Investigation of Gas Diffusion Time Dynamics at the Bottom Hole Under Convection–Diffusion Coupling Mechanisms
Abstract
:1. Introduction
2. Basic Theory of Gas Diffusion
2.1. Fick’s First Law
2.2. Fick’s Second Law
2.3. Influencing Factors of Gas Diffusion Coefficient
2.4. Chapman–Enskog Theory
3. Calculation of Pressure and Temperature in the Annular Diffusion Process of Raw Gas Oil Jacket
4. Establishment and Solution of Mathematical Model
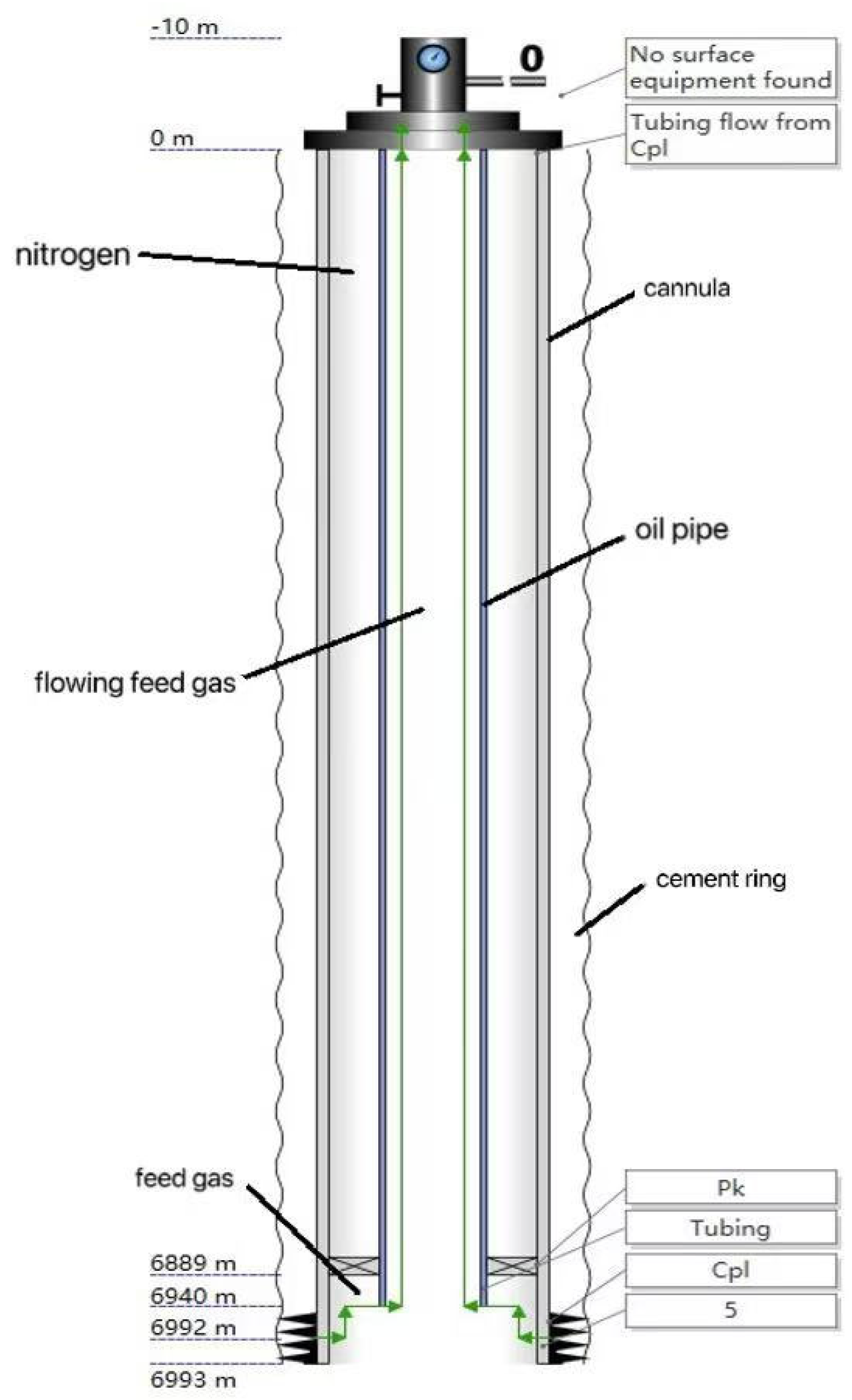
4.1. Well Body Structure
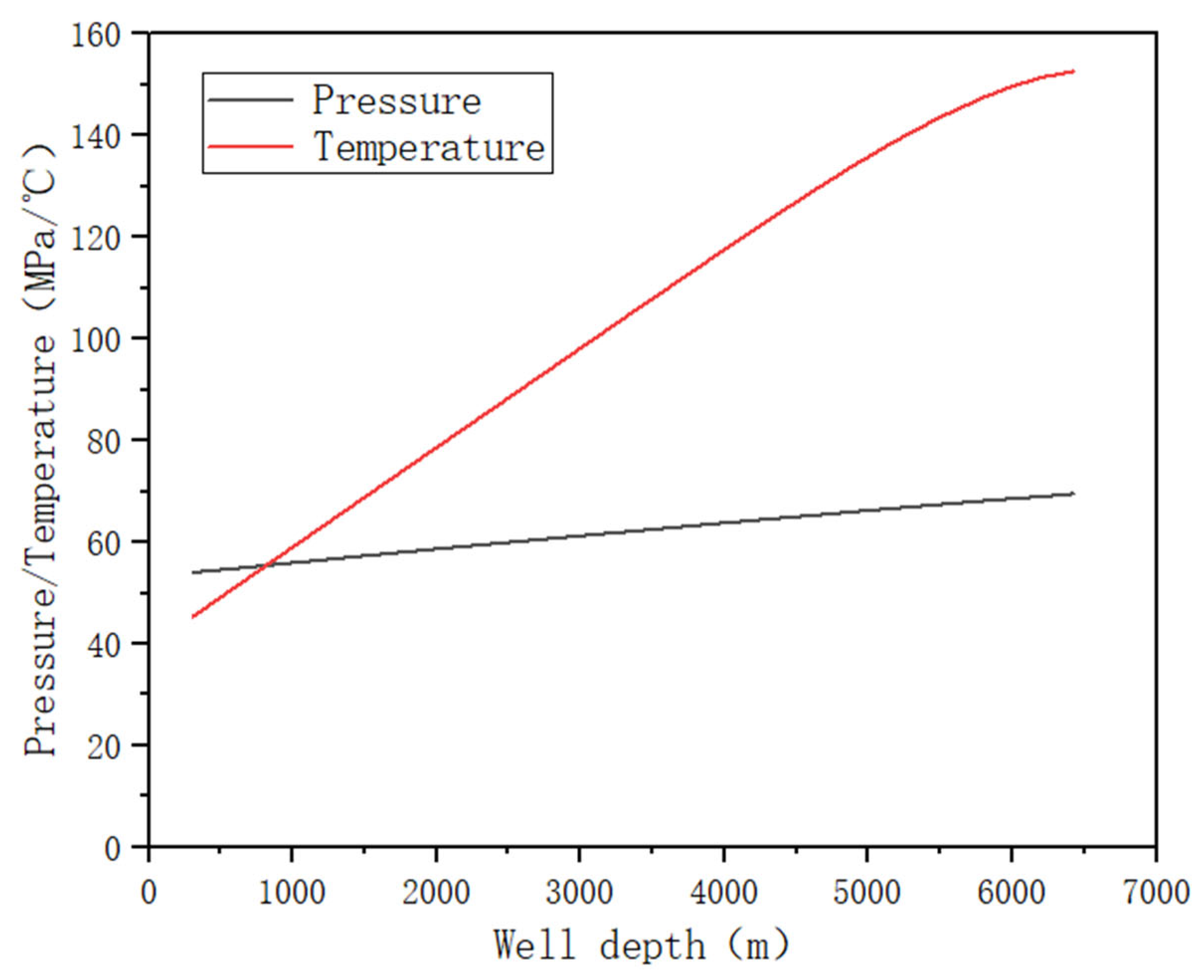
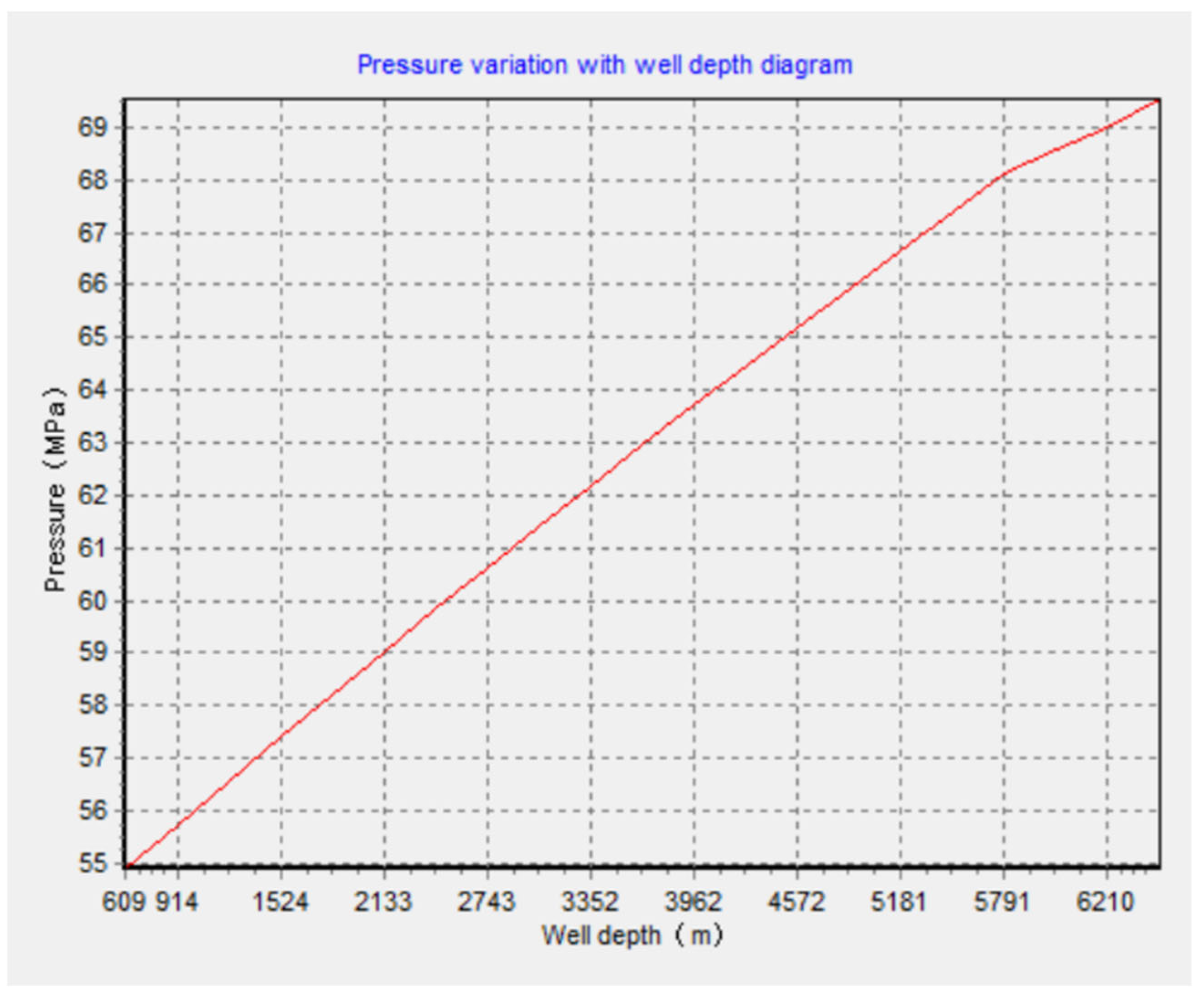
4.2. Pressure and Temperature Processing in Stages
4.3. The Convection–Diffusion Coupling Equation Is Established
4.4. Solution of Convection–Diffusion Coupling Equation
5. Numerical Simulation of Diffusion Process Based on COMSOL
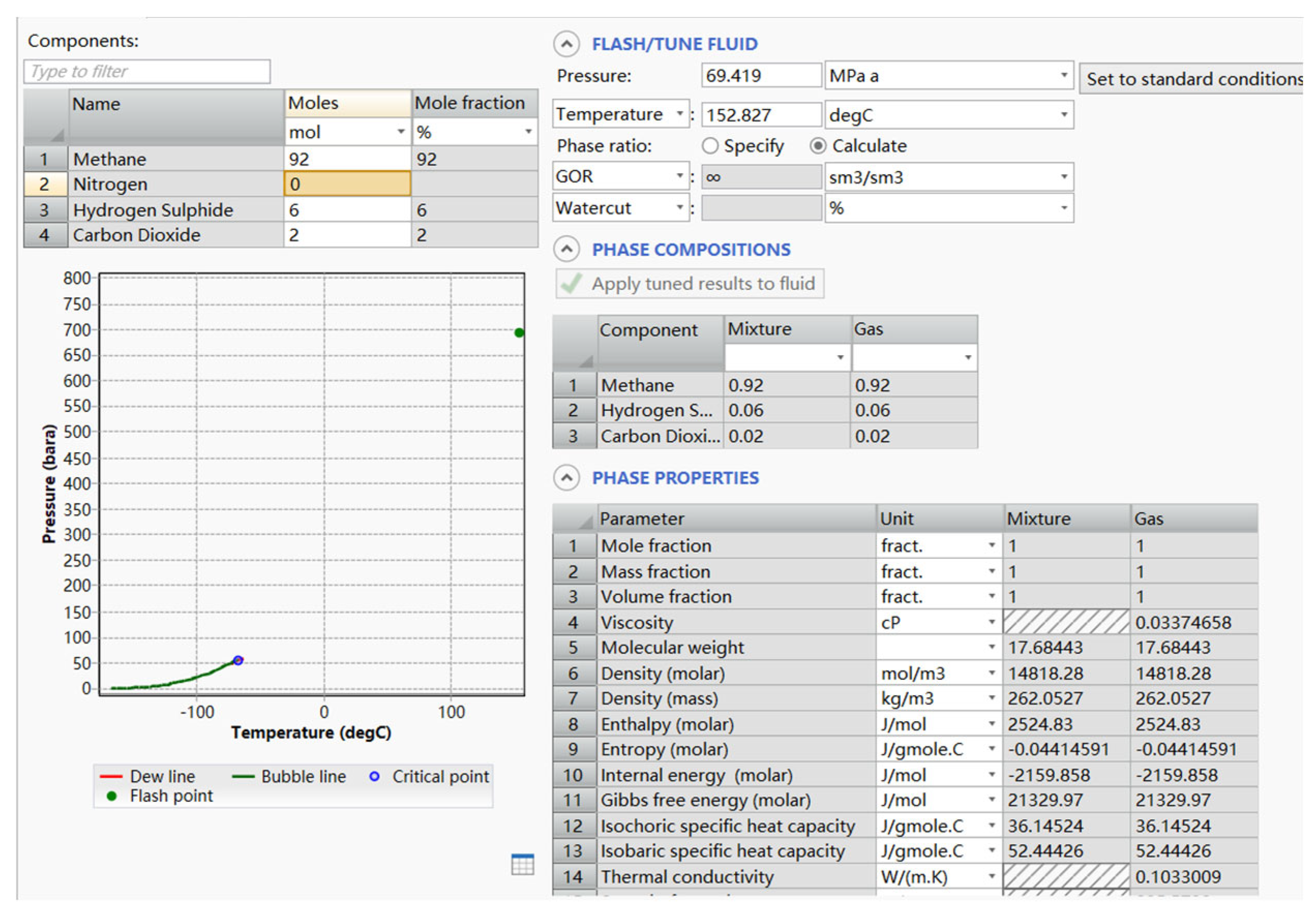
5.1. Phase State Prediction of Annular Diffusion Process of Raw Gas Oil Jacket
5.2. Calculation of Diffusion Coefficient
5.3. Local Diffusion Model
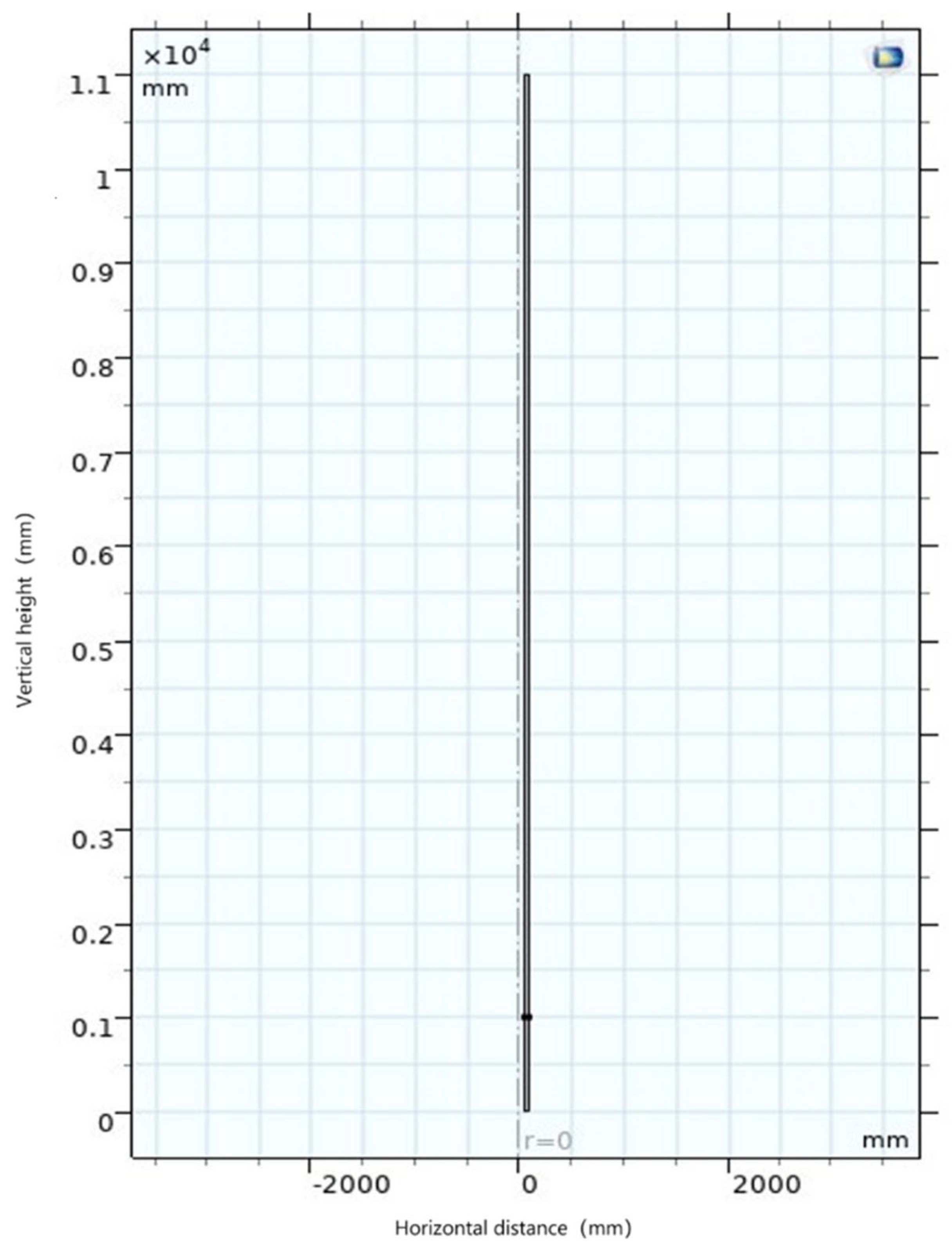
5.3.1. Establishment of Geometric Model
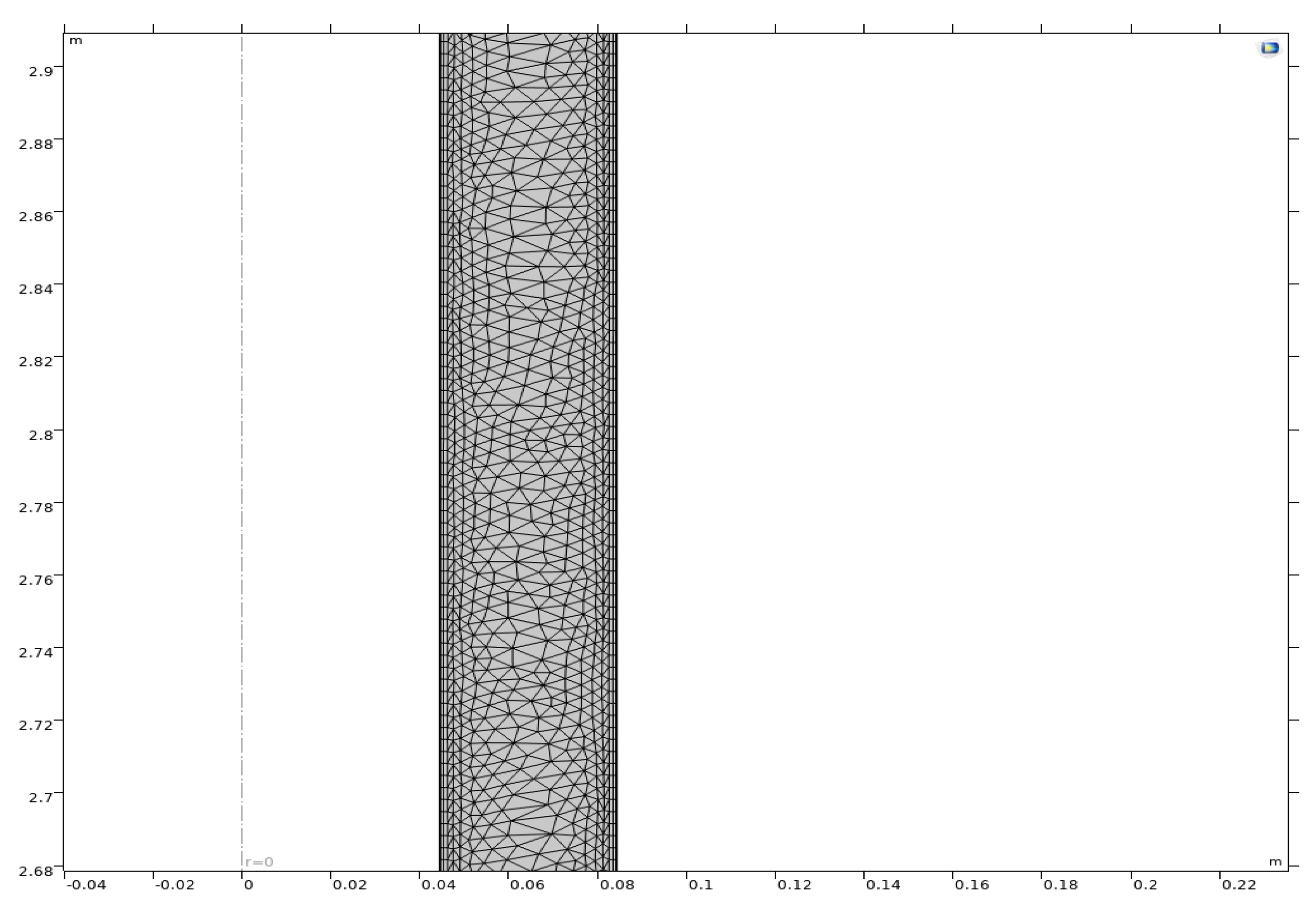
5.3.2. Grid Division
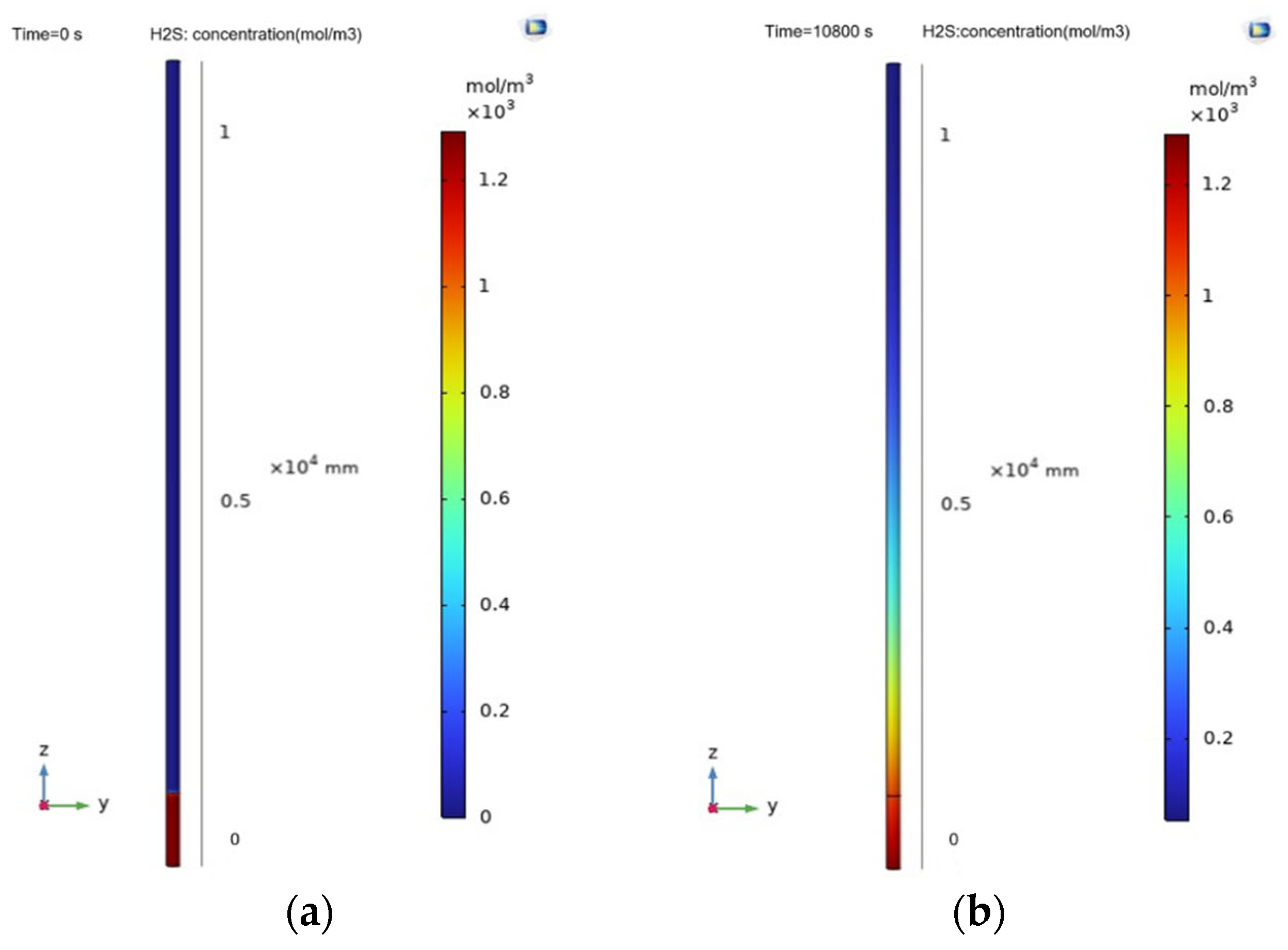
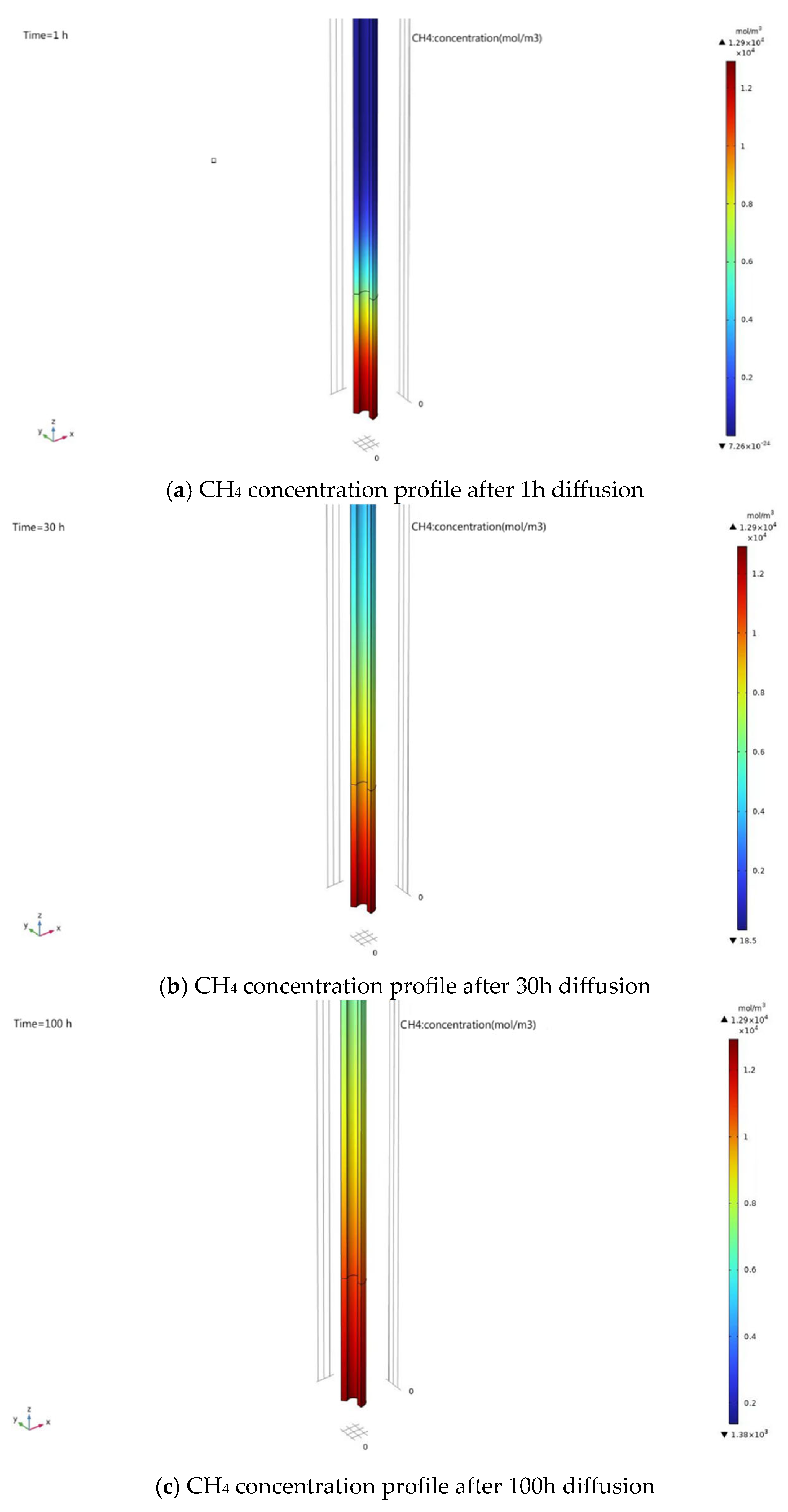
5.3.3. Local Diffusion Model Simulation Results
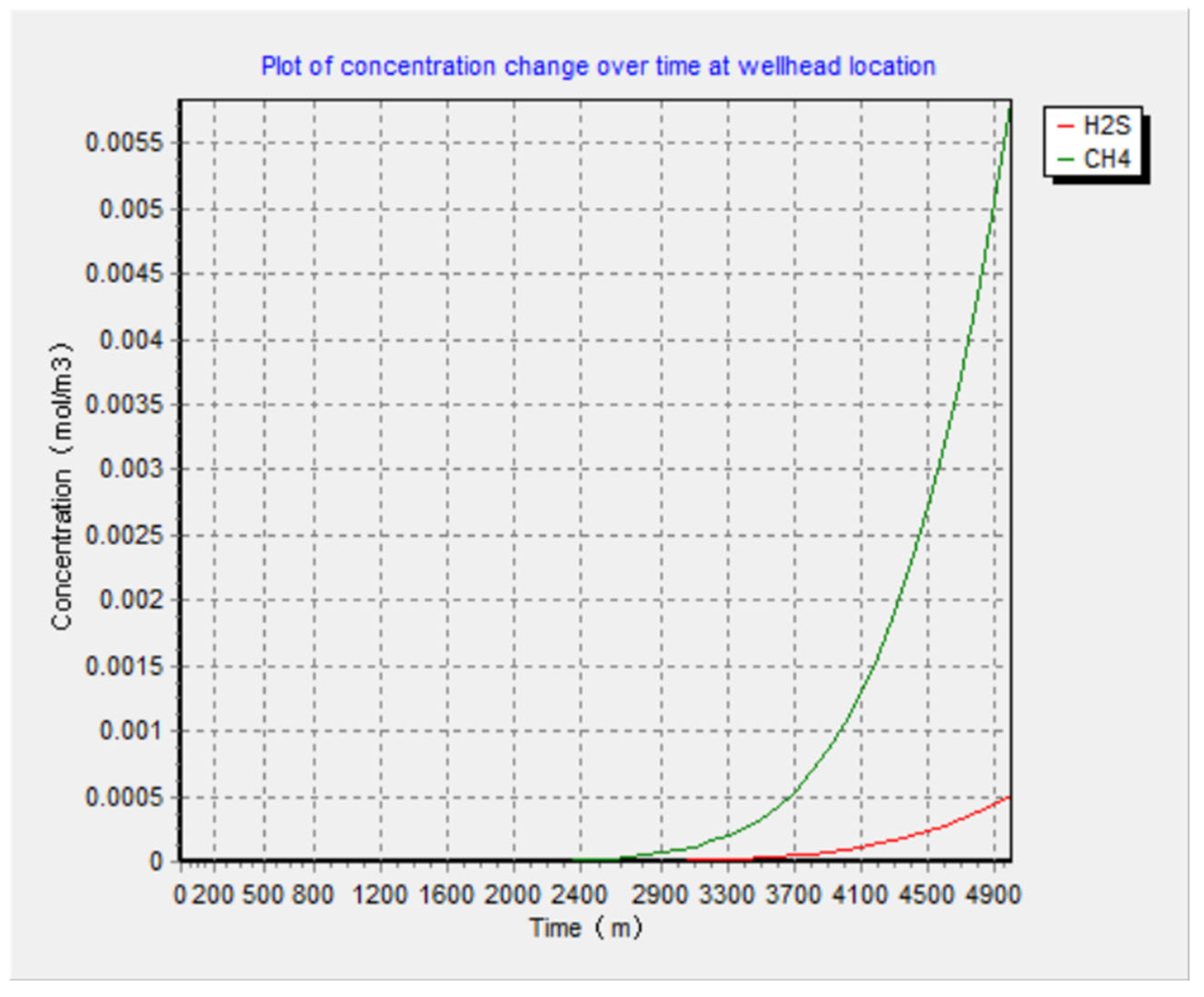
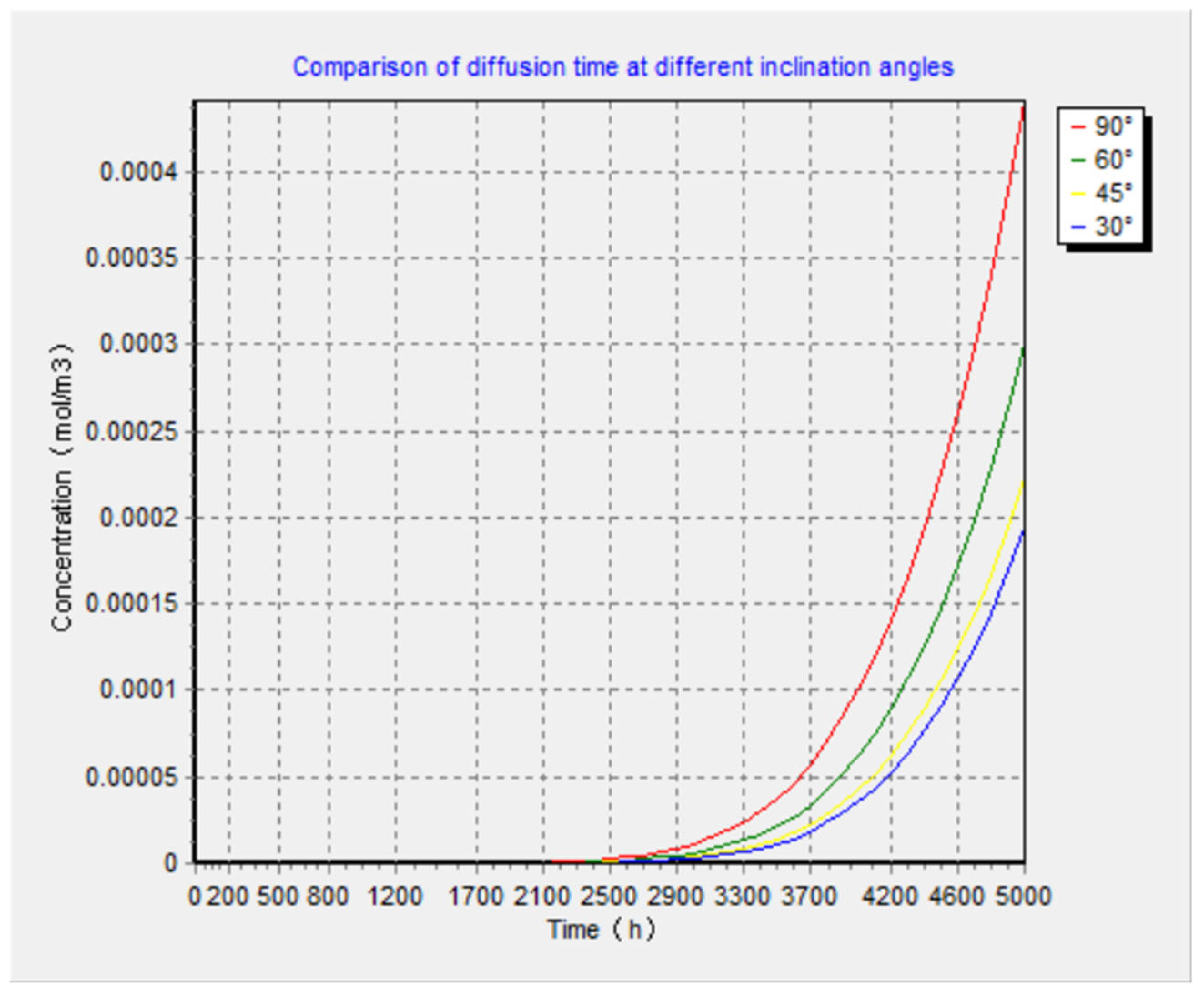
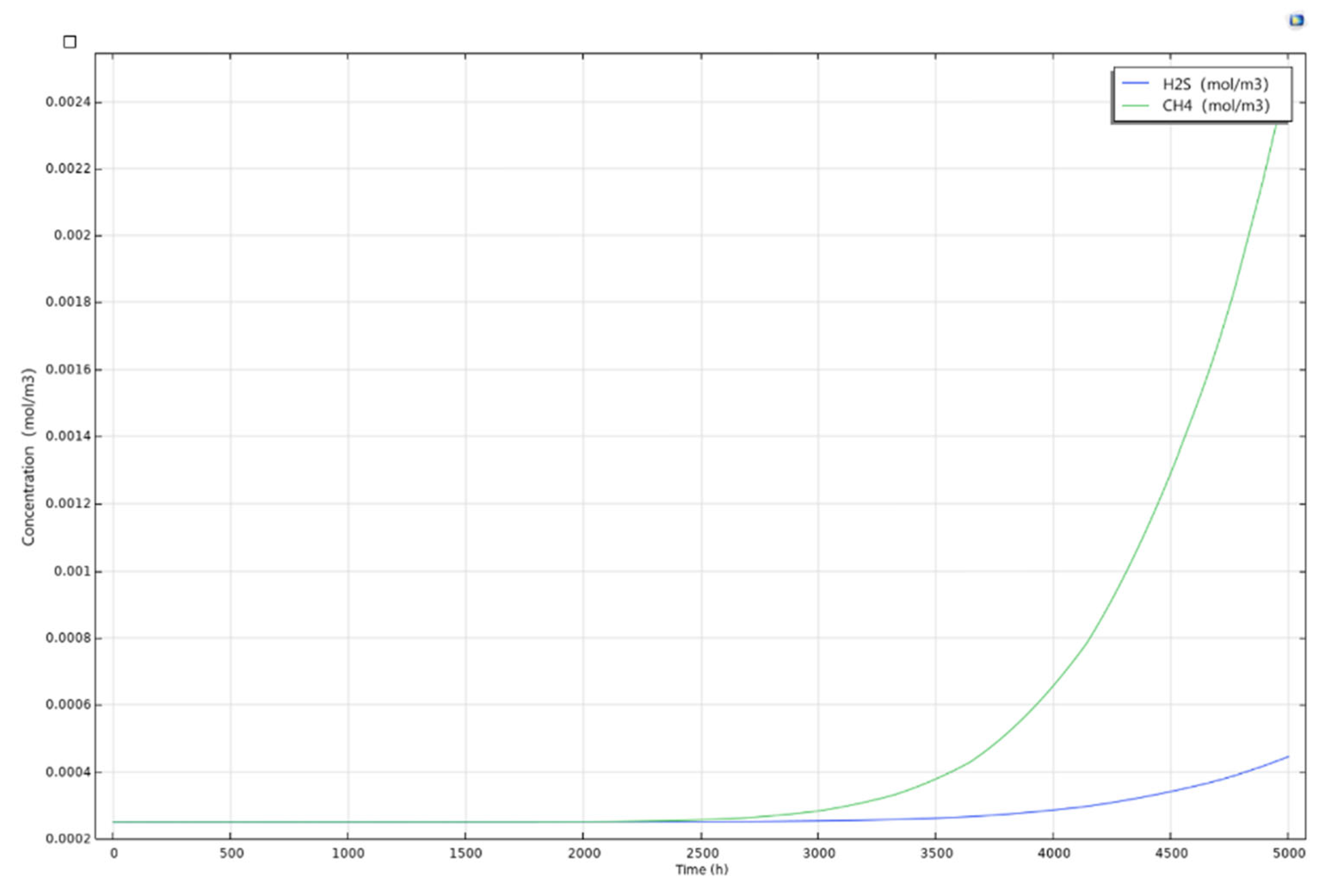
5.4. Results of the Complete Diffusion Model
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhi, S.J.; Xu, H.F.; Liu, L.Q.; Xue, S.L.; Peng, S.Y.; Zhang, X.B. Characteristics of small hole leakage and diffusion in hydrogen-doped natural gas High Pressure pipeline. J. Petrochem. Univ. 2024, 37, 25–32. [Google Scholar]
- Chen, Y.; Zhao, J.H.; Zhang, Z.D. Study on leakage and diffusion characteristics of natural gas pipelines. Pet. Chem. Equip. 2022, 25, 8–10. [Google Scholar]
- Shedid, S.A.; Zekri, A.Y. Sensitivity analysis of horizontal well productivity under steady-state conditions. In Proceedings of the SPE International Improved Oil Recovery Conference in Asia Pacific, Kuala Lumpur, Malaysia, 8–9 October 2001; SPE: Houston, TX, USA, 2001. SPE-72121-MS. [Google Scholar]
- Yang, D.D. Offshore Platform Sulfurous Gas Leakage Accident Consequence Chain Prediction and Protection Optimization. Ph.D. Thesis, China University of Petroleum (East China), Dongying, China, 2021. [Google Scholar]
- Chang, A.; Sun, H.G.; Zheng, C.; Lu, B.; Lu, C.; Ma, R.; Zhang, Y. A time fractional convection–diffusion equation to model gas transport through heterogeneous soil and gas reservoirs. Phys. A Stat. Mech. Appl. 2018, 502, 356–369. [Google Scholar] [CrossRef]
- Luan, Y.; Huo, F.; Lu, M.; Li, W.; Wu, T. Molecular thermal motion harvester for electricity conversion. APL Mater. 2023, 11, 101118. [Google Scholar] [CrossRef]
- Liu, A.; Liu, P.; Liu, S. Gas diffusion coefficient estimation of coal: A dimensionless numerical method and its experimental validation. Int. J. Heat Mass Transf. 2020, 162, 120336. [Google Scholar] [CrossRef]
- Lou, H.; Luo, P.; Xue, P.; Yang, W. Quantitative simulation of influencing factors of natural gas diffusion coefficient. Inn. Mong. Petrochem. Ind. 2012, 38, 5–6. [Google Scholar]
- Li, L.; Huang, W.; Ji, Y.; Zhou, N.; Wang, Y.; Wang, Z. Gas diffusion coefficient of test equipment development and application. J. Exp. Technol. Manag. 2016, 2, 77–80+86. [Google Scholar]
- Zhang, L.; Guo, J.Y.; Xie, Z.Y.; Liu, A.G.; Yang, C.L. Analysis on technical details of hydrocarbon gas diffusion coefficient determination in rocks. Pet. Chem. Equip. 2019, 22, 28–31. (In Chinese) [Google Scholar]
- Liu, Y.H. Research on Leakage Model of Natural Gas Pipeline Containing H2S. Master’s Thesis, Southwest Petroleum University, Chengdu, China, 2018. [Google Scholar]
- Birch, A.D.; Brown, D.R.; Dodson, M.G.; Swaffield, F. The structure and concentration decay of high pressure jets of natural gas. Combust. Sci. Technol. 1984, 36, 249–261. [Google Scholar] [CrossRef]
- Su, Y.; Li, J.; Yu, B.; Zhao, Y. Numerical investigation on the leakage and diffusion characteristics of hydrogen-blended natural gas in a domestic kitchen. Renew. Energy 2022, 189, 899–916. [Google Scholar] [CrossRef]
- Liu, G.D.; Zhao, Z.Y.; Sun, M.L.; Li, J.; Hu, G.Y.; Wang, X.B. A new understanding of the diffusion coefficient of natural gas in rocks. Pet. Explor. Dev. 2012, 39, 559–565. [Google Scholar] [CrossRef]
- Mi, L.-D.; Jiang, H.Q.; Li, J.J.; Tian, Y. Gas diffusion mechanism of shale reservoir. Daqing Pet. Geol. Dev. 2014, 33, 154–159. [Google Scholar]
- Etiope, G. Natural Gas Seepage: The Earth’s Hydrocarbon Degassing; Springer International Publishing: Cham, Switzerland, 2015; p. 17. [Google Scholar]
- Yin, Z.H.; Zhao, X.C.; Liu, Z.D. Development of a measuring device for diffusion coefficient of natural gas. Pet. Instrum. 2014, 28, 19–21+16. [Google Scholar]
- Wang, C. Laser Measurement System and Simulation of Binary Gas Diffusion Coefficient at High Temperature and Pressure. Master’s Thesis, Huazhong University of Science and Technology, Wuhan, China, 2012. (In Chinese). [Google Scholar]
- Xu, H.F.; Wang, G.X.; Shi, J.; Lu, L. Binary gas diffusion coefficient of a measuring method. J. Dalian Jiaotong Univ. 2012, 5, 90–92. [Google Scholar]
- Yang, M.; Huang, S.; Zhao, F.; Sun, H.; Chen, X.; Yang, C. Estimation of gas diffusion coefficient for gas/oil-saturated porous media systems by use of early-time pressure-decay data: An experimental/numerical approach. Phys. Fluids 2024, 36, 107134. [Google Scholar] [CrossRef]
- Ratnakar, R.R.; Dindoruk, B. The Role of diffusivity in oil and gas industries: Fundamentals, measurement, and correlative techniques. Processes 2022, 10, 1194. [Google Scholar] [CrossRef]
- Phan, B.K.; Shen, K.H.; Gurnani, R.; Tran, H.; Lively, R.; Ramprasad, R. Gas permeability, diffusivity, and solubility in polymers: Simulation-experiment data fusion and multi-task machine learning. npj Comput. Mater. 2024, 10, 186. [Google Scholar] [CrossRef]
- Yuan, M.; Jing, Y.; Lanetc, Z.; Zhuravljov, A.; Soleimani, F.; Si, G.; Armstrong, R.T.; Mostaghimi, P. Modelling multicomponent gas diffusion and predicting the concentration-dependent effective diffusion coefficient of coal with application to carbon geo-sequestration. Fuel 2023, 339, 127255. [Google Scholar] [CrossRef]
- Mao, H.Z. Marine Oil and Gas Production Platform Gas Leakage Diffusion Numerical Simulation and Control Study. Master’s Thesis, Hebei University of Engineering, Handan, China, 2022. [Google Scholar]
- Shi, Y.X.; Liu, F. Effect analysis of offshore oil platform reconstruction on gas diffusion. China Pet. Chem. Stand. Qual. 2019, 39, 151–152. [Google Scholar]
- Bird, R.B.; Klingenberg, D.J. Multicomponent diffusion—A brief review. Adv. Water Resour. 2013, 62, 238–242. [Google Scholar] [CrossRef]
- Qian, X.L.; Yan, X.Y.; Zhao, J.P. Simulation of gas pipeline leakage and diffusion in underground integrated pipeline corridor. China Prod. Saf. Sci. Technol. 2017, 13, 85–89. [Google Scholar]
- Zhang, B.Y.; Ma, G.Y.; Wang, K.; Huang, M.J.; Chen, S.J. Numerical Analysis of leakage diffusion in Buried Natural Gas Pipeline based on CFD. J. Liaoning Shihua Univ. 2019, 39, 39–43. [Google Scholar]
- Qian, X.L.; Yan, X.Y.; Zhao, J.P. Study on simulation of leakage and diffusion for natural gas pipeline in underground utility tunne. J. Saf. Sci. Technol. 2017, 13, 85–89. [Google Scholar]
- Wang, Y.; Li, X.Q.; Sun, J.Y.; Zhao, Z.C.; Zhou, R. Simulation of slope natural gas pipeline rupture leakage and diffusion based on Fluent. China Prod. Saf. Sci. Technol. 2014, 10, 89–93. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhao, C.; Wu, Q.; Zhang, X. Investigation of Gas Diffusion Time Dynamics at the Bottom Hole Under Convection–Diffusion Coupling Mechanisms. Processes 2025, 13, 1153. https://doi.org/10.3390/pr13041153
Wang Y, Zhao C, Wu Q, Zhang X. Investigation of Gas Diffusion Time Dynamics at the Bottom Hole Under Convection–Diffusion Coupling Mechanisms. Processes. 2025; 13(4):1153. https://doi.org/10.3390/pr13041153
Chicago/Turabian StyleWang, Yabin, Chunli Zhao, Qiang Wu, and Xinghua Zhang. 2025. "Investigation of Gas Diffusion Time Dynamics at the Bottom Hole Under Convection–Diffusion Coupling Mechanisms" Processes 13, no. 4: 1153. https://doi.org/10.3390/pr13041153
APA StyleWang, Y., Zhao, C., Wu, Q., & Zhang, X. (2025). Investigation of Gas Diffusion Time Dynamics at the Bottom Hole Under Convection–Diffusion Coupling Mechanisms. Processes, 13(4), 1153. https://doi.org/10.3390/pr13041153




