Pharmaceutical Contamination by Biofilms Formed of the Burkholderia cepacia Complex: Public Health Risks
Abstract
1. Introduction
2. The Genus Burkholderia
3. Biofilms: Resistance Strategy
4. Adaptation System: From Susceptible Planktonic to Resistant Sessile
4.1. Phase 1: Initial Adhesion
4.2. Phase 2: Growth and Intercellular Communication
4.3. Phase 3: Maturation
4.4. Phase 4: Dispersion and Recycling
4.5. Temperature Variation
5. Extracellular Matrix: Complex Structure
6. Molecular Mechanisms Associated with Substrate Attachment
6.1. Flagellum: Motility and Immunomodulation
6.2. Pili: Connecting Motility and Adhesion
6.3. Type III Secretion System: Pathogen–Host Interaction
6.4. Lipopolysaccharides: Adhesion and Immune Evasion
6.5. Exopolysaccharides: Structural Diversity and Implications in Pathogenesis
6.6. Extracellular DNA: Structural Component and Resistance
7. Population Density Sensing Mechanism
7.1. Quorum Sensing
7.2. Quorum Sensing Through Acyl-Homoserine Lactone
7.3. Quorum Sensing Through Cis-2-Unsaturated Fatty Acids
7.4. Quorum Sensing in Burkholderia mallei and Burkholderia pseudomallei
7.5. Quorum sensing Between Pseudomonas aeruginosa and Burkholderia cenocepacia
8. Biofilms Associated with the Healthcare Environment
8.1. Infections Associated with Biofilms in Cystic Fibrosis
8.2. Infections Associated with Biofilms in Chronic Granulomatous Disease
8.3. Infections Associated with Biofilms in Melioidosis
9. Biofilms Associated with the Pharmaceutical Industry
10. Resistance to Antibiotics
10.1. Resistance of Planktonic Cell
10.2. Resistance of Sessile Cells
11. Resistance to Biocides
12. Frequent Resistance Mechanisms
12.1. Heterogeneity
12.2. Efflux Pumps
13. Therapeutic Methods Against Biofilm-Induced Infections
13.1. Antibiotic Therapy: Antimicrobial Synergy
13.2. Association Between Mechanical Force and Antibiotic Therapy
13.3. Synthetic and Natural Peptides
13.4. Extracellular Polymeric Substances as a Therapeutic Target
13.5. Immunological Modulation in Enhancing Defense
13.6. Recombinant Bacteriophages: A Promising Alternative
13.7. Inhibition of Quorum Sensing: Innovative Therapy
13.8. Innovative Approaches: Nanoparticles and Electric Currents
14. Nosocomial Outbreaks Caused by Contaminated Pharmaceutical Products
15. Control and Prevention of Contamination
16. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahenthiralingam, E.; Urban, T.A.; Goldberg, J.B. The multifarious, multireplicon Burkholderia cepacia complex. Nat. Rev. Microbiol. 2005, 3, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Sfeir, M.M. Burkholderia cepacia complex infections: More complex than the bacterium name suggests. J. Infect. 2018, 77, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Valvano, M.A. Intracellular survival of Burkholderia cepacia complex in phagocytic cells. Can. J. Microbiol. 2015, 61, 607–615. [Google Scholar] [CrossRef]
- LiPuma, J.J. Update on the Burkholderia cepacia complex. Curr. Opin. Pulm. Med. 2005, 11, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Glowicz, J.; Crist, M.; Gould, C.; Moulton-Meissner, H.; Noble-Wang, J.; de Man, T.J.B.; Perry, K.A.; Miller, Z.; Yang, W.C.; Langille, S.; et al. cepacia Investigation Workgroup. A multistate investigation of health care-associated Burkholderia cepacia complex infections related to liquid docusate sodium contamination, January–October 2016. Am. J. Infect. Control 2018, 46, 649–655. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef]
- Depoorter, E.; Bull, M.J.; Peeters, C.; Coenye, T.; Vandamme, P.; Mahenthiralingam, E. Burkholderia: An update on taxonomy and biotechnological potential as antibiotic producers. Appl. Microbiol. Biotechnol. 2016, 100, 5215–5229. [Google Scholar] [CrossRef]
- Santana, G.S.; Aguiar, A.R.; Sales, F.L.S.; Miranda, R.V.S.L.; Valadão, T.B.; Costa, L.V.; Brandão, M.L.L. Polyphasic characterization of Burkholderia cepacia complex strains isolated from a pharmaceutical industry facility. In Proceedings of the 8th International Symposium on Immunobiological, Rio de Janeiro, Brazil, 8–10 May 2024; Bio-Manguinhos: Rio de Janeiro, Brazil, 2024; p. 144. [Google Scholar]
- Eberl, L.; Vandamme, P. Members of the genus Burkholderia: Good and bad guys. F1000Research 2016, 5, 1007. [Google Scholar] [CrossRef]
- Bourtzis, K.; Miller, T.A. Insect Symbiosis; CRC Press: Boca Raton, FL, USA, 2009; Volume 3. [Google Scholar] [CrossRef]
- Barke, J.; Seipke, R.F.; Grüschow, S.; Heavens, D.; Drou, N.; Bibb, M.J.; Goss, R.J.; Yu, D.W.; Hutchings, M.I. A mixed community of actinomycetes produce multiple antibiotics for the fungus farming ant Acromyrmex octospinosus. BMC Biol. 2010, 8, 109. [Google Scholar] [CrossRef]
- Yabuuchi, E.; Kosako, Y.; Oyaizu, H.; Yano, I.; Hotta, H.; Hashimoto, Y.; Ezaki, T.; Arakawa, M. Proposal of Burkholderia gen. nov. and transfer of seven species of the genus Pseudomonas homology group II to the new genus, with the type species Burkholderia cepacia (Palleroni and Holmes 1981) comb. nov. Microbiol. Immunol. 1992, 36, 1251–1275. [Google Scholar] [CrossRef]
- Coenye, T.; Vandamme, P. Diversity and significance of Burkholderia species occupying diverse ecological niches. Environ. Microbiol. 2003, 5, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Sawana, A.; Adeolu, M.; Gupta, R.S. Molecular signatures and phylogenomic analysis of the genus Burkholderia: Proposal for division of this genus into the emended genus Burkholderia containing pathogenic organisms and a new genus Paraburkholderia gen. nov. harboring environmental species. Front. Genet. 2014, 5, 429. [Google Scholar] [CrossRef] [PubMed]
- Dobritsa, A.P.; Samadpour, M. Transfer of eleven species of the genus Burkholderia to the genus Paraburkholderia and proposal of Caballeronia gen. nov. to accommodate twelve species of the genera Burkholderia and Paraburkholderia. Int. J. Syst. Evol. Microbiol. 2016, 66, 2836–2846. [Google Scholar] [CrossRef] [PubMed]
- Dobritsa, A.P.; Linardopoulou, E.V.; Samadpour, M. Transfer of 13 species of the genus Burkholderia to the genus Caballeronia and reclassification of Burkholderia jirisanensis as Paraburkholderia jirisanensis comb. nov. Int. J. Syst. Evol. Microbiol. 2017, 67, 3846–3853. [Google Scholar] [CrossRef]
- Coenye, T. Social interactions in the Burkholderia cepacia complex: Biofilms and quorum sensing. Future Microbiol. 2010, 5, 1087–1099. [Google Scholar] [CrossRef]
- Lauman, P.; Dennis, J.J. Advances in Phage Therapy: Targeting the Burkholderia cepacia Complex. Viruses 2021, 13, 1331. [Google Scholar] [CrossRef]
- Holmes, A.; Govan, J.; Goldstein, R. Agricultural use of Burkholderia (Pseudomonas) cepacia: A threat to human health? Emerg. Infect. Dis. 1998, 4, 221–227. [Google Scholar] [CrossRef]
- Coenye, T.; Vandamme, P.; Govan, J.R.; LiPuma, J.J. Taxonomy and identification of the Burkholderia cepacia complex. J. Clin. Microbiol. 2001, 39, 3427–3436. [Google Scholar] [CrossRef]
- Tavares, M.; Kozak, M.; Balola, A.; Sá-Correia, I. Burkholderia cepacia Complex Bacteria: A Feared Contamination Risk in Water-Based Pharmaceutical Products. Clin. Microbiol. Rev. 2020, 33, e00139-19. [Google Scholar] [CrossRef]
- Holden, M.T.; Seth-Smith, H.M.; Crossman, L.C.; Sebaihia, M.; Bentley, S.D.; Cerdeño-Tárraga, A.M.; Thomson, N.R.; Bason, N.; Quail, M.A.; Sharp, S.; et al. The genome of Burkholderia cenocepacia J2315, an epidemic pathogen of cystic fibrosis patients. J. Bacteriol. 2009, 191, 261–277. [Google Scholar] [CrossRef]
- Loutet, S.A.; Valvano, M.A. A decade of Burkholderia cenocepacia virulence determinant research. Infect. Immun. 2010, 78, 4088–4100. [Google Scholar] [CrossRef] [PubMed]
- Juhas, M.; van der Meer, J.R.; Gaillard, M.; Harding, R.M.; Hood, D.W.; Crook, D.W. Genomic islands: Tools of bacterial horizontal gene transfer and evolution. FEMS Microbiol. Rev. 2009, 33, 376–393. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Rojas, F.U.; López-Sánchez, D.; Meza-Radilla, G.; Méndez-Canarios, A.; Ibarra, J.A.; Estrada-de los Santos, P. El género Burkholderia: Entre el mutualismo y la patogenicidad. Rev. Mex. Fitopatol. 2019, 37, 383–407. [Google Scholar]
- Sana, T.G.; Berni, B.; Bleves, S. The T6SSs of Pseudomonas aeruginosa Strain PAO1 and Their Effectors: Beyond Bacterial-Cell Targeting. Front. Cell. Infect. Microbiol. 2016, 6, 61. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, S.; Hood, R.D.; Mougous, J.D. What is type VI secretion doing in all those bugs? Trends Microbiol. 2010, 18, 531–537. [Google Scholar] [CrossRef]
- Smalley, N.E.; An, D.; Parsek, M.R.; Chandler, J.R.; Dandekar, A.A. Quorum Sensing Protects Pseudomonas aeruginosa against Cheating by Other Species in a Laboratory Coculture Model. J. Bacteriol. 2015, 197, 3154–3159. [Google Scholar] [CrossRef]
- Whiteley, M.; Diggle, S.P.; Greenberg, E.P. Progress in and promise of bacterial quorum sensing research. Nature 2017, 551, 313–320. [Google Scholar] [CrossRef]
- Merry, C.R.; Perkins, M.; Mu, L.; Peterson, B.K.; Knackstedt, R.W.; Weingart, C.L. Characterization of a novel two-component system in Burkholderia cenocepacia. Curr. Microbiol. 2015, 70, 556–561. [Google Scholar] [CrossRef]
- Wu, H.; Moser, C.; Wang, H.Z.; Høiby, N.; Song, Z.J. Strategies for combating bacterial biofilm infections. Int. J. Oral Sci. 2015, 7, 1–7. [Google Scholar] [CrossRef]
- Mahanta, N.; Gupta, A.; Khare, S.K. Production of protease and lipase by solvent tolerant Pseudomonas aeruginosa PseA in solid-state fermentation using Jatropha curcas seed cake as substrate. Bioresour. Technol. 2008, 99, 1729–1735. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Bioterrorism Agents/Diseases. CDC. Available online: https://www.cdc.gov/emergency/index.html (accessed on 6 February 2025).
- Meumann, E.M.; Limmathurotsakul, D.; Dunachie, S.J.; Wiersinga, W.J.; Currie, B.J. Burkholderia pseudomallei and melioidosis. Nat. Rev. Microbiol. 2024, 22, 155–169. [Google Scholar] [CrossRef]
- Currie, B.J. Melioidosis: Evolving concepts in epidemiology, pathogenesis, and treatment. Semin. Respir. Crit. Care Med. 2015, 36, 111–125. [Google Scholar] [CrossRef]
- Limmathurotsakul, D.; Golding, N.; Dance, D.A.; Messina, J.P.; Pigott, D.M.; Moyes, C.L.; Rolim, D.B.; Bertherat, E.; Day, N.P.; Peacock, S.J.; et al. Predicted global distribution of Burkholderia pseudomallei and burden of melioidosis. Nat. Microbiol. 2016, 1, 15008. [Google Scholar] [CrossRef] [PubMed]
- Wiersinga, W.J.; Virk, H.S.; Torres, A.G.; Currie, B.J.; Peacock, S.J.; Dance, D.A.B.; Limmathurotsakul, D. Melioidosis. Nat. Rev. Dis. Primers 2018, 4, 17107. [Google Scholar] [CrossRef] [PubMed]
- Currie, B.J.; Dance, D.A.; Cheng, A.C. The global distribution of Burkholderia pseudomallei and melioidosis: An update. Trans. R. Soc. Trop. Med. Hyg. 2008, 102, S1–S4. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Fazli, M.; Almblad, H.; Rybtke, M.L.; Givskov, M.; Eberl, L.; Tolker-Nielsen, T. Regulation of biofilm formation in Pseudomonas and Burkholderia species. Environ. Microbiol. 2014, 16, 1961–1981. [Google Scholar] [CrossRef]
- Pamp, S.J.; Gjermansen, M.; Johansen, H.K.; Tolker-Nielsen, T. Tolerance to the antimicrobial peptide colistin in Pseudomonas aeruginosa biofilms is linked to metabolically active cells, and depends on the pmr and mexAB-oprM genes. Mol. Microbiol. 2008, 68, 223–240. [Google Scholar] [CrossRef]
- Stewart, P.S.; Franklin, M.J. Physiological heterogeneity in biofilms. Nat. Rev. Microbiol. 2008, 6, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S. Mechanisms of antibiotic resistance in bacterial biofilms. Int. J. Med. Microbiol. 2002, 292, 107–113. [Google Scholar] [CrossRef]
- Lewis, K. Persister cells, dormancy and infectious disease. Nat. Rev. Microbiol. 2007, 5, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.Y.; Prentice, E.L.; Webber, M.A. Mechanisms of antimicrobial resistance in biofilms. NPJ Antimicrob. Resist. 2024, 2, 27. [Google Scholar] [CrossRef]
- Leid, J.G.; Willson, C.J.; Shirtliff, M.E.; Hassett, D.J.; Parsek, M.R.; Jeffers, A.K. The exopolysaccharide alginate protects Pseudomonas aeruginosa biofilm bacteria from IFN-gamma-mediated macrophage killing. J. Immunol. 2005, 175, 7512–7518. [Google Scholar] [CrossRef] [PubMed]
- Jensen, P.Ø.; Bjarnsholt, T.; Phipps, R.; Rasmussen, T.B.; Calum, H.; Christoffersen, L.; Moser, C.; Williams, P.; Pressler, T.; Givskov, M.; et al. Rapid necrotic killing of polymorphonuclear leukocytes is caused by quorum-sensing-controlled production of rhamnolipid by Pseudomonas aeruginosa. Microbiology 2007, 153, 1329–1338. [Google Scholar] [CrossRef]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef]
- Bjarnsholt, T.; Alhede, M.; Alhede, M.; Eickhardt-Sørensen, S.R.; Moser, C.; Kühl, M.; Jensen, P.Ø.; Høiby, N. The in vivo biofilm. Trends Microbiol. 2013, 21, 466–474. [Google Scholar] [CrossRef]
- Hayward, C.; Ross, K.E.; Brown, M.H.; Nisar, M.A.; Hinds, J.; Jamieson, T.; Leterme, S.C.; Whiley, H. Handwashing basins and healthcare associated infections: Bacterial diversity in biofilms on faucets and drains. Sci. Total Environ. 2024, 949, 175194. [Google Scholar] [CrossRef]
- Stewart, P.S.; Bjarnsholt, T. Risk factors for chronic biofilm-related infection associated with implanted medical devices. Clin. Microbiol. Infect. 2020, 26, 1034–1038. [Google Scholar] [CrossRef]
- Blot, S.; Ruppé, E.; Harbarth, S.; Asehnoune, K.; Poulakou, G.; Luyt, C.E.; Rello, J.; Klompas, M.; Depuydt, P.; Eckmann, C.; et al. Healthcare-associated infections in adult intensive care unit patients: Changes in epidemiology, diagnosis, prevention and contributions of new technologies. Intensive Crit. Care Nurs. 2022, 70, 103227. [Google Scholar] [CrossRef] [PubMed]
- Silva-Santana, G.; Castro, H.C.; Ferreira, B.L.A.; Aguiar-Alves, F. Staphylococcus aureus biofilm development: The urgent need for treatment alternatives. J. Glob. Biosci. 2015, 4, 2092–2107. [Google Scholar]
- Berne, C.; Ellison, C.K.; Ducret, A.; Brun, Y.V. Bacterial adhesion at the single-cell level. Nat. Rev. Microbiol. 2018, 16, 616–627. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M. Biofilm formation: A clinically relevant microbiological process. Clin. Infect. Dis. 2002, 33, 1387–1392. [Google Scholar] [CrossRef]
- Sharma, S.; Mohler, J.; Mahajan, S.D.; Schwartz, S.A.; Bruggemann, L.; Aalinkeel, R. Microbial Biofilm: A Review on Formation, Infection, Antibiotic Resistance, Control Measures, and Innovative Treatment. Microorganisms 2023, 11, 1614, Erratum in Microorganisms 2024, 12, 1961. https://doi.org/10.3390/microorganisms12101961. [Google Scholar] [CrossRef]
- Rather, M.A.; Gupta, K.; Mandal, M. Microbial biofilm: Formation, architecture, antibiotic resistance, and control strategies. Braz. J. Microbiol. 2021, 52, 1701–1718. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, M.; Yu, C.; Li, J.; Zhou, X. Biofilm formation: Mechanistic insights and therapeutic targets. Mol. Biomed. 2023, 4, 49. [Google Scholar] [CrossRef]
- Zhang, K.; Li, X.; Yu, C.; Wang, Y. Promising Therapeutic Strategies Against Microbial Biofilm Challenges. Front. Cell. Infect. Microbiol. 2020, 10, 359. [Google Scholar] [CrossRef]
- Luo, A.; Wang, F.; Sun, D.; Liu, X.; Xin, B. Formation, Development, and Cross-Species Interactions in Biofilms. Front. Microbiol. 2022, 12, 757327. [Google Scholar] [CrossRef]
- Silva-Santana, G.; Cabral-Oliveira, G.G.; Oliveira, D.R.; Nogueira, B.A.; Pereira-Ribeiro, P.M.A.; Mattos-Guaraldi, A.L. Staphylococcus aureus biofilms: An opportunistic pathogen with multidrug resistance. Rev. Med. Microbiol. 2021, 32, 12–21. [Google Scholar] [CrossRef]
- Singh, S.; Datta, S.; Narayanan, K.B.; Rajnish, K.N. Bacterial exo-polysaccharides in biofilms: Role in antimicrobial resistance and treatments. J. Genet. Eng. Biotechnol. 2021, 19, 140. [Google Scholar] [CrossRef]
- Kaplan, J.B. Biofilm dispersal: Mechanisms, clinical implications, and potential therapeutic uses. J. Dent. Res. 2010, 89, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Hirai, C.K. Microbiologia: Burkholderia cepacia. Rev. Analytica 2025. Available online: https://revistaanalytica.com.br/microbiologia-burkhoderia-cepacia/ (accessed on 6 February 2025).
- da Silva, G.L.R. Caracterização Epidemiológica e de Fatores de Virulência de Burkholderia cepacia, Isoladas de casos de Bacteremia em Pacientes e da água da Unidade de Hemodiálise. Master’s Thesis, Instituto de Biociências de Botucatu, Universidade Estadual Paulista “Júlio de Mesquita Filho”, Botucatu, Brazil, 2023. [Google Scholar]
- Branda, S.S.; Vik, S.; Friedman, L.; Kolter, R. Biofilms: The matrix revisited. Trends Microbiol. 2005, 13, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Whitchurch, C.B.; Tolker-Nielsen, T.; Ragas, P.C.; Mattick, J.S. Extracellular DNA required for bacterial biofilm formation. Science 2002, 295, 1487. [Google Scholar] [CrossRef]
- Okshevsky, M.; Meyer, R.L. The role of extracellular DNA in the establishment, maintenance and perpetuation of bacterial biofilms. Crit. Rev. Microbiol. 2015, 41, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, I. Biofilm exopolysaccharides: A strong and sticky framework. Microbiology 2001, 147, 3–9. [Google Scholar] [CrossRef]
- Das, T.; Sharma, P.K.; Busscher, H.J.; van der Mei, H.C.; Krom, B.P. Role of extracellular DNA in initial bacterial adhesion and surface aggregation. Appl. Environ. Microbiol. 2010, 76, 3405–3408. [Google Scholar] [CrossRef]
- Almatroudi, A. Investigating Biofilms: Advanced Methods for Comprehending Microbial Behavior and Antibiotic Resistance. Front. Biosci. 2024, 29, 133. [Google Scholar] [CrossRef]
- Jennings, L.K.; Storek, K.M.; Ledvina, H.E.; Coulon, C.; Marmont, L.S.; Sadovskaya, I.; Secor, P.R.; Tseng, B.S.; Scian, M.; Filloux, A.; et al. Pel is a cationic exopolysaccharide that cross-links extracellular DNA in the Pseudomonas aeruginosa biofilm matrix. Proc. Natl. Acad. Sci. USA 2015, 112, 11353–11358. [Google Scholar] [CrossRef]
- Limoli, D.H.; Whitfield, G.B.; Kitao, T.; Ivey, M.L.; Davis, M.R., Jr.; Grahl, N.; Hogan, D.A.; Rahme, L.G.; Howell, P.L.; O’Toole, G.A.; et al. Pseudomonas aeruginosa Alginate Overproduction Promotes Coexistence with Staphylococcus aureus in a Model of Cystic Fibrosis Respiratory Infection. mBio 2017, 8, e00186-17. [Google Scholar] [CrossRef]
- Hentzer, M.; Teitzel, G.M.; Balzer, G.J.; Heydorn, A.; Molin, S.; Givskov, M.; Parsek, M.R. Alginate overproduction affects Pseudomonas aeruginosa biofilm structure and function. J. Bacteriol. 2001, 183, 5395–5401. [Google Scholar] [CrossRef] [PubMed]
- Mah, T.F.; O’Toole, G.A. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001, 9, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Chua, K.L.; Chan, Y.Y.; Gan, Y.H. Flagella are virulence determinants of Burkholderia pseudomallei. Infect. Immun. 2003, 71, 1622–1629. [Google Scholar] [CrossRef]
- Essex-Lopresti, A.E.; Boddey, J.A.; Thomas, R.; Smith, M.P.; Hartley, M.G.; Atkins, T.; Brown, N.F.; Tsang, C.H.; Peak, I.R.; Hill, J.; et al. A type IV pilin, PilA, Contributes to Adherence of Burkholderia pseudomallei and virulence in vivo. Infect. Immun. 2005, 73, 1260–1264. [Google Scholar] [CrossRef]
- Boddey, J.A.; Flegg, C.P.; Day, C.J.; Beacham, I.R.; Peak, I.R. Temperature-regulated microcolony formation by Burkholderia pseudomallei requires pilA and enhances association with cultured human cells. Infect. Immun. 2006, 74, 5374–5381. [Google Scholar] [CrossRef] [PubMed]
- Lazar Adler, N.R.; Govan, B.; Cullinane, M.; Harper, M.; Adler, B.; Boyce, J.D. The molecular and cellular basis of pathogenesis in melioidosis: How does Burkholderia pseudomallei cause disease? FEMS Microbiol. Rev. 2009, 33, 1079–1099. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, F.; Smith, K.D.; Ozinsky, A.; Hawn, T.R.; Yi, E.C.; Goodlett, D.R.; Eng, J.K.; Akira, S.; Underhill, D.M.; Aderem, A. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 2001, 410, 1099–1103. [Google Scholar] [CrossRef]
- Utaisincharoen, P.; Anuntagool, N.; Arjcharoen, S.; Lengwehasatit, I.; Limposuwan, K.; Chaisuriya, P.; Sirisinha, S. Burkholderia pseudomallei stimulates low interleukin-8 production in the human lung epithelial cell line A549. Clin. Exp. Immunol. 2004, 138, 61–65. [Google Scholar] [CrossRef]
- Wiersinga, W.J.; van der Poll, T.; White, N.J.; Day, N.P.; Peacock, S.J. Melioidosis: Insights into the pathogenicity of Burkholderia pseudomallei. Nat. Rev. Microbiol. 2006, 4, 272–282. [Google Scholar] [CrossRef]
- Gan, Y.H.; Chua, K.L.; Chua, H.H.; Liu, B.; Hii, C.S.; Chong, H.L.; Tan, P. Characterization of Burkholderia pseudomallei infection and identification of novel virulence factors using a Caenorhabditis elegans host system. Mol. Microbiol. 2002, 44, 1185–1197. [Google Scholar] [CrossRef] [PubMed]
- Kofoed, E.M.; Vance, R.E. Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature 2011, 477, 592–595. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, J.; Shi, J.; Gong, Y.N.; Lu, Q.; Xu, H.; Liu, L.; Shao, F. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature 2011, 477, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Miao, E.A.; Mao, D.P.; Yudkovsky, N.; Bonneau, R.; Lorang, C.G.; Warren, S.E.; Leaf, I.A.; Aderem, A. Innate immune detection of the type III secretion apparatus through the NLRC4 inflammasome. Proc. Natl. Acad. Sci. USA 2010, 107, 3076–3080. [Google Scholar] [CrossRef]
- Broz, P.; Ruby, T.; Belhocine, K.; Bouley, D.M.; Kayagaki, N.; Dixit, V.M.; Monack, D.M. Caspase-11 increases susceptibility to Salmonella infection in the absence of caspase-1. Nature 2012, 490, 288–291. [Google Scholar] [CrossRef] [PubMed]
- Maltez, V.I.; Miao, E.A. Reassessing the Evolutionary Importance of Inflammasomes. J. Immunol. 2016, 196, 956–962. [Google Scholar] [CrossRef]
- Sajjan, U.S.; Yang, J.H.; Hershenson, M.B.; LiPuma, J.J. Intracellular trafficking and replication of Burkholderia cenocepacia in human cystic fibrosis airway epithelial cells. Cell. Microbiol. 2006, 8, 1456–1466. [Google Scholar] [CrossRef]
- McCarthy, Y.; Yang, L.; Twomey, K.B.; Sass, A.; Tolker-Nielsen, T.; Mahenthiralingam, E.; Dow, J.M.; Ryan, R.P. A sensor kinase recognizing the cell-cell signal BDSF (cis-2-dodecenoic acid) regulates virulence in Burkholderia cenocepacia. Mol. Microbiol. 2010, 77, 1220–1236. [Google Scholar] [CrossRef]
- Scoffone, V.C.; Chiarelli, L.R.; Trespidi, G.; Mentasti, M.; Riccardi, G.; Buroni, S. Burkholderia cenocepacia infections in cystic fibrosis patients: Drug resistance and therapeutic approaches. Front. Microbiol. 2017, 8, 1592. [Google Scholar] [CrossRef]
- Schwab, U.; Leigh, M.; Ribeiro, C.; Yankaskas, J.; Burns, K.; Gilligan, P.; Sokol, P.; Boucher, R. Patterns of epithelial cell invasion by different species of the Burkholderia cepacia complex in well-differentiated human airway epithelia. Infect. Immun. 2002, 70, 4547–4555. [Google Scholar] [CrossRef]
- Vilas Boas, D.; Castro, J.; Araújo, D.; Nóbrega, F.L.; Keevil, C.W.; Azevedo, N.F.; Vieira, M.J.; Almeida, C. The role of flagellum and flagellum-based motility on Salmonella Enteritidis and Escherichia coli biofilm formation. Microorganisms 2024, 12, 232. [Google Scholar] [CrossRef] [PubMed]
- Burrows, L.L. Pseudomonas aeruginosa twitching motility: Type IV pili in action. Annu. Rev. Microbiol. 2012, 66, 493–520. [Google Scholar] [CrossRef] [PubMed]
- Craig, L.; Forest, K.T.; Maier, B. Type IV pili: Dynamics, biophysics and functional consequences. Nat. Rev. Microbiol. 2019, 17, 429–440. [Google Scholar] [CrossRef]
- Sun, G.W.; Gan, Y.H. Unraveling type III secretion systems in the highly versatile Burkholderia pseudomallei. Trends Microbiol. 2010, 18, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.P.; Haque, A.; Atkins, T.; Hill, J.; Wood, M.W.; Easton, A.; Nelson, M.; Underwood-Fowler, C.; Titball, R.W.; Bancroft, G.J.; et al. Attenuated virulence and protective efficacy of a Burkholderia pseudomallei bsa type III secretion mutant in murine models of melioidosis. Microbiology 2004, 150, 2669–2676. [Google Scholar] [CrossRef]
- Galán, J.E.; Lara-Tejero, M.; Marlovits, T.C.; Wagner, S. Bacterial type III secretion systems: Specialized nanomachines for protein delivery into target cells. Annu. Rev. Microbiol. 2014, 68, 415–438. [Google Scholar] [CrossRef]
- Stevens, M.P.; Stevens, J.M.; Jeng, R.L.; Taylor, L.A.; Wood, M.W.; Hawes, P.; Monaghan, P.; Welch, M.D.; Galyov, E.E. Identification of a bacterial factor required for actin-based motility of Burkholderia pseudomallei. Mol. Microbiol. 2005, 56, 40–53. [Google Scholar] [CrossRef]
- Muangsombut, V.; Suparak, S.; Pumirat, P.; Damnin, S.; Vattanaviboon, P.; Thongboonkerd, V.; Korbsrisate, S. Inactivation of Burkholderia pseudomallei bsaQ results in decreased invasion efficiency and delayed escape of bacteria from endocytic vesicles. Arch. Microbiol. 2008, 190, 623–631. [Google Scholar] [CrossRef]
- Tomich, M.; Herfst, C.A.; Golden, J.W.; Mohr, C.D. Role of flagella in host cell invasion by Burkholderia cepacia. Infect. Immun. 2002, 70, 1799–1806. [Google Scholar] [CrossRef]
- Aubert, D.F.; Xu, H.; Yang, J.; Shi, X.; Gao, W.; Li, L.; Bisaro, F.; Chen, S.; Valvano, M.A.; Shao, F. A Burkholderia Type VI effector deamidates Rho GTPases to activate the Pyrin inflammasome and trigger inflammation. Cell Host Microbe 2016, 19, 664–674. [Google Scholar] [CrossRef]
- Aubert, D.F.; Flannagan, R.S.; Valvano, M.A. A novel sensor kinase-response regulator hybrid controls biofilm formation and type VI secretion system activity in Burkholderia cenocepacia. Infect. Immun. 2008, 76, 1979–1991. [Google Scholar] [CrossRef]
- Uehlinger, S.; Schwager, S.; Bernier, S.P.; Riedel, K.; Nguyen, D.T.; Sokol, P.A.; Eberl, L. Identification of specific and universal virulence factors in Burkholderia cenocepacia strains by using multiple infection hosts. Infect. Immun. 2009, 77, 4102–4110. [Google Scholar] [CrossRef] [PubMed]
- Vander Broek, C.W.; Stevens, J.M. Type III secretion in the melioidosis pathogen Burkholderia pseudomallei. Front. Cell. Infect. Microbiol. 2017, 7, 255. [Google Scholar] [CrossRef] [PubMed]
- Estrada-de Los Santos, P.; Palmer, M.; Chávez-Ramírez, B.; Beukes, C.; Steenkamp, E.T.; Briscoe, L.; Khan, N.; Maluk, M.; Lafos, M.; Humm, E.; et al. Whole genome analyses suggests that Burkholderia sensu lato contains two additional novel genera (Mycetohabitans gen. nov., and Trinickia gen. nov.): Implications for the evolution of diazotrophy and nodulation in the Burkholderiaceae. Genes 2018, 9, 389. [Google Scholar] [CrossRef] [PubMed]
- Ceballos-Olvera, I.; Sahoo, M.; Miller, M.A.; Del Barrio, L.; Re, F. Inflammasome-dependent pyroptosis and IL-18 protect against Burkholderia pseudomallei lung infection while IL-1β is deleterious. PLoS Pathog. 2011, 7, e1002452. [Google Scholar] [CrossRef]
- Burtnick, M.N.; Brett, P.J.; DeShazer, D. Proteomic analysis of the Burkholderia pseudomallei type II secretome reveals hydrolytic enzymes, novel proteins, and the deubiquitinase TssM. Infect. Immun. 2014, 82, 3214–3226. [Google Scholar] [CrossRef]
- Bzdyl, N.M.; Moran, C.L.; Bendo, J.; Sarkar-Tyson, M. Pathogenicity and virulence of Burkholderia pseudomallei. Virulence 2022, 13, 1945–1965. [Google Scholar] [CrossRef]
- Berbudi, A.; Rahmadika, N.; Tjahjadi, A.I.; Ruslami, R. Type 2 diabetes and its impact on the immune system. Curr. Diabetes Rev. 2020, 16, 442–449. [Google Scholar] [CrossRef]
- Cloutier, M.; Muru, K.; Ravicoularamin, G.; Gauthier, C. Polysaccharides from Burkholderia species as targets for vaccine development, immunomodulation and chemical synthesis. Nat. Prod. Rep. 2018, 35, 1251–1293. [Google Scholar] [CrossRef]
- Tuanyok, A.; Stone, J.K.; Mayo, M.; Kaestli, M.; Gruendike, J.; Georgia, S.; Warrington, S.; Mullins, T.; Allender, C.J.; Wagner, D.M.; et al. The genetic and molecular basis of O-antigenic diversity in Burkholderia pseudomallei lipopolysaccharide. PLoS Negl. Trop. Dis. 2012, 6, e1453. [Google Scholar] [CrossRef]
- Perry, M.B.; MacLean, L.L.; Schollaardt, T.; Bryan, L.E.; Ho, M. Structural characterization of the lipopolysaccharide O antigens of Burkholderia pseudomallei. Infect. Immun. 1995, 63, 3348–3352. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.B.; Dow, S.W. Development of Burkholderia mallei and pseudomallei vaccines. Front. Cell. Infect. Microbiol. 2013, 3, 10. [Google Scholar] [CrossRef]
- Wiersinga, W.J.; Currie, B.J.; Peacock, S.J. Melioidosis. N. Engl. J. Med. 2012, 367, 1035–1044. [Google Scholar] [CrossRef]
- Nyanasegran, P.K.; Nathan, S.; Firdaus-Raih, M.; Muhammad, N.A.N.; Ng, C.L. Biofilm signaling, composition and regulation in Burkholderia pseudomallei. J. Microbiol. Biotechnol. 2023, 33, 15–27. [Google Scholar] [CrossRef]
- Conway, B.A.; Chu, K.K.; Bylund, J.; Altman, E.; Speert, D.P. Production of exopolysaccharide by Burkholderia cenocepacia results in altered cell-surface interactions and altered bacterial clearance in mice. J. Infect. Dis. 2004, 190, 957–966. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.S.; Silva, I.N.; Oliveira, V.H.; Cunha, R.; Moreira, L.M. Insights into the role of extracellular polysaccharides in Burkholderia adaptation to different environments. Front. Cell. Infect. Microbiol. 2011, 1, 16. [Google Scholar] [CrossRef]
- Sousa, S.A.; Ramos, C.G.; Leitão, J.H. Burkholderia cepacia Complex: Emerging multihost pathogens equipped with a wide range of virulence factors and determinants. Int. J. Microbiol. 2011, 2011, 607575. [Google Scholar] [CrossRef] [PubMed]
- Cunha, M.V.; Pinto-de-Oliveira, A.; Meirinhos-Soares, L.; Salgado, M.J.; Melo-Cristino, J.; Correia, S.; Barreto, C.; Sá-Correia, I. Exceptionally high representation of Burkholderia cepacia among B. cepacia complex isolates recovered from the major Portuguese cystic fibrosis center. J. Clin. Microbiol. 2007, 45, 1628–1633. [Google Scholar] [CrossRef]
- Van Acker, H.; Sass, A.; Bazzini, S.; De Roy, K.; Udine, C.; Messiaen, T.; Riccardi, G.; Boon, N.; Nelis, H.J.; Mahenthiralingam, E.; et al. Biofilm-grown Burkholderia cepacia complex cells survive antibiotic treatment by avoiding production of reactive oxygen species. PLoS ONE 2013, 8, e58943. [Google Scholar] [CrossRef]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. Extracellular DNA (eDNA). A major ubiquitous element of the bacterial biofilm architecture. Int. J. Mol. Sci. 2021, 22, 9100. [Google Scholar] [CrossRef]
- Pakkulnan, R.; Anutrakunchai, C.; Kanthawong, S.; Taweechaisupapong, S.; Chareonsudjai, P.; Chareonsudjai, S. Extracellular DNA facilitates bacterial adhesion during Burkholderia pseudomallei biofilm formation. PLoS ONE 2019, 14, e0213288. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, L.; Toyofuku, M.; Hynen, A.L.; Kurosawa, M.; Pessi, G.; Petty, N.K.; Osvath, S.R.; Cárcamo-Oyarce, G.; Gloag, E.S.; Shimoni, R.; et al. Explosive cell lysis as a mechanism for the biogenesis of bacterial membrane vesicles and biofilms. Nat. Commun. 2016, 7, 11220. [Google Scholar] [CrossRef] [PubMed]
- Austin, C.R.; Goodyear, A.W.; Bartek, I.L.; Stewart, A.; Sutherland, M.D.; Silva, E.B.; Zweifel, A.; Vitko, N.P.; Tuanyok, A.; Highnam, G.; et al. A Burkholderia pseudomallei colony variant necessary for gastric colonization. mBio 2015, 6, e02462-14. [Google Scholar] [CrossRef]
- Garcia, E.C.; Perault, A.I.; Marlatt, S.A.; Cotter, P.A. Interbacterial signaling via Burkholderia contact-dependent growth inhibition system proteins. Proc. Natl. Acad. Sci. USA 2016, 113, 8296–8301. [Google Scholar] [CrossRef]
- Chiang, W.C.; Nilsson, M.; Jensen, P.Ø.; Høiby, N.; Nielsen, T.E.; Givskov, M.; Tolker-Nielsen, T. Extracellular DNA shields against aminoglycosides in Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 2013, 57, 2352–2361. [Google Scholar] [CrossRef] [PubMed]
- Pakkulnan, R.; Thonglao, N.; Chareonsudjai, S. DNase I and chitosan enhance efficacy of ceftazidime to eradicate Burkholderia pseudomallei biofilm cells. Sci. Rep. 2023, 13, 1059. [Google Scholar] [CrossRef]
- Miller, M.B.; Bassler, B.L. Quorum sensing in bacteria. Annu. Rev. Microbiol. 2001, 55, 165–199. [Google Scholar] [CrossRef]
- O’Grady, E.P.; Viteri, D.F.; Malott, R.J.; Sokol, P.A. Reciprocal regulation by the CepIR and CciIR quorum sensing systems in Burkholderia cenocepacia. BMC Genom. 2009, 10, 441. [Google Scholar] [CrossRef]
- De la Fuente, I.; Manzano-Morales, S.; Sanz, D.; Prieto, A.; Barriuso, J. Quorum sensing in bacteria: In silico protein analysis, ecophysiology, and reconstruction of their evolutionary history. BMC Genom. 2024, 25, 441. [Google Scholar] [CrossRef]
- Nealson, K.H.; Hastings, J.W. Bacterial bioluminescence: Its control and ecological significance. Microbiol. Rev. 1979, 43, 496–518. [Google Scholar] [CrossRef]
- Coulon, P.M.L.; Groleau, M.-C.; Hachani, A.; Padula, M.P.; Stinear, T.P.; Déziel, E. Quorum sensing and DNA methylation play active roles in clinical Burkholderia phase variation. J. Bacteriol. 2025, 207, e0053124. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; Yang, Q. Regulatory mechanisms and physiological impacts of quorum sensing in Gram-negative bacteria. Infect. Drug Resist. 2024, 17, 5395–5410. [Google Scholar] [CrossRef] [PubMed]
- Niu, K.M.; Lee, Y.J.; Jung, H.I.; Kothari, D.; Singh, D.; Kim, S.K. Functional analysis of quorum sensing-mediated pathogenicity in Burkholderia contaminans SK875 using transposon mutagenesis. Microb. Pathog. 2025, 200, 107332. [Google Scholar] [CrossRef]
- Le Guillouzer, S.; Groleau, M.C.; Déziel, E. The complex quorum sensing circuitry of Burkholderia thailandensis is both hierarchically and homeostatically organized. mBio 2017, 8, e01861-17. [Google Scholar] [CrossRef]
- Venturi, V.; Friscina, A.; Bertani, I.; Devescovi, G.; Aguilar, C. Quorum sensing in the Burkholderia cepacia complex. Res. Microbiol. 2004, 155, 238–244. [Google Scholar] [CrossRef]
- Venturi, V.; Subramoni, S. Future research trends in the major chemical language of bacteria. HFSP J. 2009, 3, 105–116. [Google Scholar] [CrossRef]
- McKenney, D.; Brown, K.E.; Allison, D.G. Influence of Pseudomonas aeruginosa exoproducts on virulence factor production in Burkholderia cepacia: Evidence of interspecies communication. J. Bacteriol. 1995, 177, 6989–6992. [Google Scholar] [CrossRef]
- Leadbetter, J.R.; Greenberg, E.P. Metabolism of acyl-homoserine lactone quorum-sensing signals by Variovorax paradoxus. J. Bacteriol. 2000, 182, 6921–6926. [Google Scholar] [CrossRef] [PubMed]
- Majerczyk, C.; Brittnacher, M.; Jacobs, M.; Armour, C.D.; Radey, M.; Schneider, E.; Phattarasokul, S.; Bunt, R.; Greenberg, E.P. Global analysis of the Burkholderia thailandensis quorum sensing-controlled regulon. J. Bacteriol. 2014, 196, 1412–1424. [Google Scholar] [CrossRef]
- Tseng, B.S.; Majerczyk, C.D.; Passos da Silva, D.; Chandler, J.R.; Greenberg, E.P.; Parsek, M.R. Quorum sensing influences Burkholderia thailandensis biofilm development and matrix production. J. Bacteriol. 2016, 198, 2643–2650. [Google Scholar] [CrossRef]
- Victor, I.U.; Kwiencien, M.; Tripathi, L.; Cobice, D.; McClean, S.; Marchant, R.; Banat, I.M. Quorum sensing as a potential target for increased production of rhamnolipid biosurfactant in Burkholderia thailandensis E264. Appl. Microbiol. Biotechnol. 2019, 103, 6505–6517. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, M.; Plener, L.; Armengaud, J.; Armstrong, N.; Chabrière, É.; Daudé, D. Lactonase-mediated inhibition of quorum sensing largely alters phenotypes, proteome, and antimicrobial activities in Burkholderia thailandensis E264. Front. Cell. Infect. Microbiol. 2023, 13, 1190859. [Google Scholar] [CrossRef] [PubMed]
- Ryan, R.P.; Dow, J.M. Communication with a growing family: Diffusible signal factor (DSF) signaling in bacteria. Trends Microbiol. 2011, 19, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Lim, A.; Lee, J.; Chen, S.; An, S.; Dong, Y.H.; Zhang, L.H. Diffusible signal factor (DSF) quorum sensing signal and structurally related molecules enhance the antimicrobial efficacy of antibiotics against some bacterial pathogens. BMC Microbiol. 2014, 14, 51. [Google Scholar] [CrossRef]
- Ryan, R.P.; Fouhy, Y.; Garcia, B.F.; Watt, S.A.; Niehaus, K.; Yang, L.; Tolker-Nielsen, T.; Dow, J.M. Inter-species signalling via the Stenotrophomonas maltophilia diffusible signal factor influences biofilm formation and polymyxin tolerance in Pseudomonas aeruginosa. Mol. Microbiol. 2008, 68, 75–86. [Google Scholar] [CrossRef]
- Lewenza, S.; Sokol, P.A. Regulation of ornibactin biosynthesis and N-acyl-L-homoserine lactone production by CepR in Burkholderia cepacia. J. Bacteriol. 2001, 183, 2212–2218. [Google Scholar] [CrossRef]
- Subsin, B.; Chambers, C.E.; Visser, M.B.; Sokol, P.A. Identification of genes regulated by the cepIR quorum-sensing system in Burkholderia cenocepacia by high-throughput screening of a random promoter library. J. Bacteriol. 2007, 189, 968–979. [Google Scholar] [CrossRef]
- Suppiger, A.; Schmid, N.; Aguilar, C.; Pessi, G.; Eberl, L. Two quorum sensing systems control biofilm formation and virulence in members of the Burkholderia cepacia complex. Virulence 2013, 4, 400–409. [Google Scholar] [CrossRef]
- Malott, R.J.; Baldwin, A.; Mahenthiralingam, E.; Sokol, P.A. Characterization of the cciIR quorum-sensing system in Burkholderia cenocepacia. Infect. Immun. 2005, 73, 4982–4992. [Google Scholar] [CrossRef]
- Ganesh, P.S.; Vishnupriya, S.; Vadivelu, J.; Mariappan, V.; Vellasamy, K.M.; Shankar, E.M. Intracellular survival and innate immune evasion of Burkholderia cepacia: Improved understanding of quorum sensing-controlled virulence factors, biofilm, and inhibitors. Microbiol. Immunol. 2020, 64, 87–98. [Google Scholar] [CrossRef]
- Lutter, E.; Lewenza, S.; Dennis, J.J.; Visser, M.B.; Sokol, P.A. Distribution of quorum-sensing genes in the Burkholderia cepacia complex. Infect. Immun. 2001, 69, 4661–4666. [Google Scholar] [CrossRef] [PubMed]
- Chapalain, A.; Groleau, M.C.; Le Guillouzer, S.; Miomandre, A.; Vial, L.; Milot, S.; Déziel, E. Interplay between 4-Hydroxy-3-Methyl-2-Alkylquinoline and N-Acyl-Homoserine Lactone Signaling in a Burkholderia cepacia Complex Clinical Strain. Front. Microbiol. 2017, 8, 1021. [Google Scholar] [CrossRef]
- Yao, F.; Zhou, H.; Lessie, T.G. Characterization of N-acyl homoserine lactone overproducing mutants of Burkholderia multivorans ATCC 17616. FEMS Microbiol. Lett. 2002, 206, 201–207. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Malott, R.J.; Sokol, P.A. Expression of the bviIR and cepIR quorum-sensing systems of Burkholderia vietnamiensis. J. Bacteriol. 2007, 189, 3006–3016. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Conway, B.A.; Greenberg, E.P. Quorum-sensing signals and quorum-sensing genes in Burkholderia vietnamiensis. J. Bacteriol. 2002, 184, 1187–1191. [Google Scholar] [CrossRef]
- Boon, C.; Deng, Y.; Wang, L.H.; He, Y.; Xu, J.L.; Fan, Y.; Pan, S.Q.; Zhang, L.H. A novel DSF-like signal from Burkholderia cenocepacia interferes with Candida albicans morphological transition. ISME J. 2008, 2, 27–36. [Google Scholar] [CrossRef]
- Deng, Y.; Lim, A.; Wang, J.; Zhou, T.; Chen, S.; Lee, J.; Dong, Y.H.; Zhang, L.H. Cis-2-dodecenoic acid quorum sensing system modulates N-acyl homoserine lactone production through RpfR and cyclic di-GMP turnover in Burkholderia cenocepacia. BMC Microbiol. 2013, 13, 148. [Google Scholar] [CrossRef]
- Ryan, R.P.; McCarthy, Y.; Watt, S.A.; Niehaus, K.; Dow, J.M. Intraspecies signaling involving the diffusible signal factor BDSF (cis-2-dodecenoic acid) influences virulence in Burkholderia cenocepacia. J. Bacteriol. 2009, 191, 5013–5019. [Google Scholar] [CrossRef]
- Bellich, B.; Terán, L.C.; Fazli, M.M.; Berti, F.; Rizzo, R.; Tolker-Nielsen, T.; Cescutti, P. The Bep gene cluster in Burkholderia cenocepacia H111 codes for a water-insoluble exopolysaccharide essential for biofilm formation. Carbohydr. Polym. 2023, 301, 120318. [Google Scholar] [CrossRef]
- Vallet-Gely, I.; Lemaitre, B.; Boccard, F. Bacterial strategies to overcome insect defences. Nat. Rev. Microbiol. 2008, 6, 302–313. [Google Scholar] [CrossRef]
- Wang, M.; Li, X.; Song, S.; Cui, C.; Zhang, L.H.; Deng, Y. The cis-2-Dodecenoic Acid (BDSF) Quorum Sensing System in Burkholderia cenocepacia. Appl. Environ. Microbiol. 2022, 88, e0234221. [Google Scholar] [CrossRef] [PubMed]
- Eberl, L. Quorum sensing in the genus Burkholderia. Int. J. Med. Microbiol. 2006, 296, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Bellich, B.; Jou, I.A.; Buriola, C.; Ravenscroft, N.; Brady, J.W.; Fazli, M.; Tolker-Nielsen, T.; Rizzo, R.; Cescutti, P. The biofilm of Burkholderia cenocepacia H111 contains an exopolysaccharide composed of l-rhamnose and l-mannose: Structural characterization and molecular modelling. Carbohydr. Res. 2021, 499, 108231. [Google Scholar] [CrossRef]
- Paszti, S.; Biner, O.; Liu, Y.; Bolli, K.; Jeggli, S.D.; Pessi, G.; Eberl, L. Insights into the diverse roles of the terminal oxidases in Burkholderia cenocepacia H111. Sci. Rep. 2025, 15, 2390. [Google Scholar] [CrossRef]
- Shirtliff, M.E.; Mader, J.T.; Camper, A.K. Molecular interactions in biofilms. Chem. Biol. 2002, 9, 859–871. [Google Scholar] [CrossRef]
- Vial, L.; Lépine, F.; Milot, S.; Groleau, M.C.; Dekimpe, V.; Woods, D.E.; Déziel, E. Burkholderia pseudomallei, B. thailandensis, and B. ambifaria produce 4-hydroxy-2-alkylquinoline analogues with a methyl group at the 3 position that is required for quorum-sensing regulation. J. Bacteriol. 2008, 190, 5339–5352. [Google Scholar] [CrossRef] [PubMed]
- Le Guillouzer, S.; Groleau, M.C.; Mauffrey, F.; Déziel, E. ScmR, a Global Regulator of Gene Expression, Quorum Sensing, pH Homeostasis, and Virulence in Burkholderia thailandensis. J. Bacteriol. 2020, 202, e00776-19. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, R.L.; DeShazer, D.; Brueggemann, E.E.; Hines, H.B.; Oyston, P.C.; Jeddeloh, J.A. Role of quorum sensing in the pathogenicity of Burkholderia pseudomallei. J. Med. Microbiol. 2004, 53, 1053–1064. [Google Scholar] [CrossRef]
- Chandler, J.R.; Heilmann, S.; Mittler, J.E.; Greenberg, E.P. Acyl-homoserine lactone-dependent eavesdropping promotes competition in a laboratory co-culture model. ISME J. 2012, 6, 2219–2228. [Google Scholar] [CrossRef]
- Duerkop, B.A.; Varga, J.; Chandler, J.R.; Peterson, S.B.; Herman, J.P.; Churchill, M.E.; Parsek, M.R.; Nierman, W.C.; Greenberg, E.P. Quorum-sensing control of antibiotic synthesis in Burkholderia thailandensis. J. Bacteriol. 2009, 191, 3909–3918. [Google Scholar] [CrossRef]
- Song, H.; Hwang, J.; Yi, H.; Ulrich, R.L.; Yu, Y.; Nierman, W.C.; Kim, H.S. The early stage of bacterial genome-reductive evolution in the host. PLoS Pathog. 2010, 6, e1000922. [Google Scholar] [CrossRef] [PubMed]
- Hamad, M.A.; Di Lorenzo, F.; Molinaro, A.; Valvano, M.A. Aminoarabinose is essential for lipopolysaccharide export and intrinsic antimicrobial peptide resistance in Burkholderia cenocepacia†. Mol. Microbiol. 2012, 85, 962–974. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.; Ooi, C.H.; Wang, D.; Chong, H.; Ng, K.C.; Rodrigues, F.; Lee, M.A.; Tan, P. Patterns of large-scale genomic variation in virulent and avirulent Burkholderia species. Genome Res. 2004, 14, 2295–2307. [Google Scholar] [CrossRef]
- Schell, M.A.; Ulrich, R.L.; Ribot, W.J.; Brueggemann, E.E.; Hines, H.B.; Chen, D.; Lipscomb, L.; Kim, H.S.; Mrázek, J.; Nierman, W.C.; et al. Type VI secretion is a major virulence determinant in Burkholderia mallei. Mol. Microbiol. 2007, 64, 1466–1485. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Schaefer, A.L.; Parsek, M.R.; Moninger, T.O.; Welsh, M.J.; Greenberg, E.P. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature 2000, 407, 762–764. [Google Scholar] [CrossRef]
- Geisenberger, O.; Givskov, M.; Riedel, K.; Høiby, N.; Tümmler, B.; Eberl, L. Production of N-acyl-L-homoserine lactones by Pseudomonas aeruginosa isolates from chronic lung infections associated with cystic fibrosis. FEMS Microbiol. Lett. 2000, 184, 273–278. [Google Scholar] [CrossRef]
- Riedel, K.; Hentzer, M.; Geisenberger, O.; Huber, B.; Steidle, A.; Wu, H.; Høiby, N.; Givskov, M.; Molin, S.; Eberl, L. N-acylhomoserine-lactone-mediated communication between Pseudomonas aeruginosa and Burkholderia cepacia in mixed biofilms. Microbiology 2001, 147, 3249–3262. [Google Scholar] [CrossRef]
- Venturi, V. Regulation of quorum sensing in Pseudomonas. FEMS Microbiol. Rev. 2006, 30, 274–291. [Google Scholar] [CrossRef]
- Solano, C.; Echeverz, M.; Lasa, I. Biofilm dispersion and quorum sensing. Curr. Opin. Microbiol. 2014, 18, 96–104. [Google Scholar] [CrossRef]
- Hoffman, L.R.; D’Argenio, D.A.; MacCoss, M.J.; Zhang, Z.; Jones, R.A.; Miller, S.I. Aminoglycoside antibiotics induce bacterial biofilm formation. Nature 2005, 436, 1171–1175. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilms and device-associated infections. Emerg. Infect. Dis. 2001, 7, 277–281. [Google Scholar] [CrossRef]
- Percival, S.L.; Suleman, L.; Vuotto, C.; Donelli, G. Healthcare-associated infections, medical devices and biofilms: Risk, tolerance and control. J. Med. Microbiol. 2015, 64, 323–334. [Google Scholar] [CrossRef]
- Mermel, L.A. Prevention of intravascular catheter-related infections. Ann. Intern. Med. 2000, 132, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P.; Caraher, E. Residence in biofilms allows Burkholderia cepacia complex (Bcc) bacteria to evade the antimicrobial activities of neutrophil-like dHL60 cells. Pathog. Dis. 2015, 73, ftv069. [Google Scholar] [CrossRef] [PubMed]
- Petrucca, A.; Cipriani, P.; Valenti, P.; Santapaola, D.; Cimmino, C.; Scoarughi, G.L.; Santino, I.; Stefani, S.; Sessa, R.; Nicoletti, M. Molecular characterization of Burkholderia cepacia isolates from cystic fibrosis (CF) patients in an Italian CF center. Res. Microbiol. 2003, 154, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Hentzer, M.; Givskov, M. Pharmacological inhibition of quorum sensing for the treatment of chronic bacterial infections. J. Clin. Investig. 2003, 112, 1300–1307. [Google Scholar] [CrossRef]
- Brackman, G.; Coenye, T. Quorum sensing inhibitors as anti-biofilm agents. Curr. Pharm. Des. 2015, 21, 5–11. [Google Scholar] [CrossRef]
- Drevinek, P.; Mahenthiralingam, E. Burkholderia cenocepacia in cystic fibrosis: Epidemiology and molecular mechanisms of virulence. Clin. Microbiol. Infect. 2010, 16, 821–830. [Google Scholar] [CrossRef]
- Rutherford, S.T.; Bassler, B.L. Bacterial quorum sensing: Its role in virulence and possibilities for its control. Cold Spring Harb. Perspect. Med. 2012, 2, a012427. [Google Scholar] [CrossRef]
- Chmiel, J.F.; Aksamit, T.R.; Chotirmall, S.H.; Dasenbrook, E.C.; Elborn, J.S.; LiPuma, J.J.; Ranganathan, S.C.; Waters, V.J.; Ratjen, F.A. Antibiotic management of lung infections in cystic fibrosis. I. The microbiome, methicillin-resistant Staphylococcus aureus, gram-negative bacteria, and multiple infections. Ann. Am. Thorac. Soc. 2014, 11, 1120–1129. [Google Scholar] [CrossRef]
- Ghosh, D.; Seth, M.; Mondal, P.; Mukhopadhyay, S.K. Biocontrol of biofilm forming Burkholderia cepacia using a quorum quenching crude lactonase enzyme extract from a marine Chromohalobacter sp. strain D23. Arch. Microbiol. 2023, 205, 374. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Martiniano, S.L.; Hoppe, J.E.; Sagel, S.D.; Zemanick, E.T. Advances in the diagnosis and treatment of cystic fibrosis. Adv. Pediatr. 2014, 61, 225–243. [Google Scholar] [CrossRef] [PubMed]
- Heneghan, M.; Southern, K.W.; Murphy, J.; Sinha, I.P.; Nevitt, S.J. Corrector therapies (with or without potentiators) for people with cystic fibrosis with class II CFTR gene variants (most commonly F508del). Cochrane Database Syst. Rev. 2023, 11, CD010966. [Google Scholar] [CrossRef]
- Silva Filho, L.V.R.F.D.; Athanazio, R.A.; Tonon, C.R.; Ferreira, J.C.; Tanni, S.E. Use of elexacaftor+tezacaftor+ivacaftor in individuals with cystic fibrosis and at least one F508del allele: A systematic review and meta-analysis. J. Bras. Pneumol. 2024, 49, e20230187. [Google Scholar] [CrossRef]
- Riordan, J.R.; Rommens, J.M.; Kerem, B.; Alon, N.; Rozmahel, R.; Grzelczak, Z.; Zielenski, J.; Lok, S.; Plavsic, N.; Chou, J.L.; et al. Identification of the cystic fibrosis gene: Cloning and characterization of complementary DNA. Science 1989, 245, 1066–1073. [Google Scholar] [CrossRef]
- Ratjen, F.; Döring, G. Cystic fibrosis. Lancet 2003, 361, 681–689. [Google Scholar] [CrossRef]
- Govan, J.R.; Deretic, V. Microbial pathogenesis in cystic fibrosis: Mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol. Rev. 1996, 60, 539–574. [Google Scholar] [CrossRef]
- Boucher, R.C. New concepts of the pathogenesis of cystic fibrosis lung disease. Eur. Respir. J. 2004, 23, 146–158. [Google Scholar] [CrossRef]
- LiPuma, J.J. Burkholderia cepacia epidemiology and pathogenesis: Implications for infection control. Curr. Opin. Pulm. Med. 1998, 4, 337–341. [Google Scholar] [CrossRef]
- Eberl, L.; Tümmler, B. Pseudomonas aeruginosa and Burkholderia cepacia in cystic fibrosis: Genome evolution, interactions and adaptation. Int. J. Med. Microbiol. 2004, 294, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Isles, A.; Maclusky, I.; Corey, M.; Gold, R.; Prober, C.; Fleming, P.; Levison, H. Pseudomonas cepacia infection in cystic fibrosis: An emerging problem. J. Pediatr. 1984, 104, 206–210. [Google Scholar] [CrossRef]
- Somayaji, R.; Yau, Y.C.W.; Tullis, E.; LiPuma, J.J.; Ratjen, F.; Waters, V. Clinical outcomes associated with Burkholderia cepacia complex infection in patients with cystic fibrosis. Ann. Am. Thorac. Soc. 2020, 17, 1542–1548. [Google Scholar] [CrossRef]
- Porter, L.A.; Goldberg, J.B. Influence of neutrophil defects on Burkholderia cepacia complex pathogenesis. Front. Cell. Infect. Microbiol. 2011, 1, 9. [Google Scholar] [CrossRef]
- Saiman, L.; Siegel, J. Infection control in cystic fibrosis. Clin. Microbiol. Rev. 2004, 17, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.E.; Short, P.; Carter, P.E. Species distribution of Burkholderia cepacia complex isolates in cystic fibrosis and non-cystic fibrosis patients in New Zealand. J. Cyst. Fibros. 2010, 9, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Cystic Fibrosis Foundation Patient Registry (CFFPR). Annual Data Report, 2021; CFFPR: Bethesda, MD, USA, 2021. [Google Scholar]
- UK Cystic Fibrosis Registry (UKCFR). Annual Data Report, 2021; UKCFR: London, UK, 2021. [Google Scholar]
- Mojica, M.F.; Zeiser, E.T.; Becka, S.A.; LiPuma, J.J.; Six, D.A.; Moeck, G.; Papp-Wallace, K.M. Examining the activity of cefepime-taniborbactam against Burkholderia cepacia complex and Burkholderia gladioli isolated from cystic fibrosis patients in the United States. Antimicrob. Agents Chemother. 2023, 67, e0049823. [Google Scholar] [CrossRef] [PubMed]
- Rotrosen, D.; Gallin, J.I. Disorders of phagocyte function. Annu. Rev. Immunol. 1987, 5, 127–150. [Google Scholar] [CrossRef]
- Dinauer, M.C. Chronic granulomatous disease and other disorders of phagocyte function. Hematol. Am. Soc. Hematol. Educ. Program. 2005, 2005, 89–95. [Google Scholar] [CrossRef]
- Holland, S.M. Chronic granulomatous disease. Clin. Rev. Allergy Immunol. 2010, 38, 3–10. [Google Scholar] [CrossRef]
- Kuhns, D.B.; Alvord, W.G.; Heller, T.; Feld, J.J.; Pike, K.M.; Marciano, B.E.; Uzel, G.; DeRavin, S.S.; Priel, D.A.; Soule, B.P.; et al. Residual NADPH oxidase and survival in chronic granulomatous disease. N. Engl. J. Med. 2010, 363, 2600–2610. [Google Scholar] [CrossRef] [PubMed]
- Aris, R.M.; Routh, J.C.; LiPuma, J.J.; Heath, D.G.; Gilligan, P.H. Lung transplantation for cystic fibrosis patients with Burkholderia cepacia complex. Survival linked to genomovar type. Am. J. Respir. Crit. Care Med. 2001, 164, 2102–2106. [Google Scholar] [CrossRef]
- Staudacher, O.; von Bernuth, H. Clinical presentation, diagnosis, and treatment of chronic granulomatous disease. Front. Pediatr. 2024, 12, 1384550. [Google Scholar] [CrossRef] [PubMed]
- Alexander, B.D.; Petzold, E.W.; Reller, L.B.; Palmer, S.M.; Davis, R.D.; Woods, C.W.; LiPuma, J.J. Survival after lung transplantation of cystic fibrosis patients infected with Burkholderia cepacia complex. Am. J. Transplant. 2008, 8, 1025–1030. [Google Scholar] [CrossRef] [PubMed]
- Dance, D.A. Melioidosis: The tip of the iceberg? Clin. Microbiol. Rev. 1991, 4, 52–60. [Google Scholar] [CrossRef]
- Naigowit, P.; Maneeboonyoung, W.; Wongroonsub, P.; Chaowagul, V.; Kanai, K. Serosurveillance for Pseudomonas pseudomallei infection in Thailand. Jpn. J. Med. Sci. Biol. 1992, 45, 215–230. [Google Scholar] [CrossRef][Green Version]
- Hinjoy, S.; Hantrakun, V.; Kongyu, S.; Kaewrakmuk, J.; Wangrangsimakul, T.; Jitsuronk, S.; Saengchun, W.; Bhengsri, S.; Akarachotpong, T.; Thamthitiwat, S.; et al. Melioidosis in Thailand: Present and future. Trop. Med. Infect. Dis. 2018, 3, 38. [Google Scholar] [CrossRef]
- Wuthiekanun, V.; Peacock, S.J. Management of melioidosis. Expert Rev. Anti-Infect. Ther. 2006, 4, 445–455. [Google Scholar] [CrossRef]
- Chakravorty, A.; Heath, C.H. Melioidosis: An updated review. Aust. J. Gen. Pract. 2019, 48, 327–332. [Google Scholar] [CrossRef]
- Ulett, G.C.; Ketheesan, N.; Hirst, R.G. Cytokine gene expression in innately susceptible BALB/c mice and relatively resistant C57BL/6 mice during infection with virulent Burkholderia pseudomallei. Infect. Immun. 2000, 68, 2034–2042. [Google Scholar] [CrossRef]
- Birnie, E.; Weehuizen, T.A.F.; Lankelma, J.M.; de Jong, H.K.; Koh, G.C.K.W.; van Lieshout, M.H.P.; Roelofs, J.J.T.H.; Budding, A.E.; de Vos, A.F.; van der Poll, T.; et al. Role of Toll-Like Receptor 5 (TLR5) in Experimental Melioidosis. Infect. Immun. 2019, 87, e00409-18. [Google Scholar] [CrossRef]
- De Volder, A.L.; Teves, S.; Isasmendi, A.; Pinheiro, J.L.; Ibarra, L.; Breglia, N.; Herrera, T.; Vazquez, M.; Hernandez, C.; Degrossi, J. Distribution of Burkholderia cepacia complex species isolated from industrial processes and contaminated products in Argentina. Int. Microbiol. 2021, 24, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Song, L.; Wang, Y.; Suo, J.; Bai, Y.; Xing, Y.; Xie, L.; Liu, B.; Li, L.; Luo, Y.; et al. Investigation and control of an outbreak of urinary tract infections caused by Burkholderia cepacia-contaminated anesthetic gel. Antimicrob. Resist. Infect. Control. 2021, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Bazani, V.B.; da Silva, A.C.F.; de Pádua Silva , K.; Müller, K.C. Contamination of pharmaceutical products by the Burkholderia cepacia complex and its possible impacts on healthcare and industry: A bibliographic review. Res. Soc. Dev. 2024, 13, e10313245032. [Google Scholar] [CrossRef]
- Periaiah, P.; Antony, T.; Samuel, S. Identification of Burkholderia cepacia Complex: Comparing Conventional, Automated, and Molecular Methods in a Tertiary Care Center. Cureus 2024, 16, e70847. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration (FDA). FDA Updates on Burkholderia Cepacia Contamination. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-updates-2017-burkholderia-cepacia-contamination (accessed on 6 February 2025).
- Jimenez, L. Microbial diversity in pharmaceutical product recalls and environments. PDA J. Pharm. Sci. Technol. 2007, 61, 383–399. [Google Scholar]
- Sutton, S.; Jimenez, L. A review of reported recalls involving microbiological control 2004–2011 with emphasis on FDA considerations of objectionable organisms. Am. Pharm. Rev. 2012, 15, 42–57. Available online: https://www.americanpharmaceuticalreview.com/Featured-Articles/38382-A-Review-of-Reported-Recalls-Involving-Microbiological-Control-2004-2011-with-Emphasis-on-FDA-Considerations-of-Objectionable-Organisms/ (accessed on 6 February 2025).
- Jimenez, L. Analysis of FDA Enforcement Reports (2012–2019) to Determine the Microbial Diversity in Contaminated Non-Sterile and Sterile Drugs. Am. Pharm. Rev. 2019, 21, 48–73. Available online: https://www.americanpharmaceuticalreview.com/Featured-Articles/518912-Analysis-of-FDA-Enforcement-Reports-2012-2019-to-Determine-the-Microbial-Diversity-in-Contaminated-Non-Sterile-and-Sterile-Drugs/ (accessed on 6 February 2025).
- Gama Healthcare. Contamination of Healthcare Products by Burkholderia cepacia Complex. Available online: https://gamahealthcare.com/latest/contamination-of-healthcare-products-by-burkholderia-cepacia-complex/ (accessed on 6 February 2025).
- Sales, F.L.S.; Aguiar, A.R.; Miranda, R.V.S.L.; Valadão, T.B.; Costa, L.V.; Brandão, M.L.L.; Santana, G.S. Antimicrobial resistance profile and aggregation capacity evaluation of Burkholderia cepacia complex strains isolated in a pharmaceutical facility. In Proceedings of the 8th International Symposium on Immunobiological, Rio de Janeiro, Brazil, 8–10 May 2024; Bio-Manguinhos: Rio de Janeiro, Brazil, 2024; p. 144. [Google Scholar] [CrossRef]
- Food and Drug Administration (FDA). FDA Advises Drug Manufacturers That Burkholderia cepacia Complex Poses a Contamination Risk in Non-Sterile, Water-Based Drug Products. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-advises-drug-manufacturers-burkholderia-cepacia-complex-poses-contamination-risk-non-sterile (accessed on 6 February 2025).
- Kumar, S.P.; Uthra, K.T.; Chitra, V.; Damodharan, N.; Pazhani, G.P. Challenges and mitigation strategies associated with Burkholderia cepacia complex contamination in pharmaceutical manufacturing. Arch. Microbiol. 2024, 206, 159. [Google Scholar] [CrossRef]
- Rhodes, K.A.; Schweizer, H.P. Antibiotic resistance in Burkholderia species. Drug Resist. Updat. 2016, 28, 82–90. [Google Scholar] [CrossRef]
- Nikaido, H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 2003, 67, 593–656. [Google Scholar] [CrossRef] [PubMed]
- Nair, B.M.; Cheung, K.J., Jr.; Griffith, A.; Burns, J.L. Salicylate induces an antibiotic efflux pump in Burkholderia cepacia complex genomovar III (B. cenocepacia). J. Clin. Investig. 2004, 113, 464–473. [Google Scholar] [CrossRef]
- Buroni, S.; Pasca, M.R.; Flannagan, R.S.; Bazzini, S.; Milano, A.; Bertani, I.; Venturi, V.; Valvano, M.A.; Riccardi, G. Assessment of three Resistance-Nodulation-Cell Division drug efflux transporters of Burkholderia cenocepacia in intrinsic antibiotic resistance. BMC Microbiol. 2009, 9, 200. [Google Scholar] [CrossRef] [PubMed]
- Podnecky, N.L.; Rhodes, K.A.; Schweizer, H.P. Efflux pump-mediated drug resistance in Burkholderia. Front. Microbiol. 2015, 6, 305. [Google Scholar] [CrossRef] [PubMed]
- Tseng, S.P.; Tsai, W.C.; Liang, C.Y.; Lin, Y.S.; Huang, J.W.; Chang, C.Y.; Tyan, Y.C.; Lu, P.L. The contribution of antibiotic resistance mechanisms in clinical Burkholderia cepacia complex isolates: An emphasis on efflux pump activity. PLoS ONE 2014, 9, e104986. [Google Scholar] [CrossRef]
- Frost, F.; Shaw, M.; Nazareth, D. Antibiotic therapy for chronic infection with Burkholderia cepacia complex in people with cystic fibrosis. Cochrane Database Syst. Rev. 2019, 6, CD013079. [Google Scholar] [CrossRef]
- Flemming, H.C.; Neu, T.R.; Wozniak, D.J. The EPS matrix: The “house of biofilm cells”. J. Bacteriol. 2007, 189, 7945–7947. [Google Scholar] [CrossRef]
- Sutherland, I.W. The biofilm matrix—An immobilized but dynamic microbial environment. Trends Microbiol. 2001, 9, 222–227. [Google Scholar] [CrossRef]
- Harrison, J.J.; Ceri, H.; Turner, R.J. Multimetal resistance and tolerance in microbial biofilms. Nat. Rev. Microbiol. 2007, 5, 928–938. [Google Scholar] [CrossRef]
- Brauner, A.; Fridman, O.; Gefen, O.; Balaban, N.Q. Distinguishing between resistance, tolerance and persistence to antibiotic treatment. Nat. Rev. Microbiol. 2016, 14, 320–330. [Google Scholar] [CrossRef]
- Boles, B.R.; Singh, P.K. Endogenous oxidative stress produces diversity and adaptability in biofilm communities. Proc. Natl. Acad. Sci. USA 2008, 105, 12503–12508. [Google Scholar] [CrossRef] [PubMed]
- Sousa, S.A.; Moreira, L.M.; Wopperer, J.; Eberl, L.; Sá-Correia, I.; Leitão, J.H. The Burkholderia cepacia bceA gene encodes a protein with phosphomannose isomerase and GDP-D-mannose pyrophosphorylase activities. Biochem. Biophys. Res. Commun. 2007, 353, 200–206. [Google Scholar] [CrossRef]
- Torbeck, L.; Raccasi, D.; Guilfoyle, D.E.; Friedman, R.L.; Hussong, D. Burkholderia cepacia: This Decision Is Overdue. PDA J. Pharm. Sci. Technol. 2011, 65, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Rose, H.; Baldwin, A.; Dowson, C.G.; Mahenthiralingam, E. Biocide susceptibility of the Burkholderia cepacia complex. J. Antimicrob. Chemother. 2009, 63, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Ahn, Y.; LiPuma, J.J.; Hussong, D.; Cerniglia, C.E. Survival and susceptibility of Burkholderia cepacia complex in chlorhexidine gluconate and benzalkonium chloride. J. Ind. Microbiol. Biotechnol. 2015, 42, 905–913. [Google Scholar] [CrossRef]
- Ahn, Y.; Kim, J.M.; Kweon, O.; Kim, S.J.; Jones, R.C.; Woodling, K.; Gamboa da Costa, G.; LiPuma, J.J.; Hussong, D.; Marasa, B.S.; et al. Intrinsic Resistance of Burkholderia cepacia Complex to Benzalkonium Chloride. mBio 2016, 7, e01716-16. [Google Scholar] [CrossRef]
- Hall, C.W.; Mah, T.F. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol. Rev. 2017, 41, 276–301. [Google Scholar] [CrossRef]
- Lewis, K. Riddle of biofilm resistance. Antimicrob. Agents Chemother. 2001, 45, 999–1007. [Google Scholar] [CrossRef]
- Lebeaux, D.; Ghigo, J.M.; Beloin, C. Biofilm-related infections: Bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiol. Mol. Biol. Rev. 2014, 78, 510–543. [Google Scholar] [CrossRef]
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents. 2010, 35, 322–332. [Google Scholar] [CrossRef]
- Lewis, K. Persister cells and the riddle of biofilm survival. Biochemistry 2005, 70, 267–274. [Google Scholar] [CrossRef]
- Conlon, B.P.; Rowe, S.E.; Lewis, K. Persister cells in biofilm associated infections. Adv. Exp. Med. Biol. 2015, 831, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Dhar, N.; McKinney, J.D. Microbial phenotypic heterogeneity and antibiotic tolerance. Curr. Opin. Microbiol. 2007, 10, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Fauvart, M.; De Groote, V.N.; Michiels, J. Role of persister cells in chronic infections: Clinical relevance and perspectives on anti-persister therapies. J. Med. Microbiol. 2011, 60, 699–709. [Google Scholar] [CrossRef]
- Poole, K. Efflux-mediated antimicrobial resistance. J. Antimicrob. Chemother. 2005, 56, 20–51. [Google Scholar] [CrossRef]
- Huang, L.; Wu, C.; Gao, H.; Xu, C.; Dai, M.; Huang, L.; Hao, H.; Wang, X.; Cheng, G. Bacterial Multi-drug Efflux Pumps at the Frontline of Antimicrobial Resistance: An Overview. Antibiotics 2022, 11, 520. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Z.; Nikaido, H. Efflux-mediated drug resistance in bacteria. Drugs 2004, 64, 159–204. [Google Scholar] [CrossRef]
- Wang, H.; Xu, X.; Shen, Z. Role of efflux pumps in antibiotic resistance of biofilm-forming bacteria. Front. Microbiol. 2011, 2, 229. [Google Scholar]
- Soto, S.M. Role of efflux pumps in the antibiotic resistance of bacteria embedded in a biofilm. Virulence 2013, 4, 223–229. [Google Scholar] [CrossRef]
- Ren, J.; Wang, M.; Zhou, W.; Liu, Z. Efflux pumps as potential targets for biofilm inhibition. Front. Microbiol. 2024, 15, 1315238. [Google Scholar] [CrossRef]
- Mah, T.F.; Pitts, B.; Pellock, B.; Walker, G.C.; Stewart, P.S.; O’Toole, G.A. A genetic basis for Pseudomonas aeruginosa biofilm antibiotic resistance. Nature 2003, 426, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Masuda, N.; Sakagawa, E.; Ohya, S.; Gotoh, N.; Tsujimoto, H.; Nishino, T. Substrate specificities of MexAB-OprM, MexCD-OprJ, and MexXY-OprM efflux pumps in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2000, 44, 3322–3327. [Google Scholar] [CrossRef]
- Muller, C.; Plésiat, P.; Jeannot, K. A two-component regulatory system interconnects resistance to polymyxins, aminoglycosides, fluoroquinolones, and β-lactams in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2011, 55, 1211–1221. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Z.; Nikaido, H.; Poole, K. Role of mexA-mexB-oprM in antibiotic efflux in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 1995, 39, 1948–1953. [Google Scholar] [CrossRef]
- Linares, J.F.; López, J.A.; Camafeita, E.; Albar, J.P.; Rojo, F.; Martínez, J.L. Overexpression of the multidrug efflux pumps MexCD-OprJ and MexEF-OprN is associated with a reduction of type III secretion in Pseudomonas aeruginosa. J. Bacteriol. 2005, 187, 1384–1391. [Google Scholar] [CrossRef]
- Alcalde-Rico, M.; Olivares-Pacheco, J.; Alvarez-Ortega, C.; Cámara, M.; Martínez, J.L. Role of the multidrug resistance efflux pump MexCD-OprJ in the Pseudomonas aeruginosa quorum sensing response. Front. Microbiol. 2018, 9, 2752. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Liao, J.; Petrova, O.E.; Cherny, K.E.; Sauer, K. Elevated levels of the second messenger c-di-GMP contribute to antimicrobial resistance of Pseudomonas aeruginosa. Mol. Microbiol. 2014, 92, 488–506. [Google Scholar] [CrossRef]
- Poudyal, B.; Sauer, K. The ABC of biofilm drug tolerance: The MerR-like regulator BrlR is an activator of ABC transport systems, with PA1874-77 contributing to the tolerance of Pseudomonas aeruginosa biofilms to tobramycin. Antimicrob. Agents Chemother. 2018, 62, e01981-17. [Google Scholar] [CrossRef]
- Poole, K. Multidrug efflux pumps and antimicrobial resistance in Pseudomonas aeruginosa and related organisms. J. Mol. Microbiol. Biotechnol. 2001, 3, 255–264. [Google Scholar]
- Nikaido, H.; Pagès, J.M. Broad-specificity efflux pumps and their role in multidrug resistance of Gram-negative bacteria. FEMS Microbiol. Rev. 2012, 36, 340–363. [Google Scholar] [CrossRef]
- Baugh, S.; Phillips, C.R.; Ekanayaka, A.S.; Piddock, L.J.; Webber, M.A. Inhibition of multidrug efflux as a strategy to prevent biofilm formation. J. Antimicrob. Chemother. 2014, 69, 673–681. [Google Scholar] [CrossRef]
- Saeed, M.; Ahmad, M.; Jamil, I.; Rasheed, F.; Waheed, A.; Khan, H.; Yousaf, S.; Tariq, M.; Rehman, Z.; Ali, N. Burkholderia cepacia complex in disinfectants: Challenges and strategies for outbreak control. JSM Microbiol. 2024, 10, 1061. [Google Scholar]
- Jacobs, A.C.; Thompson, M.G.; Black, C.C.; Kessler, J.L.; Clark, L.P.; McQueary, C.N.; Gancz, H.Y.; Corey, B.W.; Moon, J.K.; Si, Y.; et al. AB5075, a highly virulent isolate of Acinetobacter baumannii, as a model strain for the evaluation of pathogenesis and antimicrobial treatments. mBio 2014, 5, e01076-14. [Google Scholar] [CrossRef] [PubMed]
- Poole, K. Pseudomonas aeruginosa: Resistance to the max. Front. Microbiol. 2011, 2, 65. [Google Scholar] [CrossRef] [PubMed]
- Helmy, N.A.M.; Basyony, A.F.; Tohamy, S.T.K.; Zaki, S.A. In-vivo and In-vitro Therapeutic Perspectives in the Treatment of Burkholderia cepacia Complex Infections: A Review. Azhar Int. J. Pharm. Med. Sci. 2025, 5, 38–61. [Google Scholar] [CrossRef]
- Avgeri, S.G.; Matthaiou, D.K.; Dimopoulos, G.; Grammatikos, A.P.; Falagas, M.E. Therapeutic options for Burkholderia cepacia infections beyond co-trimoxazole: A systematic review of the clinical evidence. Int. J. Antimicrob. Agents 2009, 33, 394–404. [Google Scholar] [CrossRef]
- Aaron, S.D.; Ferris, W.; Henry, D.A.; Speert, D.P.; Macdonald, N.E. Multiple combination bactericidal antibiotic testing for patients with cystic fibrosis infected with Burkholderia cepacia. Am. J. Respir. Crit. Care Med. 2000, 161, 1206–1212. [Google Scholar] [CrossRef]
- Clark, R.B.; Pakiz, C.B.; Hostetter, M.K. Synergistic activity of aminoglycoside–beta-lactam combinations against Pseudomonas aeruginosa with an unusual aminoglycoside antibiogram. Med. Microbiol. Immunol. 1990, 179, 77–86. [Google Scholar] [CrossRef]
- Williamson, R.; Collatz, E.; Gutmann, L. Mécanismes d’action des bêta-lactamines et mécanismes de résistance non enzymatique [Mechanisms of action of beta-lactam antibiotics and mechanisms of non-enzymatic resistance]. Presse Med. 1986, 15, 2282–2289. (In French) [Google Scholar]
- Levison, M.E.; Levison, J.H. Pharmacokinetics and pharmacodynamics of antibacterial agents. Infect. Dis. Clin. N. Am. 2009, 23, 791–815. [Google Scholar] [CrossRef]
- Cheer, S.M.; Waugh, J.; Noble, S. Inhaled tobramycin (TOBI): A review of its use in the management of Pseudomonas aeruginosa infections in patients with cystic fibrosis. Drugs 2003, 63, 2501–2520. [Google Scholar] [CrossRef] [PubMed]
- Gibson, R.L.; Burns, J.L.; Ramsey, B.W. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2003, 168, 918–951. [Google Scholar] [CrossRef]
- Hooper, D.C. Mechanisms of action and resistance of older and newer fluoroquinolones. Clin. Infect. Dis. 2000, 31 (Suppl. 2), S24–S28. [Google Scholar] [CrossRef]
- Shariati, A.; Arshadi, M.; Khosrojerdi, M.A.; Abedinzadeh, M.; Ganjalishahi, M.; Maleki, A.; Heidary, M.; Khoshnood, S. The resistance mechanisms of bacteria against ciprofloxacin and new approaches for enhancing the efficacy of this antibiotic. Front. Public Health 2022, 10, 1025633. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Nation, R.L.; Turnidge, J.D.; Milne, R.W.; Coulthard, K.; Rayner, C.R.; Paterson, D.L. Colistin: The re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect. Dis. 2006, 6, 589–601. [Google Scholar] [CrossRef]
- Lomovskaya, O.; Warren, M.S.; Lee, A.; Galazzo, J.; Fronko, R.; Lee, M.; Blais, J.; Cho, D.; Chamberland, S.; Renau, T.; et al. Identification and characterization of inhibitors of multidrug resistance efflux pumps in Pseudomonas aeruginosa: Novel agents for combination therapy. Antimicrob. Agents Chemother. 2001, 45, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Fernández, L.; Hancock, R.E. Adaptive and mutational resistance: Role of porins and efflux pumps in drug resistance. Clin. Microbiol. Rev. 2012, 25, 661–681. [Google Scholar] [CrossRef]
- Wolcott, R.D.; Rhoads, D.D.; Dowd, S.E. Biofilms and chronic wound inflammation. J. Wound Care 2008, 17, 333–341. [Google Scholar] [CrossRef]
- Borges, E.L.; Spira, J.A.O.; Amorim, G.L.; Coelho, A.C.S.M. Biofilm formation in cutaneous wounds and its behavior in the face of interventions: An integrative review. Rev. Rene 2022, 23, e78112. [Google Scholar] [CrossRef]
- Kaiser, S.; Verboket, R.D.; Frank, J.; Marzi, I.; Janko, M. Effectiveness of combined local therapy with antibiotics and fibrin vs. vacuum-assisted wound therapy in soft tissue infections: A retrospective study. Eur. J. Trauma Emerg. Surg. 2024, 50, 1559–1567. [Google Scholar] [CrossRef]
- McEwan, C.; Fowley, C.; Nomikou, N.; McCaughan, B.; McHale, A.P.; Callan, J.F. Polymeric microbubbles as delivery vehicles for sensitizers in sonodynamic therapy. Langmuir 2014, 30, 14926–14930. [Google Scholar] [CrossRef] [PubMed]
- Lattwein, K.R.; Shekhar, H.; Kouijzer, J.J.P.; van Wamel, W.J.B.; Holland, C.K.; Kooiman, K. Sonobactericide: An Emerging Treatment Strategy for Bacterial Infections. Ultrasound Med. Biol. 2020, 46, 193–215. [Google Scholar] [CrossRef] [PubMed]
- McEwan, C.; Owen, J.; Stride, E.; Fowley, C.; Nesbitt, H.; Cochrane, D.; Coussios, C.C.; Borden, M.; Nomikou, N.; McHale, A.P.; et al. Oxygen carrying microbubbles for enhanced sonodynamic therapy of hypoxic tumours. J. Control. Release 2015, 203, 51–56. [Google Scholar] [CrossRef]
- Nesbitt, H.; Sheng, Y.; Kamila, S.; Logan, K.; Thomas, K.; Callan, B.; Taylor, M.A.; Love, M.; O’Rourke, D.; Kelly, P.; et al. Gemcitabine loaded microbubbles for targeted chemo-sonodynamic therapy of pancreatic cancer. J. Control. Release 2018, 279, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Sirsi, S.R.; Borden, M.A. Advances in ultrasound mediated gene therapy using microbubble contrast agents. Theranostics 2012, 2, 1208–1222. [Google Scholar] [CrossRef]
- Chapla, R.; Huynh, K.T.; Schutt, C.E. Microbubble-Nanoparticle Complexes for Ultrasound-Enhanced Cargo Delivery. Pharmaceutics 2022, 14, 2396. [Google Scholar] [CrossRef]
- Hancock, R.E.; Nijnik, A.; Philpott, D.J. Modulating immunity as a therapy for bacterial infections. Nat. Rev. Microbiol. 2012, 10, 243–254. [Google Scholar] [CrossRef]
- Li, C.H.; Chang, Y.C.; Hsiao, M.; Chan, M.H. Ultrasound and Nanomedicine for Cancer-Targeted Drug Delivery: Screening, Cellular Mechanisms and Therapeutic Opportunities. Pharmaceutics 2022, 14, 1282. [Google Scholar] [CrossRef]
- Min, K.H.; Kim, K.H.; Ki, M.R.; Pack, S.P. Antimicrobial Peptides and Their Biomedical Applications: A Review. Antibiotics 2024, 13, 794. [Google Scholar] [CrossRef]
- Lai, Y.; Gallo, R.L. AMPed up immunity: How antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 2009, 30, 131–141. [Google Scholar] [CrossRef]
- Hancock, R.E.; Sahl, H.G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006, 24, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Mba, I.E.; Nweze, E.I. Antimicrobial Peptides Therapy: An Emerging Alternative for Treating Drug-Resistant Bacteria. Yale J. Biol. Med. 2022, 95, 445–463. [Google Scholar] [PubMed]
- Falagas, M.E.; Kasiakou, S.K. Colistin: The revival of polymyxins for the management of multi-drug-resistant gram-negative bacterial infections. Clin. Infect. Dis. 2005, 40, 1333–1341. [Google Scholar] [CrossRef]
- Velkov, T.; Thompson, P.E.; Nation, R.L.; Li, J. Structure-activity relationships of polymyxin antibiotics. J. Med. Chem. 2010, 53, 1898–1916. [Google Scholar] [CrossRef]
- Bals, R. Epithelial antimicrobial peptides in host defense against infection. Respir. Res. 2000, 1, 141–150. [Google Scholar] [CrossRef]
- Nijnik, A.; Pistolic, J.; Wyatt, A.; Tam, S.; Hancock, R.E. Human cathelicidin peptide LL-37 modulates the effects of IFN-γ on APCs. J. Immunol. 2009, 183, 5788–5798. [Google Scholar] [CrossRef]
- Nizet, V.; Ohtake, T.; Lauth, X.; Trowbridge, J.; Rudisill, J.; Dorschner, R.A.; Pestonjamasp, V.; Piraino, J.; Huttner, K.; Gallo, R.L. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature 2001, 414, 454–457. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T. Defensins: Antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 2003, 3, 710–720. [Google Scholar] [CrossRef]
- Cole, A.M.; Waring, A.J. The role of defensins in lung biology and therapy. Am. J. Respir. Med. 2002, 1, 249–259. [Google Scholar] [CrossRef]
- Selsted, M.E.; Ouellette, A.J. Mammalian defensins in the antimicrobial immune response. Nat. Immunol. 2005, 6, 551–557. [Google Scholar] [CrossRef]
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef]
- Hancock, R.E.; Scott, M.G. The role of antimicrobial peptides in animal defenses. Proc. Natl. Acad. Sci. USA 2000, 97, 8856–8861. [Google Scholar] [CrossRef] [PubMed]
- Avci, F.G.; Akbulut, B.S.; Ozkirimli, E. Membrane Active Peptides and Their Biophysical Characterization. Biomolecules 2018, 8, 77. [Google Scholar] [CrossRef] [PubMed]
- Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef]
- Merrifield, R.B. Solid-phase peptide synthesis. Adv. Enzymol. Relat. Areas Mol. Biol. 1969, 32, 221–296. [Google Scholar] [CrossRef]
- Yount, N.Y.; Yeaman, M.R. Peptide antimicrobials: Cell wall as a bacterial target. Ann. N. Y. Acad. Sci. 2013, 1277, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Mahlapuu, M.; Håkansson, J.; Ringstad, L.; Björn, C. Antimicrobial peptides: An emerging category of therapeutic agents. Front. Cell. Infect. Microbiol. 2016, 6, 194. [Google Scholar] [CrossRef]
- Rumbaugh, K.P.; Sauer, K. Biofilm dispersion. Nat. Rev. Microbiol. 2020, 18, 571–586. [Google Scholar] [CrossRef]
- Koo, H.; Allan, R.N.; Howlin, R.P.; Stoodley, P.; Hall-Stoodley, L. Targeting microbial biofilms: Current and prospective therapeutic strategies. Nat. Rev. Microbiol. 2017, 15, 740–755. [Google Scholar] [CrossRef]
- Valentini, M.; Filloux, A. Biofilms and cyclic di-GMP (c-di-GMP) signaling: Lessons from Pseudomonas aeruginosa and other bacteria. J. Biol. Chem. 2016, 291, 12547–12555. [Google Scholar] [CrossRef]
- Mattick, J.S. Type IV pili and twitching motility. Annu. Rev. Microbiol. 2002, 56, 289–314. [Google Scholar] [CrossRef] [PubMed]
- Römling, U.; Galperin, M.Y.; Gomelsky, M. Cyclic di-GMP: The first 25 years of a universal bacterial second messenger. Microbiol. Mol. Biol. Rev. 2013, 77, 1–52. [Google Scholar] [CrossRef]
- Kaplan, J.B.; Ragunath, C.; Velliyagounder, K.; Fine, D.H.; Ramasubbu, N. Enzymatic detachment of Staphylococcus epidermidis biofilms. Antimicrob. Agents Chemother. 2004, 48, 2633–2636. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.B.; Velliyagounder, K.; Ragunath, C.; Rohde, H.; Mack, D.; Knobloch, J.K.; Ramasubbu, N. Genes involved in the synthesis and degradation of matrix polysaccharide in Actinobacillus actinomycetemcomitans and Actinobacillus pleuropneumoniae biofilms. J. Bacteriol. 2004, 186, 8213–8220. [Google Scholar] [CrossRef]
- Iwase, T.; Uehara, Y.; Shinji, H.; Tajima, A.; Seo, H.; Takada, K.; Agata, T.; Mizunoe, Y. Staphylococcus epidermidis Esp inhibits Staphylococcus aureus biofilm formation and nasal colonization. Nature 2010, 465, 346–349. [Google Scholar] [CrossRef]
- Tetz, V.V.; Tetz, G.V. Effect of extracellular DNA destruction by DNase I on characteristics of forming biofilms. DNA Cell Biol. 2010, 29, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.B.; Jabbouri, S.; Sadovskaya, I. Extracellular DNA-dependent biofilm formation by Staphylococcus epidermidis RP62A in response to subminimal inhibitory concentrations of antibiotics. Res. Microbiol. 2011, 162, 535–541. [Google Scholar] [CrossRef]
- Fuji, S.; Löffler, J.; Einsele, H.; Kapp, M. Immunotherapy for opportunistic infections: Current status and future perspectives. Virulence 2016, 7, 939–949. [Google Scholar] [CrossRef]
- Schoggins, J.W. Interferon-Stimulated Genes: What Do They All Do? Annu. Rev. Virol. 2019, 6, 567–584. [Google Scholar] [CrossRef]
- Cetean, S.; Căinap, C.; Constantin, A.M.; Căinap, S.; Gherman, A.; Oprean, L.; Hangan, A.; Oprean, R. The importance of the granulocyte-colony stimulating factor in oncology. Clujul Med. 2015, 88, 468–472. [Google Scholar] [CrossRef]
- Zhan, Y.; Lieschke, G.J.; Grail, D.; Dunn, A.R.; Cheers, C. Essential roles for granulocyte-macrophage colony-stimulating factor (GM-CSF) and G-CSF in the sustained hematopoietic response of Listeria monocytogenes-infected mice. Blood 1998, 91, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, D. The colony-stimulating factors and cancer. Nat. Rev. Cancer 2010, 10, 425–434. [Google Scholar] [CrossRef]
- Sarkar-Tyson, M.; Titball, R.W. Progress toward development of vaccines against melioidosis: A review. Clin. Ther. 2010, 32, 1437–1445. [Google Scholar] [CrossRef] [PubMed]
- Bondi, S.K.; Goldberg, J.B. Strategies toward vaccines against Burkholderia mallei and Burkholderia pseudomallei. Expert Rev. Vaccines 2008, 7, 1357–1365. [Google Scholar] [CrossRef]
- Wang, G.; Zarodkiewicz, P.; Valvano, M.A. Current Advances in Burkholderia Vaccines Development. Cells 2020, 9, 2671. [Google Scholar] [CrossRef] [PubMed]
- Sette, A.; Rappuoli, R. Reverse vaccinology: Developing vaccines in the era of genomics. Immunity 2010, 33, 530–541. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef]
- Liu, M.A. A Comparison of Plasmid DNA and mRNA as Vaccine Technologies. Vaccines 2019, 7, 37. [Google Scholar] [CrossRef]
- Sulakvelidze, A.; Alavidze, Z.; Morris, J.G., Jr. Bacteriophage therapy. Antimicrob. Agents Chemother. 2001, 45, 649–659. [Google Scholar] [CrossRef]
- Wright, A.; Hawkins, C.H.; Anggård, E.E.; Harper, D.R. A controlled clinical trial of a therapeutic bacteriophage preparation in chronic otitis due to antibiotic-resistant Pseudomonas aeruginosa; a preliminary report of efficacy. Clin. Otolaryngol. 2009, 34, 349–357. [Google Scholar] [CrossRef]
- Clokie, M.R.; Millard, A.D.; Letarov, A.V.; Heaphy, S. Phages in nature. Bacteriophage 2011, 1, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Kortright, K.E.; Chan, B.K.; Koff, J.L.; Turner, P.E. Phage Therapy: A Renewed Approach to Combat Antibiotic-Resistant Bacteria. Cell Host Microbe 2019, 25, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Calero-Cáceres, W.; Ye, M.; Balcázar, J.L. Bacteriophages as Environmental Reservoirs of Antibiotic Resistance. Trends Microbiol. 2019, 27, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, I.W.; Hughes, K.A.; Skillman, L.C.; Tait, K. The interaction of phage and biofilms. FEMS Microbiol. Lett. 2004, 232, 1–6. [Google Scholar] [CrossRef]
- Lin, D.M.; Koskella, B.; Lin, H.C. Phage therapy: An alternative to antibiotics in the age of multi-drug resistance. World J. Gastrointest. Pharmacol. Ther. 2017, 8, 162–173. [Google Scholar] [CrossRef]
- Górski, A.; Międzybrodzki, R.; Węgrzyn, G.; Jończyk-Matysiak, E.; Borysowski, J.; Weber-Dąbrowska, B. Phage therapy: Current status and perspectives. Med. Res. Rev. 2020, 40, 459–463. [Google Scholar] [CrossRef]
- O’Flaherty, S.; Ross, R.P.; Coffey, A. Bacteriophage and their lysins for elimination of infectious bacteria. FEMS Microbiol. Rev. 2009, 33, 801–819. [Google Scholar] [CrossRef]
- Fischetti, V.A. Bacteriophage lysins as effective antibacterials. Curr. Opin. Microbiol. 2008, 11, 393–400. [Google Scholar] [CrossRef]
- Wintachai, P.; Surachat, K.; Singkhamanan, K. Isolation and Characterization of a Novel Autographiviridae Phage and Its Combined Effect with Tigecycline in Controlling Multidrug-Resistant Acinetobacter baumannii-Associated Skin and Soft Tissue Infections. Viruses 2022, 14, 194. [Google Scholar] [CrossRef]
- Abedon, S.T.; Kuhl, S.J.; Blasdel, B.G.; Kutter, E.M. Phage treatment of human infections. Bacteriophage 2011, 1, 66–85. [Google Scholar] [CrossRef]
- Chan, B.K.; Abedon, S.T.; Loc-Carrillo, C. Phage cocktails and the future of phage therapy. Future Microbiol. 2013, 8, 769–783. [Google Scholar] [CrossRef] [PubMed]
- Manohar, P.; Nachimuthu, R.; Lopes, B.S. The therapeutic potential of bacteriophages targeting gram-negative bacteria using Galleria mellonella infection model. BMC Microbiol. 2018, 18, 97. [Google Scholar] [CrossRef]
- Furfaro, L.L.; Payne, M.S.; Chang, B.J. Bacteriophage Therapy: Clinical Trials and Regulatory Hurdles. Front. Cell. Infect. Microbiol. 2018, 8, 376. [Google Scholar] [CrossRef] [PubMed]
- Loc-Carrillo, C.; Abedon, S.T. Pros and cons of phage therapy. Bacteriophage 2011, 1, 111–114. [Google Scholar] [CrossRef]
- Sahoo, K.; Meshram, S. The Evolution of Phage Therapy: A Comprehensive Review of Current Applications and Future Innovations. Cureus 2024, 16, e70414. [Google Scholar] [CrossRef]
- Davies, D.G.; Marques, C.N. A fatty acid messenger is responsible for inducing dispersion in microbial biofilms. J. Bacteriol. 2009, 191, 1393–1403. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.H.; Zhang, L.H. Quorum sensing and quorum-quenching enzymes. J. Microbiol. 2005, 43, 101–109. [Google Scholar]
- Fetzner, S. Quorum quenching enzymes. J. Biotechnol. 2015, 201, 2–14. [Google Scholar] [CrossRef]
- LaSarre, B.; Federle, M.J. Exploiting quorum sensing to confuse bacterial pathogens. Microbiol. Mol. Biol. Rev. 2013, 77, 73–111. [Google Scholar] [CrossRef]
- Rasmussen, T.B.; Givskov, M. Quorum sensing inhibitors: A bargain of effects. Microbiology 2006, 152, 895–904. [Google Scholar] [CrossRef]
- Papenfort, K.; Bassler, B.L. Quorum sensing signal-response systems in Gram-negative bacteria. Nat. Rev. Microbiol. 2016, 14, 576–588. [Google Scholar] [CrossRef]
- Hentzer, M.; Riedel, K.; Rasmussen, T.B.; Heydorn, A.; Andersen, J.B.; Parsek, M.R.; Rice, S.A.; Eberl, L.; Molin, S.; Høiby, N.; et al. Inhibition of quorum sensing in Pseudomonas aeruginosa biofilm bacteria by a halogenated furanone compound. Microbiology 2002, 148, 87–102. [Google Scholar] [CrossRef] [PubMed]
- Kalia, V.C. Quorum sensing inhibitors: An overview. Biotechnol. Adv. 2013, 31, 224–245. [Google Scholar] [CrossRef]
- Slachmuylders, L.; Van Acker, H.; Brackman, G.; Sass, A.; Van Nieuwerburgh, F.; Coenye, T. Elucidation of the mechanism behind the potentiating activity of baicalin against Burkholderia cenocepacia biofilms. PLoS ONE 2018, 13, e0190533. [Google Scholar] [CrossRef] [PubMed]
- Faleye, O.S.; Sathiyamoorthi, E.; Lee, J.H.; Lee, J. Inhibitory Effects of Cinnamaldehyde Derivatives on Biofilm Formation and Virulence Factors in Vibrio Species. Pharmaceutics 2021, 13, 2176. [Google Scholar] [CrossRef] [PubMed]
- Brackman, G.; Celen, S.; Hillaert, U.; Van Calenbergh, S.; Cos, P.; Maes, L.; Nelis, H.J.; Coenye, T. Structure-activity relationship of cinnamaldehyde analogs as inhibitors of AI-2 based quorum sensing and their effect on virulence of Vibrio spp. PLoS ONE 2011, 6, e16084. [Google Scholar] [CrossRef]
- Ragab, A.; Fouad, S.A.; Ammar, Y.A.; Aboul-Magd, D.S.; Abusaif, M.S. Antibiofilm and Anti-Quorum-Sensing Activities of Novel Pyrazole and Pyrazolo [1,5-a]pyrimidine Derivatives as Carbonic Anhydrase I and II Inhibitors: Design, Synthesis, Radiosterilization, and Molecular Docking Studies. Antibiotics 2023, 12, 128. [Google Scholar] [CrossRef]
- Husain, F.M.; Perveen, K.; Qais, F.A.; Ahmad, I.; Alfarhan, A.H.; El-Sheikh, M.A. Naringin inhibits the biofilms of metallo-β-lactamases (MβLs) producing Pseudomonas species isolated from camel meat. Saudi J. Biol. Sci. 2021, 28, 333–341. [Google Scholar] [CrossRef]
- Rasko, D.A.; Sperandio, V. Anti-virulence strategies to combat bacteria-mediated disease. Nat. Rev. Drug Discov. 2010, 9, 117–128. [Google Scholar] [CrossRef]
- Brackman, G.; Cos, P.; Maes, L.; Nelis, H.J.; Coenye, T. Quorum sensing inhibitors increase the susceptibility of bacterial biofilms to antibiotics in vitro and in vivo. Antimicrob. Agents Chemother. 2011, 55, 2655–2661. [Google Scholar] [CrossRef]
- Defoirdt, T.; Brackman, G.; Coenye, T. Quorum sensing inhibitors: How strong is the evidence? Trends Microbiol. 2013, 21, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Beyth, N.; Houri-Haddad, Y.; Domb, A.; Khan, W.; Hazan, R. Alternative antimicrobial approach: Nano-antimicrobial materials. Evid. Based Complement. Alternat. Med. 2015, 2015, 246012. [Google Scholar] [CrossRef]
- Li, Q.; Mahendra, S.; Lyon, D.Y.; Brunet, L.; Liga, M.V.; Li, D.; Alvarez, P.J. Antimicrobial nano-materials for water disinfection and microbial control: Potential applications and implications. Water Res. 2008, 42, 4591–4602. [Google Scholar] [CrossRef] [PubMed]
- Hajareh Haghighi, F.; Mercurio, M.; Cerra, S.; Salamone, T.A.; Bianymotlagh, R.; Palocci, C.; Romano Spica, V.; Fratoddi, I. Surface modification of TiO2 nanoparticles with organic molecules and their biological applications. J. Mater. Chem. B 2023, 11, 2334–2366. [Google Scholar] [CrossRef]
- Rabea, E.I.; Badawy, M.E.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef]
- Fathil, M.A.; Faris Taufeq, F.Y.; Suleman Ismail Abdalla, S.; Katas, H. Roles of chitosan in synthesis, antibacterial and anti-biofilm properties of bionano silver and gold. RSC Adv. 2022, 12, 19297–19312. [Google Scholar] [CrossRef]
- Shehabeldine, A.M.; Salem, S.S.; Ali, O.M.; Abd-Elsalam, K.A.; Elkady, F.M.; Hashem, A.H. Multifunctional silver nanoparticles based on chitosan: Antibacterial, antibiofilm, antifungal, antioxidant, and wound-healing activities. J. Fungi 2022, 8, 612. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, E.M.P.; Martins, C.C.B.; de Oliveira Santos, J.V.; da Silva, W.R.C.; Silva, S.B.C.; Pelagio-Flores, M.A.; Galembeck, A.; Cavalcanti, I.M.F. Silver nanoparticles-chitosan composites activity against resistant bacteria: Tolerance and biofilm inhibition. J. Nanopart. Res. 2021, 23, 196. [Google Scholar] [CrossRef]
- Mocanu, G.; Nichifor, M.; Picton, L.; About-Jaudet, E.; Le Cerf, D. Preparation and characterization of anionic pullulan thermoassociative nanoparticles for drug delivery. Carbohydr. Polym. 2014, 111, 892–900. [Google Scholar] [CrossRef]
- Murthy, P.S.; Murali Mohan, Y.; Varaprasad, K.; Sreedhar, B.; Mohana Raju, K. First successful design of semi-IPN hydrogel-silver nanocomposites: A facile approach for antibacterial application. J. Colloid Interface Sci. 2008, 318, 217–224. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Q.; Han, N.; Bai, L.; Li, J.; Liu, J.; Che, E.; Hu, L.; Zhang, Q.; Jiang, T.; et al. Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomedicine 2015, 11, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S.; Costerton, J.W. Antibiotic resistance of bacteria in biofilms. Lancet 2001, 358, 135–138. [Google Scholar] [CrossRef]
- Caubet, R.; Pedarros-Caubet, F.; Chu, M.; Freye, E.; de Belém Rodrigues, M.; Moreau, J.M.; Ellison, W.J. A radio frequency electric current enhances antibiotic efficacy against bacterial biofilms. Antimicrob. Agents Chemother. 2004, 48, 4662–4664. [Google Scholar] [CrossRef]
- Niepa, T.H.; Snepenger, L.M.; Wang, H.; Sivan, S.; Gilbert, J.L.; Jones, M.B.; Ren, D. Sensitizing Pseudomonas aeruginosa to antibiotics by electrochemical disruption of membrane functions. Biomaterials 2016, 74, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Seelman, S.L.; Bazaco, M.C.; Wellman, A.; Hardy, C.; Fatica, M.K.; Huang, M.J.; Brown, A.M.; Garner, K.; Yang, W.C.; Norris, C.; et al. Burkholderia cepacia complex outbreak linked to a no-rinse cleansing foam product, United States—2017–2018. Epidemiol. Infect. 2022, 150, e154. [Google Scholar] [CrossRef]
- Martin, M.; Christiansen, B.; Caspari, G.; Hogardt, M.; von Thomsen, A.J.; Ott, E.; Mattner, F. Hospital-wide outbreak of Burkholderia contaminans caused by prefabricated moist washcloths. J. Hosp. Infect. 2011, 77, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Omar, N.; Abd El Raouf, H.; Okasha, H.; Nabil, N. Microbiological assessment of Burkholderia cepacia complex (Bcc) isolates in Alexandria Main University Hospital. Alex. J. Med. 2015, 51, 41–46. [Google Scholar] [CrossRef]
- Lima, J.R.O.; Marques, S.G.; Gonçalves, A.G.; Salgado Filho, N.; Nunes, P.C. Burkholderia cepacia contamination in hemodialysis units in Brazil. Braz. J. Nephrol. 2005, 27, 89–94. [Google Scholar] [CrossRef]
- Coutinho, C.P.; Barreto, C.; Pereira, L.; Lito, L.; Melo Cristino, J.; Sá-Correia, I. Incidence of Burkholderia contaminans at a cystic fibrosis centre with an unusually high representation of Burkholderia cepacia during 15 years of epidemiological surveillance. J. Med. Microbiol. 2015, 64, 927–935. [Google Scholar] [CrossRef]
- El Chakhtoura, N.G.; Saade, E.; Wilson, B.M.; Perez, F.; Papp-Wallace, K.M.; Bonomo, R.A. A 17-Year Nationwide Study of Burkholderia cepacia Complex Bloodstream Infections Among Patients in the United States Veterans Health Administration. Clin. Infect. Dis. 2017, 65, 1253–1259. [Google Scholar] [CrossRef]
- Dolan, S.A.; Dowell, E.; LiPuma, J.J.; Valdez, S.; Chan, K.; James, J.F. An outbreak of Burkholderia cepacia complex associated with intrinsically contaminated nasal spray. Infect. Control Hosp. Epidemiol. 2011, 32, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Lalitha, P.; Das, M.; Purva, P.S.; Karpagam, R.; Geetha, M.; Lakshmi Priya, J.; Naresh Babu, K. Postoperative endophthalmitis due to Burkholderia cepacia complex from contaminated anaesthetic eye drops. Br. J. Ophthalmol. 2014, 98, 1498–1502. [Google Scholar] [CrossRef] [PubMed]
- Moehring, R.W.; Lewis, S.S.; Isaacs, P.J.; Schell, W.A.; Thomann, W.R.; Althaus, M.M.; Hazen, K.C.; Dicks, K.V.; Lipuma, J.J.; Chen, L.F.; et al. Outbreak of bacteremia due to Burkholderia contaminans linked to intravenous fentanyl from an institutional compounding pharmacy. JAMA Intern. Med. 2014, 174, 606–612. [Google Scholar] [CrossRef]
- Zurita, J.; Mejia, L.; Zapata, S.; Trueba, G.; Vargas, A.C.; Aguirre, S.; Falconi, G. Healthcare-associated respiratory tract infection and colonization in an intensive care unit caused by Burkholderia cepacia isolated in mouthwash. Int. J. Infect. Dis. 2014, 29, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, L.; Xia, S.; Wu, H.; Zhang, R.; Fan, M.; Wang, T. An investigation on surgical-site infection among post-cesarean section patients with Burkholderia cepacia contaminated ultrasonic couplant. Zhonghua Liu Xing Bing Xue Za Zhi 2014, 35, 566–568. [Google Scholar] [CrossRef]
- Ko, S.; An, H.S.; Bang, J.H.; Park, S.W. An outbreak of Burkholderia cepacia complex pseudobacteremia associated with intrinsically contaminated commercial 0.5% chlorhexidine solution. Am. J. Infect. Control 2015, 43, 266–268. [Google Scholar] [CrossRef]
- Nannini, E.C.; Ponessa, A.; Muratori, R.; Marchiaro, P.; Ballerini, V.; Flynn, L.; Limansky, A.S. Polyclonal outbreak of bacteremia caused by Burkholderia cepacia complex and the presumptive role of ultrasound gel. Braz. J. Infect. Dis. 2015, 19, 543–545. [Google Scholar] [CrossRef]
- Singhal, T.; Shah, S.; Naik, R. Outbreak of Burkholderia cepacia complex bacteremia in a chemotherapy day care unit due to intrinsic contamination of an antiemetic drug. Indian J. Med. Microbiol. 2015, 33, 117–119. [Google Scholar] [CrossRef]
- Rolón Ortiz, A.; Rios-González, C.M. Outbreak of Burkholderia cepacia in a hemodialysis unit of Paraguay, 2014. J. Infect. Public Health 2017, 10, 688–689. [Google Scholar] [CrossRef]
- Nazik, S.; Topal, B.; Şahin, A.R.; Ateş, S. Nosocomial Burkholderia cepacia infection in a tertiary hospital; five-year surveillance: A retrospective cross-sectional study. Urology 2018, 6, 10–13. [Google Scholar] [CrossRef]
- Tempo. Anvisa Suspende uso de Sete Lotes de Antisséptico Bucal. 2014. Available online: https://www.otempo.com.br/brasil/anvisa-suspense-uso-de-sete-lotes-de-antisseptico-bucal-1.935778 (accessed on 6 February 2025).
- Wang, L.; Wang, M.; Zhang, J.; Wu, W.; Lu, Y.; Fan, Y. An outbreak of Burkholderia stabilis colonization in a nasal ward. Int. J. Infect. Dis. 2015, 33, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Paul, L.M.; Hegde, A.; Pai, T.; Shetty, S.; Baliga, S.; Shenoy, S. An outbreak of Burkholderia cepacia bacteremia in a neonatal intensive care unit. Indian J. Pediatr. 2016, 83, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Montaño-Remacha, C.; Márquez-Cruz, M.D.; Hidalgo-Guzmán, P.; Sánchez-Porto, A.; Téllez-Pérez, F.P. Brote de bacteriemia por Burkholderia cepacia en una unidad de hemodiálisis de Cádiz, 2014 [An outbreak of Burkholderia cepacia bacteremia in a hemodialysis unit, Cadiz, 2014]. Enferm. Infecc. Microbiol. Clin. 2015, 33, 646–650. [Google Scholar] [CrossRef]
- Shrivastava, B.; Sriram, A.; Shetty, S.; Doshi, R.; Varior, R. An unusual source of Burkholderia cepacia outbreak in a neonatal intensive care unit. J. Hosp. Infect. 2016, 94, 358–360. [Google Scholar] [CrossRef] [PubMed]
- Mali, S.; Dash, L.; Gautam, V.; Shastri, J.; Kumar, S. An outbreak of Burkholderia cepacia complex in the paediatric unit of a tertiary care hospital. Indian J. Med. Microbiol. 2017, 35, 216–220. [Google Scholar] [CrossRef]
- Guo, L.; Li, G.; Wang, J.; Zhao, X.; Wang, S.; Zhai, L.; Jia, H.; Cao, B. Suspicious outbreak of ventilator-associated pneumonia caused by Burkholderia cepacia in a surgical intensive care unit. Am. J. Infect. Control 2017, 45, 660–666. [Google Scholar] [CrossRef]
- Sommerstein, R.; Führer, U.; Lo Priore, E.; Casanova, C.; Meinel, D.M.; Seth-Smith, H.M.; Kronenberg, A.; Anresis; Koch, D.; Senn, L.; et al. Burkholderia stabilis outbreak associated with contaminated commercially-available washing gloves, Switzerland, May 2015 to August 2016. Euro. Surveill. 2017, 22, 17–00213. [Google Scholar] [CrossRef]
- Shaban, R.Z.; Maloney, S.; Gerrard, J.; Collignon, P.; Macbeth, D.; Cruickshank, M.; Hume, A.; Jennison, A.V.; Graham, R.M.A.; Bergh, H.; et al. Outbreak of health care-associated Burkholderia cenocepacia bacteremia and infection attributed to contaminated sterile gel used for central line insertion under ultrasound guidance and other procedures. Am. J. Infect. Control 2017, 45, 954–958. [Google Scholar] [CrossRef]
- Leong, L.E.X.; Lagana, D.; Carter, G.P.; Wang, Q.; Smith, K.; Stinear, T.P.; Shaw, D.; Sintchenko, V.; Wesselingh, S.L.; Bastian, I.; et al. Burkholderia lata infections from intrinsically contaminated chlorhexidine mouthwash, Australia, 2016. Emerg. Infect. Dis. 2018, 24, 2109–2111. [Google Scholar] [CrossRef]
- Antony, B.; Cherian, E.V.; Boloor, R.; Shenoy, K.V. A sporadic outbreak of Burkholderia cepacia complex bacteremia in pediatric intensive care unit of a tertiary care hospital in coastal Karnataka, South India. Indian J. Pathol. Microbiol. 2016, 59, 197–199. [Google Scholar] [CrossRef]
- Marquez, L.; Jones, K.N.; Whaley, E.M.; Koy, T.H.; Revell, P.A.; Taylor, R.S.; Bernhardt, M.B.; Wagner, J.L.; Dunn, J.J.; LiPuma, J.J.; et al. An outbreak of Burkholderia cepacia complex infections associated with contaminated liquid docusate. Infect. Control Hosp. Epidemiol. 2017, 38, 567–573. [Google Scholar] [CrossRef]
- Abdelfattah, R.; Al-Jumaah, S.; Al-Qahtani, A.; Al-Thawadi, S.; Barron, I.; Al-Mofada, S. Outbreak of Burkholderia cepacia bacteremia in a tertiary care centre due to contaminated ultrasound probe gel. J. Hosp. Infect. 2018, 98, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Song, J.E.; Kwak, Y.G.; Um, T.H.; Cho, C.R.; Kim, S.; Park, I.S.; Hwang, J.H.; Kim, N.; Oh, G.B. Outbreak of Burkholderia cepacia pseudobacteremia caused by intrinsically contaminated commercial 0.5% chlorhexidine solution in neonatal intensive care units. J. Hosp. Infect. 2018, 98, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Crist, M.B. Multistate Outbreaks of Burkholderia cepacia Complex Infections Due to Contaminated Medical Products. Apresentação na AFDO Annual Educational Conference. Available online: https://www.afdo.org/wp-content/uploads/2020/09/1530-1610_Matthew-Crist_AFDO-B-cepacia.pdf (accessed on 6 February 2025).
- Brooks, R.B.; Mitchell, P.K.; Miller, J.R.; Vasquez, A.M.; Havlicek, J.; Lee, H.; Quinn, M.; Adams, E.; Baker, D.; Greeley, R.; et al. Multistate Outbreak of Burkholderia cepacia Complex Bloodstream Infections After Exposure to Contaminated Saline Flush Syringes: United States, 2016–2017. Clin. Infect. Dis. 2019, 69, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Nimri, L.; Sulaiman, M.; Hani, O.B. Community-acquired urinary tract infections caused by Burkholderia cepacia complex in patients with no underlying risk factor. JMM Case Rep. 2017, 4, e005081. [Google Scholar] [CrossRef]
- Wong, J.K.; Chambers, L.C.; Elsmo, E.J.; Jenkins, T.L.; Howerth, E.W.; Sánchez, S.; Sakamoto, K. Cellulitis caused by the Burkholderia cepacia complex associated with contaminated chlorhexidine 2% scrub in five domestic cats. J. Vet. Diagn. Investig. 2018, 30, 763–769. [Google Scholar] [CrossRef]
- Rastogi, N.; Khurana, S.; Veeraraghavan, B.; Yesurajan Inbanathan, F.; Rajamani Sekar, S.K.; Gupta, D.; Goyal, K.; Bindra, A.; Sokhal, N.; Panda, A.; et al. Epidemiological investigation and successful management of a Burkholderia cepacia outbreak in a neurotrauma intensive care unit. Int. J. Infect. Dis. 2019, 79, 4–11. [Google Scholar] [CrossRef]
- Gleeson, S.; Mulroy, E.; Bryce, E.; Fox, S.; Taylor, S.L.; Talreja, H. Burkholderia cepacia: An Outbreak in the Peritoneal Dialysis Unit. Perit. Dial. Int. 2019, 39, 92–95. [Google Scholar] [CrossRef]
- Becker, S.L.; Berger, F.K.; Feldner, S.K.; Karliova, I.; Haber, M.; Mellmann, A.; Schäfers, H.J.; Gärtner, B. Outbreak of Burkholderia cepacia complex infections associated with contaminated octenidine mouthwash solution, Germany, August to September 2018. Eurosurveillance 2018, 23, 1800540. [Google Scholar] [CrossRef]
- Zou, Q.; Li, N.; Liu, J.; Li, X.; Wang, Z.; Ai, X.; Tao, F.; Qu, M.; Cai, M.; Hu, Y. Investigation of an outbreak of Burkholderia cepacia infection caused by drug contamination in a tertiary hospital in China. Am. J. Infect. Control 2020, 48, 199–203. [Google Scholar] [CrossRef]
- Wong, S.C.Y.; Wong, S.C.; Chen, J.H.K.; Poon, R.W.S.; Hung, D.L.L.; Chiu, K.H.Y.; So, S.Y.C.; Leung, W.S.; Chan, T.M.; Yap, D.Y.H.; et al. Polyclonal Burkholderia cepacia Complex Outbreak in Peritoneal Dialysis Patients Caused by Contaminated Aqueous Chlorhexidine. Emerg. Infect. Dis. 2020, 26, 1987–1997. [Google Scholar] [CrossRef] [PubMed]
- Plastlabor. Complexo Burkholderia cepacia. Available online: https://plastlabor.com.br/wp-content/uploads/2020/06/PlastLabor.USP_60_Bcc._Junho_2020-1.pdf (accessed on 6 February 2025).
- Association pour les Produits Propres et Parentéraux—Association for Clean and Parenteral Products (A3P Association). Burkholderia Cepacia Strikes Again. Available online: https://www.a3p.org/en/burkholderia-cepacia/ (accessed on 6 February 2025).
- Parenteral Drug Association (PDA). Exclusion of Objectionable Microorganisms from Nonsterile Pharmaceuticals, Over-the-Counter (OTC) Products, Medical Devices, and Cosmetics. Technical Report No. 67. 2023. Available online: https://www.pda.org/bookstore/product-detail/2456-tr-67-exclusion-of-objectionable-microorganisms (accessed on 6 February 2025).
- Jones, A.M.; Dodd, M.E.; Webb, A.K. Burkholderia cepacia: Current clinical issues, environmental controversies and ethical dilemmas. Eur. Respir. J. 2001, 17, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Agência Nacional de Vigilância Sanitária (ANVISA). Resolução da Diretoria Colegiada—RDC nº 658, de 2022. In Dispõe sobre as Boas Práticas de Fabricação de Medicamentos; Diário Oficial da União: Brasília, Brazil, 2022. [Google Scholar]
- United States Pharmacopeia (USP). USP 61: Microbial Enumeration Tests. In United States Pharmacopeia and National Formulary (USP 42-NF 37); United States Pharmacopeial Convention: Rockville, MD, USA, 2019. [Google Scholar]
- United States Pharmacopeia (USP). USP 62: Tests for Specified Microorganisms. In United States Pharmacopeia and National Formulary (USP 42-NF 37); United States Pharmacopeial Convention: Rockville, MD, USA, 2019. [Google Scholar]
- Jimenez, L.; Smalls, S.; Jimenez, L.; Smalls, S. Molecular detection of Burkholderia cepacia in toiletry, cosmetic, and pharmaceutical raw materials and finished products. J. AOAC Int. 2000, 83, 963–966. [Google Scholar] [CrossRef] [PubMed]
- ASTM E2756-19; Standard Terminology Relating to Antimicrobial and Antiviral Agents. ASTM International: West Conshohocken, PA, USA, 2019.
- ASTM E2871-19; Standard Test Methods for Identification of Burkholderia cepacia Complex in Water-Miscible Cosmetics. ASTM International: West Conshohocken, PA, USA, 2019.
- Zhang, L.; Tolan, J.; Lavigne, N.; Montei, C.; Donofrio, R.; Biswas, P. Soleris® Automated System for the Rapid Detection of Burkholderia cepacia Complex in Cosmetic Products. J. AOAC Int. 2022, 106, 171–178. [Google Scholar] [CrossRef]
- Cosmetic, Toiletry, and Fragrance Association (CTFA). Microbiological Guidelines for Cosmetics; CTFA: Washington, DC, USA, 2007. [Google Scholar]
- Cosmetic, Toiletry and Fragrance (CTFA). Guidelines for Microbial Limits in Cosmetics; CTFA: Washington, DC, USA, 2007. [Google Scholar]
- Food and Drug Administration (FDA). (1981/2011). Burkholderia cepacia: This Decision Is Overdue. Available online: https://www.fda.gov/files/about%20fda/published/Burkholderia-cepacia--This-Decision-Is-Overdue-%28PDF-%E2%80%93-285KB%29.pdf (accessed on 6 February 2025).
- Welsh, M.J.; Smith, A.E. Molecular mechanisms of CFTR chloride channel dysfunction in cystic fibrosis. Cell 1993, 73, 1251–1254. [Google Scholar] [CrossRef]
- Ali, M. Burkholderia cepacia in Pharmaceutical Industries. Int. J. Vaccines Vaccin. 2016, 3, 00064. [Google Scholar] [CrossRef]

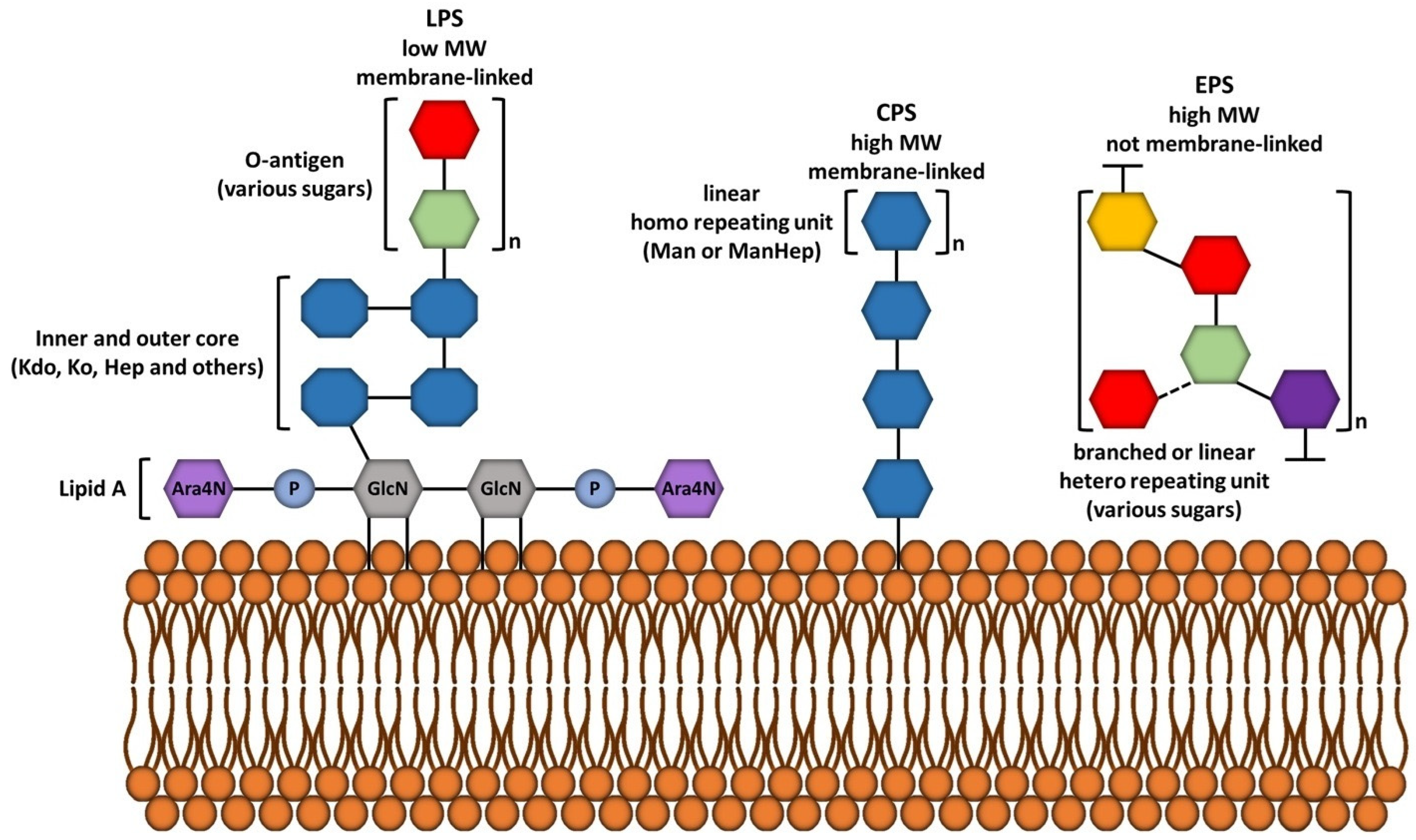

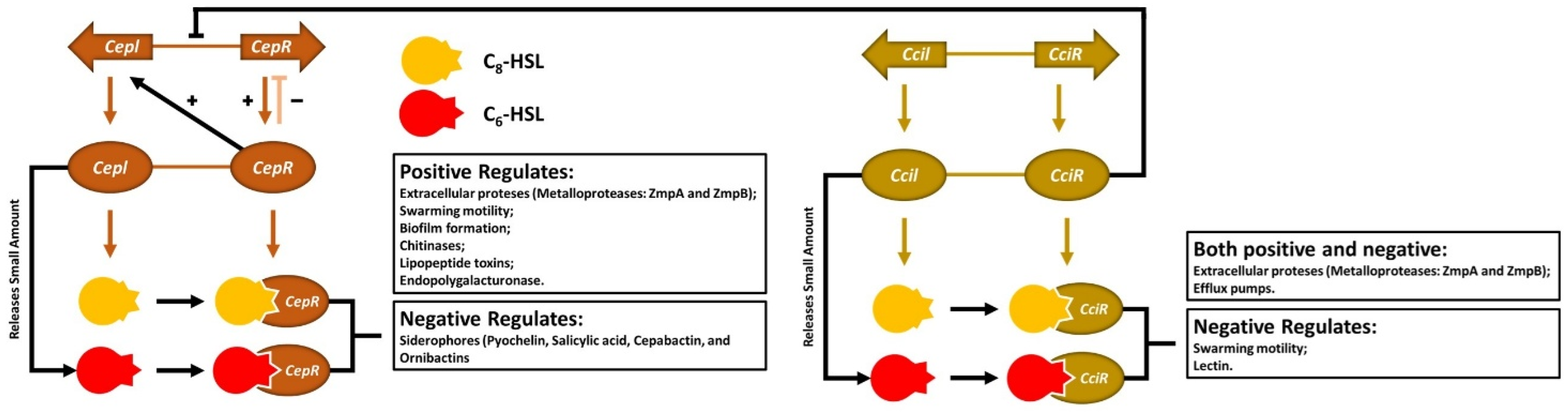
| Period | Country | Location | Product | Species | N° of Infected | Clinical Data | Underlying Conditions | References |
|---|---|---|---|---|---|---|---|---|
| ND | Portugal | CF Center | ND | B. cepacia (62.5%) B. multivorans (25.0%) B. cenocepacia (12.5%) | 32 | CRTI and DPF | CF | [122] |
| 1995–2009 | Portugal | CF Center | ND | B. cepacia (56.5%) B. contaminans (18.5%) B. multivorans (14.8%) B. cenocepacia (7.4%) | 81 | CRTI and DPF | CF | [399] |
| 1999–2015 | USA | VHA | CVC | Bcc | 248 | CVC (41%), PNM (20%), D at 14 days (16%), at 30 days (25%), and at 90 days (36%) | NCDs | [400] |
| ND | Brazil | HDU | Dialysis solution | B. cepacia | ND | ND | CKD, IMS, CVC, and IMDU | [398] |
| 2007–2008 | USA | UH | Nasal spray | Bcc | 36 | ARTC, ARTI, and PNM | IMS and CRIs | [401] |
| 2008–2009 | Germany | TH | Wet wipes | B. contaminans | 29 | ARTI and SW | NCDs, IMS, IMDU, and SW | [396] |
| 2011 | India | OC | Anesthetic eye drops | Bcc | 12 | RDN, Ed, OP, PD, BV, AE, and VL | OS, CAT, and CT | [402] |
| 2012 | USA | TH | Intravenous fentanyl | B. contaminans | 6 | FV, LKC, TCH, and HPT | NCDs and IMDU | [403] |
| 2013 | Ecuador | ICU | Mouthwash | B. cepacia | 10 | ARTC and ARTI | MV and SMC | [404] |
| 2013 | China | TH | Ultrasound coupling gel | B. cepacia | 8 | RDN, Ed, ISP, PD, and FV | PCS | [405] |
| 2013 | South Korea | BMC | CHX 0.5% | Bcc | 21 | SIS | VMC | [406] |
| 2013 | Argentina | ICU, NICU | Ultrasound coupling gel | Bcc | 6 | FV, LKC, and BCT | NCDs and IMDU | [407] |
| 2013 | India | ACU | Antiemetic (granisetron) | Bcc | 6 | FV, Ch, and BCT | IMS and CAN | [408] |
| 2013–2014 | Paraguay | HDU | Heparin solutions | B. cepacia | 12 | FV, Ch, and SIS | HTN and DM, | [409] |
| 2013–2018 | Turkey | TH | Medical devices | B. cepacia | 45 | UTI, ARTI, and BCT | NCDs, IMS, and IMDU | [410] |
| 2014 | Brazil | ND | Mouthwash | B. cepacia | ND | MI, LP, OI, and TI | ND | [411] |
| 2014 | China | NPD Ward | Saline solution | B. stabilis | 15 | AS | CNDs | [412] |
| 2014 | India | NICU | Dextrose 5.0%, NS, and CPAP | B. cepacia | 10 | FV, LKC, TCH, HPT, and Seps | PT and SMC | [413] |
| 2014 | Spain | HDU | Heparin solutions | B. cepacia | 10 | FV, Ch, and SIS | HTN, DM, and CKD | [414] |
| 2015 | India | NICU | Liquid soap | B. cepacia | 6 | FV, LKC, TCH, HPT, and NS | PT and SMC | [415] |
| 2015 | India | TH, PEDU | Injection vials (AMK) | Bcc | 15 | ARTI, FV, LKC, and Seps | NCDs, IMS, and IMDU | [416] |
| 2015 | China | SICU | Ventilators | B. cepacia | 10 | CPD, FV, LKC, PI, and VAP | NCDs, IMS, and IMDU | [417] |
| 6 months | Egypt | UH | ND | Bcc | 35 | PD and SB | ND | [397] |
| 2015–2016 | Switzerland | 9HCs | Washing gloves | B. stabilis | 46 | BCT and OIs | ND | [418] |
| 2015–2016 | Australia | 4HCs | Gel packed in sachets (SUPC) | B. cenocepacia | 15 | AC, ARTI, and BCT | NCDs, IMS, and IMDU | [419] |
| 2016 | Australia | H and HC | Mouthwash (CHX) | B. lata | 12 | ARTC and ARTI | CLDs, IMS, and IMDU | [420] |
| ND | India | TH, PICU | Distilled water | Bcc | 3 | VSD, PNM, PTX, and RMS | IC/BSAT | [421] |
| 2016 | USA | TH, PEDU | Liquid sodium docusate | Bcc | 12 | AC, ARTI, and BCT | NCDs, IMS, and IMDU | [422] |
| 2016 | USA | 16HCs | Liquid sodium docusate | Bcc | 63 | AC, ARTI, and BCT | NCDs, IMS, and IMDU | [5] |
| 2016 | Saudi Arabia | TH | Ultrasound coupling gel | B. cepacia | 12 | FV, Ch, and SIS | NCDs, IMS, and IMDU | [423] |
| 2016 | South Korea | NICUs | CHX 0.5% | B. cepacia | 10 | RF | PT and SMC | [424] |
| 2016 | USA | TH, ICU, PEDU | Liquid sodium docusate | Bcc | 15 | UTIs, ARTI, and BCT | ND | [425] |
| 2016–2017 | USA | 59HCs | Syringes with saline solution | Bcc | 162 | AC, ARTI, and BCT | NCDs, IMS, and IMDU | [426] |
| ND | Jordan | UCs | ND | Bcc | 2 | PLR, Dys, and FV | ND | [427] |
| 2017 | USA | VH | CHX 2% | B. cenocepacia | 5 | Ed, LP, FV, and CLL | VMC | [428] |
| 2017 | India | NTICU | CVC | B. cepacia | 12 | AC, ARTI, and BCT | NCDs, IMS, CST, and IMDU | [429] |
| 2017 | Canada | PDU | Dialysis solution | B. cepacia | 6 | AC, BCT, and PRT | HTN, DM, and CKD | [430] |
| 2017–2018 | USA | Multiple HCs | No-rinse cleansing foam | B. cenocepacia B. vietnamiensis | 59 | AC, ARTI, and BCT | NCDs, IMS, and IMDU | [395] |
| 2018 | Germany | CTICU | Mouthwash (Oct) | Bcc | 12 | AC, ARTI, and BCT | NCDs, IMS, and IMDU | [431] |
| 2018 | China | TH | Anesthetic gel, and injectables | B. cepacia | 12 | UTI and BCT | NCDs, IMS, and IMDU | [432] |
| 2019 | China | TH | CHX 0.5% | B. cepacia | 10 | AC, BCT, and PRT | HTN, DM, and CKD | [433] |
| 2020 | China | TH | Anesthetic gel (cystoscopy) | B. cepacia | 8 | Dys, UTI, and FV | VMC | [229] |
| ND | Argentina | PhI | IS, PP, and MM | B. aenigmatica B. arboris B. cenocepacia B. cepacia B. contaminans B. multivorans B. vietnamiensis | 135 isolates | NA | NA | [228] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva-Santana, G.; Sales, F.L.S.; Aguiar, A.R.; Brandão, M.L.L. Pharmaceutical Contamination by Biofilms Formed of the Burkholderia cepacia Complex: Public Health Risks. Processes 2025, 13, 1270. https://doi.org/10.3390/pr13051270
Silva-Santana G, Sales FLS, Aguiar AR, Brandão MLL. Pharmaceutical Contamination by Biofilms Formed of the Burkholderia cepacia Complex: Public Health Risks. Processes. 2025; 13(5):1270. https://doi.org/10.3390/pr13051270
Chicago/Turabian StyleSilva-Santana, Giorgio, Francisca Letícia Sousa Sales, Alícia Ribeiro Aguiar, and Marcelo Luiz Lima Brandão. 2025. "Pharmaceutical Contamination by Biofilms Formed of the Burkholderia cepacia Complex: Public Health Risks" Processes 13, no. 5: 1270. https://doi.org/10.3390/pr13051270
APA StyleSilva-Santana, G., Sales, F. L. S., Aguiar, A. R., & Brandão, M. L. L. (2025). Pharmaceutical Contamination by Biofilms Formed of the Burkholderia cepacia Complex: Public Health Risks. Processes, 13(5), 1270. https://doi.org/10.3390/pr13051270






