Synergistic Removal of Hazardous Dyes Using a Clay/Carbon Composite Derived from Spent Bleaching Earth: Optimization Using Response Surface Methodology
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of the Adsorbent
2.3. Adsorption Experiments
2.4. Analysis Methods
2.5. Experimental Design and Optimization Using RSM
3. Results and Discussion
3.1. Characterization
3.1.1. Morphology of SBE and SBE/C (500 °C)
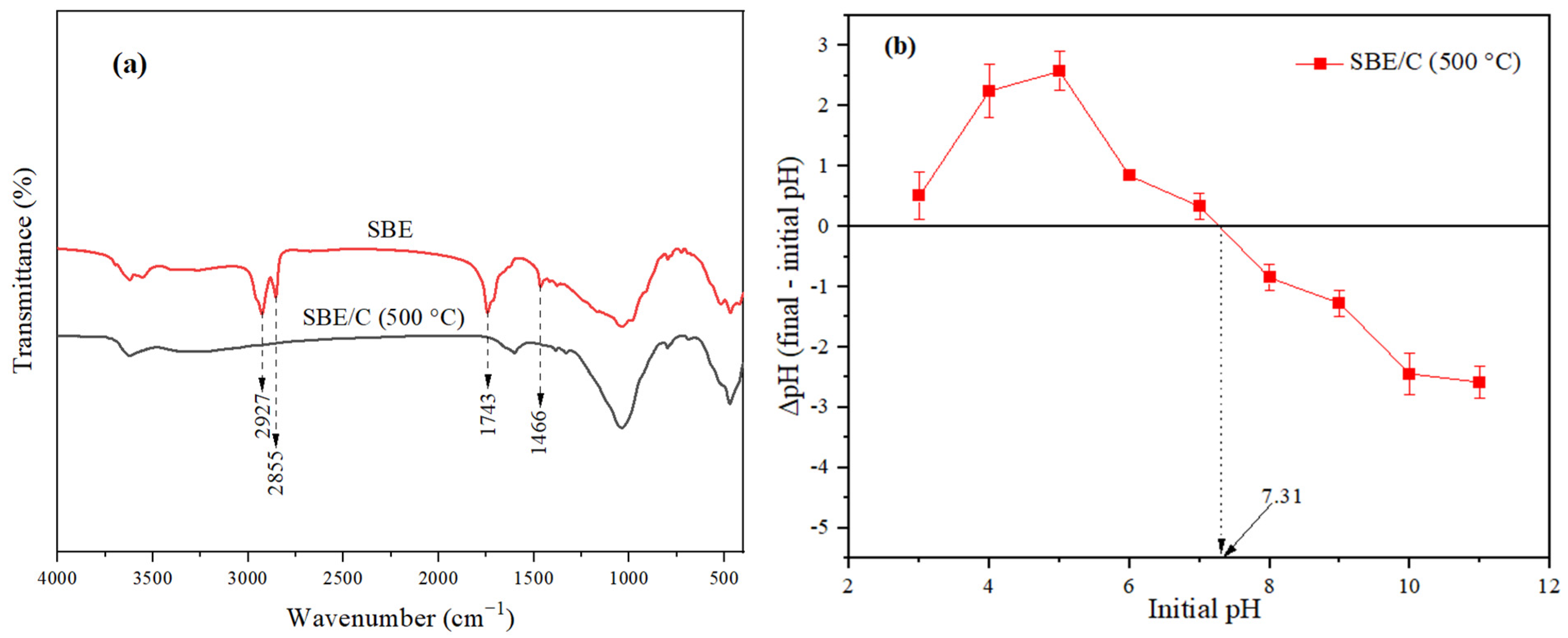
3.1.2. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
3.1.3. pHpzc of SBE/C (500 °C)
3.2. Response Surface Methodology
3.3. ANOVA Analysis
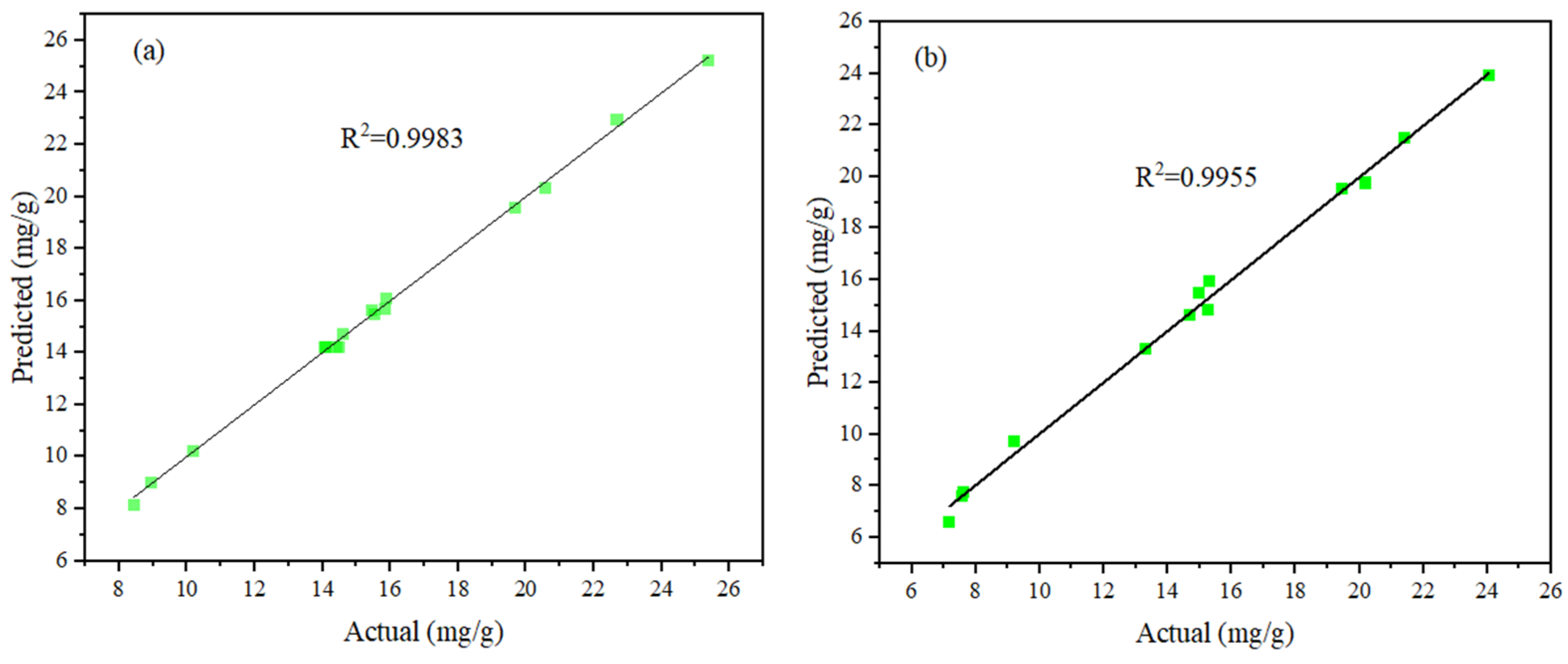
3.4. Statistical Analysis
3.5. Interaction Effects of Factors
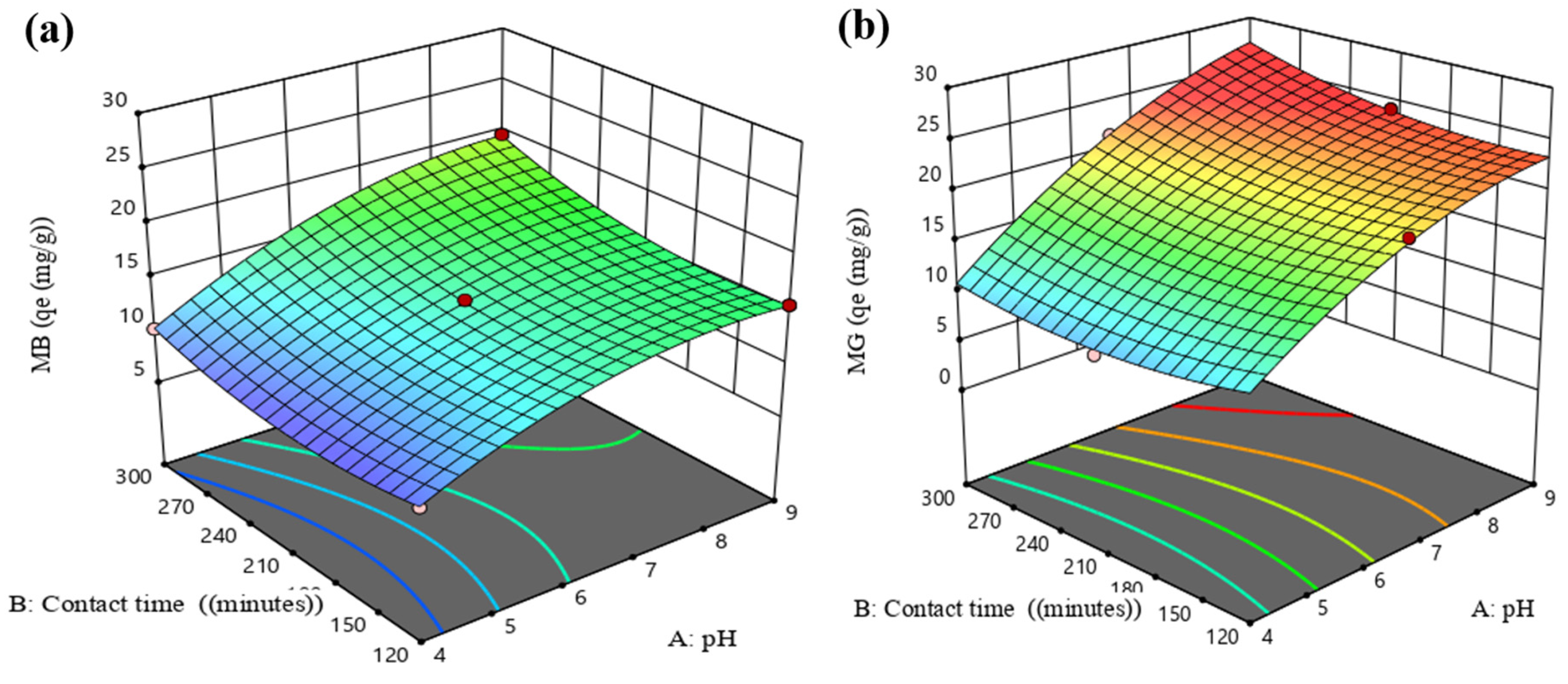
3.5.1. Interaction Effect of MB and MG AB (pH and Contact Time (min))
3.5.2. Interaction Effects of MB and MG BC (Contact Time (min) and Adsorbent Dosage (g/L))
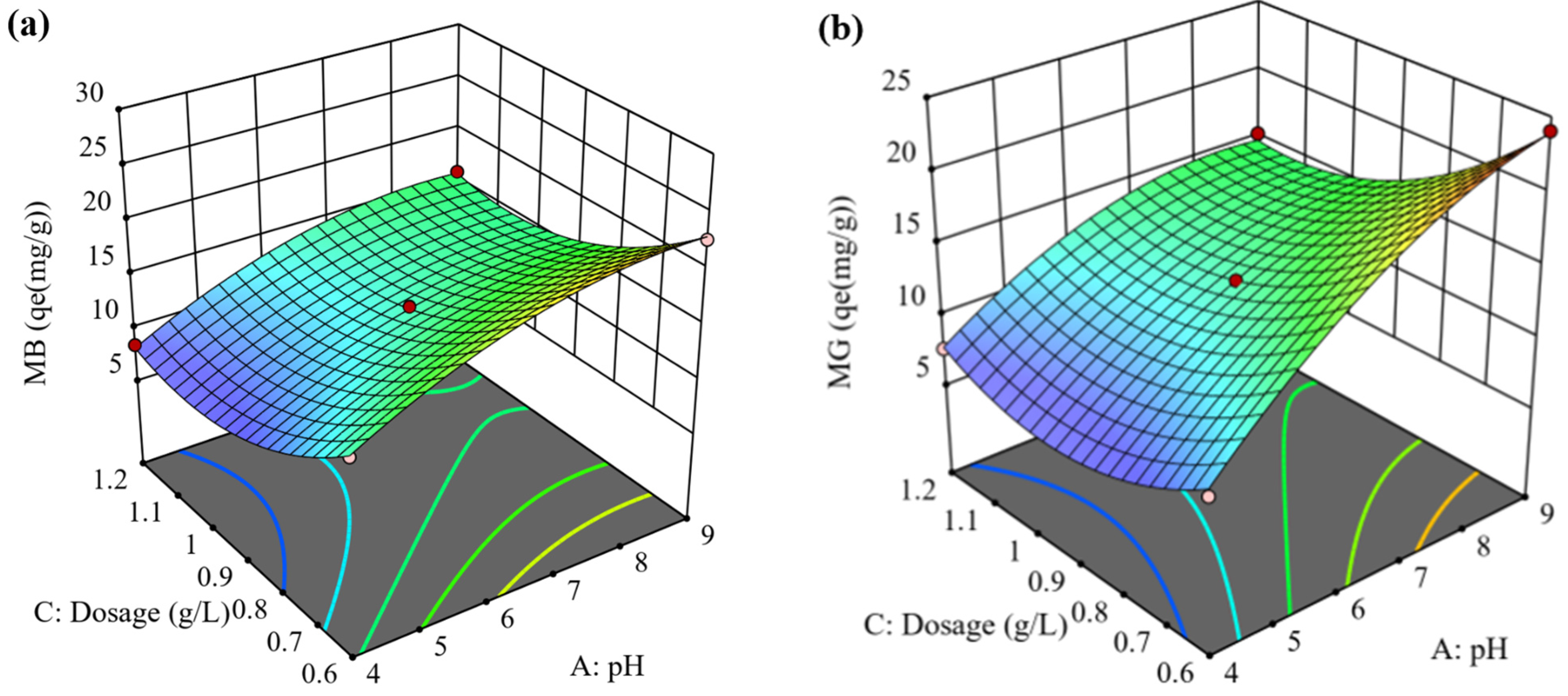
3.5.3. Interaction Effects of MB and MG AC (pH and Adsorbent Dosage)
3.6. Process Optimization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ali, S.; Ijaz, H.; Ahmad, M.U.; Rukhma; Ullah, N.; Sarwar, A.; Khan, M.J.; Aziz, T.; Shami, A.; Al-Asmari, F. Photocatalytic Removal of Textile Wastewater-Originated Methylene Blue and Malachite Green Dyes Using Spent Black Tea Extract-Coated Silver Nanoparticles. Sci. Rep. 2025, 15, 1851. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.; Ahmad, I.; Abd El-Gawad, H.H.; Alshahrani, W.A.; Alqarni, N.D.; El-Bahy, Z.M.; Ikram, S. Appraisal of the Adsorption Potential of Novel Modified Gellan Gum Nanocomposite for the Confiscation of Methylene Blue and Malachite Green. Int. J. Biol. Macromol. 2024, 259, 129221. [Google Scholar] [CrossRef]
- Sharma, J.; Sharma, S.; Soni, V. Toxicity of Malachite Green on Plants and Its Phytoremediation: A Review. Reg. Stud. Mar. Sci. 2023, 62, 102911. [Google Scholar] [CrossRef]
- Liu, X.; Han, B.; He, P.; Wang, Q.; Chen, Z. Modeling Competitive Biosorption for Methylene Blue Removal on Rape Straw Powders Using Response Surface Methodology in a Ternary Dye Aqueous Solution. Int. J. Phytoremediation 2024, 26, 1453–1464. [Google Scholar] [CrossRef] [PubMed]
- Alharby, N.F.; Almutairi, R.S.; Mohamed, N.A. Adsorption Behavior of Methylene Blue Dye by Novel CrossLinked O-CM-Chitosan Hydrogel in Aqueous Solution: Kinetics, Isotherm and Thermodynamics. Polymers 2021, 13, 3659. [Google Scholar] [CrossRef]
- Moradihamedani, P. Recent Advances in Dye Removal from Wastewater by Membrane Technology: A Review. Polym. Bull. 2022, 79, 2603–2631. [Google Scholar] [CrossRef]
- Elgarahy, A.M.; Elwakeel, K.Z.; Mohammad, S.H.; Elshoubaky, G.A. A Critical Review of Biosorption of Dyes, Heavy Metals and Metalloids from Wastewater as an Efficient and Green Process. Clean. Eng. Technol. 2021, 4, 100209. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Y.; Shi, Y.; Wan, D.; Chen, J.; Xiao, S. Adsorption of Toxic Dye Eosin Y from Aqueous Solution by Clay/Carbon Composite Derived from Spent Bleaching Earth. Water Environ. Res. 2021, 93, 159–169. [Google Scholar] [CrossRef]
- Muhamad Ng, S.N.; Idrus, S.; Ahsan, A.; Tuan Mohd Marzuki, T.N.; Mahat, S.B. Treatment of Wastewater from a Food and Beverage Industry Using Conventional Wastewater Treatment Integrated with Membrane Bioreactor System: A Pilot-Scale Case Study. Membranes 2021, 11, 456. [Google Scholar] [CrossRef]
- Ramutshatsha-Makhwedzha, D.; Munyengabe, A.; Mavhungu, M.L.; Mbaya, R.; Baloyi, J. Breakthrough Studies for the Sorption of Methylene Blue Dye from Wastewater Samples Using Activated Carbon Derived from Waste Banana Peels. Biomass Convers. Biorefinery 2024, 14, 21757–21769. [Google Scholar] [CrossRef]
- Guo, X.; Wu, Z.; Lu, Z.; Wang, Z.; Li, S.; Madhau, F.; Guo, T.; Huo, R. Preparation and Characterization of Chitosan-Modified Bentonite Hydrogels and Application for Tetracycline Adsorption from Aqueous Solution. Gels 2024, 10, 503. [Google Scholar] [CrossRef] [PubMed]
- Futalan, C.M.; Kan, C.C.; Dalida, M.L.; Hsien, K.J.; Pascua, C.; Wan, M.W. Comparative and Competitive Adsorption of Copper, Lead, and Nickel Using Chitosan Immobilized on Bentonite. Carbohydr. Polym. 2011, 83, 528–536. [Google Scholar] [CrossRef]
- Hasanpour, M.; Hatami, M. Application of Three Dimensional Porous Aerogels as Adsorbent for Removal of Heavy Metal Ions from Water/Wastewater: A Review Study. Adv. Colloid Interface Sci. 2020, 284, 102247. [Google Scholar] [CrossRef]
- Ramutshatsha-Makhwedzha, D.; Mavhungu, A.; Moropeng, M.L.; Mbaya, R. Activated Carbon Derived from Waste Orange and Lemon Peels for the Adsorption of Methyl Orange and Methylene Blue Dyes from Wastewater. Heliyon 2022, 8, e09930. [Google Scholar] [CrossRef] [PubMed]
- Salah Omer, A.; A.El Naeem, G.; Abd-Elhamid, A.I.; Farahat, O.O.M.; El-Bardan, A.A.; Soliman, H.M.A.; Nayl, A.A. Adsorption of Crystal Violet and Methylene Blue Dyes Using a Cellulose-Based Adsorbent from Sugercane Bagasse: Characterization, Kinetic and Isotherm Studies. J. Mater. Res. Technol. 2022, 19, 3241–3254. [Google Scholar] [CrossRef]
- Adeyemo, A.A.; Adeoye, I.O.; Bello, O.S. Adsorption of Dyes Using Different Types of Clay: A Review. Appl. Water Sci. 2017, 7, 543–568. [Google Scholar] [CrossRef]
- Amalina, F.; Razak, A.S.A.; Krishnan, S.; Zularisam, A.W.; Nasrullah, M. Dyes Removal from Textile Wastewater by Agricultural Waste as an Absorbent—A Review. Clean. Waste Syst. 2022, 3, 100051. [Google Scholar] [CrossRef]
- Abdelbasir, S.M.; Shehab, A.I.; Khalek, M.A.A. Spent Bleaching Earth; Recycling and Utilization Techniques: A Review. Resour. Conserv. Recycl. Adv. 2023, 17, 200124. [Google Scholar] [CrossRef]
- Tsai, W.; Chen, H.; Hsieh, M.; Sun, H.; Chien, S. Regeneration of Spent Bleaching Earth by Pyrolysis in a Rotary Furnace. J. Anal. Appl. Pyrolysis 2002, 63, 157–170. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Wu, L.; Shi, Y.; He, Q.; Chen, J.; Wan, D. Magnetic Spent Bleaching Earth Carbon (Mag-SBE@C) for Efficient Adsorption of Tetracycline Hydrochloride: Response Surface Methodology for Optimization and Mechanism of Action. Sci. Total Environ. 2020, 722, 137817. [Google Scholar] [CrossRef]
- Wan, D.; Wu, L.; Liu, Y.; Chen, J.; Zhao, H.; Xiao, S. Enhanced Adsorption of Aqueous Tetracycline Hydrochloride on Renewable Porous Clay-Carbon Adsorbent Derived from Spent Bleaching Earth via Pyrolysis. Langmuir 2019, 35, 3925–3936. [Google Scholar] [CrossRef]
- Rasdei, S.S.; Yatim, N.I.; Ali, N.; Kasan, N.A. Optimization of the Direct Synthesis of Hydroxyapatite from Aquaculture Wastewater Using Response Surface Methodology. AQUA—Water Infrastruct. Ecosyst. Soc. 2024, 73, 1789–1800. [Google Scholar] [CrossRef]
- Mitadar, S.P.; Adi, V.K. Adsorption and Statistical Analysis of Textile Effluent Contaminated with Congo Red On Orange Peel. Int. J. Innov. Technol. Explor. Eng. 2019, 8, 2607–2615. [Google Scholar] [CrossRef]
- Jawad, A.H.; Mohd Firdaus Hum, N.N.; Abdulhameed, A.S.; Mohd Ishak, M.A. Mesoporous Activated Carbon from Grass Waste via H3PO4-Activation for Methylene Blue Dye Removal: Modelling, Optimisation, and Mechanism Study. Int. J. Environ. Anal. Chem. 2022, 102, 6061–6077. [Google Scholar] [CrossRef]
- Box, G.E.P.; Behnken, D.W. Some New Three Level Designs for the Study of Quantitative Variables. Technometrics 1960, 2, 455–475. [Google Scholar] [CrossRef]
- Peng, L.; Gao, X.; Wang, L.; Zhu, A.; Cai, X.; Li, P.; Li, W. Design of Experiment Techniques for the Optimization of Chromatographic Analysis Conditions: A Review. Electrophoresis 2022, 43, 1882–1898. [Google Scholar] [CrossRef] [PubMed]
- Madhau, F.; Wu, Z.; Shi, Y.; Wan, D.; Tichapondwa, S.; Wang, Y.; Wang, J.; Wan, H. Adsorption of Methylene Blue onto Clay/Carbon Composite: Kinetics and Isotherms Study. Water Pract. Technol. 2024, 19, 4267–4281. [Google Scholar] [CrossRef]
- Tripathi, P.; Srivastava, V.C.; Kumar, A. Optimization of an Azo Dye Batch Adsorption Parameters Using Box-Behnken Design. Desalination 2009, 249, 1273–1279. [Google Scholar] [CrossRef]
- Ahmed, F.S.; AbdulRazak, A.A.; Alsaffar, M.A. Modelling and Optimization of Methylene Blue Adsorption from Wastewater Utilizing Magnetic Marble Dust Adsorbent: A Response Surface Methodology Approach. Mater. Today Proc. 2022, 60, 1676–1688. [Google Scholar] [CrossRef]
- Zhao, W.; Hao, C.; Guo, Y.; Shao, W.; Tian, Y.; Zhao, P. Optimization of Adsorption Conditions Using Response Surface Methodology for Tetracycline Removal by MnFe2O4/Multi-Wall Carbon Nanotubes. Water 2023, 15, 2392. [Google Scholar] [CrossRef]
- Hock, P.E.; Faizal, A.N.M.; Sirajo, L.; Zaini, M.A.A. Insight into Kinetics, Equilibrium, and Thermodynamics of Malachite Green Adsorption onto Banana Peel Adsorbents. Biomass Convers. Biorefinery 2024, 14, 17405–17421. [Google Scholar] [CrossRef]
- Sanou, I.; Bamogo, H.; Sanou, A.; Ouedraogo, M.; Saadi, L.; Waqif, M.; Millogo, Y. Adsorption of Methylene Blue in Aqueous Medium by Activated Carbon from Peanut Shells. Chem. Africa 2024, 7, 2777–2794. [Google Scholar] [CrossRef]
- Nandoost, A.; Bahramifar, N.; Moghadamnia, A.A.; Kazemi, S. Adsorption of Malachite Green (MG) as a Cationic Dye on Amberlyst 15, an Ion-Exchange Resin. J. Environ. Public Health 2022, 2022, 4593835. [Google Scholar] [CrossRef]
- Mohammad, A.-T.; Abdulhameed, A.S.; Jawad, A.H. Box-Behnken Design to Optimize the Synthesis of New Crosslinked Chitosan-Glyoxal/TiO2 Nanocomposite: Methyl Orange Adsorption and Mechanism Studies. Int. J. Biol. Macromol. 2019, 129, 98–109. [Google Scholar] [CrossRef]
- Mittal, A. Adsorption Kinetics of Removal of a Toxic Dye, Malachite Green, from Wastewater by Using Hen Feathers. J. Hazard. Mater. 2006, 133, 196–202. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Shitu, A.; Ibrahim, A. Removal of Methylene Blue Using Low Cost Adsorbent: A Review. Res. J. Chem. Sci. 2014, 4, 91–102. [Google Scholar]
- Ibrahim, E.S.; Moustafa, H.; El-Molla, S.A.; Halim, S.A.; Ibrahim, S.M. Integrated Experimental and Theoretical Insights for Malachite Green Dye Adsorption from Wastewater Using Low Cost Adsorbent. Water Sci. Technol. 2021, 84, 3833–3858. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, M.T. Preparation and Characterization of Low-Cost Adsorbents for the Efficient Removal of Malachite Green Using Response Surface Modeling and Reusability Studies. Sci. Rep. 2023, 13, 4493. [Google Scholar] [CrossRef]
- Mana, M.; Ouali, M.S.; Lindheimer, M.; Menorval, L.C. de Removal of Lead from Aqueous Solutions with a Treated Spent Bleaching Earth. J. Hazard. Mater. 2008, 159, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Inam, M.A.; Khan, R.; Yeom, I.T.; Buller, A.S.; Akram, M.; Inam, M.W. Optimization of Antimony Removal by Coagulation-Flocculation-Sedimentation Process Using Response Surface Methodology. Processes 2021, 9, 117. [Google Scholar] [CrossRef]
- Boumya, W.; Khnifira, M.; Machrouhi, A.; Abdennouri, M.; Achak, M.; Qourzal, S.; Tounsadi, H.; Barka, N. Box–Behnken Design for Understanding of Adsorption Behaviors of Cationic and Anionic Dyes by Activated Carbon. Desalin. WATER Treat. 2021, 212, 204–211. [Google Scholar] [CrossRef]
- Bonyadi, Z.; Nasoudari, E.; Ameri, M.; Ghavami, V.; Shams, M.; Sillanpää, M. Biosorption of Malachite Green Dye over Spirulina Platensis Mass: Process Modeling, Factors Optimization, Kinetic, and Isotherm Studies. Appl. Water Sci. 2022, 12, 167. [Google Scholar] [CrossRef]
- Igder, A.; Fazlavi, A.; Allahkarami, E.; Dehghanipour, A. Optimization of Ni(II) & Co(II) Removal from Wastewater and Statistical Studies on the Results of Experimental Designs. Geosystem Eng. 2019, 22, 91–100. [Google Scholar] [CrossRef]
- Zekavat, S.R.; Raouf, F.; Talesh, S.S.A. Simultaneous Adsorption of Cu2+ and Cr (VI) Using HDTMA-Modified Zeolite: Isotherm, Kinetic, Mechanism, and Thermodynamic Studies. Water Sci. Technol. 2020, 82, 1808–1824. [Google Scholar] [CrossRef]
- Wannahari, R.; Mohamad, M.; Kamal, A.A.A.; Ai, J.Y.S. Removal of Malachite Green Dye by Peanut (Arachis Hypogaea) Shell Biochar: Optimisation Using Response Surface Methodology. Int. J. Sci. Technol. Res. 2019, 8, 2564–2569. [Google Scholar]
- Khelifi, O.; Affoune, A.M.; Nacef, M.; Chelaghmia, M.L.; Laksaci, H. Response Surface Modeling and Optimization of Ni(II) and Cu(II) Ions Competitive Adsorption Capacity by Sewage Sludge Activated Carbon. Arab. J. Sci. Eng. 2022, 47, 5797–5809. [Google Scholar] [CrossRef]
- Ba Mohammed, B.; Hsini, A.; Abdellaoui, Y.; Abou Oualid, H.; Laabd, M.; El Ouardi, M.; Ait Addi, A.; Yamni, K.; Tijani, N. Fe-ZSM-5 Zeolite for Efficient Removal of Basic Fuchsin Dye from Aqueous Solutions: Synthesis, Characterization and Adsorption Process Optimization Using BBD-RSM Modeling. J. Environ. Chem. Eng. 2020, 8, 104419. [Google Scholar] [CrossRef]
- Biradar, V.R.; Charde, M.S.; Chakole, R.D. QBD approach based development of validated analytical method for estimation of clarithromycin by RP-HPLC. J. Adv. Sci. Res. 2023, 14, 15–24. [Google Scholar] [CrossRef]
- Inam, M.A.; Khan, R.; Lee, K.H.; Babar, Z.B.; Yeom, I.T. Competitive Removal of Antimony and Humic Acid by Ferric Chloride: Optimization of Coagulation Process Using Response Surface Methodology. Water 2023, 15, 1676. [Google Scholar] [CrossRef]
- Jaafari, J.; Yaghmaeian, K. Response Surface Methodological Approach for Optimizing Heavy Metal Biosorption by the Blue-Green Alga Chroococcus Disperses. Desalin. Water Treat. 2019, 142, 225–234. [Google Scholar] [CrossRef]
- Bulut, Y.; Aydın, H. A Kinetics and Thermodynamics Study of Methylene Blue Adsorption on Wheat Shells. Desalination 2006, 194, 259–267. [Google Scholar] [CrossRef]
- Chemingui, H.; Rezma, S.; Lafi, R.; Alhalili, Z.; Missaoui, T.; Harbi, I.; Smiri, M.; Hafiane, A. Investigation of Methylene Blue Adsorption from Aqueous Solution onto ZnO Nanoparticles: Equilibrium and Box-Behnken Optimisation Design. Int. J. Environ. Anal. Chem. 2023, 103, 2716–2741. [Google Scholar] [CrossRef]
- Minale, M.; Gu, Z.; Guadie, A.; Li, Y.; Wang, Y.; Meng, Y.; Wang, X. Hydrous Manganese Dioxide Modified Poly(Sodium Acrylate) Hydrogel Composite as a Novel Adsorbent for Enhanced Removal of Tetracycline and Lead from Water. Chemosphere 2021, 272, 129902. [Google Scholar] [CrossRef] [PubMed]
- Beakou, B.H.; El Hassani, K.; Houssaini, M.A.; Belbahloul, M.; Oukani, E.; Anouar, A. A Novel Biochar from Manihot Esculenta Crantz Waste: Application for the Removal of Malachite Green from Wastewater and Optimization of the Adsorption Process. Water Sci. Technol. 2017, 76, 1447–1456. [Google Scholar] [CrossRef]
- Gürses, A.; Doǧar, Ç.; Karaca, S.; Açikyildiz, M.; Bayrak, R. Production of Granular Activated Carbon from Waste Rosa Canina Sp. Seeds and Its Adsorption Characteristics for Dye. J. Hazard. Mater. 2006, 131, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Al-Asadi, S.T.; Al-Qaim, F.F.; Al-Saedi, H.F.S.; Deyab, I.F.; Kamyab, H.; Chelliapan, S. Adsorption of Methylene Blue Dye from Aqueous Solution Using Low-Cost Adsorbent: Kinetic, Isotherm Adsorption, and Thermodynamic Studies. Environ. Monit. Assess. 2023, 195, 676. [Google Scholar] [CrossRef]
- Gebre Meskel, A.; Kwikima, M.M.; Meshesha, B.T.; Habtu, N.G.; Naik, S.V.C.S.; Vellanki, B.P. Malachite Green and Methylene Blue Dye Removal Using Modified Bagasse Fly Ash: Adsorption Optimization Studies. Environ. Chall. 2024, 14, 100829. [Google Scholar] [CrossRef]
- Gul, S.; Afsar, S.; Shah, T.A.; Gul, H.; Aziz, T.; Zahra, N.; Alhomrani, M.; Alsanie, W.F.; Alamri, A.S. White Clover Components as an Effective Biosorbent for the Elimination of Toxic Malachite Green from Wastewater. Biomass Convers. Biorefinery 2025. [Google Scholar] [CrossRef]


| Real Values | Coded Values | |||||
|---|---|---|---|---|---|---|
| Variable | Low | Center | High | Low | Center | High |
| pH (A) | 4 | 6.5 | 9 | −1 | 0 | +1 |
| Adsorbent dosage (g/L) (B) | 0.6 | 0.9 | 1.2 | −1 | 0 | +1 |
| Contact time (min) (C) | 120 | 210 | 300 | −1 | 0 | +1 |
| Run | pH (A) | Contact Time, min (B) | Adsorbent Dosage (g/L) (C) | MB Adsorption Capacity (mg/g) | MB Adsorption Capacity (mg/g) | MG Adsorption Capacity (mg/g) | MG Adsorption Capacity (mg/g) |
|---|---|---|---|---|---|---|---|
| Experimental | Predicted | Experimental | Predicted | ||||
| 1 | 9 | 300 | 0.9 | 19.68 | 19.59 | 19.46 | 19.49 |
| 2 | 9 | 210 | 0.6 | 22.68 | 22.95 | 24.05 | 23.92 |
| 3 | 6.5 | 210 | 0.9 | 14.42 | 14.23 | 13.30 | 13.30 |
| 4 | 9 | 120 | 0.9 | 15.52 | 15.46 | 15.31 | 15.91 |
| 5 | 4 | 210 | 1.2 | 8.43 | 8.16 | 7.61 | 7.73 |
| 6 | 4 | 210 | 0.6 | 14.59 | 14.71 | 9.21 | 9.71 |
| 7 | 6.5 | 210 | 0.9 | 14.08 | 14.23 | 13.30 | 13.30 |
| 8 | 6.5 | 210 | 0.9 | 14.49 | 14.23 | 13.30 | 13.30 |
| 9 | 6.5 | 120 | 1.2 | 15.46 | 15.64 | 14.72 | 14.63 |
| 10 | 4 | 300 | 0.9 | 10.18 | 10.24 | 7.18 | 6.58 |
| 11 | 6.5 | 120 | 0.6 | 20.55 | 20.33 | 20.21 | 19.74 |
| 12 | 6.5 | 210 | 0.9 | 14.07 | 14.23 | 13.30 | 13.30 |
| 13 | 6.5 | 300 | 1.2 | 15.88 | 16.10 | 15.00 | 15.48 |
| 14 | 6.5 | 210 | 0.9 | 14.08 | 14.23 | 13.30 | 13.30 |
| 15 | 6.5 | 300 | 0.6 | 25.39 | 25.21 | 21.40 | 21.49 |
| 16 | 9 | 210 | 1.2 | 15.83 | 15.70 | 15.29 | 14.79 |
| 17 | 4 | 120 | 0.9 | 8.93 | 9.03 | 7.59 | 7.56 |
| Source | SD | R2 | Adj-R2 | Pred-R2 | Comment |
| MB | |||||
| Linear | 2.6200 | 0.7246 | 0.6610 | 0.4499 | |
| 2FI | 2.8600 | 0.7467 | 0.5947 | −0.1917 | |
| Quadratic | 0.2769 | 0.9983 | 0.9962 | 0.9811 | Suggested |
| Cubic | 0.2075 | 0.9995 | 0.9979 | Aliased | |
| MG | |||||
| Linear | 2.6300 | 0.7644 | 0.7100 | 0.5201 | |
| 2FI | 2.6800 | 0.8122 | 0.6995 | 0.0901 | |
| Quadratic | 0.4966 | 0.9955 | 0.9896 | 0.9275 | Suggested |
| Cubic | 0.0000 | 1.0000 | 1.0000 | Aliased |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | Remarks |
|---|---|---|---|---|---|---|
| Model | 322.57 | 9 | 35.84 | 467.56 | <0.0001 | Significant |
| A-pH | 124.67 | 1 | 124.67 | 1626.30 | <0.0001 | Significant |
| B-contact time | 14.26 | 1 | 14.26 | 185.98 | <0.0001 | Significant |
| C-dosage | 95.19 | 1 | 95.19 | 1241.81 | <0.0001 | Significant |
| AB | 2.13 | 1 | 2.13 | 27.76 | 0.0012 | Significant |
| AC | 0.1221 | 1 | 0.1221 | 1.59 | 0.2473 | Not significant |
| BC | 4.89 | 1 | 4.89 | 63.81 | <0.0001 | Significant |
| A2 | 22.17 | 1 | 22.17 | 289.15 | <0.0001 | Significant |
| B2 | 11.41 | 1 | 11.41 | 148.85 | <0.0001 | Significant |
| C2 | 50.08 | 1 | 50.08 | 653.27 | <0.0001 | Significant |
| Residual | 0.5366 | 7 | 0.0767 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | Remarks |
|---|---|---|---|---|---|---|
| Model | 379.35 | 9 | 42.15 | 170.94 | <0.0001 | Significant |
| A-pH | 226.09 | 1 | 226.09 | 916.88 | <0.0001 | Significant |
| B-contact time | 3.39 | 1 | 3.39 | 13.74 | 0.0076 | Significant |
| C-dosage | 61.82 | 1 | 61.82 | 250.71 | <0.0001 | Significant |
| AB | 5.20 | 1 | 5.20 | 21.07 | 0.0025 | Significant |
| AC | 12.80 | 1 | 12.80 | 51.92 | 0.0002 | Significant |
| BC | 0.2042 | 1 | 0.2042 | 0.8283 | 0.3930 | Not Significant |
| A2 | 23.37 | 1 | 23.37 | 94.76 | <0.0001 | Significant |
| B2 | 8.72 | 1 | 8.72 | 35.36 | 0.0006 | Significant |
| C2 | 40.27 | 1 | 40.27 | 163.32 | <0.0001 | Significant |
| Residual | 1.73 | 7 | 0.2466 |
| Co (MB and MG) | T °C | A | B | C | qe (mg/g) | |||
|---|---|---|---|---|---|---|---|---|
| Pre (MB) | Exp (MB) | Pre (MG) | Exp (MG) | |||||
| 20 | 45 | 9 | 300 | 0.6 | 27.769 | 25.39 | 27.379 | 24.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madhau, F.; Wu, Z.; Shi, Y.; Guo, D.; Wan, D.; Tichapondwa, S.; Wang, Y.; Chisadza, B.; Zhu, B. Synergistic Removal of Hazardous Dyes Using a Clay/Carbon Composite Derived from Spent Bleaching Earth: Optimization Using Response Surface Methodology. Processes 2025, 13, 1217. https://doi.org/10.3390/pr13041217
Madhau F, Wu Z, Shi Y, Guo D, Wan D, Tichapondwa S, Wang Y, Chisadza B, Zhu B. Synergistic Removal of Hazardous Dyes Using a Clay/Carbon Composite Derived from Spent Bleaching Earth: Optimization Using Response Surface Methodology. Processes. 2025; 13(4):1217. https://doi.org/10.3390/pr13041217
Chicago/Turabian StyleMadhau, Freeman, Zhenjun Wu, Yahui Shi, Dongli Guo, Dongjin Wan, Shepherd Tichapondwa, Yangyang Wang, Bright Chisadza, and Beibei Zhu. 2025. "Synergistic Removal of Hazardous Dyes Using a Clay/Carbon Composite Derived from Spent Bleaching Earth: Optimization Using Response Surface Methodology" Processes 13, no. 4: 1217. https://doi.org/10.3390/pr13041217
APA StyleMadhau, F., Wu, Z., Shi, Y., Guo, D., Wan, D., Tichapondwa, S., Wang, Y., Chisadza, B., & Zhu, B. (2025). Synergistic Removal of Hazardous Dyes Using a Clay/Carbon Composite Derived from Spent Bleaching Earth: Optimization Using Response Surface Methodology. Processes, 13(4), 1217. https://doi.org/10.3390/pr13041217





