Study on the Evolution Law and Theoretical Solution of a Freezing Temperature Field in Transcritical CO2 Ultra-Low Temperature Formation
Abstract
1. Introduction
2. Design of Laboratory Model Tests
2.1. Measurement of Physical and Thermodynamic Parameters
2.2. Similarity Criteria for Model Tests
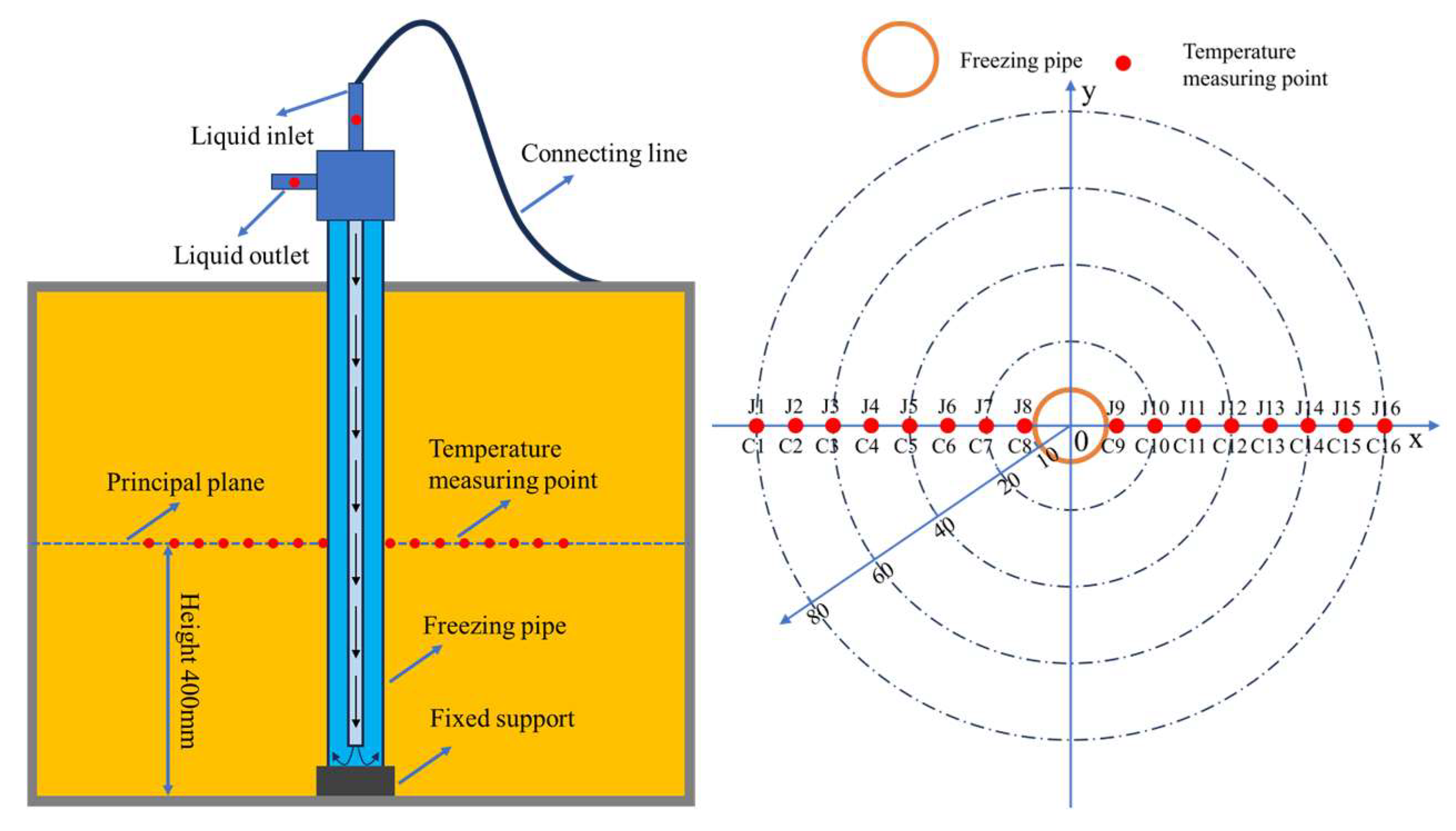
2.3. Model Test System
2.3.1. Test Box
2.3.2. Freezing System
2.3.3. Temperature Measuring System
2.3.4. Test Operation Steps
3. Analysis of Laboratory Model Test Results
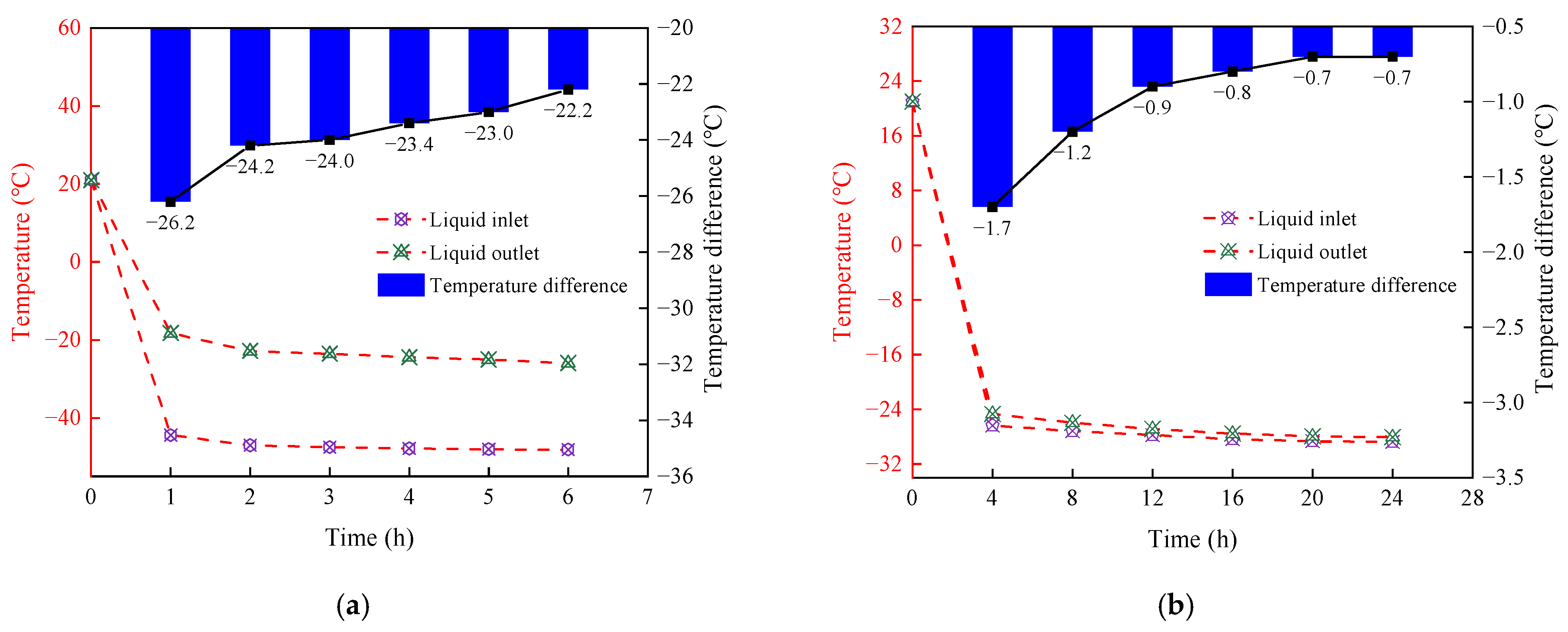
3.1. Analysis of Temperature Difference Between Liquid Inlet and Outlet
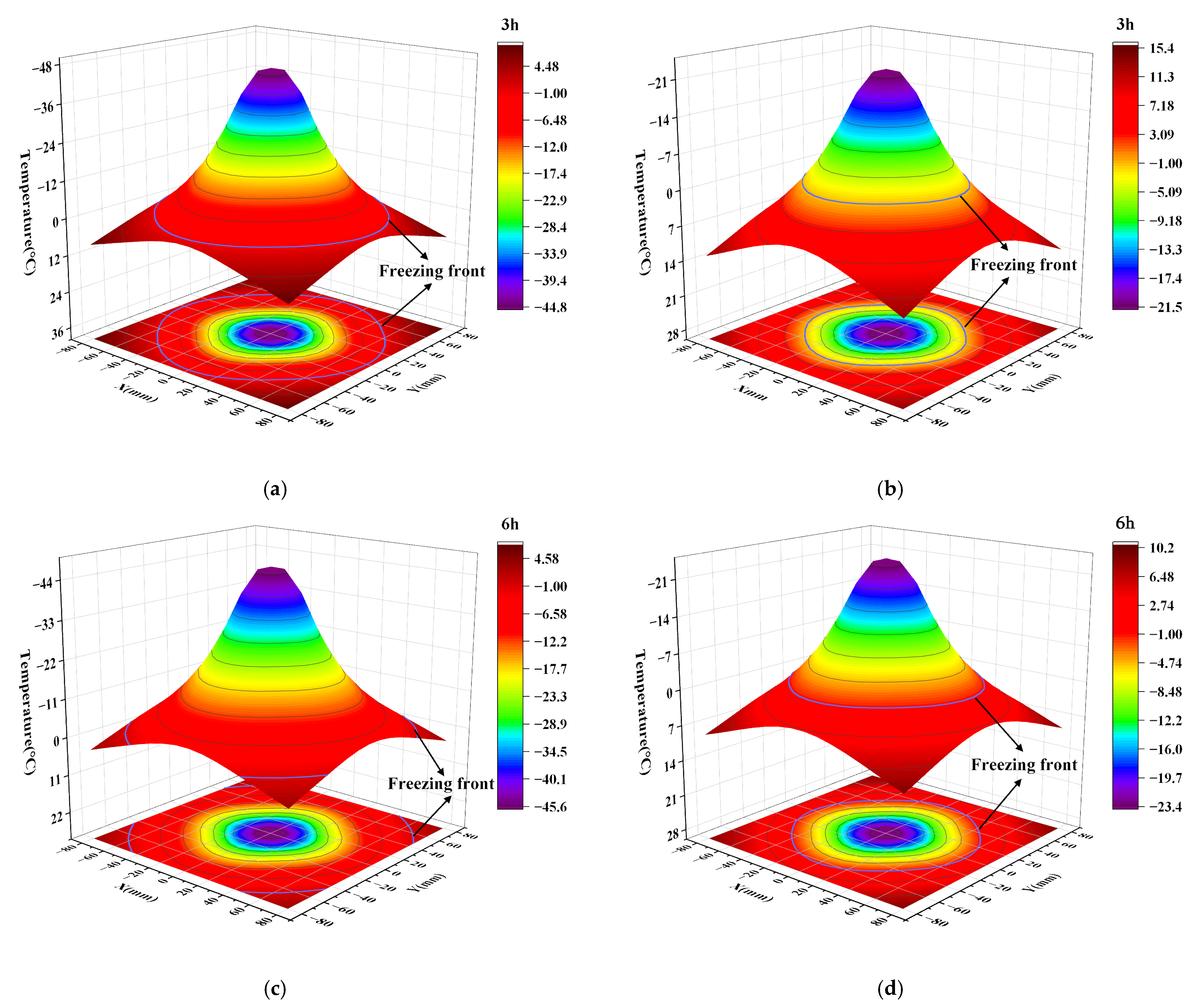
3.2. Temperature Field Analysis
3.2.1. Temperature Change Rule of Measuring Points
3.2.2. Spatial Temperature Field Distribution Characteristics
4. Formula Derivation and Verification Analysis of Transcritical CO2 Single-Pipe Freezing Temperature Field
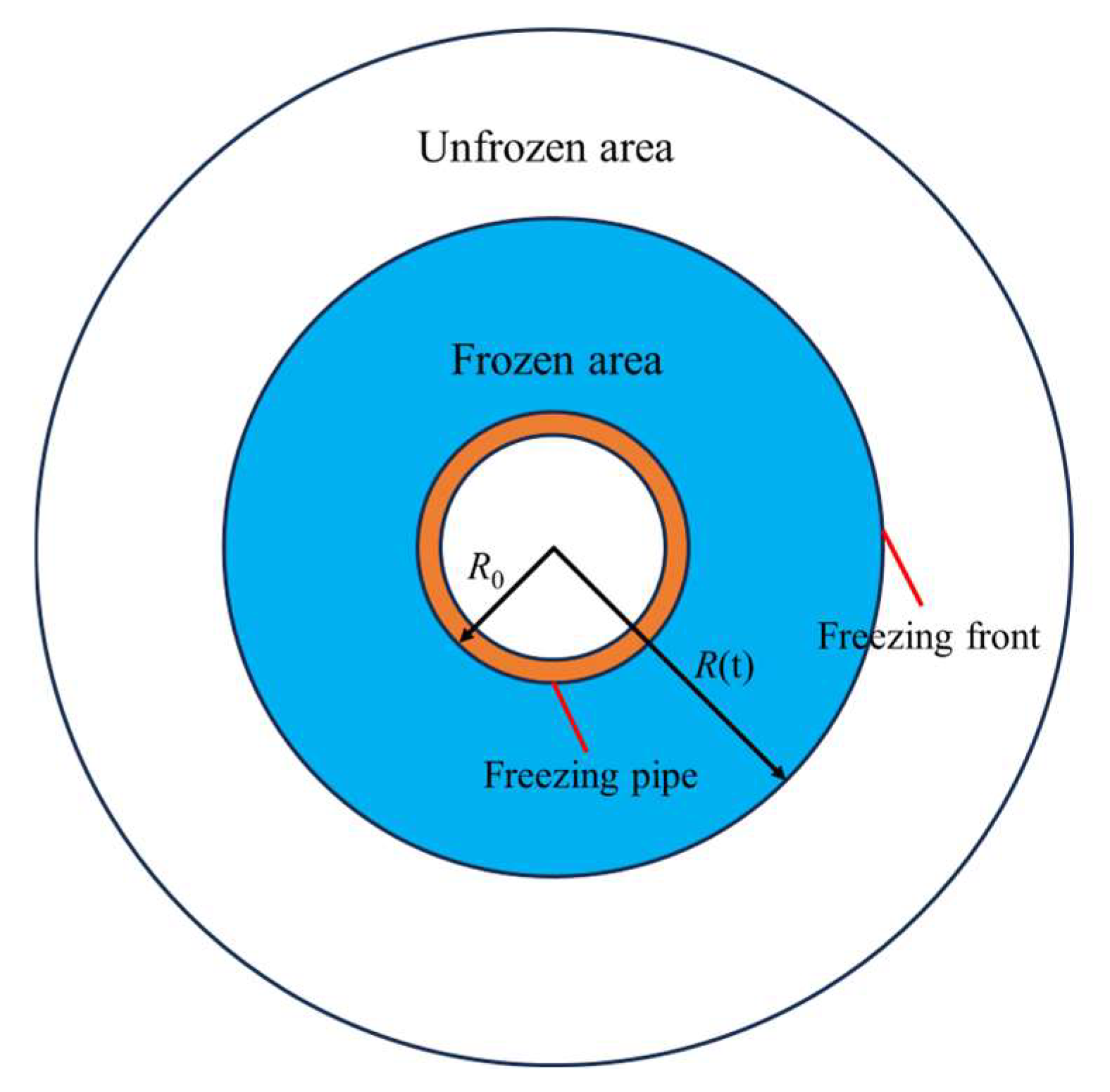
4.1. Basic Assumption
- (1)
- The frozen soil is a homogeneous material, and its thermodynamic parameters remain constant during the freezing process.
- (2)
- The plane heat conduction problem is simplified to a single pipe axisymmetric problem.
- (3)
- The temperature of the freezing pipe wall remains unchanged during freezing.
- (4)
- The freezing front spreads evenly around to form obvious frozen and unfrozen areas, as shown in Figure 9, where R0 is the radius of the freezing pipe and R(t) is the radius of the freezing front.
4.2. Derivation of Transient Temperature Field Formula of Transcritical CO2 Single-Pipe Freezing
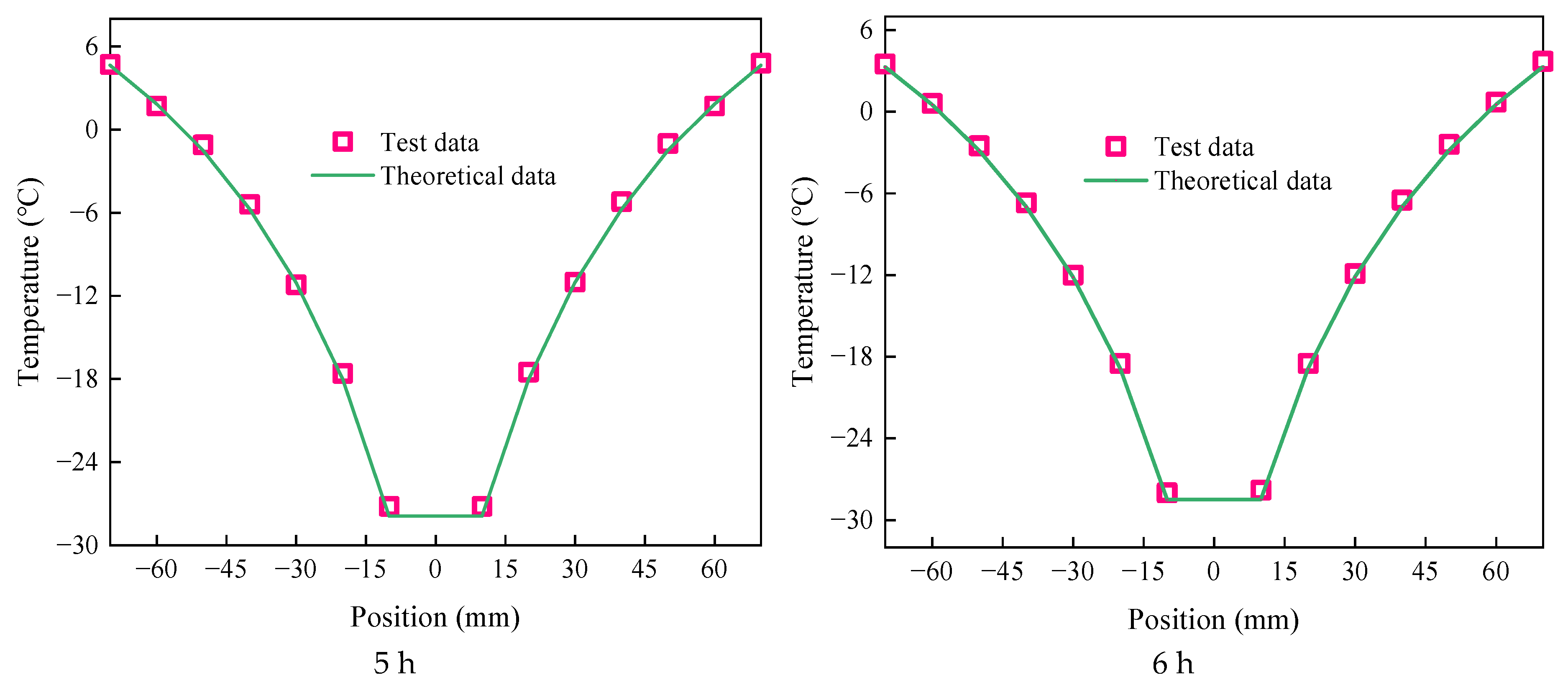
4.3. Comparative Analysis of Theoretical Formula Calculation and Experimental Results
5. Theoretical Formula Calculation and Analysis
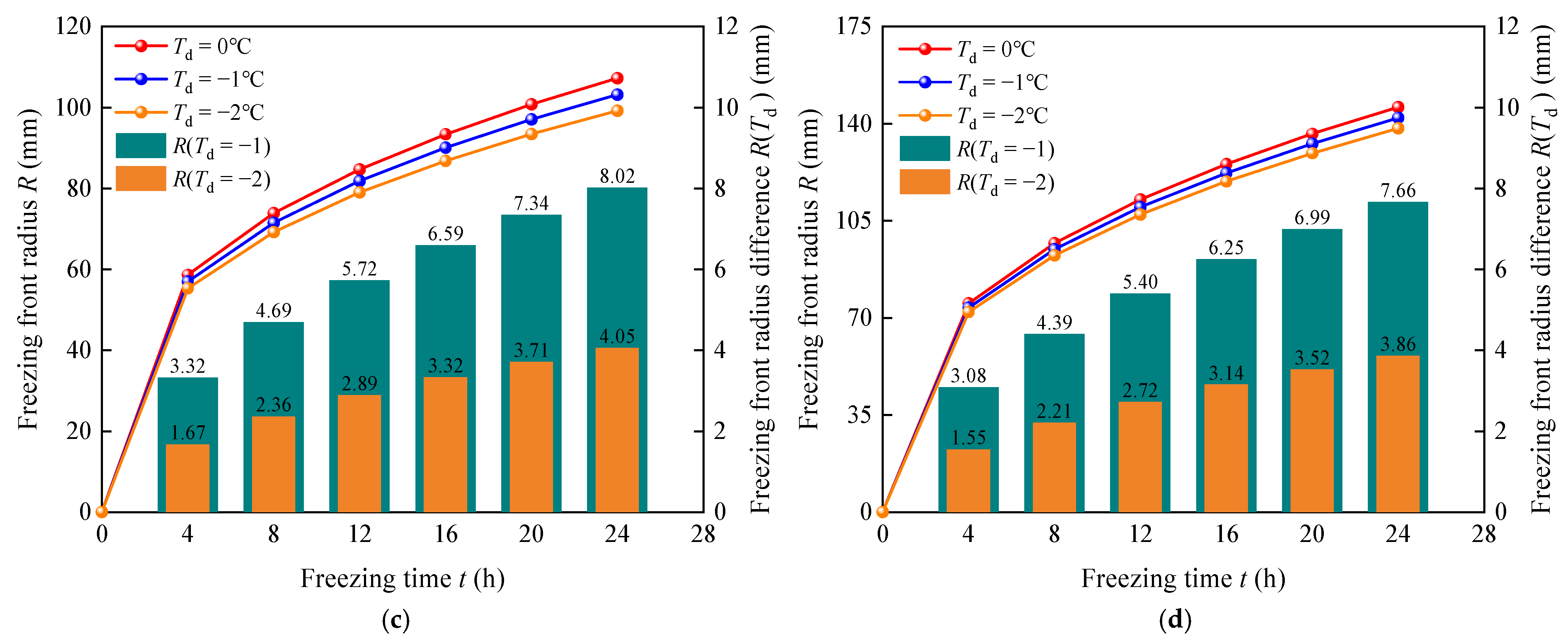
5.1. Effect of Freezing Temperature Td on Freezing Front
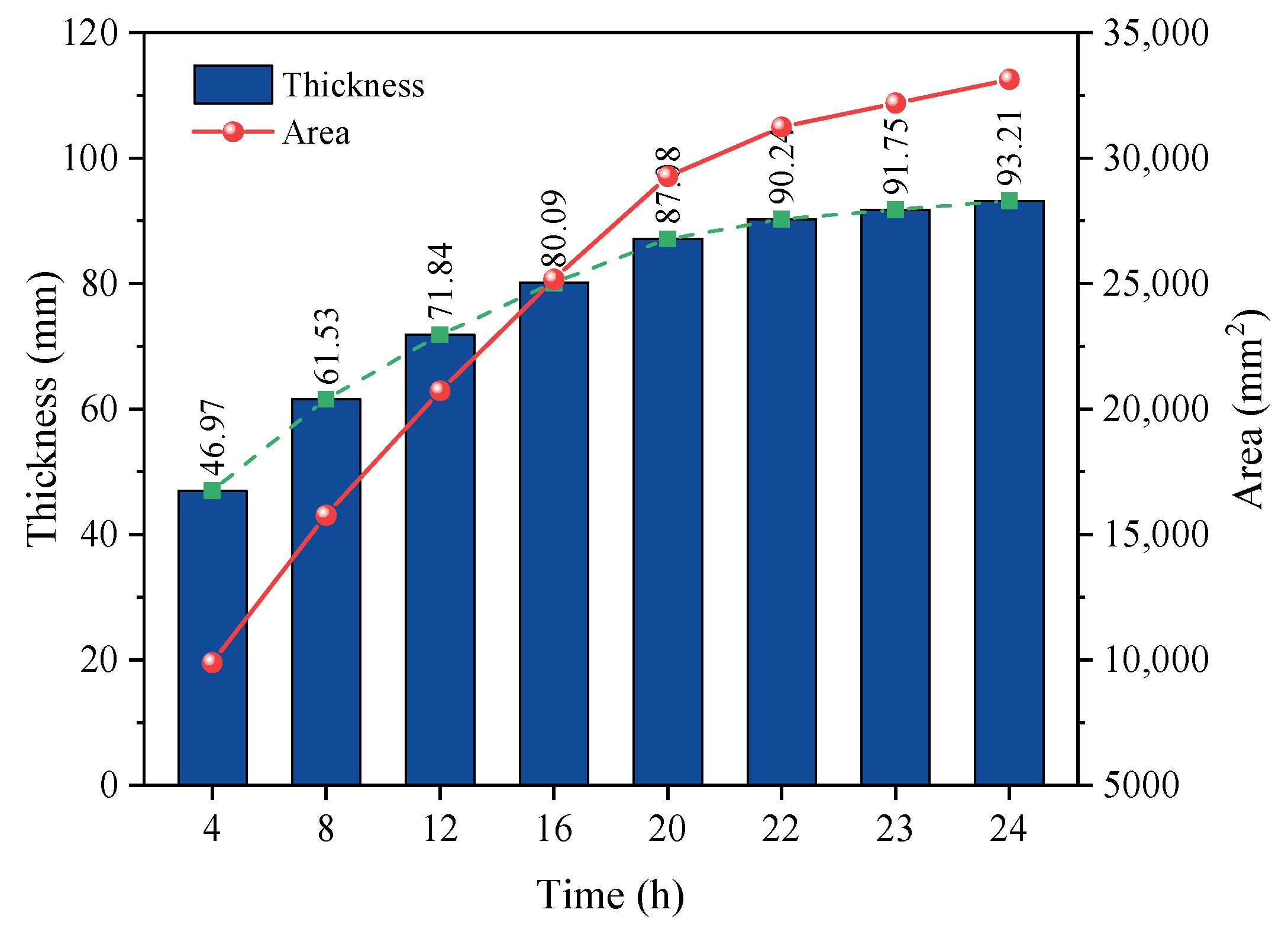
5.2. Development Law of Transcritical CO2 Frozen Wall
6. Conclusions
- (1)
- Based on the model tests, the differences in freezing efficiency between transcritical CO2 and alcohol were compared and analyzed. The test results showed that the temperature range of the measuring point can reach −28 °C–3.5 °C by using transcritical CO2 freezing for 6 h. The temperature span of the alcohol measuring point was only maintained at −12.6 °C~8.8 °C. The comparison shows that the temperature field of transcritical CO2 was significantly lower than that of alcohol, with the maximum temperature difference reaching 15.4 °C. In addition, the freezing front radius of the transcritical CO2 freezing test reached 60 mm, while it took 24 h to reach the same radius as alcohol freezing.
- (2)
- An error analysis comparing the test data with theoretical calculations of transcritical CO2 was conducted, verifying the accuracy of the theoretical model. The results demonstrate a strong agreement between the two temperature field distributions, with only a few measurement points exhibiting a temperature deviation of 1.6 °C. As freezing time increased, the difference showed a decreasing trend.
- (3)
- The influence of freezing temperature Td on the freezing front was explored based on the theoretical formula derived in this study. The results showed that the higher the freezing temperature Td under the same freezing conditions, the larger the corresponding radius of the freezing front, and that this effect was significant with freezing time. After 24 h of transcritical CO2 freezing, the calculated difference in the freezing front radius R(Td = −2) reached 8.02 mm when the freezing temperature Td was −2 °C and 0 °C. In addition, a comparison of the data for different refrigerants showed that the difference in the freezing front radius caused by the freezing temperature Td was within the range of 1.5–8.1 mm, and the change in refrigerant type did not lead to a significant difference.
- (4)
- The formation law of the frozen wall during the freezing process of transcritical CO2 was calculated and analyzed by theoretical formula. It was found that the thickness of the frozen wall rapidly increased during the initial 12 h of freezing. During 12–22 h, the growth rate gradually decreased. Within 22–24 h, the thickness of the frozen wall gradually stabilized. After 24 h of freezing, the frozen wall area finally reached 33,134.23 mm2.
- (5)
- Based on the single-pipe transcritical CO2 freezing test, this study initially explored the freezing theory, but some limitations were encountered; the influence of seepage conditions on the freezing process was not considered in the test, and homogeneous round sand was used to simulate the formation, whereas the soil in the actual project mostly showed heterogeneity, cracks, or layered structures, which led to deviations between the uniformity of the freezing front and the distribution of the temperature field. The multi-pipe interaction, long-term stability of the frozen wall, and economy of the refrigerant require further study. In the future, it will be necessary to improve the theoretical system through multi-physical field coupling models and field tests and focus on breaking through the multi-refrigerant joint freezing technology, building an efficient freezing technology system that adapts to complex geological conditions, and promoting engineering applications.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xiang, L.; Wang, F.; Jin, B.; Ouyang, O. Study on temperature field distribution of connecting passage construction in red sandstone strata by freezing method. J. Civ. Eng. 2020, 53, 306–311. [Google Scholar]
- Alzoubi, M.A.; Xu, M.; Hassani, F.P.; Poncet, S.; Sasmito, A.P. Artificial ground freezing: A review of thermal and hydraulic aspects. Tunn. Undergr. Space Technol. 2020, 104, 103534. [Google Scholar] [CrossRef]
- Gao, G.; Guo, W.; Zhu, X. Degree of artificial freezing analyses under high seepage condition. Int. J. Heat Mass Transf. 2025, 236, 126352. [Google Scholar] [CrossRef]
- Wu, W.; Yan, Q.; Zhang, C.; Yang, K.; Xu, Y. A novel method to study the energy conversion and utilization in artificial ground freezing. Energy 2021, 234, 121066. [Google Scholar] [CrossRef]
- Yang, Y.; Lei, D.; Chen, Y.; Cai, C.; Hou, S. Coupled thermal-hydro-mechanical model of deep artificial freezing clay. Cold Reg. Sci. Technol. 2022, 198, 103534. [Google Scholar] [CrossRef]
- Li, F.; Fang, L.; Wang, L.; Ding, H. Research on frost heave deformation control of close tunnel on operating station. J. Civ. Eng. 2020, 53, 292–299. [Google Scholar]
- Hou, S.; Yang, Y.; Chen, Y.; Lei, D.; Cai, C. Analysis of Coupled Seepage and Temperature Fields of Fractured Porous Rock Mass under Brine–Liquid Nitrogen Freezing. J. Mar. Sci. Eng. 2022, 10, 787. [Google Scholar] [CrossRef]
- Li, B.; Li, L.; Huang, L.; Lv, X. The Temperature field evolution and water migration law of coal under low-temperature freezing conditions. Int. J. Environ. Res. Public Health 2021, 18, 13188. [Google Scholar] [CrossRef]
- Nikolaev, P.; Jivkov, A.P.; Margetts, L.; Sedighi, M. Modelling artificial ground freezing subjected to high velocity seepage. Int. J. Heat Mass Transf. 2024, 221, 125084. [Google Scholar] [CrossRef]
- Alzoubi, M.A.; Madiseh, A.; Hassani, F.P.; Sasmito, A.P. Heat transfer analysis in artificial ground freezing under high seepage: Validation and heatlines visualization. Int. J. Therm. Sci. 2019, 139, 232–245. [Google Scholar] [CrossRef]
- Liu, B.; Han, Q.; Han, Y.; Li, T.; Ma, S.; Zhang, B. An optimized method for analysis of existing tunnels undercrossed by new tunnels in rich-water strata. Transp. Geotech. 2024, 49, 101436. [Google Scholar] [CrossRef]
- Kang, Y.; Hou, C.; Li, K.; Liu, B.; Sang, H. Evolution of temperature field and frozen wall in sandy cobble stratum using LN2 freezing method. Appl. Therm. Eng. 2021, 185, 116334. [Google Scholar] [CrossRef]
- Yi, W.B.; Choi, K.H.; Yoon, J.I.; Son, C.H.; Ha, S.J.; Jeon, M.J. Exergy characteristics of R404A indirect refrigeration system using CO2 as a secondary refrigerant. Heat Mass Transf. 2019, 55, 1133–1142. [Google Scholar] [CrossRef]
- Wang, J.; Evans, M.; Belusko, M.; Zhao, C.; Liu, M.; Bruno, F. Subcooling effect on the optimal performance for a transcritical CO2 heat pump with cold thermal energy storage. Heat Mass Transf. 2022, 59, 1257–1275. [Google Scholar] [CrossRef]
- Zhou, X.; Ren, X.; Ye, X.; Tao, L.; Zeng, Y.; Liu, X. Temperature field and anti-freezing system for cold-region tunnels through rock with high geotemperatures. Tunn. Undergr. Space Technol. 2021, 111, 103843. [Google Scholar] [CrossRef]
- Zhang, S.; Zhou, X.-M.; Ma, W.; Liu, Y.; Lu, C. Longitudinal temperature acquisition system of ground freezing and its application in solving temperature field. Cold Reg. Sci. Technol. 2023, 213, 103911. [Google Scholar] [CrossRef]
- Yan, Q.; Wu, W.; Zhong, H.; Zhao, Z.; Zhang, C. Temporal and spatial variation of temperature and displacement fields throughout cross-passage artificial ground freezing. Cold Reg. Sci. Technol. 2023, 209, 103817. [Google Scholar] [CrossRef]
- Chen, W.; Wan, W.; He, H.; Liao, D.; Liu, J. Temperature field distribution and numerical simulation of improved freezing scheme for shafts in loose and soft stratum. Rock Mech. Rock Eng. 2024, 57, 2695–2725. [Google Scholar] [CrossRef]
- Zhang, S.; Yue, Z.; Sun, T.; Zhang, J.; Huang, B. Analytical determination of the soil temperature distribution and freezing front position for linear arrangement of freezing pipes using the undetermined coefficient method. Cold Reg. Sci. Technol. 2021, 185, 103253. [Google Scholar] [CrossRef]
- Zhou, J.; Zhao, W.; Tang, Y. Practical prediction method on frost heave of soft clay in artificial ground freezing with field experiment. Tunn. Undergr. Space Technol. 2021, 107, 103647. [Google Scholar] [CrossRef]
- Rong, C.; Sun, S.; Cheng, H.; Duan, Y.; Yang, F. Experimental Study on Temporal and Spatial Evolutions of Temperature Field of Double-Pipe Freezing in Saline Stratum with a High Velocity. Energies 2022, 15, 1308. [Google Scholar] [CrossRef]
- Sun, L.; Shi, P.; Lang, R.; Shang, A. Laboratory model test on artificial freezing behavior under seepage. Chin. J. Rock Mech. Eng. 2024, 43, 3530–3542. [Google Scholar]
- Wang, B.; Rong, C.; Cheng, H. Analytical solution of steady-state temperature field of asymmetric frozen curtain induced by directional seepage. Eng. Sci. Technol. 2022, 54, 76–87. [Google Scholar]
- Zhang, S.; Zhou, X.; Zhang, J.; Sun, Q.; Liu, Y.; Ma, W.; Xu, H. Method for solving temperature field of single row pipe based on temperature Rise Formula of freezer. J. Railw. Sci. Eng. 2024, 21, 1102–1113. [Google Scholar]
- Hong, Z.; Fu, S.; Hu, X.; Wang, B. Analytical solution of artificial freezing temperature field under adiabatic boundary. J. Tongji Univ. (Nat. Sci.) 2024, 52, 749–758. (In Chinese) [Google Scholar]
- Cai, H.; Xu, L.; Yang, Y.; Li, L. Analytical solution and numerical simulation of the liquid nitrogen freezing-temperature field of a single pipe. AIP Adv. 2018, 8, 055119. [Google Scholar] [CrossRef]
- Long, W.; Rong, C.; Duan, Y.; Guo, K. Numerical calculation of temperature field by pipe curtain freezing method in Gongbei tunnel. Coal Geol. Explor. 2020, 48, 160–168. [Google Scholar]
- Cui, Z.D.; Zhang, L.J.; Xu, C. Numerical simulation of freezing temperature field and frost heave deformation for deep foundation pit by AGF. Cold Reg. Sci. Technol. 2023, 213, 103908. [Google Scholar] [CrossRef]
- Yi, Z.; Meng, Q.; Lin, C. Numerical study of supercritical CO2 heat transfer characteristics in twisted oval tubes. Heat Mass Transf. 2025, 61, 8. [Google Scholar] [CrossRef]
- Lin, W.; Du, Z.; Gu, A. Analysis on heat transfer correlations of supercritical CO2 cooled in horizontal circular tubes. Heat Mass Transf. 2012, 48, 705–711. [Google Scholar] [CrossRef]
- Xu, J.; Zhai, C.; Sang, S.; Sun, Y.; Cong, Y.; Zheng, Y.; Tang, W. Evolution of pore characteristics of coal fractured by liquid CO2 cycle based on low field nuclear magnetic resonance technology. J. China Coal Soc. 2021, 46, 3578–3589. [Google Scholar]
- Zhou, X.; Zhou, L.; Jiang, Y.; Bai, G.; Liu, T.; Wang, X. Experimental study on the influence of multiple factors on liquid CO2 freeze-thaw cracking coal. China Saf. Sci. J. 2023, 33, 58–67. [Google Scholar]
- Hong, R.; Yuan, B.; Du, W. Analysis of heat transfer deterioration mechanism of supercritical carbon dioxide in vertical riser pipe. Acta Chemologica Sin. 2023, 74, 339–3319. [Google Scholar]
- Wang, B.; Liang, X.; Zhang, Z.; Cai, H.; Rong, C. Elastoplastic stress analysis of multi-refrigerant heterogeneous artificial frozen wall. Coal Geol. Explor. 2024, 52, 106–120. [Google Scholar]
- Wu, W.; Ding, Z.; Yan, Q.; Zhao, Z. Modification on constant temperature boundary considering conjugate heat transfer of brine turbulent flow in brine artificial ground freezing method. Int. Commun. Heat Mass Transf. 2025, 160, 108387. [Google Scholar] [CrossRef]
- Bai, Y.; Sun, Y.; Liu, W.; Sun, P.; Xiao, S. Model test study on the formation of basin-shaped freezing range in sandy gravel stratum under seepage conditions. Therm. Sci. Eng. Prog. 2024, 51, 102601. [Google Scholar] [CrossRef]
- GB/T50123-2019; Standard for Geotechnical Test Methods. Water Conservancy and Hydropower Engineering Construction Quality Monitoring Station: Xinyang, China, 2021.







| Status | Argument | Numerical Value |
|---|---|---|
| Unfrozen soil | Particle size (mm) | 1 ± 0.15 |
| Dry density (kg/m3) | 1612 | |
| Saturation density (kg/m3) | 2480 | |
| Water content | 28% | |
| Thermal diffusivity (m2/d) | 0.1028 | |
| Thermal conductivity (kcal/m·d·°C) | 35.93 | |
| Frozen soil | Latent heat of icing | 25,663.04 |
| Freezing temperature (°C) | −1 | |
| Unfrozen water content | 8% | |
| Thermal diffusivity (m2/d) | 0.0651 | |
| Thermal conductivity (kcal/m·d·°C) | 43.36 |
| R0 | Td | T0 | Tc |
|---|---|---|---|
| 10 mm | −1 °C | 28 °C | −45 °C |
| Time (h) | Thickness (mm) | Area (mm2) |
|---|---|---|
| 4 | 46.97 | 9877.12 |
| 8 | 61.53 | 15,751.94 |
| 12 | 71.84 | 20,717.05 |
| 16 | 80.09 | 25,170.89 |
| 20 | 87.08 | 29,279.01 |
| 22 | 90.24 | 31,236.90 |
| 23 | 91.75 | 32,194.62 |
| 24 | 93.21 | 33,134.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Wang, B.; Liang, X.; Rong, C.; Ye, Z. Study on the Evolution Law and Theoretical Solution of a Freezing Temperature Field in Transcritical CO2 Ultra-Low Temperature Formation. Processes 2025, 13, 1154. https://doi.org/10.3390/pr13041154
Zhang Z, Wang B, Liang X, Rong C, Ye Z. Study on the Evolution Law and Theoretical Solution of a Freezing Temperature Field in Transcritical CO2 Ultra-Low Temperature Formation. Processes. 2025; 13(4):1154. https://doi.org/10.3390/pr13041154
Chicago/Turabian StyleZhang, Zihao, Bin Wang, Xiuling Liang, Chuanxin Rong, and Zhongbao Ye. 2025. "Study on the Evolution Law and Theoretical Solution of a Freezing Temperature Field in Transcritical CO2 Ultra-Low Temperature Formation" Processes 13, no. 4: 1154. https://doi.org/10.3390/pr13041154
APA StyleZhang, Z., Wang, B., Liang, X., Rong, C., & Ye, Z. (2025). Study on the Evolution Law and Theoretical Solution of a Freezing Temperature Field in Transcritical CO2 Ultra-Low Temperature Formation. Processes, 13(4), 1154. https://doi.org/10.3390/pr13041154






