The Development of an Alginate Drilling Fluid Treatment Agent for Shale and a Study on the Mechanism of Wellbore Stability Sealing
Abstract
:1. Introduction
2. Experiments and Testing
2.1. Test Materials
| Reagents Name | Manufacturer | Specifications |
|---|---|---|
| Bentonite | Industrial grade | Jianping Rongchang Mining Co., Ltd. (Chaoyang, China) |
| Sodium chloride | Analytical pure | Beijing Baiao Innovation Technology Co., Ltd. (Beijing, China) |
| Potassium chloride | Analytical pure | Beijing Baiao Innovation Technology Co., Ltd. (Beijing, China) |
| Sodium hydroxide | Analytical pure | Jinan Zhengkang Chemical Co., Ltd. (Jinan, China) |
| Anhydrous sodium carbonate | Analytical pure | Wuxi Jingke Chemical Co., Ltd. (Wuxi, China) |
| Sodium alginate | Analytical pure | Shandong Fengtai Biotechnology Co., Ltd. (Jinan, China) |
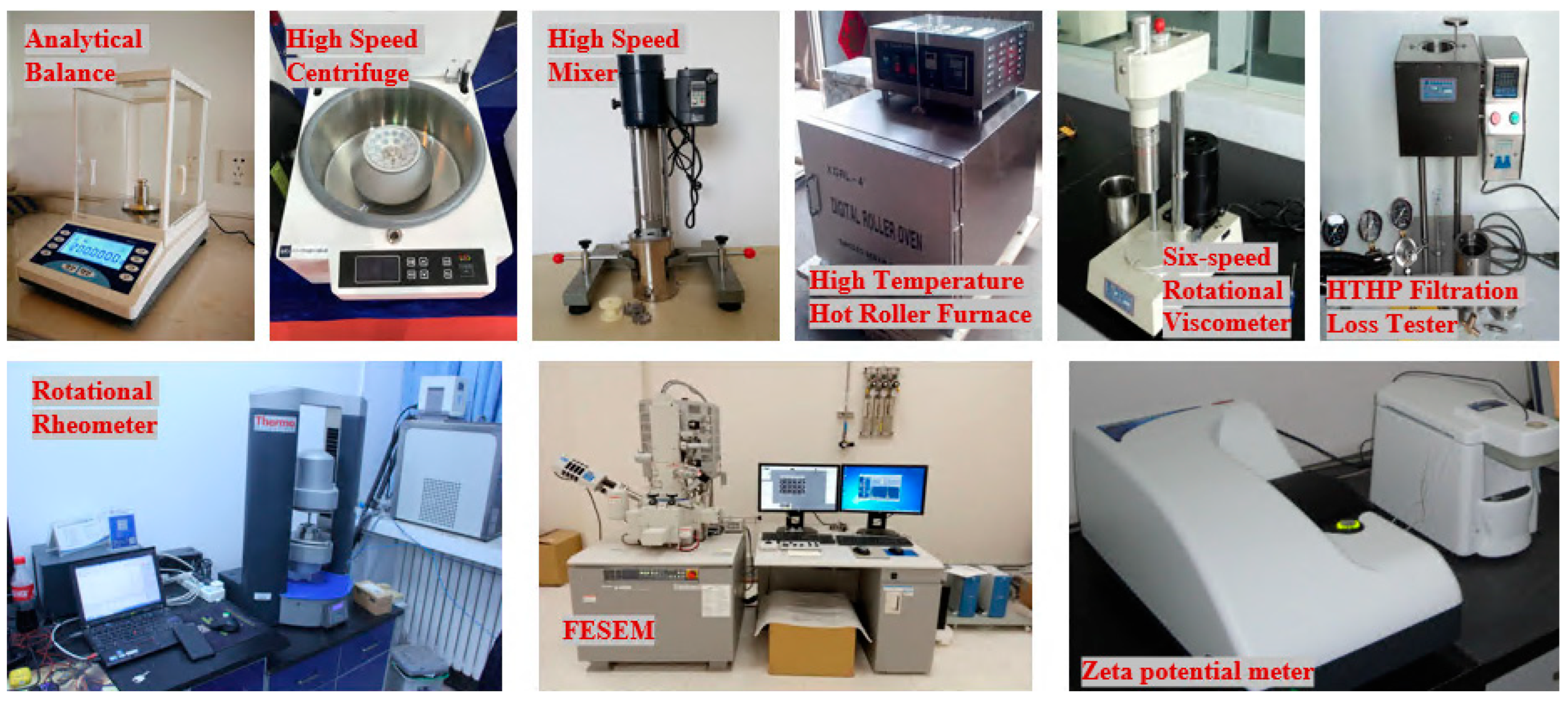
| Equipment Name | Model | Manufacturer |
|---|---|---|
| Analytical balance | FA2004 | Mettler Toledo Instruments Shanghai Company |
| High-speed centrifuge | GT10-2 | Beijing New Era Beili Medical Equipment Co. |
| High-speed mixer | GJS-B12K | Tianjin Hengxing Chemical Reagent Manufacturing Co., Ltd. |
| High-temperature hot rolling furnace | XGRL-4 | Qingdao Senxin Co., Ltd. |
| Six speed rotational viscometer | ZNN-D6B | Qingdao Shande Petroleum Instrument Co., Ltd. |
| High-temperature and high-pressure filtration instrument | STA-449F3 | Germany Naichi Instrument Manufacturing Co., Ltd. |
| Rotational rheometer | DHR-2 | Waters Corporation, Milford, MA, USA |
| SEM | S-4800 | Hitachi, Ltd., Tokyo, Japan |
| Zeta potential meter | NS-90Z | Zhuhai Ouaike Instrument Co., Ltd. |
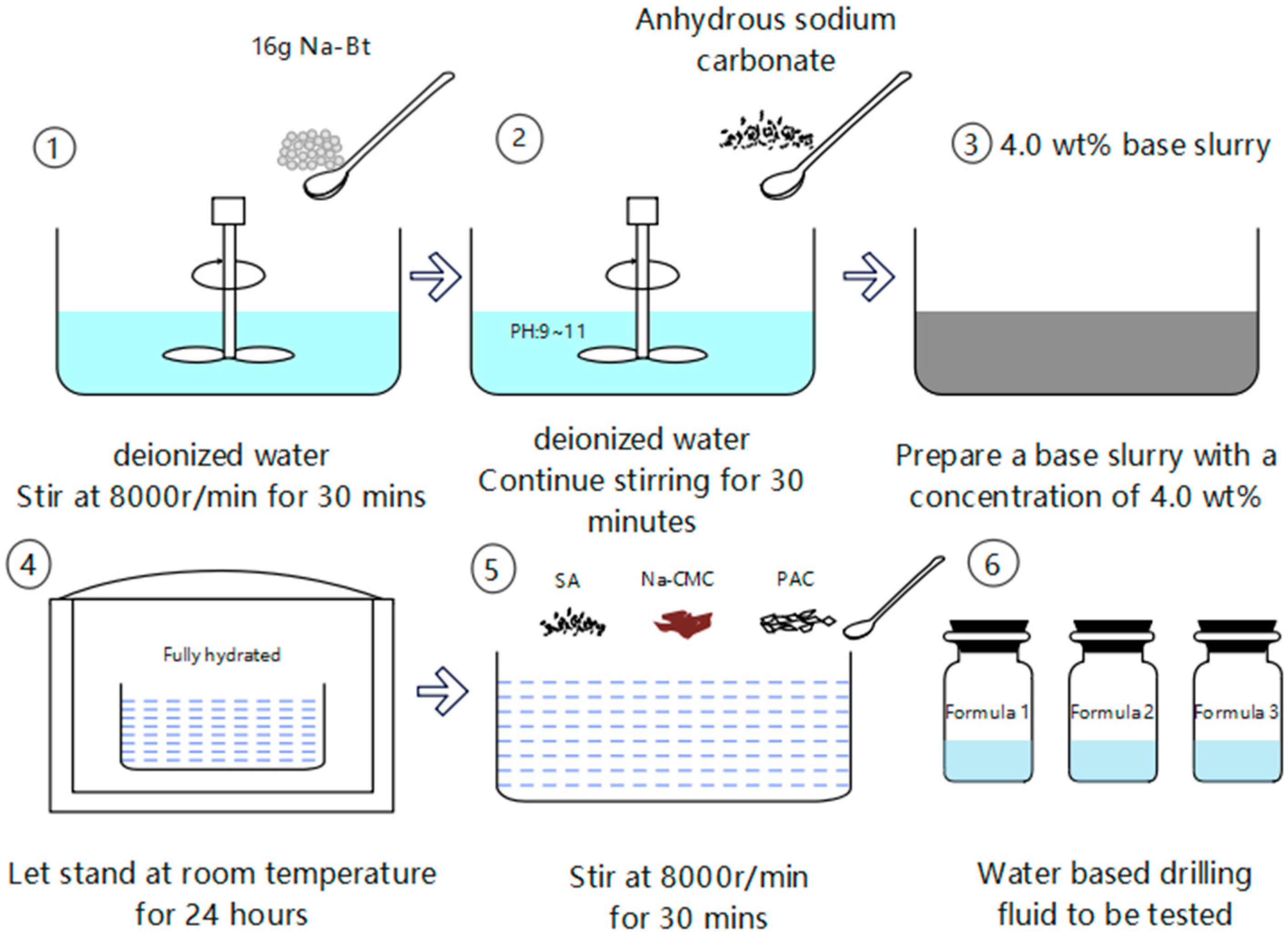
2.2. Preparation of Drilling Fluid
2.3. Performance Testing
2.3.1. Rheological Performance Testing
2.3.2. Filtration Performance Test
2.3.3. Temperature Resistance Performance Test
3. Results and Discussion
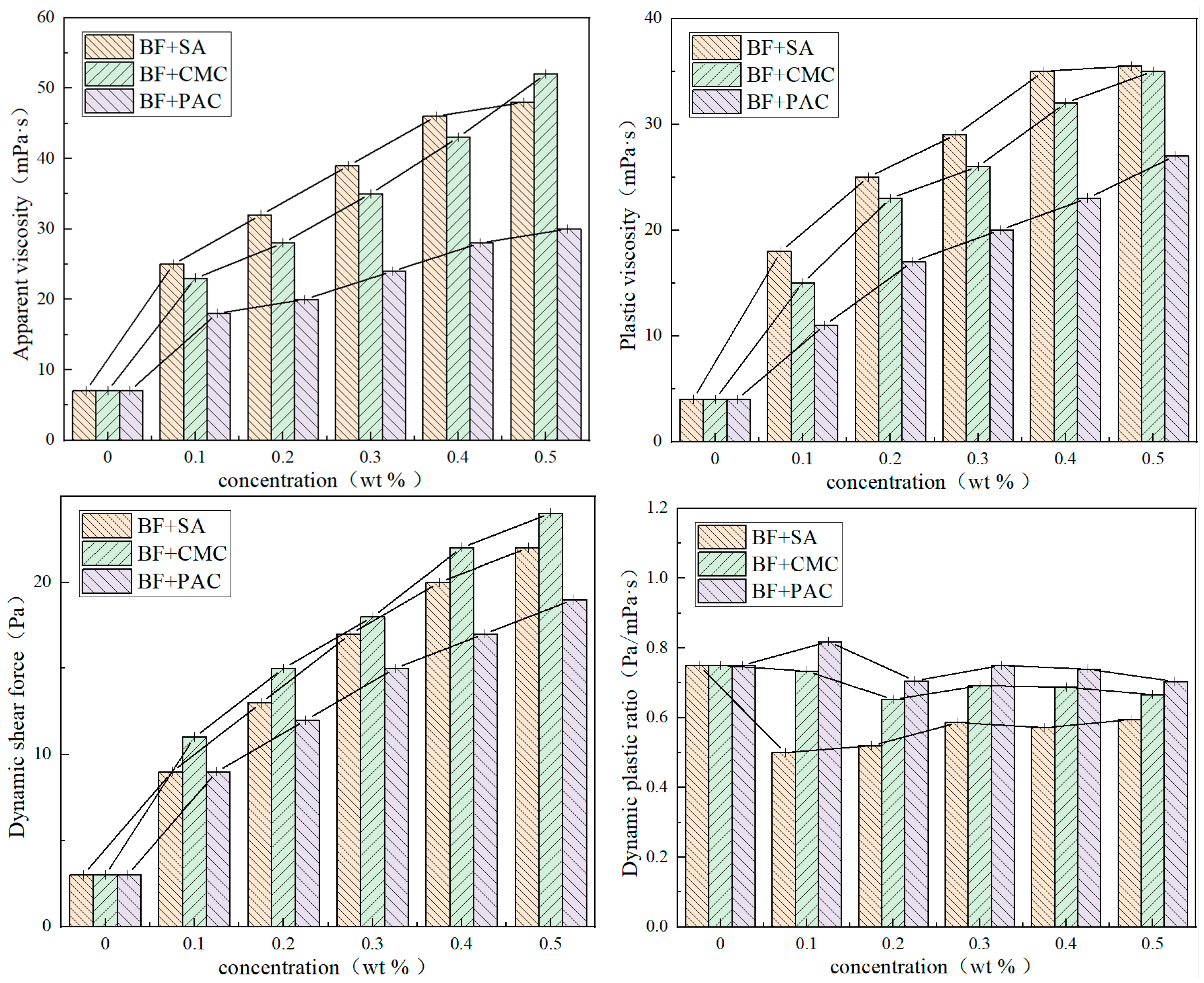
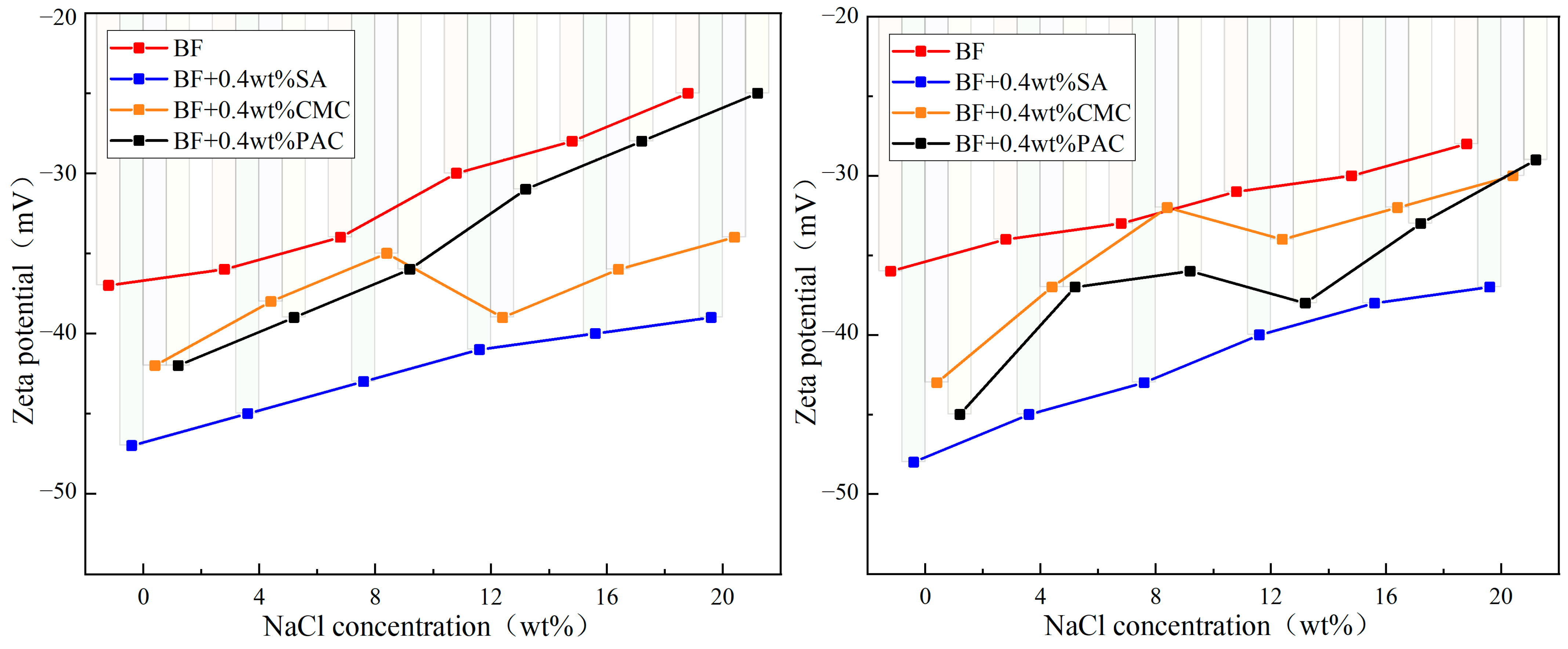
3.1. Rheological Performance
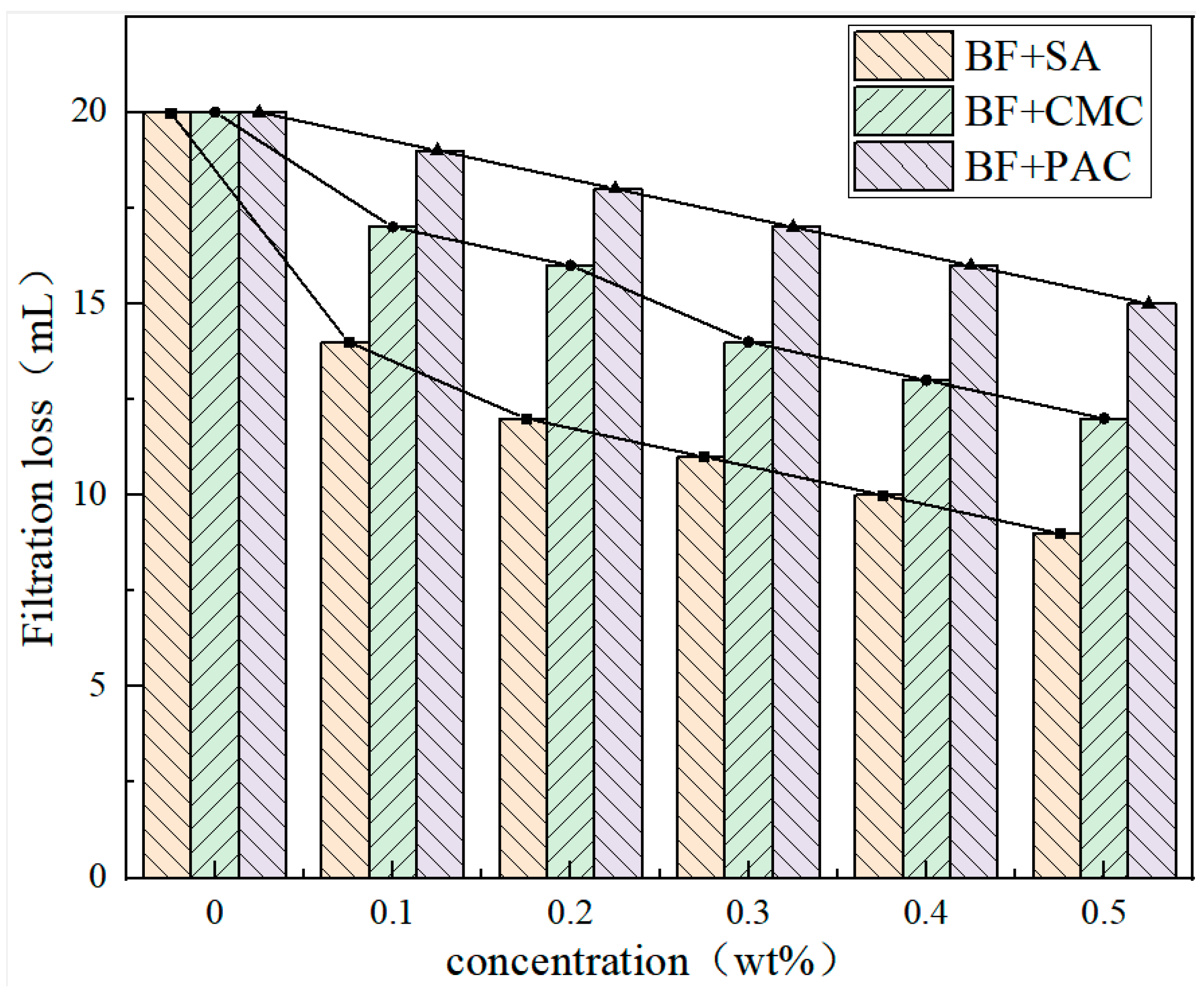
3.2. Filtration Performance
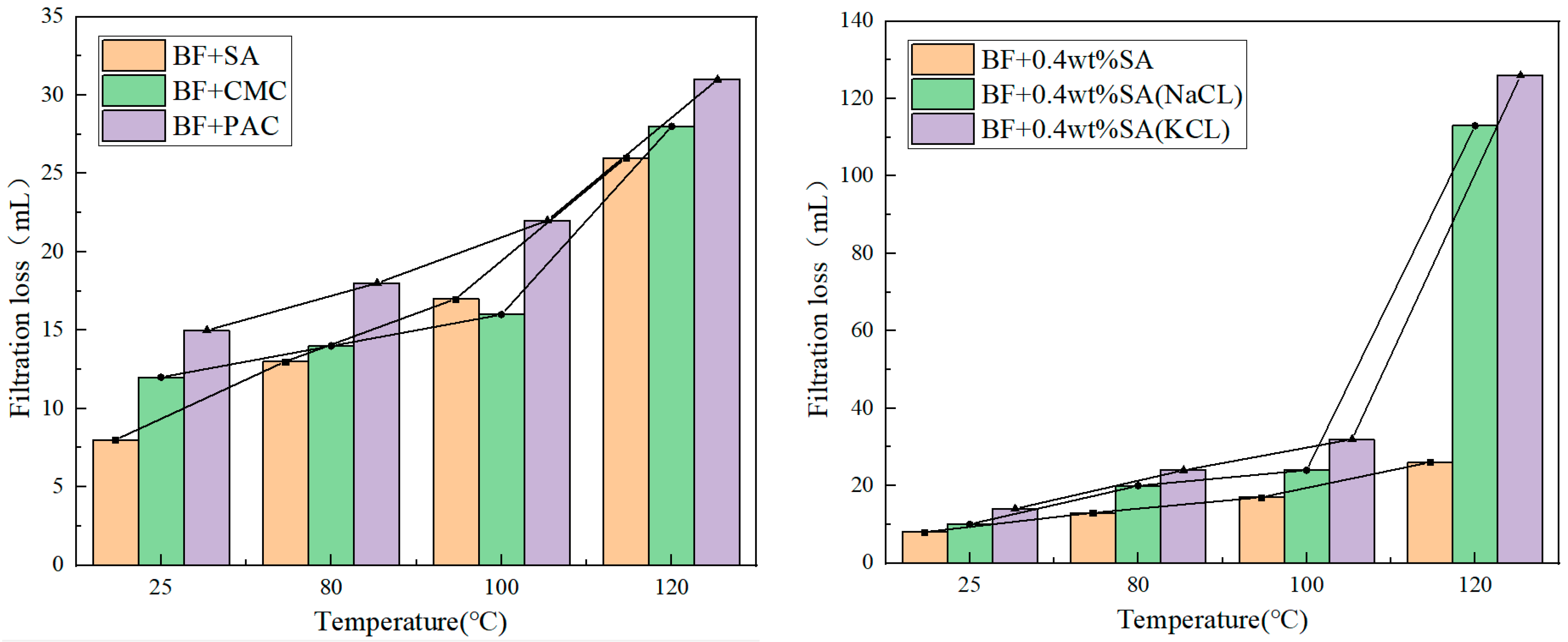
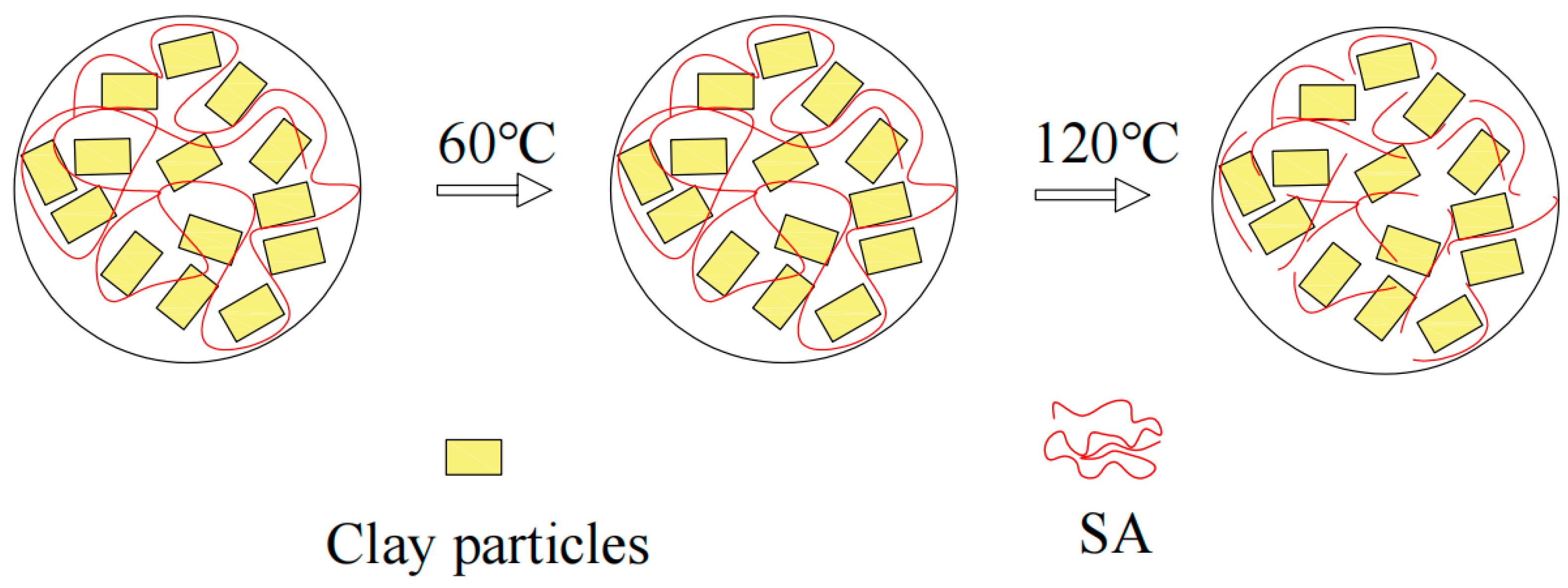
3.3. Temperature Resistance Performance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chang, L.; Wang, H.; Zhuo, L.; Huang, H.; Zou, L.; Wang, Q.; Li, H.; Yu, J. Analysis of wellbore stability considering the interaction between fluid and shale. Geofluids 2023, 2023, 4488607. [Google Scholar] [CrossRef]
- Zheng, D.; Ozbayoglu, E.; Miska, S.; Zhang, J. Experimental study of anisotropic strength properties of shale. In Proceedings of the 57th U.S. Rock Mechanics/Geomechanics Symposium, Atlanta, GA, USA, 25–28 June 2023; ARMA: Karaikal, India, 2023; p. ARMA–2023-0128. [Google Scholar]
- Rafieepour, S.; Zheng, D.; Miska, S.; Ozbayoglu, E.; Takach, N.; Yu, M.; Zhang, J. Combined experimental and well log evaluation of anisotropic mechanical properties of shales: An application to wellbore stability in bakken formation. In Proceedings of the SPE Annual Technical Conference and Exhibition, Virtual, 26–29 October 2020; SPE: Richardson, TX, USA, 2020; p. D021S015R006. [Google Scholar]
- Gautam, S.; Guria, C.; Rajak, V.K. A state of the art review on the performance of high-pressure and high-temperature drilling fluids: Towards understanding the structure-property relationship of drilling fluid additives. J. Pet. Sci. Eng. 2022, 213, 110318. [Google Scholar] [CrossRef]
- Yang, J.; Sun, J.; Wang, R.; Qu, Y. Treatment of drilling fluid waste during oil and gas drilling: A review. Environ. Sci. Pollut. Res. 2023, 30, 19662–19682. [Google Scholar] [CrossRef] [PubMed]
- Al-Shargabi, M.; Davoodi, S.; Wood, D.A.; Al-Musai, A.; Rukavishnikov, V.S.; Minaev, K.M. Nanoparticle applications as beneficial oil and gas drilling fluid additives: A review. J. Mol. Liq. 2022, 352, 118725. [Google Scholar] [CrossRef]
- Lalji, S.M.; Ali, S.I.; Sohail, H.; Misbah, A.R.; Azam, K.; Navaid, N. Combine effect of graphene oxide, pure-bore and sodium alginate on rheological and filtration properties and cutting carrying capacity of water-based drilling fluid. Chem. Pap. 2022, 76, 6461–6473. [Google Scholar] [CrossRef]
- Lalji, S.M.; Ali, S.I.; Yousufi, M.M.; Sultan, M.A.; Fatima, K.; Misbah, A.R. Factorial analysis for the impact on filtration properties of water-based drilling fluid after the addition of graphene oxide, pure-bore, and sodium alginate. Arab. J. Geosci. 2023, 16, 132. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, M.; Shan, W.; Guo, M.; Li, Y.; Qin, W.; Li, K.; An, Y.; Bo, K. Synergistic effects of potassium alginate and silicates co-inhibition performance in shale hydration. J. Mol. Liq. 2024, 393, 123538. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, M.; Li, Y.; An, Y.; Li, K.; Bo, K.; Guo, M. Sodium alginate as an eco-friendly rheology modifier and salt-tolerant fluid loss additive in water-based drilling fluids. RSC Adv. 2022, 12, 29852–29864. [Google Scholar] [CrossRef]
- Zhang, X.; Deng, J.N.; Yang, K.; Li, Q.; Meng, S.Y.; Sun, X.X.; Song, Z.-Z.; Tian, Y.-D.; Zhang, S.-A.; Liu, X.-J.; et al. High-strength and self-degradable sodium alginate/polyacrylamide preformed particle gels for conformance control to enhance oil recovery. Pet. Sci. 2022, 19, 3149–3158. [Google Scholar] [CrossRef]
- Lalji, S.M.; Ali, S.I.; Khan, M.A. Study of rheological characteristics of a water-based drilling fluid in presence of biopolymers, synthetic polymer, and modified natural polymer. Pet. Chem. 2023, 63, 906–916. [Google Scholar] [CrossRef]
- Brahmi, M.; Essifi, K.; Elbachiri, A.; Tahani, A. Adsorption of sodium alginate onto sodium montmorillonite. Mater. Today Proc. 2021, 45, 7789–7793. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, T.; Sun, Y.; Lin, D.; Feng, X.; Wang, F. Insights into the high temperature-induced failure mechanism of bentonite in drilling fluid. Chem. Eng. J. 2022, 445, 136680. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, X.; Ji, C.; Zhan, Q.; Li, Z.; Guan, J.; Huang, J. Modification method of high-efficiency organic bentonite for drilling fluids: A review. Molecules 2023, 28, 7866. [Google Scholar] [CrossRef]
- Li, J.; Wen, M.; Jiang, Z.; Xian, L.; Liu, J.; Chen, J. Development and characterization of a surfactant responsive to redox conditions for gas recovery in foam drainage. Sci. Rep. 2025, 15, 511. [Google Scholar] [CrossRef]
- El-Bana, A.A.; Barakat, N.M.; Abdelghany, A.M.; Meikhail, M.S. Effect of surfactants addition on physical, structure and antimicrobial activity of (Na-CMC/Na–Alg) biofilms. Polym. Bull. 2023, 80, 2883–2909. [Google Scholar] [CrossRef]
- Li, J.; Wen, M.; Jiang, Z.; Gao, S.; Xiao, X.; Xiang, C.; Tao, J. Formulation and characterization of surfactants with antibacterial and corrosion-inhibiting properties for enhancing shale gas drainage and production. Sci. Rep. 2025, 15, 2376. [Google Scholar] [CrossRef]
- Shi, F.J.; Feng, S.J.; Zheng, Q.T.; Zhang, X.L.; Chen, H.X. Effect of polyanionic cellulose modification on properties and microstructure of calcium bentonite. Appl. Clay Sci. 2022, 228, 106633. [Google Scholar] [CrossRef]
- Li, J.; Wen, M.; Yang, J.; Liu, Y.; Jiang, Z.; Chen, J. Synthesis and analysis of magnetic nanoparticles within foam matrix for foam drainage gas production. Geoenergy Sci. Eng. 2024, 238, 212887. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, L.; Wan, X.; Tang, Y.; Liu, Q.; Li, W.; Liao, J. Synthesis and Characterization of a Temperature-Sensitive Microcapsule Gelling Agent for High-Temperature Acid Release. ACS Omega 2024, 9, 20849–20858. [Google Scholar] [CrossRef]
- Lei, M.; Sun, J.; Zhu, H.; Huang, W.; Cai, Z. Synthesis and characterization of natural rosin-modified silica nanocomposite and its green multifunctional applications for drilling fluid. Colloids Surf. A Physicochem. Eng. Asp. 2024, 702, 134994. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Jaafar, M.Z.; Yusof, M.A.M.; Shye, C.A.; Suardi, H.; Hussin, M.F.M.; Razali, A.Z.; Idris, A.K. The influence of nanoparticle size, concentration, and functionalization on drilling fluid filtration properties. Colloids Surf. A Physicochem. Eng. Asp. 2024, 693, 134020. [Google Scholar] [CrossRef]
- ANSI/API SPEC 5L:2018; Specification for Line Pipe. American Petroleum Institute (API): Washington, DC, USA, 2018.
- Zha, H.; Fu, H.; Zeng, L.; Zhu, X.; Jia, C. Use of sodium alginate as a novel cementitious material to improve the engineering properties of disintegrated carbonaceous mudstone. Bull. Eng. Geol. Environ. 2022, 81, 431. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.; Mu, L.; Gong, X. The Development of an Alginate Drilling Fluid Treatment Agent for Shale and a Study on the Mechanism of Wellbore Stability Sealing. Processes 2025, 13, 1250. https://doi.org/10.3390/pr13041250
Huang C, Mu L, Gong X. The Development of an Alginate Drilling Fluid Treatment Agent for Shale and a Study on the Mechanism of Wellbore Stability Sealing. Processes. 2025; 13(4):1250. https://doi.org/10.3390/pr13041250
Chicago/Turabian StyleHuang, Cheng, Liping Mu, and Xuefeng Gong. 2025. "The Development of an Alginate Drilling Fluid Treatment Agent for Shale and a Study on the Mechanism of Wellbore Stability Sealing" Processes 13, no. 4: 1250. https://doi.org/10.3390/pr13041250
APA StyleHuang, C., Mu, L., & Gong, X. (2025). The Development of an Alginate Drilling Fluid Treatment Agent for Shale and a Study on the Mechanism of Wellbore Stability Sealing. Processes, 13(4), 1250. https://doi.org/10.3390/pr13041250






