber, process control and display system, and cooling system. High-voltage generators are often used in this process to produce alternating currents that result in the destruction of biological cells. These generators transform normal voltages into high-intensity voltages. A power supply is utilized to charge capacitors that store a significant amount of energy, with a key designed to discharge electrical energy from capacitors into the food [46]. The advantages of this method over conventional extraction methods include increased extraction efficiency of bioactive compounds, reduced extraction time and nutrient damage, decreased waste and energy generation, and environmental friendliness [47,48]. The disadvantages of this method include the formation of bubbles during treatment, which can lead to operational problems and non-uniform treatment. Additionally, commercial PEF units are not widely available in many regions around the world [47,49]. Factors affecting the efficiency of electric field extraction include product factors (such as chemical composition, product temperature, and electrical conductivity) and process parameters including electric field strength, number of pulses, pulse frequency, pulse waveform, pulse width, type of chamber, flow conditions, and flow rate [47]. A study titled “Challenges and Opportunities for Pilot Scaling-Up Extraction of Olive Oil Assisted by Pulsed Electric Fields: Process, Product, and Economic Evaluation” showed that the use of PEF in olive oil production reduced the malaxation step by 33%, without affecting the main characteristics of the olive oil. It also increased the oil extraction efficiency by 12.3% and raised the net profit by 36.8%. The study concluded that for small-scale production, the significant initial investment required for PEF equipment may be a challenge in terms of return on investment. However, given the seasonal nature of olive oil production, in medium-to-large-scale production, investing in PEF is a viable option. With an equipment cost of EUR 450,000 and a production output of 5 tons per hour, an annual return of 20% is possible [50]. Table 2 lists some studies on the application of pulsed electric fields in oil extraction. Studies have shown that using a pulsed electric field during oil extraction results in an increase in tocopherols, particularly alpha-tocopherols. This is attributed to the low temperature of the process and the enhanced release of these compounds into the oil [51,52]. Conversely, it has been demonstrated that unsaturated fatty acids, specifically oleic acid, are impacted by the pulsed electric field, leading to a reduction in their levels. However, the levels of other fatty acids remain unaffected by the pulsed electric field [29,51].

Table 2.
Studies conducted in the field of oil extraction using pulsed electric fields.
4. Use of Ultrasound Waves in Oil Extraction
Ultrasound is a mechanical wave that requires an elastic medium and consists of sound waves with various frequencies to disperse. Sounds fall within the frequency range of human hearing (16 Hz to 20–16 kHz), while ultrasound waves have frequencies higher than human hearing but lower than microwave frequencies (20 kHz to 10 MHz). Ultrasound waves with a frequency of less than 100 kHz and a power greater than one watt per square centimeter are known as power ultrasound waves. These waves are extensively utilized in the food industry for various processes such as cutting, degassing, filtration, drying, homogenization, meat crisping, egg quality determination, emulsion formation, surface cleaning, equipment sterilization, fat crystallization, acceleration of oxidation reactions, inhibition of enzyme and microbial activity, and extraction of aroma and taste compounds. Furthermore, acoustic technologies are employed in food evaluation (crispness, firmness, and texture), determination of physical properties (size, shape, volume, and moisture), and grading and separation of food [60,61]. The primary mechanism of ultrasound extraction is associated with cavitation. When liquid is sonicated, the sound waves create cycles of compression and low pressure, leading to the cavitation phenomenon. This intense mixing of solvent particles facilitates solid extraction. The bubbles formed during cavitation cause microcirculation and collision of particles, enhancing internal and eddy diffusion [61,62,63].
The advantages of ultrasound extraction include increased extraction speed and efficiency, reduced processing time, enhanced yield, low solvent consumption, compatibility with various solvents, broad applicability to different plants, improved quality of extracted materials (especially in terms of antioxidant properties), reduced temperature requirements, and the ability to extract heat-sensitive compounds at a low operational cost. However, drawbacks include wave weakening in the presence of a dispersed phase, the need for filtration and separation of the extract from the plant material, limited active ultrasound range within the extractor, potential corrosion of equipment over time, and potential destruction of compounds at high intensities and prolonged exposure to ultrasound [64,65].
Key factors influencing oil extraction via ultrasound include the nature of the sample, sample and solvent matrix, temperature, time, solvent-to-sample ratio, type of solvent, solvent viscosity, solvent vapor pressure, and ultrasonic power [64,66]. Considering the non-polarity of oily compounds, the highest oil extraction performance is achieved using non-polar solvents. The greater the polarity percentage of a solvent, the lower the extraction efficiency. Hexane and petroleum ether, both non-polar solvents, have the most significant impact on increasing oil extraction efficiency. These solvents exhibit only weak van der Waals forces of attraction between particles, resulting in their low boiling points and volatility. A key feature of this method that makes it a green process is that its use reduces the consumption of oxidizing chemicals, mineral acids, iron salts, and other compounds during various processes [67]. Due to the reduced time and energy, as well as the improved oil extraction efficiency, this method is a good candidate for industrializing oil extraction from vegetable seeds [68]. In an analysis presented by Grillo et al. (2022) to calculate the economic cost of olive oil extraction using a combined ultrasound and pulsed electric field method, it was found that the unit costs and energy consumption of both conventional and ultrasonic processes for industrial-scale operations did not exhibit any significant differences in total costs and energy consumption for start-up. However, the ultrasonic process eliminates the need to add water during the separation of olive pulp in the counter, resulting in less olive mill wastewater being produced compared to the conventional method. Water savings of 0.21 L per kilogram of treated olive paste are achieved, significantly reducing waste treatment costs for the entire oil production chain. Additionally, this method requires less space for the installation of large malaxons [69]. Non-polar solute particles are only influenced by van der Waals forces, allowing for the formation of a solution. Therefore, all particles in the solution are affected by this force, facilitating solution formation [10,70,71].
A study investigating the extraction of pomegranate seed oil using ultrasonic waves examined the impact of various solvents on extraction efficiency. The highest efficiency was observed with petroleum ether solvent, followed by hexane, ethyl acetate, diethyl ether, acetone, and isopropanol solvents [72]. Another study conducted on the extraction of safrole oil using ultrasonic waves found that n-hexane had the greatest effect on increasing oil extraction performance, followed by ethyl acetate, petroleum ether, and ethanol [73]. The use of mixed solvents enhances the productivity of suitable oil compounds in ultrasonic extraction [74]. Additionally, increasing the ratio of solvent to sample enhances the efficiency of oil extraction. At high solvent ratios, the reduction in solution viscosity promotes the diffusion of the solvent into the sample tissue, leading to an increase in solvent concentration towards the sample matrix and ultimately enhancing extraction performance [64,75]. Other influential factors in extracting oil from oilseeds using ultrasonic waves include extraction time, process temperature, and ultrasonic wave power. With increased extraction time, the cell wall is disrupted due to the energy generated by the cavitation phenomenon, facilitating the penetration of oily compounds into the solvent. However, as extraction time continues to increase, the concentration of oily compounds in the cell tissue and the solvent reaches equilibrium, resulting in a decrease in the release and penetration of these compounds into the solvent and a subsequent decrease in extraction performance. As the temperature rises, the number of cavitation-induced bubbles also increases, reducing the surface tension and viscosity of the solvent. This decrease in viscosity allows the solvent to more easily penetrate the cell tissue, thereby increasing extraction performance through enhanced mass transfer. The power of ultrasound affects the cavitation phenomenon. As the power of ultrasound waves increases, the temperature of the process also increases. By keeping the process time constant, the viscosity and surface tension of the solvent decrease and the vapor pressure increases. At higher temperatures, the cavitation threshold is lowered and bubble formation is better, ultimately increasing the extraction performance [72,73,76]. In Table 3, some studies that have used ultrasound in oil extraction are provided. Studies have shown that polyunsaturated fatty acids, such as linoleic acid (18:2 n-6c), γ-linolenic acid (18:3 n-6), and α-linolenic acid (18:3 n-3), are increased when ultrasound is used in the extraction of oil from flaxseed [68]. However, in more stable oils like sunflower oil, the use of this process does not significantly affect the amount of fatty acids [77]. A study conducted to investigate the effect of ultrasound on virgin olive oil showed that the use of ultrasound did not significantly affect the nutritional composition of this oil, including phenols and tocopherols [78].

Table 3.
Studies conducted in the field of oil extraction using ultrasound.
5. Use of Supercritical Fluid in Oil Extraction
Supercritical fluid is a substance that is placed at temperatures and pressures above the critical point. In this condition, the substance is not a gas or a liquid, but an intermediate state between the two, combining the permeability properties of gases and the dissolution power of liquids. The solubility of substances in this phase changes with density, controlled by temperature and pressure. After the desired substance penetrates the phase, at the end of extraction, by adjusting temperature and pressure, the supercritical phase can be removed as a gas, leaving behind the extract without solvent [88]. In this method, the choice of solvent is crucial. Carbon dioxide is commonly used for extracting non-polar compounds like hydrocarbons due to its affordability, low toxicity, low flammability, selectivity, potential for extracting heat-sensitive compounds, and favorable critical conditions (temperature 304 degrees Kelvin and pressure 7.3 MPa) [89].
The moisture content of the plant material is significant in this method. Water in the plant material can freeze, creating a physical barrier against the supercritical liquid. Additionally, the ice can partially enter the supercritical phase, leading to the dissolution of unwanted compounds. The particle size of the samples also affects extraction efficiency, similar to other methods. Increasing the flow rate of carbon dioxide only reduces extraction time, without significantly increasing the yield [89,90]. Advantages of this method over conventional extraction methods include high extraction speed, low or no organic solvent, no solvent residue, preservation of heat-sensitive compounds, tunability of solvent density resulting in selective extraction of compounds, and, ultimately, low operating costs. Disadvantages of this method include high startup costs, a need for technical knowledge, and considerations of the solvent used (e.g., phase behavior, cross-linking zone) [91]. While the production of CO2 in this method is harmful to the environment, the use of CO2 as an extraction solvent is a key feature that allows this method to contribute to the circular economy cycle. For instance, to mitigate CO2 emissions, technologies such as carbon capture and storage (CCS) and carbon capture and utilization (CCU) are being developed. CCS involves capturing emitted CO2, compressing it, and injecting it into deep geological, oceanic, or mining sites. In contrast, CCU enables the recovery and reuse of captured CO2 rather than simply storing it. Therefore, incorporating CO2 into the development of products and services can help reduce global greenhouse gas emissions. This approach allows for the direct capture of CO2 from industrial off-gases or even from the atmosphere. In this context, supercritical fluid systems can utilize CO2 from these technologies to recover valuable compounds while simultaneously removing CO2 from the atmosphere and reducing the carbon footprint. Thus, extraction aligns with the principles of the circular economy [92]. There are currently over 200 commercial supercritical fluid extraction plants worldwide, many of which are used to extract natural compounds from plant products [93]. Numerous techno-economic feasibility studies have been conducted on this method for extracting natural products, detailing the production costs of various essential oils [94]. In a study by Chañi-Paucar et al. (2021), economic calculations regarding the extraction of oil from Baru seeds using this method revealed that the operational cost per kilogram of oil from the seed ranges from USD 87 to USD 118. When subtracting this cost from the price of the seed (considering its oil percentage of 22–29%) and the price of its oil-free meal, it was determined that this method is cost-effective for extracting this specific oil. However, given these prices and the lower prices of conventional oils (such as soybean and rapeseed), it appears that this method is not currently a suitable option for extracting conventional oils, except for specific oils [95]. Table 4 presents the effects of supercritical fluid on various properties of oils extracted from different products. As shown in this table, utilizing this method results in an elevation of low-level compounds such as phenolic compounds and tocopherols in the oil [96,97]. Conversely, through the optimization of this process, the oil can attain the highest levels of monounsaturated and polyunsaturated fatty acids [98].

Table 4.
Studies conducted in the field of oil extraction using supercritical fluid.
6. Enzymatic Extraction
Enzyme-assisted extraction is considered an alternative to conventional techniques for extracting valuable compounds using water as a solvent instead of an organic solvent. To extract lipid reserves stored in cells, it must be possible to cross several barriers: first, the extra cell walls (or secondary cell walls), then the cell wall, and finally the oleosomes. Each cell wall has its own components, which are sometimes organized into a complex structure and are naturally synthesized and broken down by specific enzymes. For example, the secondary cell wall of rapeseed is composed of 39% pectin, 29% hemicellulose, 22% cellulose, and 8% arabinogalactans, and constitutes 20% to 28% of the whole grain. The primary cell wall contains 10% glycoprotein, which is very rich in hydroxyproline; it was originally called extensin, but is now called HRGP (hydroxyproline-rich glycoprotein) [104]. Enzymes such as pectinase, β-gluconase, β-glucosidase, cellulase, and xylanase degrade the polysaccharides of the cell wall and thus release the related compounds. This technique is mainly used when phytochemicals are dispersed in the cell cytoplasm and hydrophobic or hydrogen bonds are present in the matrix, which makes it difficult to extract bioactive compounds by traditional techniques. Apart from that, the biological origin, environmentally friendly nature, and higher extraction efficiency of this method have increased the demand of this method [105]. However, since this method takes longer and is more expensive, and separating emulsion and drying protein and meal can be challenging, it is rarely used [106]. On the other hand, this method has the ability to separate oil and protein simultaneously, and also removes the gumming step from oil extraction [107]. Enzyme-assisted extraction offers advantages such as high efficiency and mild conditions, while ensuring the preservation of extraction properties and stability [108]. This method is also considered one of the green processing methods compared to traditional extraction methods. The type of enzyme, the dosage and conditions required by the enzymes, the process time and temperature, the properties of the plant material such as particle size, water content, and chemical composition, and the solvent-to-solid ratio are all factors that affect the rate of enzyme-assisted extraction [109]. In a study conducted by Cheng et al. (2019) on the operating cost of oil extraction from soybeans, it was found that this method is an innovative process for oil extraction. However, operational costs remain the main issue preventing the use of this method on an industrial scale, accounting for only 27% of total revenue [110]. However, it seems that using this method for olive oil extraction is economically viable due to the different extraction method [111]. In Table 5, the effect of extraction with the help of enzymes on some properties of extracted oils from different sources is given. Enzyme-assisted oil extraction is considered an extraction method based on the concepts of “green chemistry” and “green engineering”. The extracted oil has superior physicochemical parameters and nutrients, such as tocopherols, squalene, and polyunsaturated fatty acids, compared to pressing and solvent methods [107].

Table 5.
Studies conducted in the field of oil extraction using enzymatic extraction.
7. Ohmic Extraction Method
In recent years, heating techniques have undergone significant improvements. Some notable methods include resistance heating, dielectric heating (such as microwave and radio frequency heating), and induction heating. In all of these methods, heat is generated within the food itself, leading to increased efficiency [120]. The resistance heating method, also known as Joule heating, is a volumetric heating method where the food material acts as a resistor, generating heat when an electric current passes through it [121]. Due to its numerous advantages, resistance heating can be seen as an alternative to traditional methods. Its speed and short heating time, along with energy savings, help preserve the quality of the final product [122]. Other advantages of this method include reduced maintenance costs (due to the absence of moving parts), reduced risk of sedimentation during heat transfer, immediate shutdown of the system, and environmental friendliness. Disadvantages of this method include the lack of general information, the need to constantly adjust the device according to the variable properties of plant compounds, monitoring and control of the process, and the complexity of the temperature gradient between the products [123]. This process uses electricity instead of thermal energy obtained directly from the combustion of fossil fuels and can play a key role in the transition to a decarbonized industry, and thus does not have a significant impact on the environment [124]. The electrical conductivity of the food compounds, the current intensity, voltage, and frequency used, and the shape and dimensions of the food are factors that affect the extraction efficiency of this method [125]. The efficiency and speed of extraction are enhanced in this process by the rupture of plant cells caused by the current passing through them. This shortened process time also prevents the extraction of heavy compounds [126]. Several researchers have investigated industrial applications of ohmic heating, including aseptic processing, sterilization, pasteurization, drying, and distillation. However, oil extraction using this method has been less studied. Table 6 illustrates the impact of ohmic extraction on various properties of oils extracted from different sources. The effect of ohmic heating on the vitamin and antioxidant content of oils can vary. While this method generally preserves the natural antioxidants found in oils, such as tocopherols, prolonged or excessive heating may result in the loss of these heat-sensitive compounds. However, compared to traditional extraction methods that involve higher temperatures and longer processing times, ohmic heating is generally considered more favorable for preserving the vitamin and antioxidant content of oils. On the other hand, ohmic heating can help preserve phytochemicals and bioactive compounds found in oils, such as phenolic compounds and phytosterols. The gentle and precise heat provided by ohmic heating helps reduce the degradation and loss of these beneficial compounds, preserving their nutritional properties. Studies have shown that oil extracted using this method has higher levels of omega-3 and omega-6 fatty acids than solvent- and press-extraction methods [127].

Table 6.
Studies conducted in the field of oil extraction using ohmic extraction.
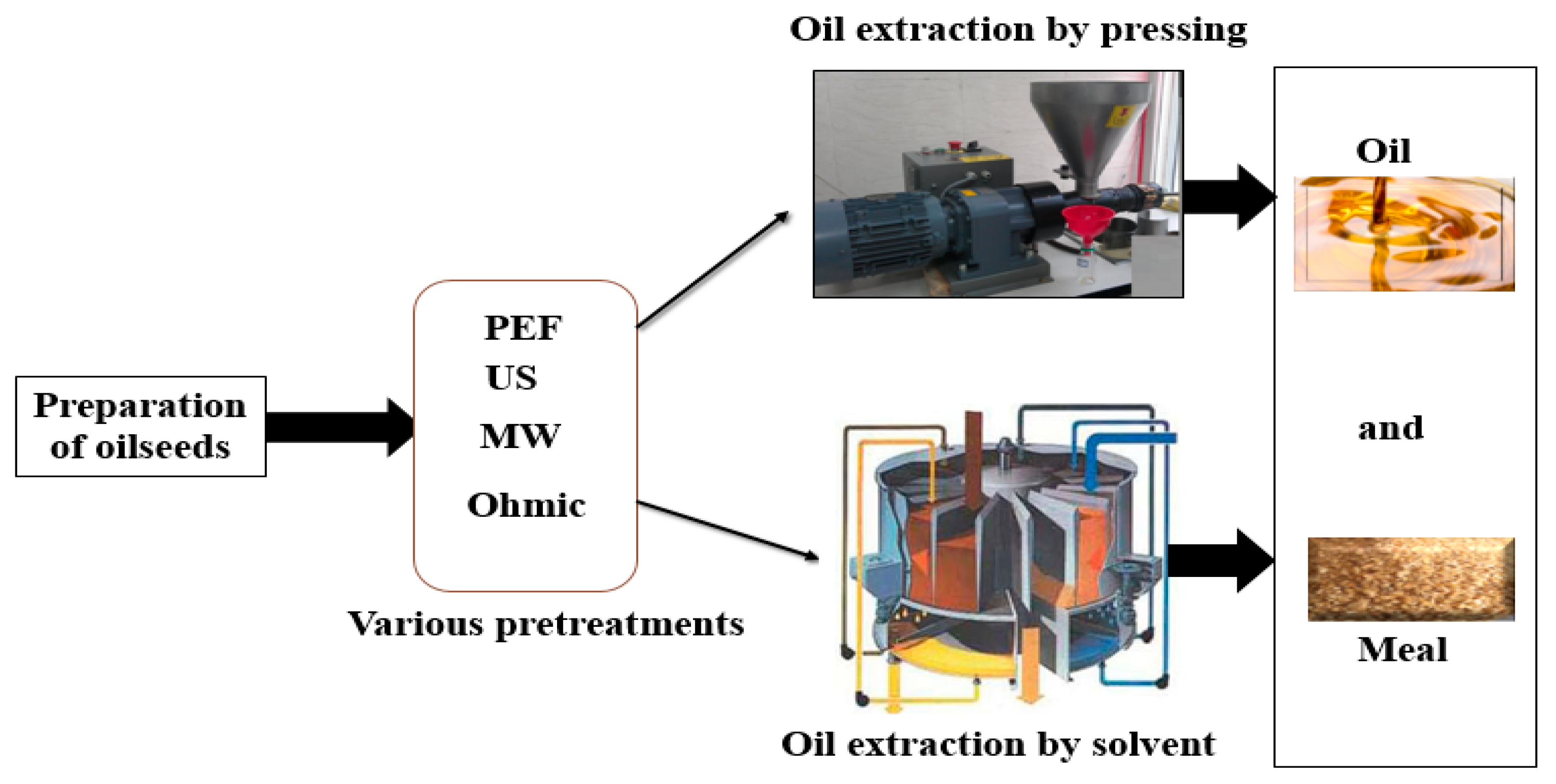
Figure 2 shows a schematic of the use of pulse electric field, microwave, ultrasound, and ohmic methods in the pretreatment of oil extraction from oilseeds.
Figure 2.
Schematic of the use of electric field, microwave, ultrasound, and ohmic pretreatments in oil extraction from oilseeds.
8. Oil Extraction Using Combined Processes
Choosing the right extraction process is crucial for maximizing compounds from tissues. Extraction efficiency is influenced by several critical elements, including the extraction technique, matrix properties of plant components, the solvent used, temperature, pressure, and time [134]. Researchers sometimes opt to combine these methods to capitalize on the simultaneous benefits outlined in this article. This approach results in oil with more bioactive compounds and improved physicochemical properties [135]. Table 7 provides examples of studies that have utilized a combined method for oil extraction. As shown in this table, utilizing combined processes can increase the levels of bioactive compounds and unsaturated fatty acids, depending on the specific type of process [136,137].

Table 7.
Studies conducted in the field of oil extraction using combined processes.
9. General Conclusions
This study aimed to introduce new methods of oil extraction from various plant and animal sources. Recent research in the field has shown the positive impact of these methods on the physicochemical properties of extracted oils, particularly in terms of extraction efficiency and bioactive compounds. On the other hand, studies have shown that certain methods such as ultrasound, pulsed electric field, and enzyme use have the potential to be industrialized for specific sources like olives. Additionally, almost all of the methods studied for oil extraction did not have a significant impact on environmental pollution levels. Therefore, considering various factors that were discussed in this study, such as increasing oil extraction efficiency, it is hoped that these methods will also be researched and utilized industrially for other oilseeds. However, further comprehensive studies on the use of different extraction methods to determine the best method for extracting oil from specific materials are necessary.
Author Contributions
H.B., M.G. (Mohammad Ganje), M.G. (Mehdi Gharekhani) and T.M.-M.: Conceptualization, Writing—Original Draft, Review and Editing, C.A. and A.M.: Methodology, Supervision and Project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data Availability Statement
No data were used for the research described in the article.
Acknowledgments
The authors would like to thank UTE University for its intellectual and financial assistance and support in carrying out this project.
Conflicts of Interest
All authors declare that they have no conflicts of interest.
References
- Bakhshabadi, H.; Mirzaei, H.; Ghodsvali, A.; Jafari, S.M.; Ziaiifar, A.M.; Farzaneh, V. The effect of microwave pretreatment on some physico-chemical properties and bioactivity of Black cumin seeds’ oil. Ind. Crops Prod. 2017, 97, 1–9. [Google Scholar] [CrossRef]
- Frankel, E.N. Lipid Oxidation, 2nd ed.; Oily Press Lipid Library Series; Oily Press: Buckinghamshire, UK, 2012. [Google Scholar] [CrossRef]
- Pan, D.A.; Hulbert, A.; Storlien, L. Dietary fats, membrane phospholipids and obesity. J. Nutr. 1994, 124, 1555–1565. [Google Scholar] [CrossRef] [PubMed]
- Siger, A.; Nogala-kalucka, M.; Lampart-Szczapa, E. The content and antioxidant activity of phenolic compounds in cold-pressed plant oils. J. Food Lipids 2008, 15, 137–149. [Google Scholar] [CrossRef]
- Anderson, D. A primer on oils processing technology. Bailey’s Ind. Oil Fat Prod. 2005, 5, 1–56. [Google Scholar]
- Azadmard-Damirchi, S.; Habibi-Nodeh, F.; Hesari, J.; Nemati, M.; Achachlouei, B.F. Effect of pretreatment with microwaves on oxidative stability and nutraceuticals content of oil from rapeseed. Food Chem. 2010, 121, 1211–1215. [Google Scholar] [CrossRef]
- Bargale, P.C. Mechanical Oil Expression from Selected Oilseeds Under Uniaxial Compression. Ph.D. Thesis, University of Saskatchewan, Saskatoon, SK, Canada, 1997. [Google Scholar]
- De Castro, M.L.; Garcıa-Ayuso, L. Soxhlet extraction of solid materials: An outdated technique with a promising innovative future. Anal. Chim. Acta 1998, 369, 1–10. [Google Scholar] [CrossRef]
- Mamidipally, P.K.; Liu, S.X. First approach on rice bran oil extraction using limonene. Eur. J. Lipid Sci. Technol. 2004, 106, 122–125. [Google Scholar] [CrossRef]
- Rostami, M.; Farzaneh, V.; Boujmehrani, A.; Mohammadi, M.; Bakhshabadi, H. Optimizing the extraction process of sesame seed’s oil using response surface method on the industrial scale. Ind. Crops Prod. 2014, 58, 160–165. [Google Scholar] [CrossRef]
- Shejawale, D.D.; Murugesh, C.; Rastogi, N.; Subramanian, R. Effect of feed particle size and solvent flow rate on soybean oil extraction in a percolation type extractor. J. Food Sci. Technol. 2022, 59, 4723–4730. [Google Scholar] [CrossRef]
- Shittu, S.; Mari, H.; Dangora, N. Statistical model for solvent oil extraction from soybean (Glycine max (L.)). Food Res. 2019, 3, 182–187. [Google Scholar] [CrossRef]
- Rodrigues, C.E.; Longo, N.M.; Silva, C.C.; Aracava, K.K.; Garavazo, B.R. Ethanolic extraction of soybean oil: Oil solubility equilibria and kinetic studies. Chem. Eng. Trans. 2011, 24, 811–816. [Google Scholar]
- Toda, T.A.; Sawada, M.M.; Rodrigues, C.E. Kinetics of soybean oil extraction using ethanol as solvent: Experimental data and modeling. Food Bioprod. Process. 2016, 98, 1–10. [Google Scholar] [CrossRef]
- Savoire, R.; Lanoisellé, J.-L.; Vorobiev, E. Mechanical continuous oil expression from oilseeds: A review. Food Bioprocess Technol. 2013, 6, 1–16. [Google Scholar] [CrossRef]
- De Oliveira, R.C.; De Barros, S.T.D.; Gimenes, M.L. The extraction of passion fruit oil with green solvents. J. Food Eng. 2013, 117, 458–463. [Google Scholar] [CrossRef]
- Mandal, V.; Mohan, Y.; Hemalatha, S. Microwave assisted extraction—An innovative and promising extraction tool for medicinal plant research. Pharmacogn. Rev. 2007, 1, 7–18. [Google Scholar]
- Aguilera, J.M.; Stanley, D.W. Microstructural Principles of Food Processing and Engineering; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1999. [Google Scholar]
- Tomasi, I.T.; Santos, S.C.; Boaventura, R.A.; Botelho, C.M. Optimization of microwave-assisted extraction of phenolic compounds from chestnut processing waste using response surface methodology. J. Clean. Prod. 2023, 395, 136452. [Google Scholar] [CrossRef]
- Zandi, P.; Bimakr, M.; Ganjloo, A. Optimization of microwave-assisted extraction of bioactive compounds from aerial parts of Catharanthus roseus L. Innov. Food Technol. 2023, 10, 153–169. [Google Scholar]
- Kumar, S.J.; Garlapati, V.K.; Gujjala, L.K.S.; Banerjee, R. Technologies for oil extraction from oilseeds and oleaginous microbes. In Three Phase Partitioning; Elsevier: Amsterdam, The Netherlands, 2021; pp. 243–266. [Google Scholar]
- Gallego-Schmid, A.; Mendoza, J.M.F.; Azapagic, A. Environmental assessment of microwaves and the effect of European energy efficiency and waste management legislation. Sci. Total Environ. 2018, 618, 487–499. [Google Scholar] [CrossRef]
- Souza, D.E.S.; Melo, J.J.C.d.; Santos, F.F.d.; Vasconcelos, A.L.d.S.; Jesus, A.d.S.d.; Freitas, L.d.S.; Souza, R.L.d.; Soares, C.M.F. Microwave-Assisted vs. Conventional Extraction of Moringa oleifera Seed Oil: Process Optimization and Efficiency Comparison. Foods 2024, 13, 3141. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Zhang, W. Techno-economic feasibility analysis of microwave-assisted biorefinery of multiple products from Australian lobster shells. Food Bioprod. Process. 2020, 124, 419–433. [Google Scholar] [CrossRef]
- Crawford, L.M. Food irradiation’s advantages will not escape public attention. Food Technol. 1998, 52, 55. [Google Scholar]
- Senphan, T.; Benjakul, S.; Sukketsiri, W.; Chotphruethipong, L.; Sriket, C. Comparative studies on characterizations and cytotoxicity of oil extracted from Lingzhi (Ganoderma lucidum) G2 spore using Soxhlet extraction and microwave-assisted extraction. Appl. Food Res. 2024, 4, 100483. [Google Scholar] [CrossRef]
- Fathi-Achachlouei, B.; Azadmard-Damirchi, S.; Zahedi, Y.; Shaddel, R. Microwave pretreatment as a promising strategy for increment of nutraceutical content and extraction yield of oil from milk thistle seed. Ind. Crops Prod. 2019, 128, 527–533. [Google Scholar] [CrossRef]
- Rezig, L.; Harzalli, Z.; Gharsallah, K.; Mahfoudhi, N.; Chouaibi, M.; Majdoub, H.; Oueslati, I. Microwave and roasting impact on pumpkin seed oil and its application in full-fat mayonnaise formula. Foods 2022, 11, 2732. [Google Scholar] [CrossRef]
- Bakhshabadi, H.; Mirzaei, H.; Ghodsvali, A.; Jafari, S.M.; Ziaiifar, A.M.; Big Babaie, A. Effects of pulsed electric field and microwave pre-treatments on some the black cumin seeds oil characterises. Innov. Food Technol. 2017, 4, 21–29. [Google Scholar]
- Boyapati, T.; Rana, S.S.; Ghosh, P. Microwave-assisted extraction of dragon fruit seed oil: Fatty acid profile and functional properties. J. Saudi Soc. Agric. Sci. 2023, 22, 149–157. [Google Scholar] [CrossRef]
- Zhang, W.; Fu, Q.; Jiang, H.; Tang, H.; Li, X.; Xie, Y.; Cao, X.; Liu, Q.; Yuan, Y. Insight into the microwave pretreatment of rapeseeds on the flavor characteristics of rapeseed oils. Lwt 2023, 184, 115045. [Google Scholar] [CrossRef]
- Gabe, A.M.; da Silva, P.R.S. Effect of microwave pre-treatment on the yield of soybean oil extraction process: Kinetic study, mathematical modeling and optimization. J. Eng. Exact Sci. 2022, 8, 13692-01. [Google Scholar] [CrossRef]
- Gotama, B.; Rahman, A.; Ahmad, A.; Hariyadi, A. Extraction of rice bran oil using microwave-assisted extraction and green solvents. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Yogyakarta, Indonesia, 23–24 April 2022; p. 012052. [Google Scholar]
- Zamanhuri, N.A.; Abd Rahman, N.; Bakar, N.F.A. Effect of microwave power and extraction time on crude palm oil quality using microwave-assisted extraction process. Int. J. Renew. Energy Dev. 2021, 10, 495. [Google Scholar] [CrossRef]
- Rezvankhah, A.; Emam-Djomeh, Z.; Safari, M.; Askari, G.; Salami, M. Microwave-assisted extraction of hempseed oil: Studying and comparing of fatty acid composition, antioxidant activity, physiochemical and thermal properties with Soxhlet extraction. J. Food Sci. Technol. 2019, 56, 4198–4210. [Google Scholar] [CrossRef]
- Hu, H.; Liu, H.; Shi, A.; Liu, L.; Fauconnier, M.L.; Wang, Q. The effect of microwave pretreatment on micronutrient contents, oxidative stability and flavor quality of peanut oil. Molecules 2018, 24, 62. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.C.; Benal, M.; Prasad, B.D.; Krupashankara, M.; Kulkarni, R.; Siddaligaswamy, N. Microwave assisted extraction of oil from pongamia pinnata seeds. Mater. Today Proc. 2018, 5, 2960–2964. [Google Scholar] [CrossRef]
- Bakhshabadi, H.; Mirzaei, H.; Ghodsvali, A.; Jafari, S.M.; Ziaiifar, A.M.; Bigbabaie, A. Optimizing the Extraction Process of Oil from Black Cumin Seeds by Using Pulsed Electric Field (PEF) Pretreatment. Res. Innov. Food Sci. Technol. 2017, 6, 221–234. [Google Scholar] [CrossRef]
- Pourzaki, A.; Mirzaee, H. New high voltage pulse generators. Recent Pat. Electr. Electron. Eng. (Former. Recent Pat. Electr. Eng.) 2009, 2, 65–76. [Google Scholar] [CrossRef]
- Schroeder, S.; Buckow, R.; Knoerzer, K. Numerical simulation of pulsed electric field (PEF) processing for chamber design and optimization. In Proceedings of the 17th International Conference on CFD in the Minerads and Process Industries CSIRO, Melbourne, Australia, 9–11 December 2009. [Google Scholar]
- Barbosa-Canovas, G.V.; Zhang, Q.H. Pulsed Electric Fields in Food Processing: Fundamental Aspects and Applications; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Asavasanti, S.; Ristenpart, W.; Stroeve, P.; Barrett, D.M. Permeabilization of plant tissues by monopolar pulsed electric fields: Effect of frequency. J. Food Sci. 2011, 76, E98–E111. [Google Scholar] [CrossRef] [PubMed]
- Salehi, M.; Omidvari, A. PEF application on optimization of energy consumption in extraction of sugar from sugar beet. Energy Eng. Manag. 2023, 5, 26–33. [Google Scholar]
- Bakhshabadi, H.; Mirzaei, H.; Ghodsvali, A.; Jafari, S.M.; Ziaiifar, A.M. The influence of pulsed electric fields and microwave pretreatments on some selected physicochemical properties of oil extracted from black cumin seed. Food Sci. Nutr. 2018, 6, 111–118. [Google Scholar] [CrossRef]
- Knorr, D.; Geulen, M.; Grahl, T.; Sitzmann, W. Food application of high electric field pulses. Trends Food Sci. Technol. 1994, 5, 71–75. [Google Scholar] [CrossRef]
- Barsotti, L.; Cheftel, J. Traitement des aliments par champs electriques pulses. 2. Aspects biologiques. Sci. Des Aliment. 1999, 19, 3–33. [Google Scholar]
- Taha, A.; Casanova, F.; Šimonis, P.; Stankevič, V.; Gomaa, M.A.; Stirkė, A. Pulsed electric field: Fundamentals and effects on the structural and techno-functional properties of dairy and plant proteins. Foods 2022, 11, 1556. [Google Scholar] [CrossRef]
- Nowosad, K.; Sujka, M.; Pankiewicz, U.; Kowalski, R. The application of PEF technology in food processing and human nutrition. J. Food Sci. Technol. 2021, 58, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Gómez, B.; Munekata, P.E.; Gavahian, M.; Barba, F.J.; Martí-Quijal, F.J.; Bolumar, T.; Campagnol, P.C.B.; Tomasevic, I.; Lorenzo, J.M. Application of pulsed electric fields in meat and fish processing industries: An overview. Food Res. Int. 2019, 123, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Dias, S.; Pino-Hernández, E.; Gonçalves, D.; Rego, D.; Redondo, L.; Alves, M. Challenges and Opportunities for Pilot Scaling-Up Extraction of Olive Oil Assisted by Pulsed Electric Fields: Process, Product, and Economic Evaluation. Appl. Sci. 2024, 14, 3638. [Google Scholar] [CrossRef]
- Yang, S.; Li, S.; Li, G.; Li, C.; Li, W.; Bi, Y.; Wei, J. Pulsed electric field treatment improves the oil yield, quality, and antioxidant activity of virgin olive oil. Food Chem. X 2024, 22, 101372. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Liu, J.; Tian, Y.; Liu, J.; Chang, Z. Effects of Pulsed Electric Field on Oil Extraction Rate and Tocopherol in Peony Seeds. Appl. Sci. 2024, 14, 3299. [Google Scholar] [CrossRef]
- Kermani, M.; Samimi, A.; Mohebbi-Kalhori, D.; Beigmoradi, R.; Shokrollahzadeh, S.; Xia, A.; Sun, C.; Sun, F.; Ashori, A.; Madadi, M. Pulsed Electric Field Treatment for Efficient oil Extraction from Nannochloropsis salina Microalgae: A Green and Sustainable Approach. J. Polym. Environ. 2024, 32, 5888–5901. [Google Scholar] [CrossRef]
- Mazroei Seydani, L.; Gharachorloo, M.; Asadi, G. Use of pulsed electric field to extract rapeseed oil and investigation of the qualitative properties of oils. J. Food Process Eng. 2022, 45, e14149. [Google Scholar] [CrossRef]
- Leone, A.; Tamborrino, A.; Esposto, S.; Berardi, A.; Servili, M. Investigation on the effects of a pulsed electric field (PEF) continuous system implemented in an industrial olive oil plant. Foods 2022, 11, 2758. [Google Scholar] [CrossRef]
- Tamborrino, A.; Urbani, S.; Servili, M.; Romaniello, R.; Perone, C.; Leone, A. Pulsed electric fields for the treatment of olive pastes in the oil extraction process. Appl. Sci. 2019, 10, 114. [Google Scholar] [CrossRef]
- Haji-Moradkhani, A.; Rezaei, R.; Moghimi, M. Optimization of pulsed electric field-assisted oil extraction from cannabis seeds. J. Food Process Eng. 2019, 42, e13028. [Google Scholar] [CrossRef]
- Shorstkii, I.; Mirshekarloo, M.; Koshevoy, E. Application of pulsed electric field for oil extraction from sunflower seeds: Labscale parametersoptimization. Proceedings of IRC Conference on Science, Engineering and TechnologyIRC-SET, Singapore, 13 May 2015. [Google Scholar]
- Sarkis, J.R.; Boussetta, N.; Tessaro, I.C.; Marczak, L.D.F.; Vorobiev, E. Application of pulsed electric fields and high voltage electrical discharges for oil extraction from sesame seeds. J. Food Eng. 2015, 153, 20–27. [Google Scholar] [CrossRef]
- Bonrath, W. Ultrasound supported catalysis. Ultrason. Sonochem. 2005, 12, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Gallo, M.; Ferrara, L.; Naviglio, D. Application of ultrasound in food science and technology: A perspective. Foods 2018, 7, 164. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Chutia, H.; Mahanta, C.L. Green ultrasound and microwave extraction of carotenoids from passion fruit peel using vegetable oils as a solvent: Optimization, comparison, kinetics, and thermodynamic studies. Innov. Food Sci. Emerg. Technol. 2021, 67, 102547. [Google Scholar] [CrossRef]
- Shen, L.; Pang, S.; Zhong, M.; Sun, Y.; Qayum, A.; Liu, Y.; Rashid, A.; Xu, B.; Liang, Q.; Ma, H. A comprehensive review of ultrasonic assisted extraction (UAE) for bioactive components: Principles, advantages, equipment, and combined technologies. Ultrason. Sonochem. 2023, 101, 106646. [Google Scholar] [CrossRef]
- Carreira-Casais, A.; Otero, P.; Garcia-Perez, P.; Garcia-Oliveira, P.; Pereira, A.G.; Carpena, M.; Soria-Lopez, A.; Simal-Gandara, J.; Prieto, M.A. Benefits and drawbacks of ultrasound-assisted extraction for the recovery of bioactive compounds from marine algae. Int. J. Environ. Res. Public Health 2021, 18, 9153. [Google Scholar] [CrossRef]
- Chemat, F.; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef]
- De Andrade, F.; Augusti, R.; De Lima, G. Ultrasound for the remediation of contaminated waters with persistent organic pollutants: A short review. Ultrason. Sonochem. 2021, 78, 105719. [Google Scholar] [CrossRef]
- Gutte, K.B.; Sahoo, A.K.; Ranveer, R.C. Effect of ultrasonic treatment on extraction and fatty acid profile of flaxseed oil. OCL 2015, 22, D606. [Google Scholar] [CrossRef]
- Grillo, G.; Boffa, L.; Calcio Gaudino, E.; Binello, A.; Rego, D.; Pereira, M.; Martínez, M.; Cravotto, G. Combined ultrasound and pulsed electric fields in continuous-flow industrial olive-oil production. Foods 2022, 11, 3419. [Google Scholar] [CrossRef] [PubMed]
- Fornasari, C.H.; Secco, D.; Santos, R.F.; da Silva, T.R.B.; Lenz, N.B.G.; Tokura, L.K.; Lenz, M.L.; de Souza, S.N.M.; Junior, L.A.Z.; Gurgacz, F. Efficiency of the use of solvents in vegetable oil extraction at oleaginous crops. Renew. Sustain. Energy Rev. 2017, 80, 121–124. [Google Scholar] [CrossRef]
- Rezaie, M.; Farhoosh, R.; Iranshahi, M.; Sharif, A.; Golmohamadzadeh, S. Ultrasonic-assisted extraction of antioxidative compounds from Bene (Pistacia atlantica subsp. mutica) hull using various solvents of different physicochemical properties. Food Chem. 2015, 173, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Xu, Z.; Zheng, B.; Lo, Y.M. Optimization of ultrasonic-assisted extraction of pomegranate (Punica granatum L.) seed oil. Ultrason. Sonochem. 2013, 20, 202–208. [Google Scholar] [CrossRef]
- Hu, A.-j.; Zhao, S.; Liang, H.; Qiu, T.-Q.; Chen, G. Ultrasound assisted supercritical fluid extraction of oil and coixenolide from adlay seed. Ultrason. Sonochem. 2007, 14, 219–224. [Google Scholar] [CrossRef]
- Jalili, F.; Jafari, S.M.; Emam-Djomeh, Z.; Malekjani, N.; Farzaneh, V. Optimization of ultrasound-assisted extraction of oil from canola seeds with the use of response surface methodology. Food Anal. Methods 2018, 11, 598–612. [Google Scholar] [CrossRef]
- Rodrigues, G.d.M.; Mello, B.T.F.d.; dos Santos Garcia, V.A.; Silva, C.d. Ultrasound-assisted extraction of oil from macauba pulp using alcoholic solvents. J. Food Process Eng. 2017, 40, e12530. [Google Scholar] [CrossRef]
- Gayas, B.; Kaur, G. Novel oil extraction methods in food industry: A review. J. Oilseed Brassica 2017, 1, 1–11. [Google Scholar]
- Moradi, N.; Rahimi, M.; Moeini, A.; Parsamoghadam, M.A. Impact of ultrasound on oil yield and content of functional food ingredients at the oil extraction from sunflower. Sep. Sci. Technol. 2018, 53, 261–276. [Google Scholar] [CrossRef]
- Gila, A.; Sánchez-Ortiz, A.; Jiménez, A.; Beltrán, G. The ultrasound application does not affect to the thermal properties and chemical composition of virgin olive oils. Ultrason. Sonochem. 2021, 70, 105320. [Google Scholar] [CrossRef]
- Dar, I.H.; Junaid, P.M.; Ahmad, S.; Shams, R.; Dash, K.K.; Shaikh, A.M.; Béla, K. Optimization of ultrasound-assisted extraction of Nigella sativa seed oil for enhancement of yield and antioxidant activity. Discov. Appl. Sci. 2024, 6, 104. [Google Scholar] [CrossRef]
- Matei, P.L.; Deleanu, I.; Brezoiu, A.M.; Chira, N.A.; Busuioc, C.; Isopencu, G.; Cîlțea-Udrescu, M.; Alexandrescu, E.; Stoica-Guzun, A. Ultrasound-assisted extraction of blackberry seed oil: Optimization and oil characterization. Molecules 2023, 28, 2486. [Google Scholar] [CrossRef]
- Gasparini, A.; Ferrentino, G.; Angeli, L.; Morozova, K.; Zatelli, D.; Scampicchio, M. Ultrasound assisted extraction of oils from apple seeds: A comparative study with supercritical fluid and conventional solvent extraction. Innov. Food Sci. Emerg. Technol. 2023, 86, 103370. [Google Scholar] [CrossRef]
- Esposito, M.; Piazza, L. Ultrasound-assisted extraction of oil from hempseed (Cannabis sativa L.): Part 1. J. Sci. Food Agric. 2022, 102, 732–739. [Google Scholar] [CrossRef]
- Garofalo, S.F.; Demichelis, F.; Mancini, G.; Tommasi, T.; Fino, D. Conventional and ultrasound-assisted extraction of rice bran oil with isopropanol as solvent. Sustain. Chem. Pharm. 2022, 29, 100741. [Google Scholar] [CrossRef]
- Tamborrino, A.; Taticchi, A.; Romaniello, R.; Perone, C.; Esposto, S.; Leone, A.; Servili, M. Assessment of the olive oil extraction plant layout implementing a high-power ultrasound machine. Ultrason. Sonochem. 2021, 73, 105505. [Google Scholar] [CrossRef]
- Stevanato, N.; da Silva, C. Radish seed oil: Ultrasound-assisted extraction using ethanol as solvent and assessment of its potential for ester production. Ind. Crops Prod. 2019, 132, 283–291. [Google Scholar] [CrossRef]
- Moghimi, M.; Farzaneh, V.; Bakhshabadi, H. The effect of ultrasound pretreatment on some selected physicochemical properties of black cumin (Nigella Sativa). Nutrire 2018, 43, 18. [Google Scholar] [CrossRef]
- Perrier, A.; Delsart, C.; Boussetta, N.; Grimi, N.; Citeau, M.; Vorobiev, E. Effect of ultrasound and green solvents addition on the oil extraction efficiency from rapeseed flakes. Ultrason. Sonochem. 2017, 39, 58–65. [Google Scholar] [CrossRef]
- Sihvonen, M.; Järvenpää, E.; Hietaniemi, V.; Huopalahti, R. Advances in supercritical carbon dioxide technologies. Trends Food Sci. Technol. 1999, 10, 217–222. [Google Scholar] [CrossRef]
- Uwineza, P.A.; Waśkiewicz, A. Recent advances in supercritical fluid extraction of natural bioactive compounds from natural plant materials. Molecules 2020, 25, 3847. [Google Scholar] [CrossRef] [PubMed]
- Yıldırım, M.; Erşatır, M.; Poyraz, S.; Amangeldinova, M.; Kudrina, N.O.; Terletskaya, N.V. Green Extraction of Plant Materials Using Supercritical CO2: Insights into Methods, Analysis, and Bioactivity. Plants 2024, 13, 2295. [Google Scholar] [CrossRef] [PubMed]
- Khaw, K.-Y.; Parat, M.-O.; Shaw, P.N.; Falconer, J.R. Solvent supercritical fluid technologies to extract bioactive compounds from natural sources: A review. Molecules 2017, 22, 1186. [Google Scholar] [CrossRef]
- Vardanega, R.; Osorio-Tobón, J.F.; Duba, K. Contributions of supercritical fluid extraction to sustainable development goal 9 in South America: Industry, innovation, and infrastructure. J. Supercrit. Fluids 2022, 188, 105681. [Google Scholar] [CrossRef]
- Prado, J.M.; Prado, G.H.; Meireles, M.A.A. Scale-up study of supercritical fluid extraction process for clove and sugarcane residue. J. Supercrit. Fluids 2011, 56, 231–237. [Google Scholar] [CrossRef]
- De Melo, M.; Silvestre, A.; Silva, C. Supercritical fluid extraction of vegetable matrices: Applications, trends and future perspectives of a convincing green technology. J. Supercrit. Fluids 2014, 92, 115–176. [Google Scholar]
- Chañi-Paucar, L.O.; Osorio-Tobón, J.F.; Johner, J.C.; Meireles, M.A.A. A comparative and economic study of the extraction of oil from Baru (Dipteryx alata) seeds by supercritical CO2 with and without mechanical pressing. Heliyon 2021, 7, e05971. [Google Scholar] [CrossRef]
- Albakry, Z.; Karrar, E.; Mohamed Ahmed, I.A.; Ali, A.A.; Al-Maqtari, Q.A.; Zhang, H.; Wu, G.; Wang, X. A comparative study of black cumin seed (Nigella sativa L.) oils extracted with supercritical fluids and conventional extraction methods. J. Food Meas. Charact. 2023, 17, 2429–2441. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, T.; Xie, L.; Karrar, E.; Shi, L.; Jin, J.; Wang, X.; Jin, Q. Physicochemical characteristics of Actinostemma lobatum Maxim. kernel oil by supercritical fluid extraction and conventional methods. Ind. Crops Prod. 2020, 152, 112516. [Google Scholar] [CrossRef]
- Salinas, F.; Vardanega, R.; Espinosa-Álvarez, C.; Jimenez, D.; Munoz, W.B.; Ruiz-Domínguez, M.C.; Meireles, M.A.A.; Cerezal-Mezquita, P. Supercritical fluid extraction of chañar (Geoffroea decorticans) almond oil: Global yield, kinetics and oil characterization. J. Supercrit. Fluids 2020, 161, 104824. [Google Scholar] [CrossRef]
- Chen, X.; Li, Z.; Smith, S.A.; Chen, M.; Liu, H.; Zhang, J.; Tang, L.; Li, J.; Liu, Q.; Wu, X. Optimization of supercritical CO2 extraction of Moringa oleifera seed oil using response surface methodological approach and its antioxidant activity. Front. Nutr. 2022, 8, 829146. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Shi, J.; Scanlon, M.; Xue, S.J.; Lu, J. Optimization of supercritical-CO2 process for extraction of tocopherol-rich oil from canola seeds. Lwt 2021, 145, 111435. [Google Scholar] [CrossRef]
- Amani, M.; Ardestani, N.S.; Honarvar, B. Experimental optimization and modeling of supercritical fluid extraction of oil from Pinus gerardiana. Chem. Eng. Technol. 2021, 44, 578–588. [Google Scholar] [CrossRef]
- Durante, M.; Ferramosca, A.; Treppiccione, L.; Di Giacomo, M.; Zara, V.; Montefusco, A.; Piro, G.; Mita, G.; Bergamo, P.; Lenucci, M. Application of response surface methodology (RSM) for the optimization of supercritical CO2 extraction of oil from patè olive cake: Yield, content of bioactive molecules and biological effects in vivo. Food Chem. 2020, 332, 127405. [Google Scholar] [CrossRef] [PubMed]
- Cvjetko, M.; Jokić, S.; Vidović, S.; Marić, B.; Redovniković, I.R. Optimization of the Supercritical CO2 Extraction of Oil from Rapeseed Using Response Surface Methodology. Food Technol. Biotechnol. 2012, 50, 208. [Google Scholar]
- Ricochon, G.; Muniglia, L. Influence of enzymes on the oil extraction processes in aqueous media. Oléagineux Corps Gras Lipides 2010, 17, 356–359. [Google Scholar] [CrossRef]
- Iqbal, A.; Schulz, P.; Rizvi, S.S. Valorization of bioactive compounds in fruit pomace from agro-fruit industries: Present Insights and future challenges. Food Biosci. 2021, 44, 101384. [Google Scholar] [CrossRef]
- Liu, J.-j.; Gasmalla, M.A.A.; Li, P.; Yang, R. Enzyme-assisted extraction processing from oilseeds: Principle, processing and application. Innov. Food Sci. Emerg. Technol. 2016, 35, 184–193. [Google Scholar] [CrossRef]
- Gao, Y.; Ding, Z.; Liu, Y.; Xu, Y.-J. Aqueous enzymatic extraction: A green, environmentally friendly and sustainable oil extraction technology. Trends Food Sci. Technol. 2023, 144, 104315. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, W.; Wang, C.; Yang, X.; Lou, Y.; Xia, X.; Xu, H. Optimization of enzyme-assisted extraction and purification of flavonoids from Pinus koraiensis nut-coated film and antioxidant activity evaluation. Molecules 2021, 26, 1950. [Google Scholar] [CrossRef]
- Shinwari, K.J. Emerging technologies for the recovery of bioactive compounds from saffron species. In Saffron; Academic Press: Cambridge, MA, USA, 2021; pp. 143–182. [Google Scholar]
- Cheng, M.-H.; Rosentrater, K.A.; Sekhon, J.; Wang, T.; Jung, S.; Johnson, L.A. Economic feasibility of soybean oil production by enzyme-assisted aqueous extraction processing. Food Bioprocess Technol. 2019, 12, 539–550. [Google Scholar] [CrossRef]
- Ferro, M.D.; Cabrita, M.J.; Herrera, J.M.; Duarte, M.F. A new laboratory scale olive oil extraction method with comparative characterization of phenolic and fatty acid composition. Foods 2023, 12, 380. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Wu, R.; Chen, B.; Zhang, F.; Chen, Y.; Cao, F.; Yu, P.; Su, E. Development of an efficient procedure for preparing high quality Camellia oleifera seed oil by enzymatic extraction and demulsification. Ind. Crops Prod. 2024, 212, 118392. [Google Scholar] [CrossRef]
- Piseskul, J.; Suttisansanee, U.; Chupeerach, C.; Khemthong, C.; Thangsiri, S.; Temviriyanukul, P.; Sahasakul, Y.; Santivarangkna, C.; Chamchan, R.; Aursalung, A. Optimization of Enzyme-Assisted Mechanical Extraction Process of Hodgsonia heteroclita Oilseeds and Physical, Chemical, and Nutritional Properties of the Oils. Foods 2023, 12, 292. [Google Scholar] [CrossRef] [PubMed]
- González-Gómez, D.; Ayuso-Yuste, M.C.; Blanco-Roque, C.; Bernalte-García, M.J. Optimization of enzyme-assisted aqueous method for the extraction of oil from walnuts using response surface methodology. J. Food Process. Preserv. 2019, 43, e14218. [Google Scholar] [CrossRef]
- Ribeiro, S.A.O.; Nicacio, A.E.; Zanqui, A.B.; Biondo, P.B.F.; de Abreu-Filho, B.A.; Visentainer, J.V.; Gomes, S.T.M.; Matsushita, M. Improvements in the quality of sesame oil obtained by a green extraction method using enzymes. LWT-Food Sci. Technol. 2016, 65, 464–470. [Google Scholar] [CrossRef]
- Bisht, T.S.; Sharma, S.K.; Sati, R.C.; Rao, V.K.; Yadav, V.K.; Dixit, A.K.; Sharma, A.K.; Chopra, C.S. Improvement of efficiency of oil extraction from wild apricot kernels by using enzymes. J. Food Sci. Technol. 2015, 52, 1543–1551. [Google Scholar] [CrossRef]
- Nyam, K.L.; Tan, C.P.; Lai, O.M.; Long, K.; Man, Y.B.C. Enzyme-assisted aqueous extraction of Kalahari melon seed oil: Optimization using response surface methodology. J. Am. Oil Chem. Soc. 2009, 86, 1235–1240. [Google Scholar] [CrossRef]
- Najafian, L.; Ghodsvali, A.; Khodaparast, M.H.; Diosady, L. Aqueous extraction of virgin olive oil using industrial enzymes. Food Res. Int. 2009, 42, 171–175. [Google Scholar] [CrossRef]
- Moreau, R.A.; Johnston, D.B.; Powell, M.J.; Hicks, K.B. A comparison of commercial enzymes for the aqueous enzymatic extraction of corn oil from corn germ. J. Am. Oil Chem. Soc. 2004, 81, 1071–1075. [Google Scholar] [CrossRef]
- Kaur, N.; Singh, A. Ohmic heating: Concept and applications—A review. Crit. Rev. Food Sci. Nutr. 2016, 56, 2338–2351. [Google Scholar] [CrossRef] [PubMed]
- De Alwis, A.; Fryer, P. The use of direct resistance heating in the food industry. J. Food Eng. 1990, 11, 3–27. [Google Scholar] [CrossRef]
- Anderson, D.R. Ohmic Heating as an Alternative Food Processing Technology. 2008. Available online: https://krex.k-state.edu/items/7b3a597a-db4c-464a-b968-a533ad658bd1 (accessed on 16 April 2008).
- Sakr, M.; Liu, S. A comprehensive review on applications of ohmic heating (OH). Renew. Sustain. Energy Rev. 2014, 39, 262–269. [Google Scholar] [CrossRef]
- Paini, A.; Romei, S.; Stefanini, R.; Vignali, G. Comparative life cycle assessment of ohmic and conventional heating for fruit and vegetable products: The role of the mix of energy sources. J. Food Eng. 2023, 350, 111489. [Google Scholar] [CrossRef]
- Sivashankari, M.; Sadvatha, R.; Karunanithi, S. Ohmic Heating and Its Impact on Oil Extraction from Food Processing By-Products. In Emerging Methods for Oil Extraction from Food Processing Waste; CRC Press: Boca Raton, FL, USA, 2025; pp. 126–149. [Google Scholar]
- Asl, R.M.Z.; Niakousari, M.; Gahruie, H.H.; Saharkhiz, M.J.; Khaneghah, A.M. Study of two-stage ohmic hydro-extraction of essential oil from Artemisia aucheri Boiss.: Antioxidant and antimicrobial characteristics. Food Res. Int. 2018, 107, 462–469. [Google Scholar]
- Zandi, M.; Ganjloo, A.; Bimakr, M.; Nasiri, M. Ohmic heating extraction of radish (Raphanus sativus L.) leaf phenolic extract: Numerical optimization and kinetic modelling. J. Food Sci. Technol. 2023, 20, 141–157. [Google Scholar]
- Hamad, R.; Chakraborty, S.K.; Kumar, A.; Kate, A. Effect of Ohmic Heating Pre-treatment on Millable Oil Extraction and Physicochemical Properties of Mustard (Brassica juncea) Oil. Biol. Forum-Int. J. 2023, 7, 7. [Google Scholar]
- Sangpradab, J.; Kamonpatana, P.; Suwannaporn, P.; Huang, T.-C. Ohmic heating-aided mechanical extraction of gamma-oryzanol and phytosterols in rice bran oil. Food Bioprocess Technol. 2021, 14, 1542–1554. [Google Scholar] [CrossRef]
- Esmaeilzadeh Kenari, R.; Dehghan, B. Optimization of hemp (Canabis sativa L.) seed oil extraction process by ohmic process and response surface methodology. Innov. Food Technol. 2020, 7, 581–596. [Google Scholar]
- Karunanithi, S.; Pare, A.; Sunil, C.; Loganathan, M. Optimization of process parameters of ohmic heating for improving yield and quality of tomato seed oil. Int. J. Pure Appl. Biosci. 2019, 7, 104–114. [Google Scholar] [CrossRef]
- Aamir, M.; Jittanit, W. Ohmic heating treatment for Gac aril oil extraction: Effects on extraction efficiency, physical properties and some bioactive compounds. Innov. Food Sci. Emerg. Technol. 2017, 41, 224–234. [Google Scholar] [CrossRef]
- Kumari, K.; Mudgal, V.; Viswasrao, G.; Srivastava, H. Studies on the effect of ohmic heating on oil recovery and quality of sesame seeds. J. Food Sci. Technol. 2016, 53, 2009–2016. [Google Scholar] [CrossRef]
- Jha, A.K.; Sit, N. Extraction of bioactive compounds from plant materials using combination of various novel methods: A review. Trends Food Sci. Technol. 2022, 119, 579–591. [Google Scholar] [CrossRef]
- Chen, Q.; Dong, W.; Wei, C.; Hu, R.; Long, Y. Combining integrated ultrasonic-microwave technique with ethanol to maximise extraction of green coffee oil from Arabica coffee beans. Ind. Crops Prod. 2020, 151, 112405. [Google Scholar] [CrossRef]
- Saghali, M.; Bakhshabadi, H.; Rezaei, R.; Farmani, M. Optimization of oil extraction from sunflower seeds using the microwave-ultrasound pretreatment. Food Res. J. 2021, 30, 151–167. [Google Scholar]
- Mohseni, N.M.; Mirzaei, H.; Moghimi, M. Optimized extraction and quality evaluation of Niger seed oil via microwave-pulsed electric field pretreatments. Food Sci. Nutr. 2020, 8, 1383–1393. [Google Scholar] [CrossRef]
- Qin, Z.; Chang, Y.-L.; Chen, Z.-M.; Wang, Y.-G.; Fan, W.; Gu, L.-B.; Qin, Z.; Liu, H.-M.; Zhu, X.-L.; Mei, H.-X. A novel strategy for preparing lignan-rich sesame oil from cold-pressed sesame seed cake by combining enzyme-assisted treatment and subcritical fluid extraction. Ind. Crops Prod. 2024, 218, 119041. [Google Scholar] [CrossRef]
- Karunanithi, S.; Gupta, R.K. Aqueous extraction of tomato seed oil using combination of ohmic heating and microwave heating as pretreatment. Food Phys. 2024, 1, 100018. [Google Scholar] [CrossRef]
- Mohseni, N.M.; Mirzaei, H.O.; Moghimi, M. Optimization of producing oil and meal from canola seeds using microwave− pulsed electric field pretreatment. OCL 2020, 27, 2. [Google Scholar] [CrossRef]
- Shah, S.; Sharma, A.; Gupta, M. Extraction of oil from Jatropha curcas L. seed kernels by combination of ultrasonication and aqueous enzymatic oil extraction. Bioresour. Technol. 2005, 96, 121–123. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).