Abstract
This study models the electrical conductivity (EC) of passion fruit juice during ohmic heating under voltage gradients of 10, 20, and 30 V/cm, considering temperature (25–85 °C) and total soluble solids (TSS: 11.5, 15.5, and 19.5 °Brix). EC was measured using a laboratory-scale ohmic heating system, and three empirical models were developed using non-linear regression with the Levenberg–Marquardt algorithm. The second-order polynomial model showed the highest accuracy (R2 = 0.9974; RMSE = 0.0191; χ2 = 0.0112). EC increased with temperature, which enhanced ion mobility and decreased viscosity, while its relationship with TSS was non-linear: EC rose at low to moderate TSS but declined at higher concentrations, attributed to reduced free water and ion solute interactions. The validated model offers a reliable tool for real-time process control in industrial scale pasteurization and evaporation of tropical fruit juices.
1. Introduction
Heating is a critical operation for food safety, extended shelf life, and product stability. Traditional heating methods such as conduction and convection are usually beset with inefficiencies such as non-uniform heating, long processing time, and product degradation, particularly for heat-sensitive products such as fruit juice [1,2]. These inefficiencies have driven growing focus on other technologies that can heat faster, uniformly, and with less impact on nutritional and sensory attributes.
Ohmic heating, an advanced thermal processing method, applies electric current directly on the food for the sole purpose of inducing energy dissipation internally. Ohmic heating offers a number of advantages relative to conventional processes, for example, volumetric heating, reduced surface degradation, and improved retention of color, flavor, and antioxidant content [3,4]. Ohmic heating has been effectively applied to a wide range of liquid and semi-liquid foods, making it a promising technology for high-quality thermal processing in the juice industry [5]. One of the most significant factors governing the performance of ohmic heating is the electrical conductivity (EC) of the food matrix. EC determines the generation of heat and is a function of the physicochemical factors such as temperature, ionic content, viscosity, and compositional factors [6]. EC is increased with increasing temperature due to greater mobility of ions and lower viscosity [7]. EC is not linearly related to total soluble solids (TSS), which include soluble acids, minerals, and sugars. Below TSS contents, the rise in free ion concentration enhances conductivity. Above higher TSS contents, it has been reported that conductivity decreases non-linearly—due to enhanced viscosity, less free water available, and ion–solute interactions. Such effects, as explained by Afraz et al. [3], along with electrostatic crowding, slow down charged particles’ mobility and decrease conductivity [7,8].
Although earlier research has looked at EC variation in fruit juices, most concentrated on either temperature or solute concentration as separate elements and often used simplified linear models that overlook non-linear interactions and dynamic heating behavior [9,10,11,12]. Particularly in complicated tropical juice systems like passion fruit, which have different electrochemical characteristics because of high organic acid and mineral content, these models usually assume a fixed composition and have limited applicability under varying ohmic heating conditions [13,14]. Moreover, limited research has looked at the combined impacts of voltage gradient, temperature, and total soluble solids (TSS) in a thorough modeling framework, hence limiting their applicability for real-time process optimization in industrial environments. This study develops and validates a second-order polynomial model to capture the interactive, non-linear effects of TSS and temperature during ohmic heating across three voltage gradients (10, 20, and 30 V/cm). This work fills in these gaps. Its great acidity and ionic complexity, which present difficulties for precise EC prediction and provide a demanding test case for modeling, led to the choice of passion fruit juice (Passiflora edulis) as a model system. The resulting empirical model, backed by low prediction errors (RMSE and χ2) and high R2 values (up to 0.9974), provides improved understanding of the electrothermal behavior of fruit juices and is a useful tool for regulating pasteurization and evaporation processes in juice processing.
2. Materials and Methods
2.1. Materials
The passion fruit used in this study was developed from the Royal Project Development Center in Mae Hae, Mae Hae District, Chiang Mai Province, Thailand. The juice was filtered and extracted using thoroughly washed fruits. A digital refractometer (Hanna Instruments, HI 96801, Woonsocket, RI, USA) was used to measure total soluble solids (TSS), which first registered at 11.5°Brix. The juice was subsequently concentrated to 15.5 and 19.5 °Brix using a rotary vacuum evaporator (Heidolph Instruments, Hei-VAP Core, Schwabach, Germany) operated at 40 °C. Until utilization, juice samples were kept at −18 °C in 5-L aluminum foil bags.
2.2. Ohmic Heating System
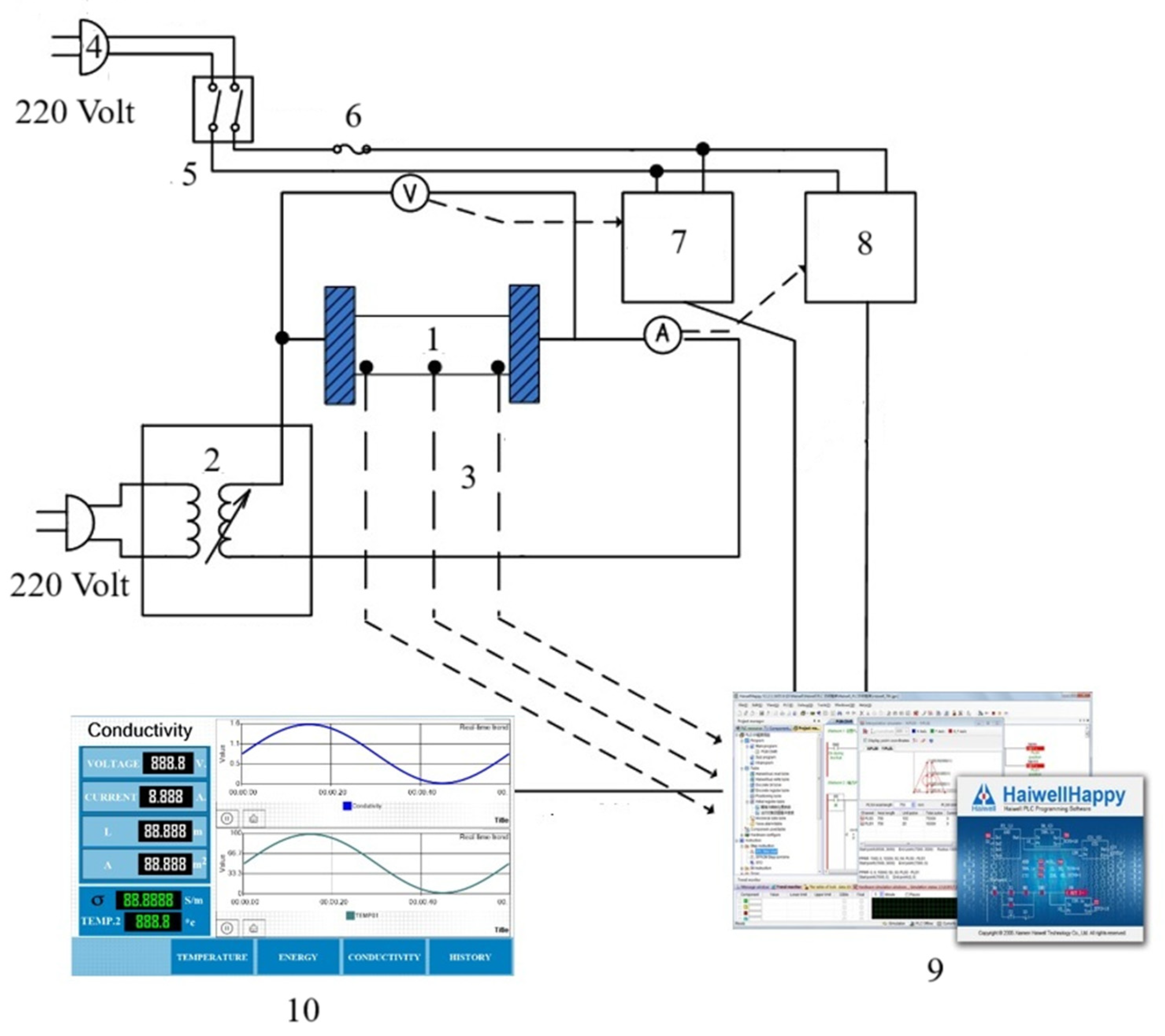
A laboratory scale ohmic heating system was developed at the smart farm engineering and agricultural innovation program; school of renewable energy, Maejo University, Thailand, as illustrated in Figure 1. The system consists of a voltage regulator (0–220 V, 50 Hz), a digital ammeter (Hioki 3286-20, Hioki E.E. Corporation, Nagano, Japan; accuracy ±0.5%) for current measurement, and SUS316 stainless steel electrodes (60 × 150 × 1 mm). The ohmic cell was made of acrylic (3 mm thickness) with an inner diameter of 25 mm and a length of 10 mm. Temperature was monitored using three K-type thermocouples (±0.5 °C), positioned at the center and adjacent to each electrode. A programmable logic controller (Haiwell model D7-G, Xiamen, China) recorded voltage, current, and temperature in real-time at 1 s intervals. Thermal insulation surrounded the cell to minimize heat loss and maintain near-adiabatic conditions.
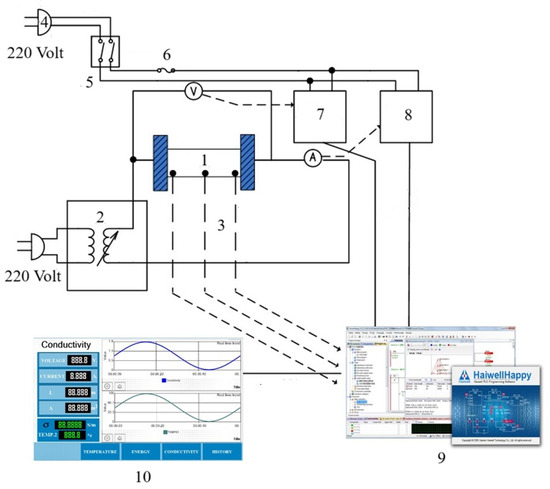
Figure 1.
Diagram of conductivity measurement system during ohmic heating. (1) Ohmic cell, (2) voltage variable, (3) thermocouple, (4) power supply, (5) circuit breaker, (6) fuse, (7) voltage transmitter, (8) current transmitter, (9) control processing unit and I/O, (10) programmable logic controller.
2.3. Electrical Conductivity of Fruit Juices Under Different Conditions
The measurement of electrical conductivity during the ohmic heating of fruit juices has attracted attention due to its implications for the efficiency of the heating. Studies indicate that conductivity changes are related to thermal processes, improving the conservation of fruit juice [6,15]. The application of an electric field induces ion migration within an electrolyte toward electrodes of opposite charge, resulting in Joule heating. Similarly, when an alternating current is applied to a food sample positioned between two electrodes, internal heat generation occurs due to ionic movement. Electrical conductivity (σ, S/m) is a fundamental parameter in this process and can be determined from voltage and current measurements using the following Equation (1):
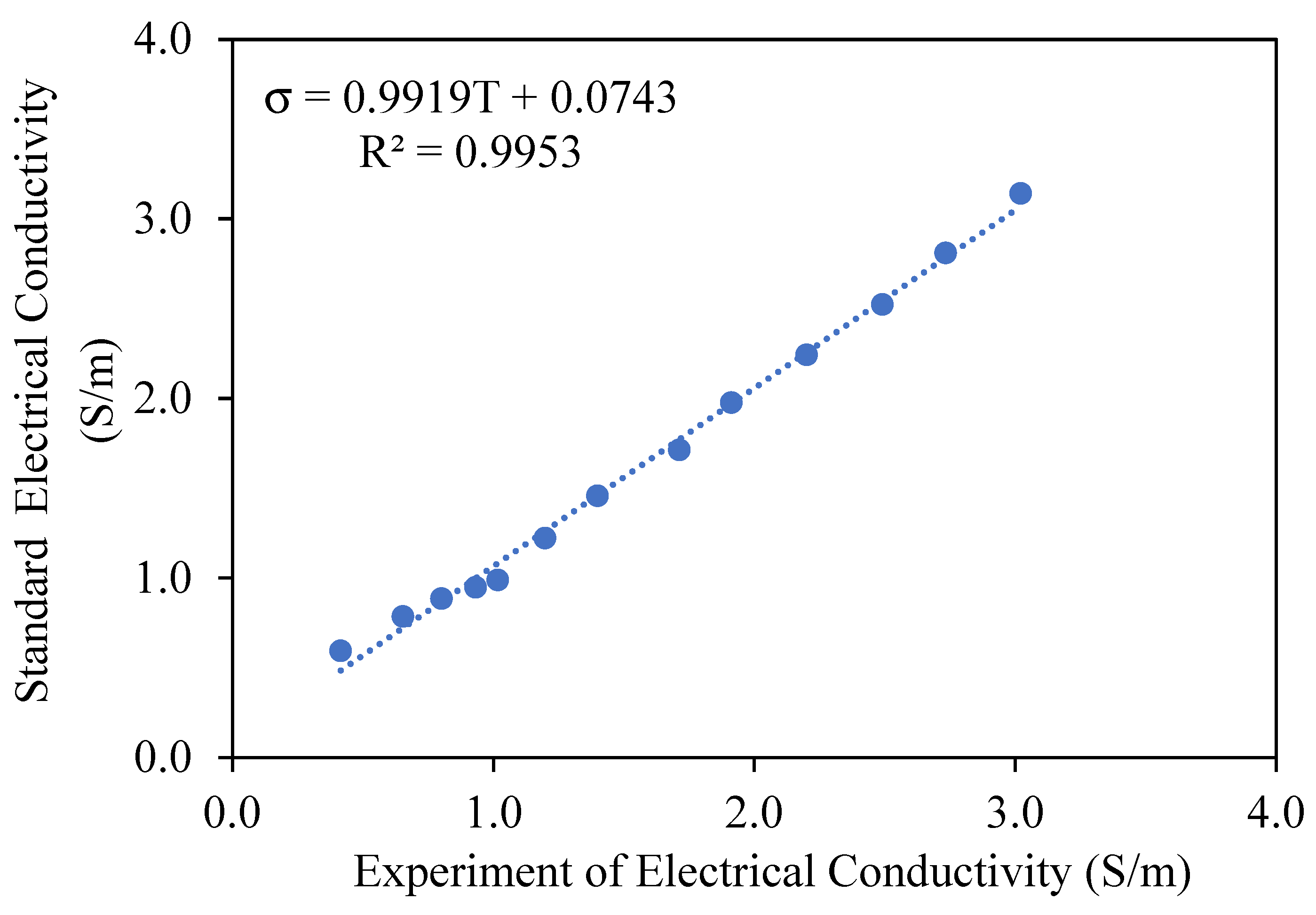
where I is the current (A), V is the applied voltage (V), L is the distance between electrodes (m), and A is the cross-sectional area of contact between the electrodes and the juice sample (m2). The geometric parameters used were L = 0.01 m, diameter = 0.025 m, thus A = 0.00049 m2. The system was validated using a 0.1 M NaCl standard solution to compare experimental and theoretical EC values across 25 to 85 °C, yielding a calibration curve with R2 = 0.9953 and error below 2.5% within this range.
To guarantee measurement reliability and eliminate confounding variables, several critical factors were regulated during the assessment of conductivity. All passion fruit juice samples were equilibrated to room temperature (25 °C) prior to heating, and their pH was stabilized at 3.2 ± 0.05 to reduce variability in ionization behavior. A constant sample volume of 20 mL was utilized, and the juice was introduced into the ohmic cell to a uniform height to standardize the electrode contact area. Each experiment was performed in triplicate, and the order of runs was randomized. Between each trial, the cell was rinsed with deionized water and dried to avert cross-contamination.
2.4. Electrical Conductivity Model
A mathematical model was developed to predict the electrical conductivity of passion fruit juice during ohmic heating, as a function of temperature (25–85 °C), TSS (11.5, 15.5, and 19.5 °Brix), and voltage gradients (10, 20, and 30 V/cm). Non-linear regression analysis was performed using SPSS Statistics 20.0 (IBM Corp., Armonk, NY, USA) under a licensed agreement with Maejo University, applying three empirical models as follows:
where A, B, C, D, and E are the coefficients of mathematical models were estimated utilizing the Levenberg–Marquardt algorithm.
Linear: σ (mS/cm) = A + B (T) + C (TSS)
Two-Factor Interaction: σ (mS/cm) = A + B (T) + C (TSS) + D (T) (TSS)
Polynomial: σ (mS/cm) = A + B (T) + C (TSS) + D (T) (TSS) + E (TSS)2
The performance of developed models was determined in terms of three statistical parameters widely used in food process modeling: R2, χ2, and RMSE [12,13]. R2 reflects the fraction of variance in observed data explained by the model with an R2 value close to 1.0 being indicative of high correlativity between predicted and experimental values. χ2 calculates squared differences between observed versus predicted values normalized by prediction, such that smaller values represent better fit to the models [12]. RMSE represents the average size of prediction errors, such that smaller values represent higher accuracy [13]. The parameters were estimated by Equations (5)–(7). The best predictor of electrical conductivity was determined by selecting the model that had maximum R2 value and minimum χ2 and RMSE value.
where, σexperiment is the experimentally observed electrical conductivity (mS/cm), σprediction is the predicted electrical conductivity, N is the number of observations, and np is the number of constants in the model. These metrics have been widely used in previous studies for model performance evaluation in ohmic heating of juices and other food matrices [9,12,13].
2.5. Statistical Evaluation
Non-linear regression analysis employing the Levenberg–Marquardt algorithm was utilized to develop empirical models for forecasting electrical conductivity (EC) based on temperature (T) and total soluble solids (TSS). Three models were compared: linear regression, two-factor interaction, and second-order polynomial fitting. Statistical fitting was conducted using SPSS Statistics 20.0 (IBM Corp., Armonk, NY, USA) under a licensed agreement with Maejo University, across three voltage gradients (10, 20, and 30 V/cm), to evaluate the model’s robustness. The model exhibiting the highest coefficient of determination (R2), along with the lowest root mean square error (RMSE) and chi-square (χ2), was selected as the optimal model. To visualize the interaction effects of T and TSS on EC, three-dimensional surface and contour plots were generated using the validated polynomial model in Design-Expert® version 13.0 (Stat-Ease, Inc., Minneapolis, MN, USA).
3. Results
3.1. Ohmic Heating Electrical Conductivity Calibration and Accuracy
Electrical conductivity is a critical characteristic of food systems, directly affecting the efficacy of ohmic heating. The accuracy of electrical conductivity measurement is crucial for optimizing thermal processing conditions, ensuring consistent heating, and enhancing energy efficiency. This study examined the validation of conductivity measurement precision utilizing a 0.1 M NaCl standard solution, a thoroughly characterized electrolyte with consistent conductive properties. The experiment entailed evaluating conductivity values within a temperature spectrum of 25–85 °C, juxtaposing experimental measurements with theoretical values to ascertain the relative percentage error, as illustrated in Figure 2. The results demonstrated a linear increase in conductivity with temperature, attributed to improved ion mobility and reduced solution resistance, as described by the equation σ = 0.9919T + 0.0743 (R2 = 0.9953). At temperatures below 20 °C, the relative percentage error surpassed 10%, due to diminished ion kinetic energy and heightened viscosity, which impeded ionic mobility and measurement precision. The results indicate that ohmic heating is an effective method for assessing electrical conductivity in liquid food systems, especially within the 20–85 °C range, where measurement precision markedly enhanced, decreasing the relative percentage error to 2.21%. The findings align with prior studies [7,16], confirming that temperature significantly influences the accuracy of conductivity measurements. This study emphasizes the importance of accurate calibration methods and temperature regulation to reduce errors, particularly at low temperatures where hydration effects and heightened viscosity affect conductivity. The validated methodology endorses the use of ohmic heating as an accurate instrument for real-time monitoring of conductivity variations in thermal processing [17,18].
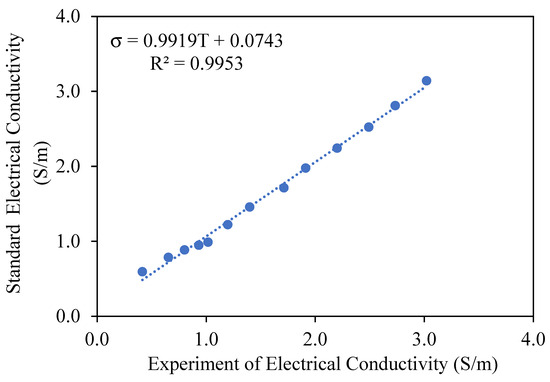
Figure 2.
Evaluation of the precision of conductivity measurements during ohmic heating utilizing a standard solution, 0.1 M saline solution.
3.2. Determination Modeling of Electrical Conductivity During Ohmic Heating
Ohmic heating is a thermal processing technique that employs electric current to traverse food materials, leading to swift and uniform internal heating. The electrical conductivity (EC) of the food matrix is a crucial factor affecting the efficiency of this process. Consequently, precise modeling of EC is crucial for optimizing energy input and ensuring efficient process control. The electrical conductivity of liquid foods is affected by several physicochemical factors, such as chemical composition, temperature, ionic concentration, and availability of free water. The interplay of temperature and total soluble solids (TSS) significantly affects electrical conductivity (EC) in fruit juices, notably in passion fruit juice, as these factors directly influence heating behavior and product quality. Ohmic heating is esteemed for its capacity to improve the electrical and physicochemical characteristics of fruit-based systems. Hardinasinta et al. [19] indicated that the rheological properties and electrical conductivity of mulberry purée significantly improved under ohmic conditions, demonstrating enhanced thermal and mass transfer. Total soluble solids (TSS), associated with sweetness and flavor intensity in passion fruit juice, also influence electrical conductivity (EC), as elevated TSS levels generally enhance ionic strength and conductivity [8]. Furthermore, Doan et al. [5] illustrated the efficacy of ohmic heating in maintaining antioxidant compounds and color stability in red climbing dragon fruit juice, underscoring the significance of temperature optimization during processing. Increased temperatures have been demonstrated to markedly augment electrical conductivity, thus enhancing heating efficiency and decreasing energy consumption [4].
Recent advancements underscore the potential of amalgamating machine learning with physicochemical measurements to forecast dielectric and electrical properties, facilitating enhanced control over processing conditions [20]. Thus, a comprehensive understanding of the interactions among TSS, temperature, and EC not only enhances predictive modeling techniques but also fosters advancements in process design and product development. Ultimately, this knowledge enhances the sensory qualities and nutritional integrity of juice products [21].
The results indicated that the electrical conductivity of passion fruit juice augmented with increasing temperature during ohmic heating at a voltage gradient of 10 V/cm. The increase in electrical conductivity was measured at 0.01983 ± 0.00082, 0.02002 ± 0.00063, and 0.01781 ± 0.00061 mS/cm for juice samples with total soluble solids (TSS) of 11.5, 15.5, and 19.5 °Brix, respectively. At elevated voltage gradients of 20 and 30 V/cm, the electrical conductivity values for samples with total soluble solids (TSS) levels of 11.5, 15.5, and 19.5 °Brix were 0.019458 ± 0.00037, 0.022185 ± 0.00091, and 0.018473 ± 0.00082 mS/cm, and 0.020613 ± 0.00041, 0.023308 ± 0.00045, and 0.01922 ± 0.00037 mS/cm, respectively.
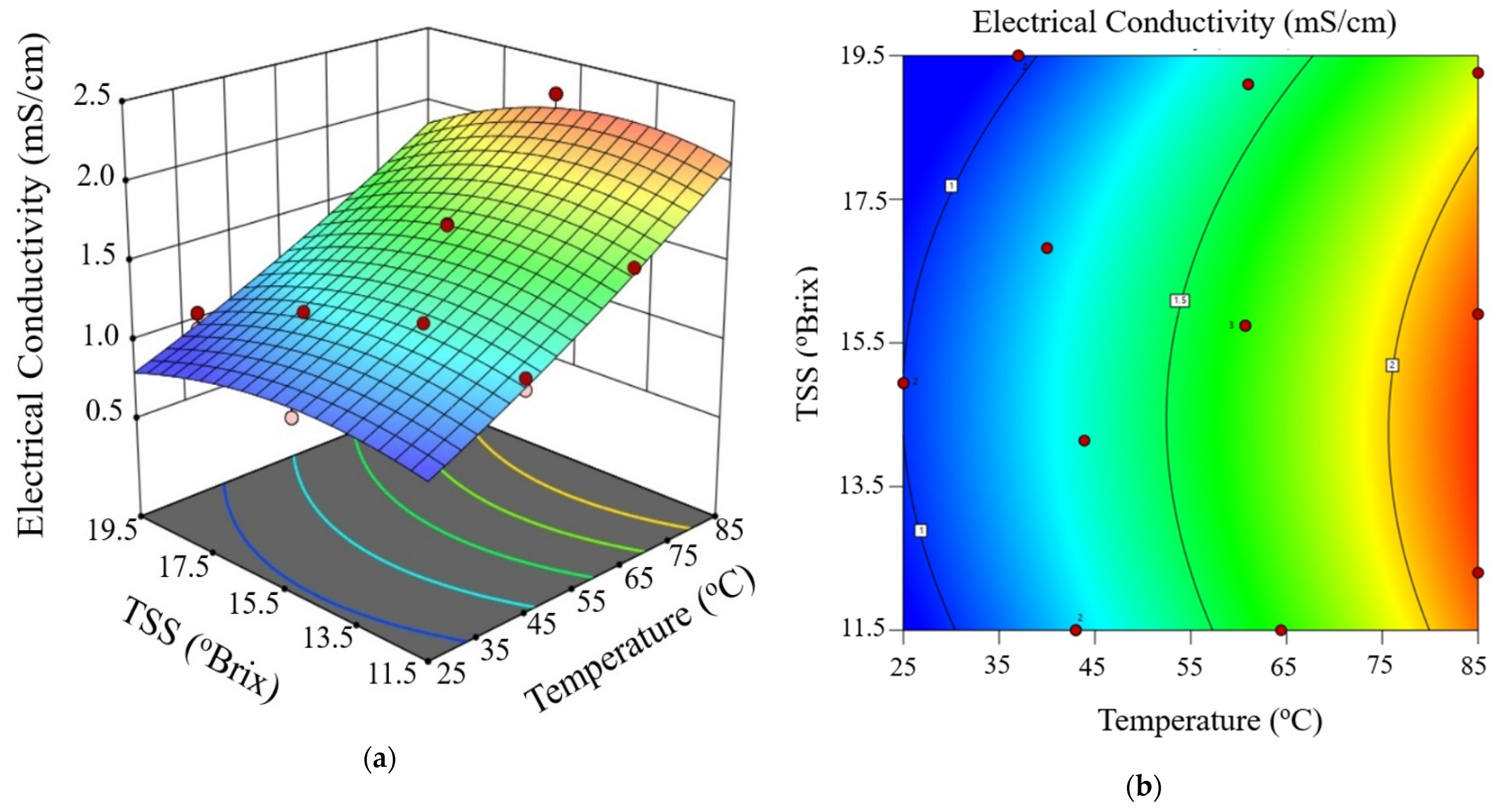
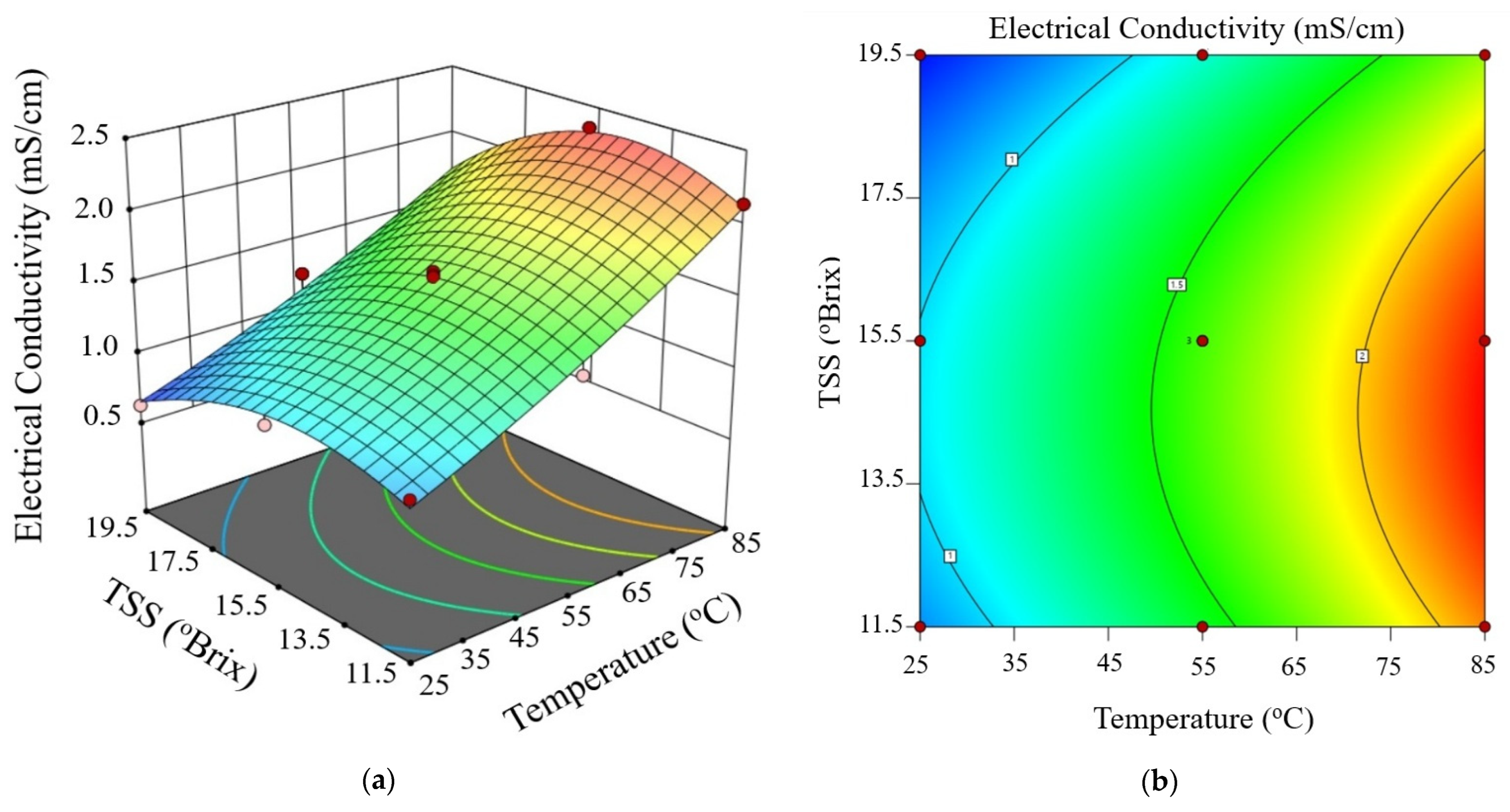
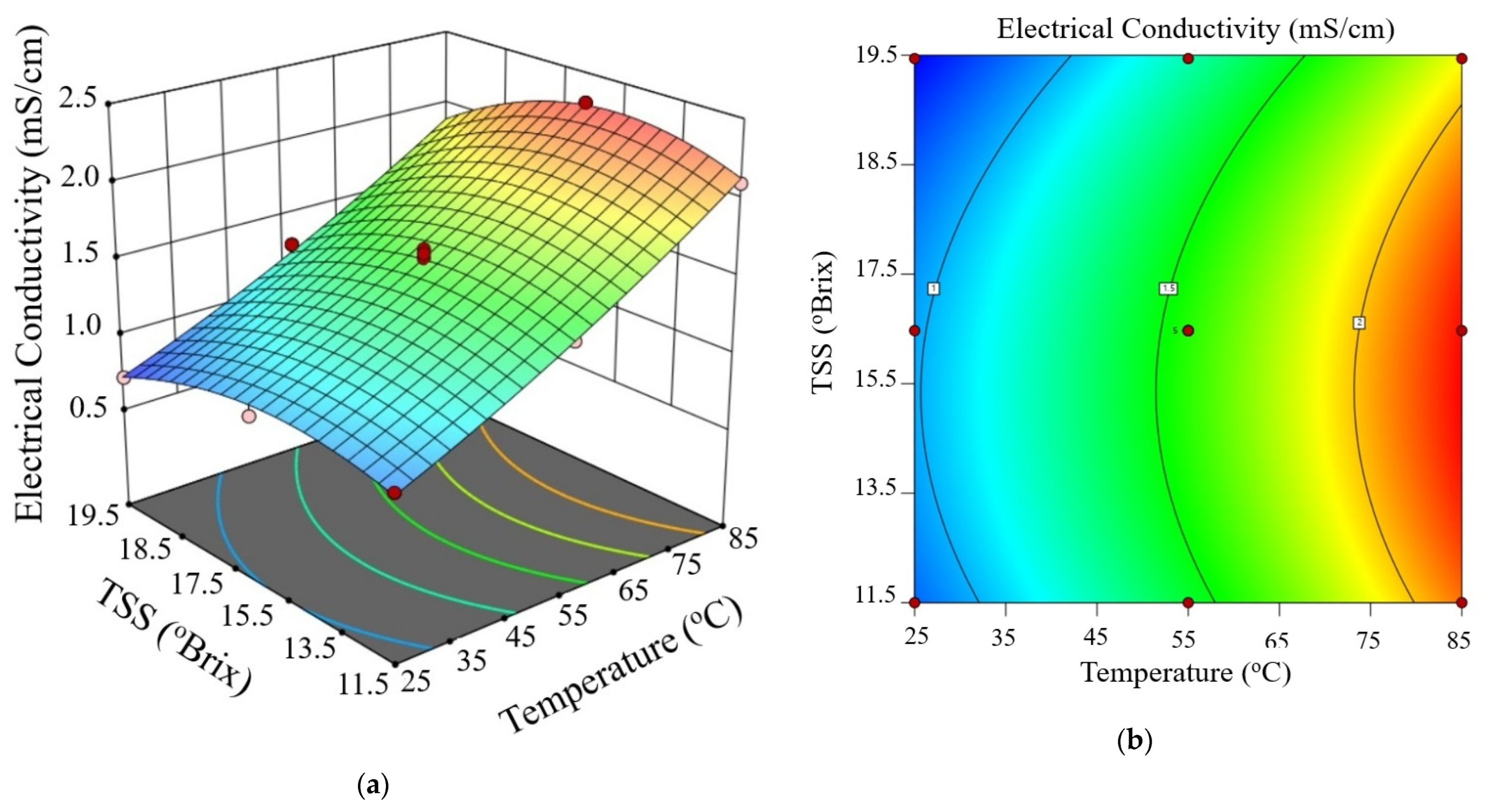
Figure 3, Figure 4 and Figure 5 show the electrical conductivity at every voltage gradient under combined influence of temperature and TSS. Increasing temperature and moderate TSS improved EC values at 10 V/cm, as seen in Figure 3. Although the effect of voltage seemed minimal at higher levels, Figure 4 and Figure 5 show comparable patterns at 20 and 30 V/cm, respectively. While augmenting the voltage gradient in ohmic heating systems can initially enhance electrical conductivity by raising temperature and improving ion mobility, the advantages typically wane at elevated voltage levels. This phenomenon can be ascribed to factors including localized ion depletion, electrochemical reactions at the electrodes, and compositional alterations within the system, which together lead to non-linear trends or reductions in conductivity. Consequently, meticulous optimization of the voltage gradient is crucial for sustaining conductivity stability and ensuring efficient and uniform thermal processing of passion fruit juice.
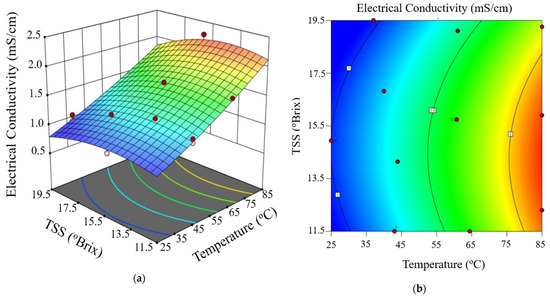
Figure 3.
Three-dimensional surface (a) and contour plot (b) illustrating the effect of temperature and total soluble solids (TSS) on electrical conductivity of passion fruit juice during ohmic heating at 10 V/cm.
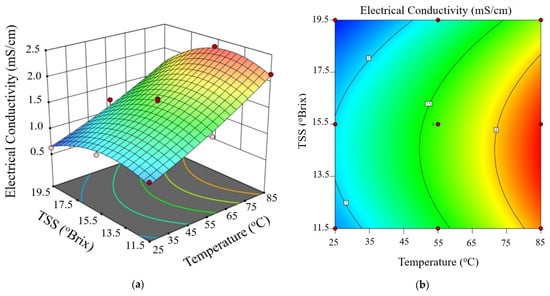
Figure 4.
Three-dimensional surface (a) and contour plot (b) illustrating the effect of temperature and total soluble solids (TSS) on electrical conductivity of passion fruit juice during ohmic heating at 20 V/cm.
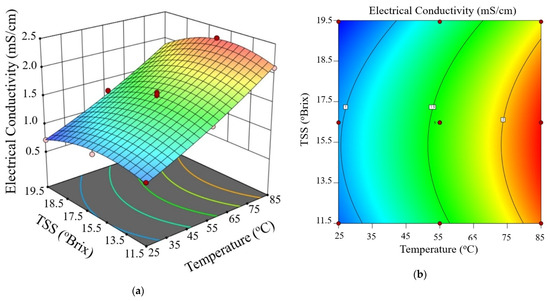
Figure 5.
Three-dimensional surface (a) and contour plot (b) illustrating the effect of temperature and total soluble solids (TSS) on electrical conductivity of passion fruit juice during ohmic heating at 30 V/cm.
Increased temperature significantly increases the electrical conductivity of passion fruit juice, mainly through mechanisms involving ion mobility, organic acid dissociation, and viscosity reduction. High temperatures facilitate ion mobility as the kinetic energy of molecules increases, leading to better conductivity [20]. This ion mobility is even more influenced by the thermodynamic properties of the organic acids present in the juice. As the temperature increases, organic acids dissociate faster, increasing the concentration of free ions and thus increasing conductivity [22]. Recent studies suggest that the dissociation of polysaccharides and other organic compounds of passion fruit can also contribute to this phenomenon [23,24]. Notably, viscosity also plays a crucial role; reduced viscosity at higher temperatures allows ions to move more freely [25]. Advances in techniques, such as pulsed electric fields and ohmic heating, correlate well with these conductivity improvements, showing the interaction between thermal and electrical properties [26,27]. Collectively, these findings emphasize the importance of temperature in optimizing conductive properties in fruit juices, particularly in passion fruit [28,29].
Total soluble solids (TSS) in passion fruit juice reflect both the sweetness and concentration of dissolved constituents, which significantly influence its electrical conductivity [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,29,30]. The ionic content, primarily derived from soluble sugars and organic acids, plays a pivotal role in determining the juice’s conductivity [14]. As TSS increases, conductivity may initially rise due to the enhanced availability of charged particles and ionizable compounds [31]. This correlation is further supported by studies examining the physicochemical properties of juice and the effects of thermal or non-thermal treatments [30,32]. However, the relationship between TSS and electrical conductivity (EC) is non-linear and varies across TSS concentration ranges. In the low to moderate TSS range (11.5–15.5 °Brix), EC increases as more dissociated ions such as hydrogen (H⁺) and citrate (C6H5O53⁻) from organic acids like citric acid become available. The dissolution of salts and minerals also elevates ionic strength, facilitating efficient charge transfer without significantly hindering ion mobility [33]. Conversely, at higher TSS levels (15.5–19.5 °Brix), EC begins to decline. This reduction is attributed to reduced free water, increased viscosity, and the formation of ion–sugar interactions that trap or slow ionic movement. Additionally, ion crowding and electrostatic shielding hinder ion transport, thereby decreasing conductivity despite the higher concentration of dissolved solids.
This study indicated that the electrical conductivity of passion fruit juice was affected by total soluble solids (TSS) and temperature. Table 1 presents the estimation model obtained from multiple linear regression analysis. These equations distinctly demonstrate that the temperature coefficient is inferior to that of TSS across all three voltage gradients, signifying a more substantial impact of TSS on the electrical conductivity values. The fluctuation in electrical conductivity of passion fruit juice can be precisely characterized through empirical correlations with temperature and total soluble solids (TSS). The models’ predictive errors were assessed using chi-square (χ2) and root mean square error (RMSE), in accordance with research on red grape juice [12], guava pulp [9], and glucose syrup [13]. The proposed mathematical model for forecasting electrical conductivity achieved the highest correlation coefficient (R2) and the lowest χ2 and RMSE values, indicating its superior predictive accuracy. In all experiments, the model performance metrics exhibited the following ranges: R2 from 0.9961 to 0.9992, χ2 from 0.0003 to 0.0051, and RMSE from 0.0173 to 0.0538, respectively.

Table 1.
Regression models and fit statistics for predicting EC of passion fruit juice under ohmic heating at different voltages.
3.3. Validation of Modeling of Electrical Conductivity
The polynomial model demonstrated the highest predictive accuracy when a mathematical equation was developed to predict the electrical conductivity (EC) of passion fruit juice based on temperature (25–85 °C) and total soluble solids (TSS: 11.5, 15.5, and 19.5 °Brix). In order to assess the generalization performance of this model, juice samples with TSS levels ranging from 14 to 16 °Brix were used for validation. The accuracy of the model was evaluated by comparing predicted values with empirical data from ohmic heating experiments. Samples of passion fruit juice were made by vacuum evaporation and adjusted to TSS levels between 14, 15, and 16 °Brix. Multiple linear regression was used to estimate the model coefficients, and Equations (5)–(7) were used to calculate the coefficient of determination (R2), chi-square (χ2), and root mean square error (RMSE) in order to assess the performance. Microsoft Excel was used for all statistical calculations. The outcomes demonstrate how well the polynomial model predicts EC in a range of TSS and voltage gradient scenarios.
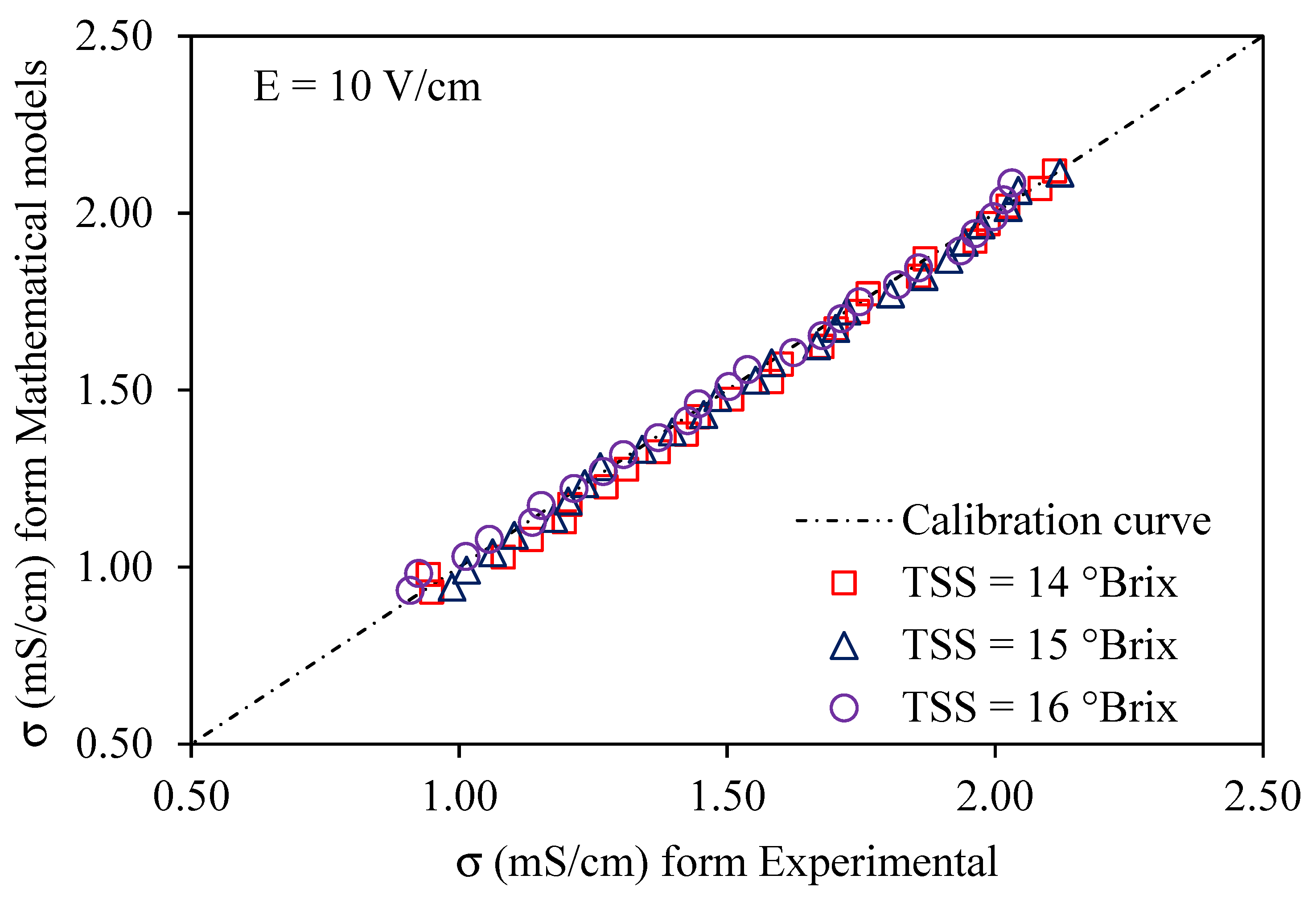
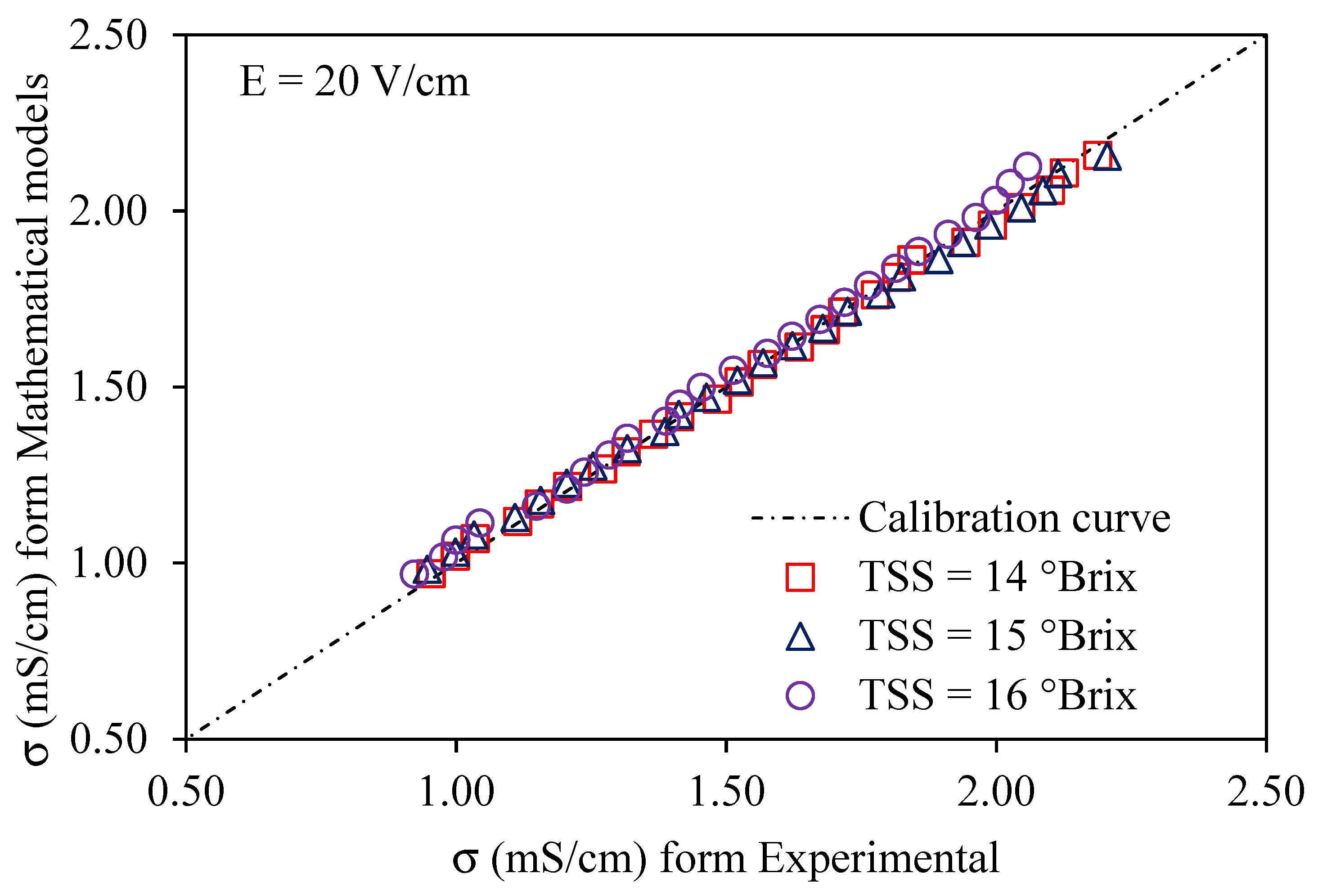
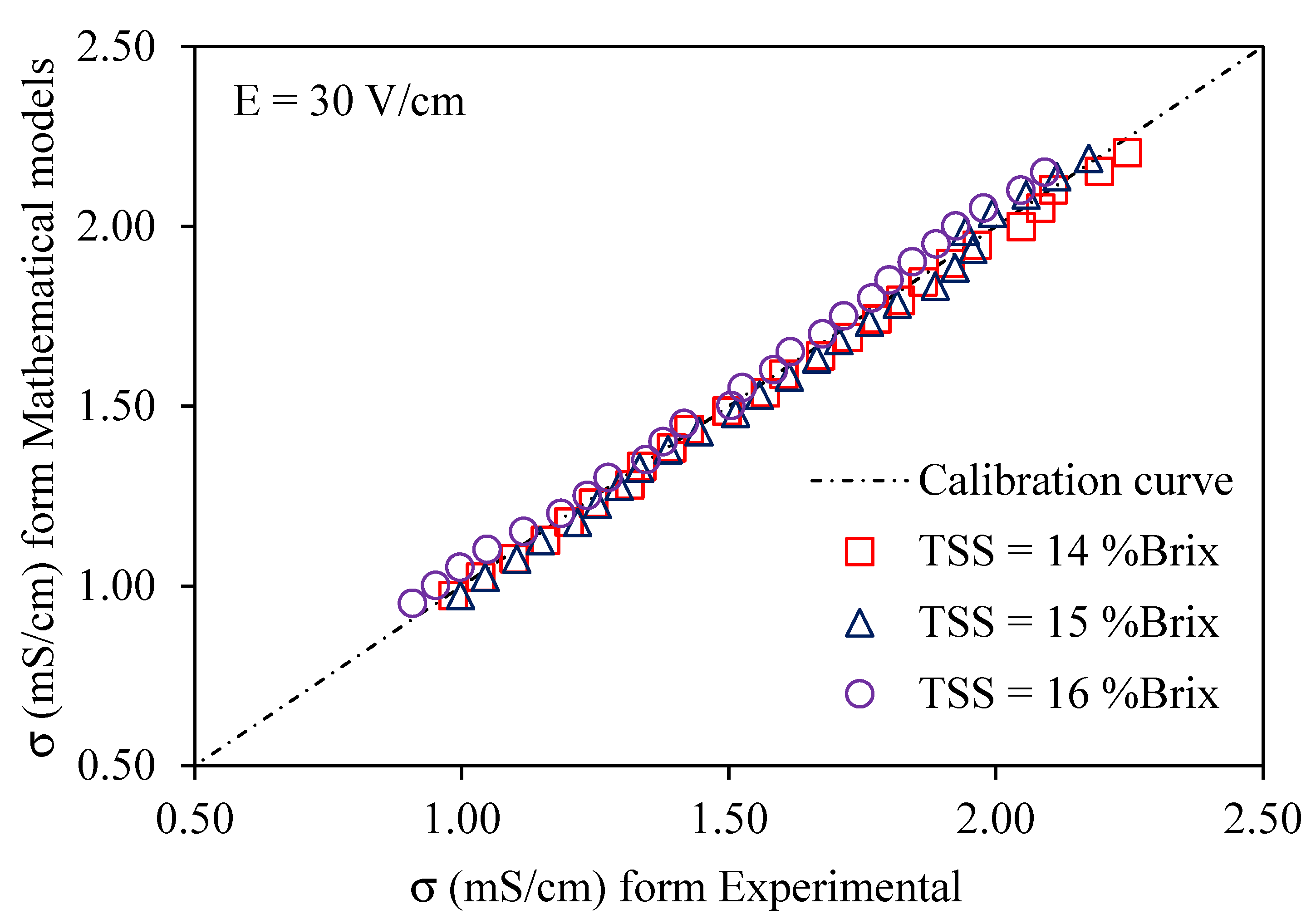
Using a voltage gradient of 10–30 V/cm, Figure 6, Figure 7 and Figure 8 depict graphs of the modeled conductivity values plotted against the experimental values for passion fruit juice. This was performed to confirm the accuracy of the model. For passion fruit juice samples of 14, 15, and 16 °Brix with a temperature range of 30–80 °C, the same modeling approach was used. The forecasted and measured data plotted along a 45° reference line through the origin confirmed the model’s ability to replicate the real variation in electrical conductivity. A substantial agreement between forecasted and measured values was suggested by the high density of data points near the identity line, which certainly supports the validity and relevance of the model. These findings confirm that the created model can be used to describe the evolution of electrical conductivity of passion fruit juice under ohmic heating and can serve as a reasonable tool for process control and optimization.
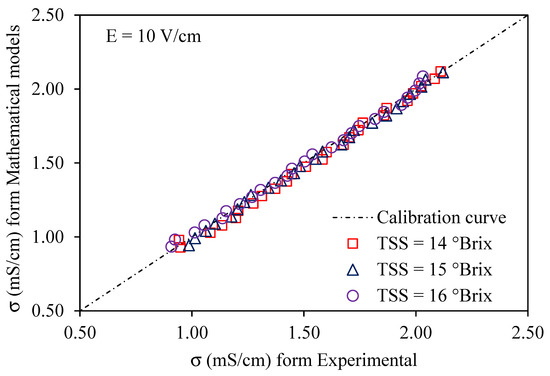
Figure 6.
Experimental vs. predicted EC of passion fruit juice (14–16 °Brix) at 10 V/cm.
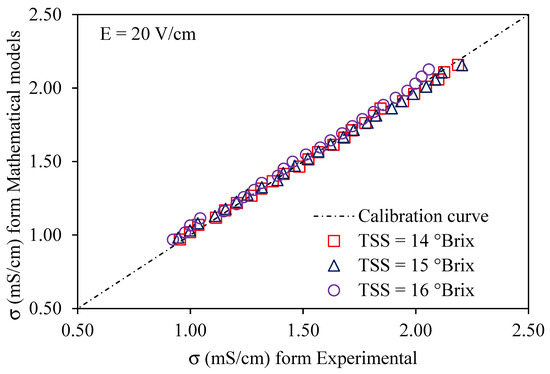
Figure 7.
Experimental vs. predicted EC of passion fruit juice (14–16 °Brix) at 20 V/cm.
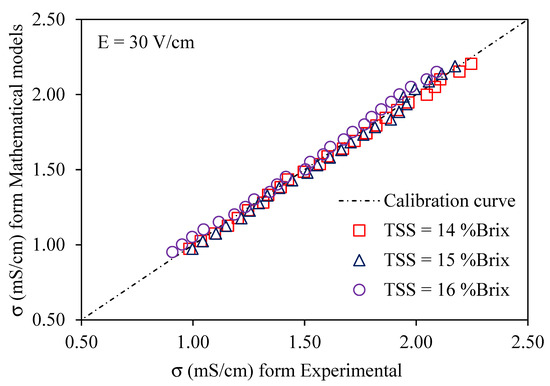
Figure 8.
Experimental vs. predicted EC of passion fruit juice (14–16 °Brix) at 30 V/cm.
The statistical metrics derived from the validation dataset, as presented in Table 2, affirm the robust reliability and generalization ability of the established polynomial model. The model showed very high R2 values (over 0.98) for all tested levels of TSS (14, 15, and 16 °Brix) and voltage gradients (10, 20, and 30 V/cm), meaning it explained more than 98% of the changes in electrical conductivity in different situations. The highest R2 (0.9967) and lowest RMSE (0.0060) were found at 20 V/cm and 14 °Brix, showing that the model worked best with medium voltage and moderate total soluble solids (TSS). This indicates that, under these conditions, ionic mobility and composition demonstrate relatively consistent electrothermal behavior. A small drop in model accuracy was observed at 30 V/cm and 16 °Brix (R2 = 0.9825; RMSE = 0.0307), likely due to increased system non-linearity and limitations caused by ion crowding and viscosity effects at higher total soluble solids (TSS) levels. The results show that the model is strong and flexible for use in the ohmic heating system, proving it is good for real-time process control, dynamic optimization, and industrial design. The low χ2 values further validate that the discrepancies between experimental and predicted EC values were statistically insignificant, thereby reinforcing the model’s relevance for processing passion fruit juice at varying concentration levels.

Table 2.
Model validation for EC prediction of passion fruit juice during ohmic heating (TSS 14–16 °Brix).
4. Discussion
This study demonstrates that ohmic heating offers precise electrical conductivity (EC) information for 25–85 °C liquid food matrices since it shows robust correlation against 0.1 M NaCl reference standards. This proven method was then utilized to characterize the electrical conductivity response of passion fruit juice under varied total soluble solids (TSS). The experimental findings facilitated the derivation of a second-order polynomial model with temperature, TSS, and their interaction. The model had excellent predictability (R2 > 0.99, lowest RMSE, and χ2), thereby confirming its suitability for modeling non-linear EC behavior as well as facilitated accurate control of processes in thermal food processing plants with ohmic heating.
5. Concluding Remarks
The assessment of electrical conductivity (EC) of passion fruit juice over the temperature spectrum of 25–85 °C using an ohmic heating apparatus showed remarkable precision in comparison to the typical NaCl 0.1 M solution. A mathematical model was created to forecast EC, expressed as a second-order polynomial model. This model had a high coefficient of determination (R2) together with low chi-square (χ2) and root mean square error (RMSE) values, signifying robust prediction accuracy. The model’s predictions closely corresponded with the experimental EC values recorded at TSS levels ranging from 14 to 16 °Brix. The electrical conductivity of passion fruit juice enhanced with temperature owing to improved ion mobility, less viscosity, and heightened dissociation of organic acids like citric acid, all of which facilitate ionic conduction. In contrast, elevating TSS from 11.5 to 19.5 °Brix produced a non-linear effect: EC rose at low to moderate TSS levels but declined beyond around 15.5 °Brix. The drop was ascribed to diminished free water availability, heightened viscosity, ion–sugar interactions, and ion crowding elements that combine to obstruct ionic mobility and restrict electrical conduction within the juice. The mathematical model for prediction conductivity variations based on these experimental results establishes a foundation for process regulation and the design of devices and systems for ohmic heating applications in future industrial contexts. These results provide a strong basis for future evolution of smart ohmic heating systems with adaptive process control and real-time EC monitoring.
Author Contributions
Conceptualization, R.A.; methodology, R.A. and S.T.; software, R.A.; validation, R.A. and S.T.; formal analysis, S.T.; investigation, R.A.; resources, R.A.; data curation, R.A.; writing—original draft preparation, R.A.; writing—review and editing, R.A.; visualization, R.A.; supervision, R.A.; project administration, R.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kaur, M.; Kumar, S.; Samota, M.K.; Lalremmawii. Ohmic Heating Technology Systems, Factors Governing Efficiency and Its Application to Inactivation of Pathogenic Microbial, Enzyme Inactivation, and Extraction of Juice, Oil, and Bioactive Compounds in the Food Sector. Food Bioprocess Technol. 2024, 17, 299–324. [Google Scholar] [CrossRef]
- Salari, S.; Jafari, S.M. The Influence of Ohmic Heating on Degradation of Food Bioactive Ingredients. Food Eng. Rev. 2020, 12, 191–208. [Google Scholar] [CrossRef]
- Sastry, S.K.; Barach, J.T. Ohmic and Inductive Heating. J. Food Sci. 2000, 65, 42–46. [Google Scholar] [CrossRef]
- Javed, T.; Oluwole-Ojo, O.; Zhang, H.; Akmal, M.; Breikin, T.; O’Brien, A. System Design, Modelling, Energy Analysis, and Industrial Applications of Ohmic Heating Technology. Food Bioprocess Technol. 2025, 18, 2195–2217. [Google Scholar] [CrossRef]
- Doan, N.K.; Lai, D.Q.; Le, T.K.P. Ohmic Heating: Its Current and Future Application in Juice Processing. Food Rev. Int. 2023, 39, 6908–6933. [Google Scholar] [CrossRef]
- Cevik, M. Electrical Conductivity and Performance Evaluation of Verjuice Concentration Process Using Ohmic Heating Method. J. Food Process Eng. 2021, 44, e13672. [Google Scholar] [CrossRef]
- Kamonpatana, P.; Sastry, S.K. Electrical Conductivity of Foods and Food Components: The Influence of Formulation Processes. J. Food Process Eng. 2022, 45, e13992. [Google Scholar] [CrossRef]
- Afraz, M.T.; Xu, X.; Zeng, X.A.; Zhao, W.; Lin, S.; Woo, M.; Han, Z. The Science behind Physical Field Technologies for Improved Extraction of Juices with Enhanced Quality Attributes. Food Phys. 2024, 1, 100008. [Google Scholar] [CrossRef]
- Athmaselvi, K.A.; Viswanathan, R.; Balasubramanian, M.; Roy, I. The Effects of Concentration and Type of Electrode on Electrical Conductivity of Guava Pulp during Ohmic Heating. J. Food Res. Technol. 2014, 2, 113–123. [Google Scholar]
- Srivastav, S.; Roy, S. Changes in Electrical Conductivity of Liquid Foods during Ohmic Heating. Int. J. Agric. Biol. Eng. 2014, 7, 133–138. [Google Scholar] [CrossRef]
- Lamsal, B.; Jindal, V. Variation in Electrical Conductivity of Selected Fruit Juices during Continuous Ohmic Heating. KMUTNB Int. J. Appl. Sci. Technol. 2014, 7, 47–56. [Google Scholar] [CrossRef][Green Version]
- Assawarachan, R. Estimation Model for Electrical Conductivity of Red Grape Juice. Int. J. Agric. Biol. Eng. 2010, 3, 52–57. [Google Scholar]
- Sabanci, S.; Kaya, K.; Göksu, A. Modeling the Electrical Conductivity Value of the Model Solution. An. Acad. Bras. Ciênc. 2023, 95, e20210062. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Militão, A.; da Silva, T.I.; do Nascimento, A.M.; da Costa, F.B.; de Castro, A.K.G.; Macêdo, L.F.; Santos, S.C.L. Storage Increases Soluble Sugars and Decreases Bioactive Compounds in Wild Passion Fruit (Passiflora cincinnata). Hortic. Environ. Biotechnol. 2025, 1, 1–16. [Google Scholar] [CrossRef]
- Icier, F.; Yildiz, H.; Sabanci, S.; Cevik, M.; Cokgezme, O.F. Ohmic Heating Assisted Vacuum Evaporation of Pomegranate Juice: Electrical Conductivity Changes. Innov. Food Sci. Emerg. Technol. 2017, 39, 241–246. [Google Scholar] [CrossRef]
- Sastry, S.K.; Li, Q. Modeling the Ohmic Heating of Solid–Liquid Mixtures: Model Development and Validation. J. Food Eng. 1996, 27, 241–260. [Google Scholar]
- Abdulstar, A.R.; Altemimi, A.; Al-HiIphy, A.R.; Watson, D.G.; Lakhssassi, N. Water Distillation Using an Ohmic Heating Apparatus. Int. J. Ambient. Energy 2022, 43, 2748–2758. [Google Scholar] [CrossRef]
- Wiroonsri, P.; Wattanachant, S. Electrical Conductivity as a Precise Method for Salt Content Estimation in Raw and Cooked Tuna Meat. J. Food Compos. Anal. 2025, 137, 106953. [Google Scholar] [CrossRef]
- Hardinasinta, G.; Salengke, S.; Mursalim, M.; Muhidong, J. Effect of Ohmic Heating on the Rheological Characteristics and Electrical Conductivity of Mulberry (Morus nigra) Puree. Pol. J. Food Nutr. Sci. 2021, 71, 289–297. [Google Scholar] [CrossRef]
- Cavalcanti, R.N.; Barbosa, V.P.; Gut, J.A.W.; Tadini, C.C. Predicting Dielectric Properties of Fruit Juices at 915 and 2450 MHz Using Machine Learning and Physicochemical Measurements. Meas. Food 2024, 14, 100158. [Google Scholar] [CrossRef]
- Zia, H.; Slatnar, A.; Košmerl, T.; Korošec, M. A Review Study on the Effects of Thermal and Non-Thermal Processing Techniques on the Sensory Properties of Fruit Juices and Beverages. Front. Food Sci. Technol. 2024, 4, 1405384. [Google Scholar] [CrossRef]
- Qiu, X.; Zhang, Y.; Zhou, Y.; Li, G.H.; Feng, X.S. Progress in Pretreatment and Analysis of Organic Acids: An Update Since 2010. Food Chem. 2021, 360, 129977. [Google Scholar] [CrossRef]
- Yu, S.; Yan, J.K.; Jin, M.Y.; Li, L.Q.; Yu, Y.H.; Xu, L. Preparation, Physicochemical and Functional Characterization of Pectic Polysaccharides from Fresh Passion Fruit Peel by Magnetic-Induced Electric Field-Assisted Three-Phase Partitioning. Food Hydrocoll. 2024, 156, 110292. [Google Scholar] [CrossRef]
- Du, H.; Olawuyi, I.F.; Said, N.S.; Lee, W.Y. Comparative Analysis of Physicochemical and Functional Properties of Pectin from Extracted Dragon Fruit Waste by Different Techniques. Polymers 2024, 16, 1097. [Google Scholar] [CrossRef] [PubMed]
- Huo, D.; Dai, J.; Yuan, S.; Cheng, X.; Pan, Y.; Wang, L.; Wang, R. Eco-Friendly Simultaneous Extraction of Pectins and Phenolics from Passion Fruit (Passiflora edulis Sims) Peel: Process Optimization, Physicochemical Properties, and Antioxidant Activity. Int. J. Biol. Macromol. 2023, 243, 125229. [Google Scholar] [CrossRef] [PubMed]
- Brito, I.P.C.; Silva, E.K. Pulsed Electric Field Technology in Vegetable and Fruit Juice Processing: A Review. Food Res. Int. 2024, 173, 114207. [Google Scholar] [CrossRef]
- Khuenpet, K.; Jittanit, W. The Effects of Pasteurization by Conventional and Ohmic Heating Methods and Concentration Processes on the Madan (Garcinia schomburgkiana Pierre) Juice Properties. Appl. Eng. Agric. 2020, 36, 205–219. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Y.; Cheng, Y.; Gao, Z.; Qu, K.; Chen, Z.; Guan, W. Effects of Pulsed Electric Field and High-Pressure Processing Treatments on the Juice Yield and Quality of Sea Buckthorn. Foods 2024, 13, 1829. [Google Scholar] [CrossRef]
- Sarbatly, R.; Sariau, J.; Krishnaiah, D. Recent Developments of Membrane Technology in the Clarification and Concentration of Fruit Juices. Food Eng. Rev. 2023, 15, 420–437. [Google Scholar] [CrossRef]
- Chutia, H.; Begum, F.; Rohilla, S.; Mahanta, C.L. Impact of Thermosonication Treatment on Passion Fruit Juice: ANN/GA Optimization, Predictive Modelling for Shelf Life and Quality Changes during Storage. Int. J. Food Eng. 2024, 20, 463–474. [Google Scholar] [CrossRef]
- Saruan, N.; Abdullah, N.; Malik, N.H.; Muhammad, N. Effect of Ultrasound Treatment on the Physicochemical Properties and Bioactive Compounds of Yellow Passion Fruit Juice. In Proceedings of the 5th International Conference on Agricultural and Food Engineering (CAFEi2022), Melaka, Malaysia, 21–23 February 2022; AIP Publishing: Melville, NY, USA, 2023; Volume 2682, p. 030012. [Google Scholar] [CrossRef]
- Angami, T.; Assumi, S.R.; Kalita, H.; Saloi, B.; Singh, K.S.; Touthang, L.; Tasung, A. Preparation and Evaluation of Fresh Pineapple, Passion Fruit and Ginger Blended Ready-to-Serve Drink. Environ. Conserv. J. 2023, 24, 63–66. [Google Scholar] [CrossRef]
- Khorshidian, N.; Yousefi, M.; Zendeboodi, F.; Mirsaeedghazi, H. Effect of Membrane Clarification on the Physicochemical Properties of Fruit Juices: A Review. Iran. J. Chem. Chem. Eng. 2022, 41, 51–66. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).