Resorcinarene-Based Polymer Conjugated for Pharmaceutical Applications
Abstract
:1. Introduction
2. Synthesis of Calix[4]resorcinarenes
2.1. Reflux Synthesis of Calix[4]resorcinarenes
2.2. Solvent-Free Synthesis of Calix[4]resorcinarenes
2.3. Synthesis of Calix[4]resorcinarenes Through Microwave Irradiation
2.4. Conformations of Calix[4]resorcinarenes
3. Functionalization and Host–Guest Interactions of Calix[4]resorcinarenes
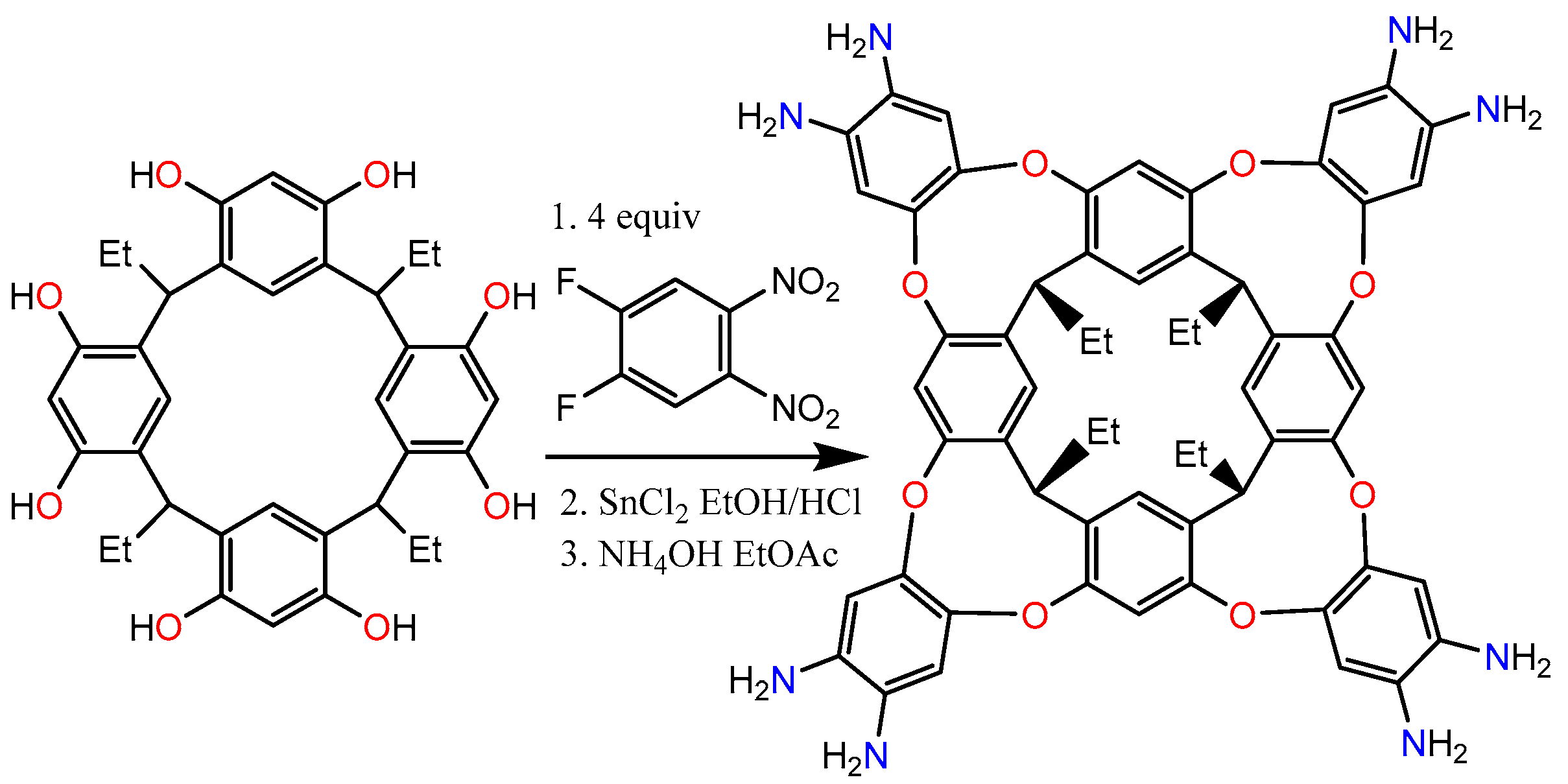
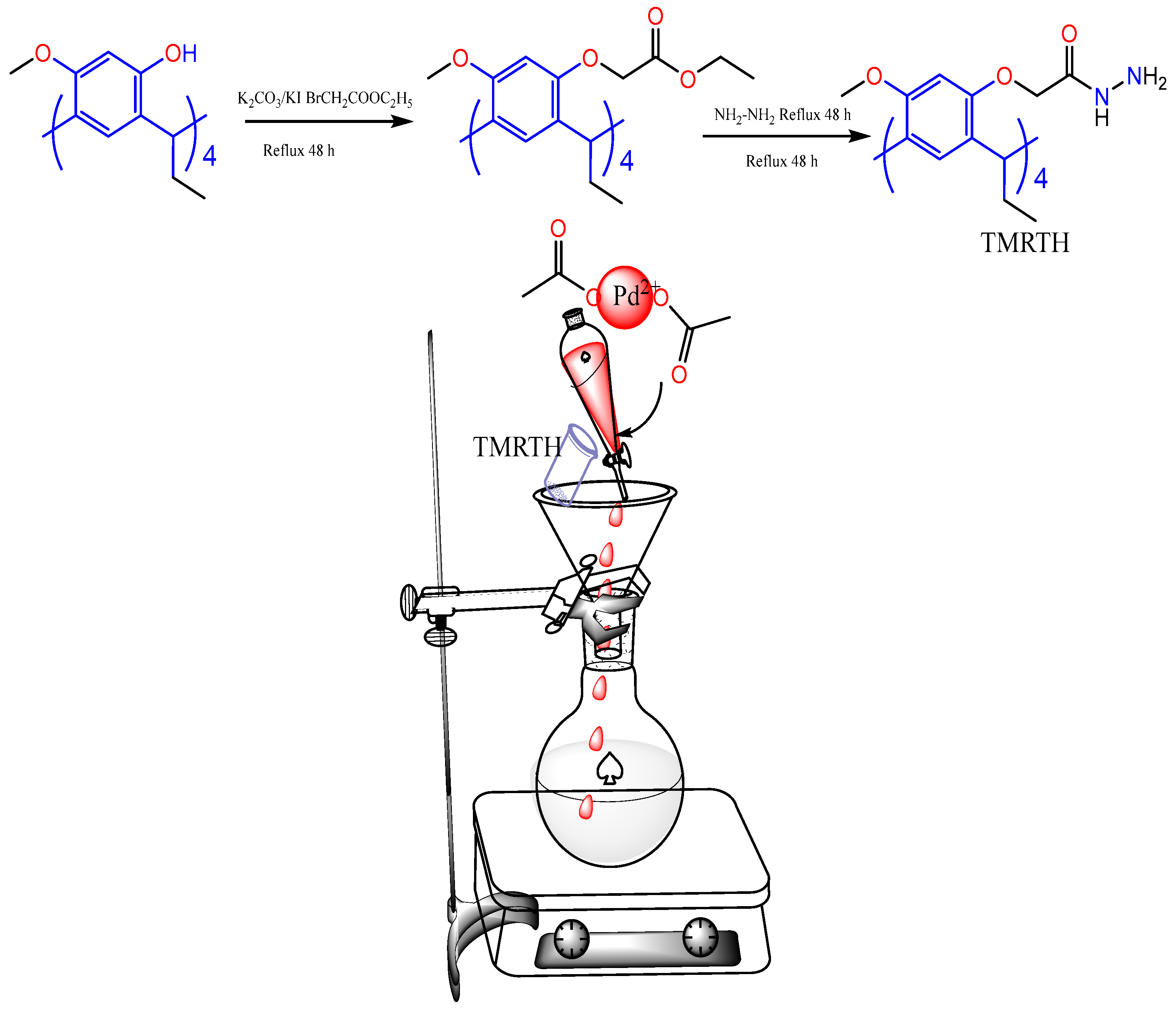
3.1. Functionalization Calix[4]resorcinarenes
- Starting from resorcinol and 4-formylbenzoic acid, two of the carboxyl groups are esterified and prevent the esterification of the other two carboxyl groups.
- Subsequently, RTH is generated via nucleophilic substitution in the carboxyl groups of the substituents of the methylene bridge. It is used to stabilize gold nanoparticles and in the detection of phenylalanine in human serum [38].
3.2. Host–Guest Interactions of Calix[4]resorcinarenes with Molecules of Biological Interest
4. Calix[4]resorcinarene Application Overview
5. Resorcinarenes in the Modification of Polymers
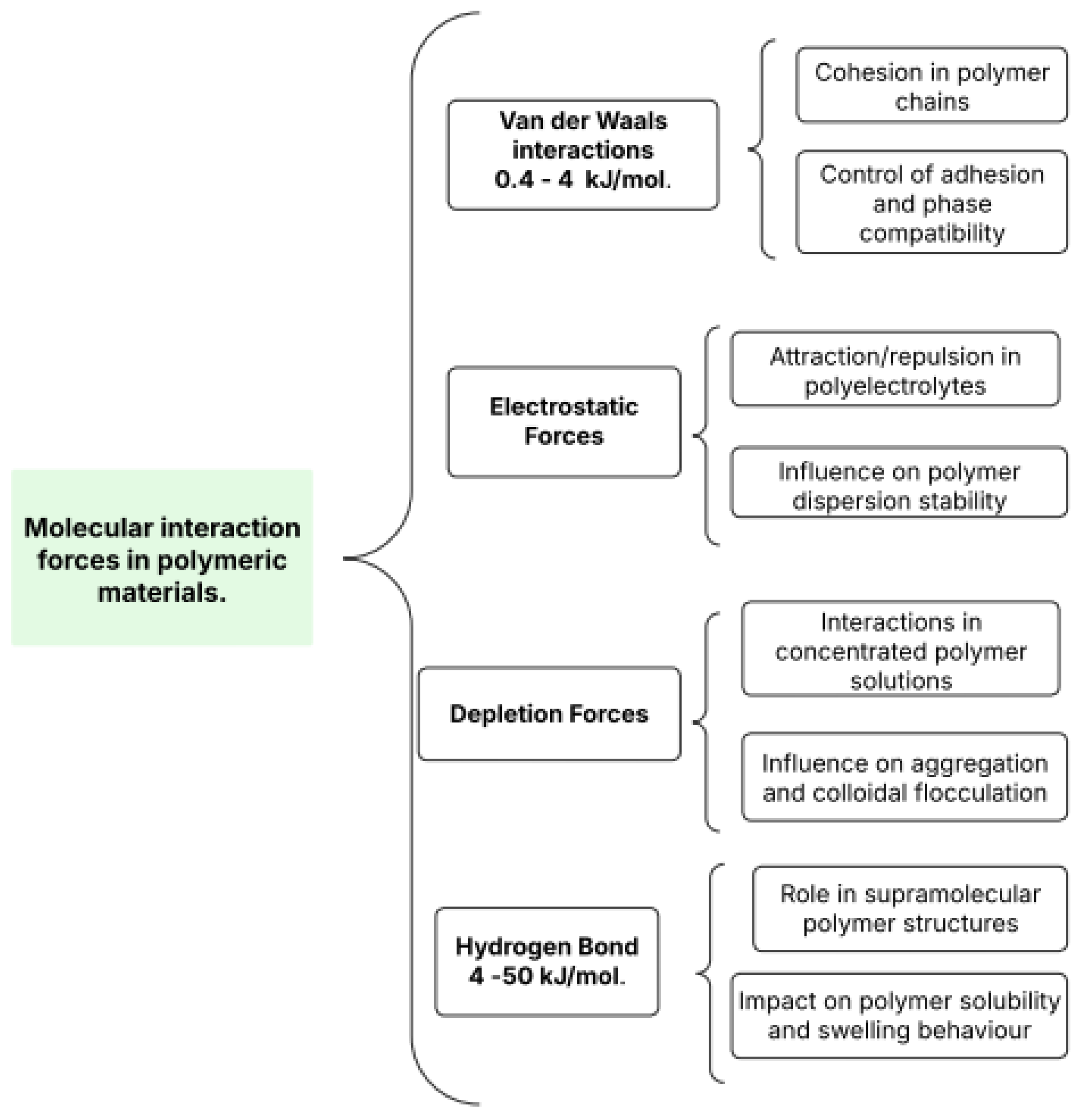
5.1. Surface Interaction Overview
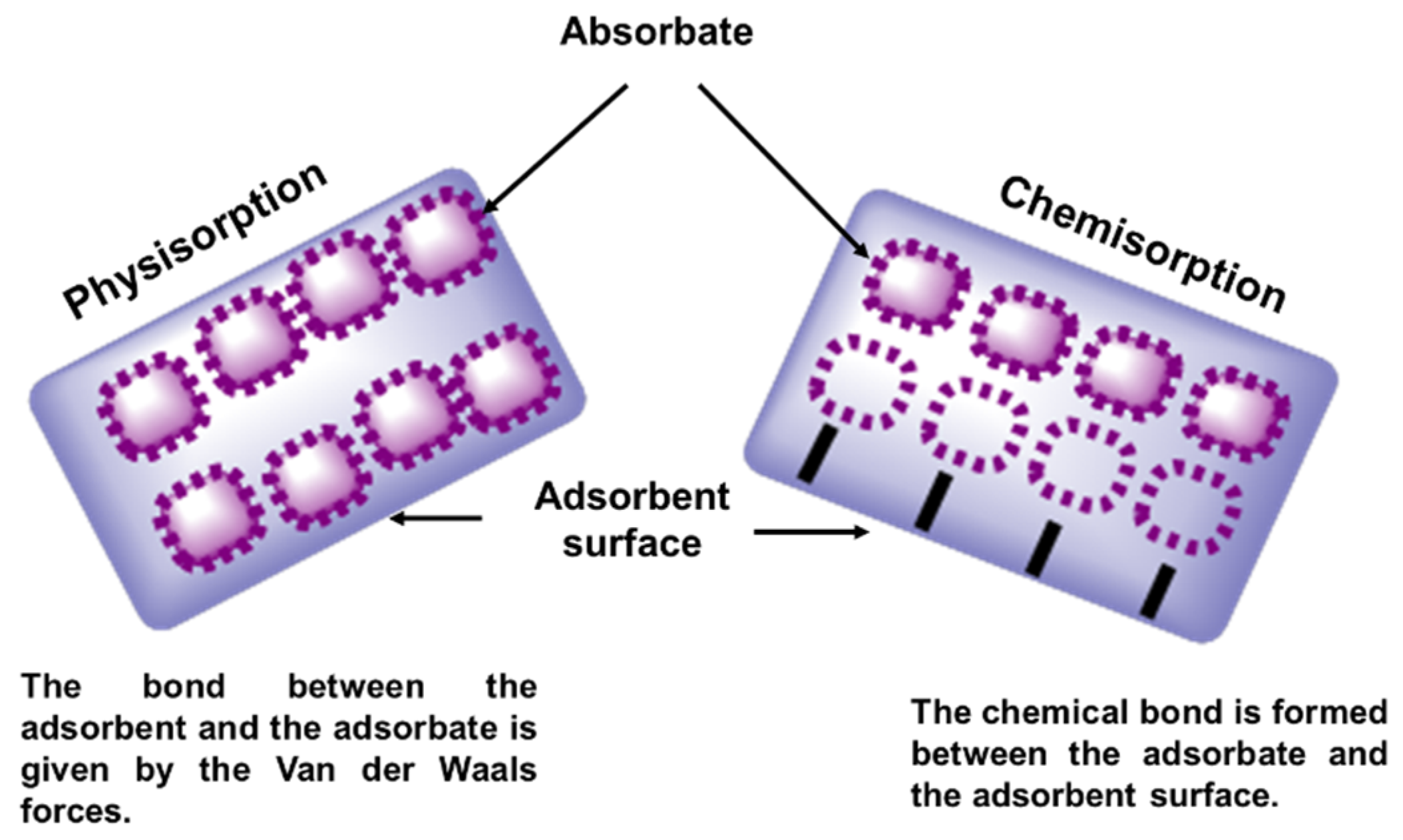
5.2. Surface-Modified Polymeric Materials in the Adsorption Process
5.3. Selectivity and Specificity in Polymeric Materials: A Molecular Perspective for Pharmaceutical Applications
6. Use of Resorcinarene-Based Polymers Conjugated for Pharmaceutical Applications
6.1. Resorcinarene-Based Polymers: Analytic Applications
6.2. Resorcinarene-Based Polymers: Delivery or Transport Systems for Drugs
6.3. Resorcinarene-Based Polymers: Therapeutic Applications
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Timmerman, P.; Verboom, W.; Reinhoudt, D.N. Resorcinarenes. Tetrahedron 1996, 52, 2663–2704. [Google Scholar] [CrossRef]
- Wang, K.; Liu, Q.; Zhou, L.; Sun, H.; Yao, X.; Hu, X.-Y. State-of-the-art and recent progress in resorcinarene-based cavitand. Chin. Chem. Lett. 2023, 34, 108559. [Google Scholar] [CrossRef]
- Boehmer, V. Calixarenes, Macrocycles with (Almost) Unlimited Possibilities. Angew. Chem. 1995, 34, 713–745. [Google Scholar] [CrossRef]
- Wang, K.; Yan, K.; Liu, Q.; Wang, Z.; Hu, X.-Y. The Versatile Applications of Calix[4]resorcinarene-Based Cavitands. Molecules 2024, 29, 5854. [Google Scholar] [CrossRef] [PubMed]
- Niederl, J.; Vogel, H. Aldeyde-Resorcinol Condensations. J. Am. Chem. Soc. 1940, 62, 2512. [Google Scholar] [CrossRef]
- Sverker, H. Two Stereoisomeric Macrocyclic Resorcinol-Acetaldehyde Condensation Products. J. Org. Chem. 1980, 45, 4498–4500. [Google Scholar]
- Yao, Y.; Sun, Y.; Han, Y.; Yan, C. Preparation of resorcinarene-functionalized gold nanoparticles and their catalytic activities for reduction of aromatic nitro compounds. Chin. J. Chem. 2010, 28, 705–712. [Google Scholar] [CrossRef]
- Shalaeva, Y.V.; Morozova, J.E.; Gubaidullin, A.T.; Saifina, A.F.; Syakaev, V.V.; Ermakova, A.M.; Nizameev, I.R.; Kadirov, M.K.; Ovsyannikov, A.S.; Konovalov, A.I. Gold nanoparticles, capped by carboxy-calix[4]resorcinarenes: Effect of structure and concentration of macrocycles on the nanoparticles size and aggregation. J. Incl. Phenom. Macrocycl. Chem. 2018, 92, 211–221. [Google Scholar] [CrossRef]
- Panchal, U.; Modi, K.; Panchal, M.; Mehta, V.; Jain, V.K. Catalytic activity of recyclable resorcinarene-protected antibacterial Pd nanoparticles in C-C coupling reactions. Chin. J. Catal. 2016, 37, 250–257. [Google Scholar] [CrossRef]
- Pedro-Hernández, L.D.; Martínez-Klimova, E.; Cortez-Maya, S.; Mendoza-Cardozo, S.; Ramírez-Ápan, T.; Martínez-García, M. Synthesis, characterization, and nanomedical applications of conjugates between resorcinarene-dendrimers and ibuprofen. Nanomaterials 2017, 7, 163. [Google Scholar] [CrossRef]
- Li, W.; Ma, J.; Zhou, Y.; Sun, X.; Gao, D. The application of sulfonated tetraphenyl calix[4] resorcinarene as a novel, multi-functional and eco-friendly ligand in zirconium tanning system. J. Clean. Prod. 2021, 280, 124337. [Google Scholar] [CrossRef]
- Harada, K.; Sekiya, R.; Haino, T. Molecular Recognition Process in Resorcinarene-based Coordination Capsules. Chem. A Eur. J. 2023, 29, e202302581. [Google Scholar] [CrossRef]
- Alshahateet, S.F.; Altarawneh, R.M.; Al-Trawneh, S.A.; Al-Saraireh, Y.M.; Al-Tawarh, W.M.; Abuawad, K.R.; Abuhalaweh, Y.M.; Zerrouk, M.; Mansour, A.A.; Salghi, R.; et al. Cheminformatics-based design and biomedical applications of a new Hydroxyphenylcalix[4] resorcinarene as anti-cancer agent. Sci. Rep. 2024, 14, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Harrison, R.G.; Lamb, J.D. Application of resorcinarene derivatives in chemical separations. J. Incl. Phenom. Macrocycl. Chem. 2014, 78, 39–60. [Google Scholar] [CrossRef]
- Giri, A.; Sahoo, A.; Dutta, T.K.; Patra, A. Cavitand and Molecular Cage-Based Porous Organic Polymers. ACS Omega 2020, 5, 28413–28424. [Google Scholar] [CrossRef]
- Han, X.; Park, J.; Wu, W.; Malagon, A.; Wang, L.; Vargas, E.; Wikramanayake, A.; Houk, K.N.; Leblanc, R.M. A resorcinarene for inhibition of Aβ fibrillation. Chem. Sci. 2017, 8, 2003–2009. [Google Scholar] [CrossRef]
- Ye, X.; Wang, Q.; Sun, M.; Chen, L.; Jia, A.; Zhang, Q. Syntheses and biological activities of calix[4]resorcinarene derivatives modified by sulfonic acid and sulfonamides. RSC Adv. 2024, 14, 25115–25119. [Google Scholar] [CrossRef]
- Menon, S.K.; Modi, N.R.; Pandya, A.; Lodha, A. Ultrasensitive and specific detection of dimethoate using a p-sulphonato-calix[4]resorcinarene functionalized silver nanoprobe in aqueous solution. RSC Adv. 2013, 3, 10623–10627. [Google Scholar] [CrossRef]
- Jeerupan, J.; Konishi, G.-I.; Nemoto, T.; Shin, D.-M.; Nakamoto, Y. Synthesis of Multifunctional Poly(calix[4]resorcinarene). Polym. J. 2007, 39, 762–763. [Google Scholar] [CrossRef]
- Jain, V.K.; Kanaiya, P.H. Chemistry of calix[4]resorcinarenes. Russ. Chem. Rev. 2011, 80, 75–102. [Google Scholar] [CrossRef]
- Morikawa, O.; Nagamatsu, Y.; Nishimura, A.; Kobayashi, K.; Konishi, H. Scandium triflate-catalyzed cyclocondensation of 1,3-dialkoxybenzenes with 1,3,5-trioxane. Formation of resorcin[4]arenes and confused resorcin[4]arenes. Tetrahedron Lett. 2006, 47, 3991–3994. [Google Scholar] [CrossRef]
- Matiz, C.; Castillo-Aguirre, A.; Maldonado, M. Synthesis of C -tetra(aryl)resorcin[4]arenes using various types of catalysts under solvent free conditions: A comparative study. Green Chem. Lett. Rev. 2024, 17, 2290847. [Google Scholar] [CrossRef]
- Roberts, B.A.; Cave, G.W.; Raston, C.L.; Scott, J.L. Solvent-free synthesis of calix[4]resorcinarenes. Green Chem. 2001, 3, 280–284. [Google Scholar] [CrossRef]
- Pfeiffer, C.R.; Feaster, K.A.; Dalgarno, S.J.; Atwood, J.L. Syntheses and characterization of aryl-substituted pyrogallol[4]arenes and resorcin[4]arenes. CrystEngComm 2015, 18, 222–229. [Google Scholar] [CrossRef]
- Sardjono, R.; Kadarohman, A.; Mardhiyah, A. Green Synthesis of Some Calix[4]Resorcinarene Under Microwave Irradiation. Procedia Chem. 2012, 4, 224–231. [Google Scholar] [CrossRef]
- Gutsche, C.D.; Bauer, L.J. Calixarenes. 13. The Conformational properties of calix[4]arenes, calix[6]arenes, Calix[8]arenes, and oxacalixarenes. J. Am. Chem. Soc. 1985, 21, 6052–6059. [Google Scholar] [CrossRef]
- Moore, D.; Watson, G.W.; Gunnlaugsson, T.; Matthews, S.E. Selective formation of the rctt chair stereoisomers of octa-O-alkyl resorcin[4]arenes using Brønsted acid catalysis. New J. Chem. 2008, 32, 994–1002. [Google Scholar] [CrossRef]
- Rawn, D.J.; Ouellette, R. Organic Chemistry: Structure, Mechanism, Synthesis. 2018. Available online: https://www.sciencedirect.com/topics/chemistry/sn2-mechanism (accessed on 17 February 2025).
- Balasubramanian, R.; Kwon, Y.-G.; Wei, A. Encapsulation and functionalization of nanoparticles in crosslinked resorcinarene shells. J. Mater. Chem. 2007, 17, 105–112. [Google Scholar] [CrossRef]
- Shalaeva, Y.V.; Yanilkin, V.V.; Morozova, Y.E.; Kazakova, E.K.; Syakaev, V.V.; Makarova, N.A.; Morozov, V.V.; Konovalov, A.I. Effect of Structure of Tetramethyl Sulfonated Calix[4]resorcinarene Aggregates on Paraquat Redox Reactions. Colloid J. 2010, 72, 262–273. [Google Scholar] [CrossRef]
- Jain, V.K.; Kanaiya, P.H.; Bhojak, N. Synthesis, spectral characterization of azo dyes derived from calix[4]resorcinarene and their application in dyeing of fibers. Fibers Polym. 2008, 9, 720–726. [Google Scholar] [CrossRef]
- Grajda, M.; Wierzbicki, M.; Cmoch, P.; Szumna, A. Inherently chiral iminoresorcinarenes through regioselective unidirectional tautomerization. J. Org. Chem. 2013, 78, 11597–11601. [Google Scholar] [CrossRef] [PubMed]
- Utomo, S.B.; Saputro, A.N.C.; Rinanto, Y. Functionalization of C-4-methoxyphenylcalix[4]resorcinarene with several ammonium compounds. IOP Conf. Ser. Mater. Sci. Eng. 2016, 107, 012042. [Google Scholar] [CrossRef]
- Kashapov, R.R.; Zakharova, L.Y.; Saifutdinova, M.N.; Kochergin, Y.S.; Gavrilova, E.L.; Sinyashin, O.G. Construction of a water-soluble form of amino acid C-methylcalix[4]resorcinarene. J. Mol. Liq. 2015, 208, 58–62. [Google Scholar] [CrossRef]
- Pineda-Castañeda, H.M.; Rivera-Monroy, Z.J.; Maldonado, M. Copper(I)-Catalyzed Alkyne–Azide Cycloaddition (CuAAC) “Click” Reaction: A Powerful Tool for Functionalizing Polyhydroxylated Platforms. ACS Omega 2023, 8, 3650–3666. [Google Scholar] [CrossRef] [PubMed]
- Wojaczyńska, E.; Ostrowska, M.; Lower, M.; Czyżyk, N.; Jakieła, A.; Marra, A. Recent Advances in Synthesis and Applications of Calixarene Derivatives Endowed with Anticancer Activity. Molecules 2024, 29, 4240. [Google Scholar] [CrossRef]
- van Velzen, E.U.T.; Engbersen, J.F.J.; de Lange, P.J.; Mahy, J.W.G.; Reinhoudt, D.N. Self-Assembled Monolayers of Resorcin[4]arene Tetrasulfides on Gold. J. Am. Chem. Soc. 1995, 117, 6853–6862. [Google Scholar] [CrossRef]
- Mishra, D.R.; Darjee, S.M.; Bhatt, K.D.; Modi, K.M.; Jain, V.K. Calix protected gold nanobeacon as turn-off fluorescent sensor for phenylalanine. J. Incl. Phenom. Macrocycl. Chem. 2015, 82, 425–436. [Google Scholar] [CrossRef]
- Khan, S.B.; Lee, S.-L. Supramolecular chemistry: Host–guest molecular complexes. Molecules 2021, 26, 3995. [Google Scholar] [CrossRef]
- Collin, S.; Parrot, A.; Marcelis, L.; Brunetti, E.; Jabin, I.; Bruylants, G.; Bartik, K.; Reinaud, O. Submerging a Biomimetic Metallo-Receptor in Water for Molecular Recognition: Micellar Incorporation or Water Solubilization? A Case Study. Chem. A Eur. J. 2018, 24, 17964–17974. [Google Scholar] [CrossRef]
- Arena, G.; Contino, A.; Fujimoto, T.; Sciotto, D.; Aoyama, Y. 1H NMR and Calorimetric Studies of the Inclusion of Trimethylammonium Cations into Water Soluble Calixresorcinarenes. Supramol. Chem. 2000, 11, 279–288. [Google Scholar] [CrossRef]
- Twum, K.; Rautiainen, J.M.; Yu, S.Y.; Truong, K.-N.K.N.; Feder, J.; Rissanen, K.; Puttreddy, R.; Beyeh, N.K. Host–Guest Interactions of Sodiumsulfonatomethyleneresorcinarene and Quaternary Ammonium Halides: An Experimental–Computational Analysis of the Guest Inclusion Properties. Cryst. Growth Des. 2020, 20, 2367–2376. [Google Scholar] [CrossRef]
- Ballester, P.; Shivanyuk, A.; Far, A.R.; Rebek, J.J. A synthetic receptor for choline and carnitine. J. Am. Chem. Soc. 2002, 124, 14014–14016. [Google Scholar] [CrossRef]
- Mustafina, A.R.; Elistratova, Y.G.; Syakaev, V.V.; Amirov, R.R.; Konovalova, A.I. Receptor properties of calix[4]resorcinarenes toward tetramethylammonium and choline cations in micellar solutions of sodium dodecyl sulfate. Russ. Chem. Bull. 2006, 55, 1419–1424. [Google Scholar] [CrossRef]
- Amrhein, P.; Shivanyuk, A.; Johnson, D.W.; Rebek, J.J. Metal-switching and self-inclusion of functional cavitands. J. Am. Chem. Soc. 2002, 124, 10349–10358. [Google Scholar] [CrossRef]
- Zeisel, S.H. Choline: An Essential Nutrient for Humans. Nutrition 2000, 16, 669–671. [Google Scholar] [CrossRef]
- Velásquez-Silva, A.; Forero, R.S.; Sanabria, E.; Pérez-Redondo, A.; Maldonado, M. Host-guest inclusion systems of tetra(alkyl)resorcin [4]arenes with choline in DMSO: Dynamic NMR studies and X-ray structural characterization of the 1:1 inclusion complex. J. Mol. Struct. 2019, 1198, 126846. [Google Scholar] [CrossRef]
- Ma, B.-Q.; Coppens, P. Transformation of a C-methylcalix[4]resorcinarene-based host–guest complex from a wave-like to a novel triangular brick-wall architecture. Chem. Commun. 2003, 9, 504–505. [Google Scholar] [CrossRef]
- Thota, S.; Rodrigues, D.A.; Crans, D.C.; Barreiro, E.J. Ru(II) Compounds: Next-Generation Anticancer Metallotherapeutics? J. Med. Chem. 2018, 61, 5805–5821. [Google Scholar] [CrossRef]
- Pinalli, R.; Brancatelli, G.; Pedrini, A.; Menozzi, D.; Hernández, D.; Ballester, P.; Geremia, S.; Dalcanale, E. The Origin of Selectivity in the Complexation of N-Methyl Amino Acids by Tetraphosphonate Cavitands. J. Am. Chem. Soc. 2016, 138, 8569–8580. [Google Scholar] [CrossRef]
- Xu, H.; Wang, S.; Wu, F.; Yuan, Q.; Guo, Y.; Zhang, Y.; Wei, X.; Zhang, J. A fully-organic polymerization carrier calix[4]resorcinarene supported cobalt ionic liquid catalyst with oxone for desulfurization. Fuel 2022, 318, 123670. [Google Scholar] [CrossRef]
- Shuai, X.; Cai, Z.; Zhao, X.; Chen, Y.; Zhang, Q.; Ma, Z.; Hu, J.; Sun, T.; Hu, S. A New Stationary Phase for Capillary Gas Chromatography: Calix[4]resorcinarene Functionalized with Imidazolium Cationic Units. Chromatographia 2021, 84, 325–333. [Google Scholar] [CrossRef]
- Hussain, K.; Umar, A.R.; Rasheed, S.; Hassan, M.; Laiche, M.H.; Muhammad, H.; Hanif, M.; Aslam, Z.; Sirajuddin; Shah, M.R. Smartphone-Integrated resorcinarene macrocycle capped silver nanoparticles (RMF-AgNPs) probe for enhanced La(III) detection in diverse environments. J. Ind. Eng. Chem. 2024, 138, 256–269. [Google Scholar] [CrossRef]
- Shaban, A.; Eddaif, L. Comparative Study of a Sensing Platform via Functionalized Calix[4]resorcinarene Ionophores on QCM Resonator as Sensing Materials for Detection of Heavy Metal Ions in Aqueous Environments. Electroanalysis 2021, 33, 336–346. [Google Scholar] [CrossRef]
- Pineda-Castañeda, H.M.; Maldonado, M.; Rivera-Monroy, Z.J. Efficient Separation of C-Tetramethylcalix[4]resorcinarene Conformers by Means of Reversed-Phase Solid-Phase Extraction. ACS Omega 2022, 8, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Diederich, F. Modern Supramolecular Chemistry: Strategies for Macrocycle Synthesis; Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008. [Google Scholar]
- Sharma, V.S.; Mali, H.; Sharma, A.S.; Thakar, S.P.; Patel, U.P.; Shrivastav, P.S. Resorcinarene appended benzothiazole based light-emitting macrocyclic compounds with nematic liquid crystalline phase and bio-imaging applications. J. Mol. Liq. 2024, 397, 124110. [Google Scholar] [CrossRef]
- Ma, M.; Wei, Q.; Meng, M.; Yin, J.; Shan, Y.; Du, L.; Zhu, X.; Soh, S.F.; Min, M.; Zhou, X.; et al. Preparation and Application of Aza-15-crown-5-capped Methylcalix[4]resorcinarene-Bonded Silica Particles for Use as Chiral Stationary Phase in HPLC. Chromatographia 2017, 80, 1007–1014. [Google Scholar] [CrossRef]
- Israelachvili, J.N. Intermolecular and Surface Forces, 3rd ed.; Academic Press: Waltham, MA, USA, 2011. [Google Scholar]
- Myshkin, N.; Kovalev, A. Adhesion and surface forces in polymer tribology—A review. Friction 2018, 6, 143–155. [Google Scholar] [CrossRef]
- Yamada, S. Molecular Interactions (Molecular and Surface Forces). In Encyclopedia of Polymeric Nanomaterials; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–7. [Google Scholar] [CrossRef]
- Berger, A.H.; Bhown, A.S. Comparing physisorption and chemisorption solid sorbents for use separating CO2 from flue gas using temperature swing adsorption. Energy Procedia 2011, 4, 562–567. [Google Scholar] [CrossRef]
- Dąbrowski, A. Adsorption—From theory to practice. Adv. Colloid Interface Sci. 2001, 93, 135–224. [Google Scholar] [CrossRef]
- Bedoya, D.A.; Figueroa, F.N.; Macchione, M.A.; Strumia, M.C. Stimuli-Responsive Polymeric systems for smart drug delivery. In Advances in Material Research and Technology; Springer: Cham, Switzerland, 2020; pp. 115–134. [Google Scholar] [CrossRef]
- Cabaleiro-Lago, C.; Hasterok, S.; Wingren, A.G.; Tassidis, H. Recent Advances in Molecularly Imprinted Polymers and Their Disease-Related Applications. Polymers 2023, 15, 4199. [Google Scholar] [CrossRef]
- Sergeeva, T.Y.; Mukhitova, R.K.; Nizameev, I.R.; Kadirov, M.K.; Klypina, P.D.; Ziganshina, A.Y.; I Konovalov, A. Closed polymer containers based on phenylboronic esters of resorcinarenes. Beilstein J. Nanotechnol. 2018, 9, 1594–1601. [Google Scholar] [CrossRef] [PubMed]
- Dria, R.D.; Goudy, B.A.; Moga, K.A.; Corbin, P.S. Synthesis and characterization of multi-armed calixarene- and resorcinarene-core polylactide star polymers. Polym. Chem. 2012, 3, 2070–2081. [Google Scholar] [CrossRef]
- Wu, R.; Al-Azemi, T.F.; Bisht, K.S. Influence of a resorcin[4]arene core structure on the spatial directionality of multi-arm poly(ε-caprolactone)s. RSC Adv. 2014, 4, 16864–16870. [Google Scholar] [CrossRef]
- Castillo-Aguirre, A.; Maldonado, M. Preparation of methacrylate-based polymers modified with chiral resorcinarenes and their evaluation as sorbents in norepinephrine microextraction. Polymers 2019, 11, 1428. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Allington, R.W.; Fréchet, J.M.J.; Svec, F. Porous polymer monoliths: An alternative to classical beads. Adv. Biochem. Eng. Biotechnol. 2002, 76, 87–125. [Google Scholar] [CrossRef]
- Ramirez, G.; Cadavid-Montoya, N.A.; Maldonado, M. Evaluation of a Resorcinarene-Based Sorbent as a Solid-Phase Extraction Material for the Enrichment of L-Carnitine from Aqueous Solutions. Processes 2023, 11, 1705. [Google Scholar] [CrossRef]
- Velásquez-Silva, B.A.; Castillo-Aguirre, A.; Rivera-Monroy, Z.J.; Maldonado, M. Aminomethylated calix[4]resorcinarenes as modifying agents for glycidyl methacrylate (GMA) rigid copolymers surface. Polymers 2019, 11, 1147. [Google Scholar] [CrossRef]
- Ruderisch, A.; Iwanek, W.; Pfeiffer, J.; Fischer, G.; Albert, K.; Schurig, V. Synthesis and characterization of a novel resorcinarene-based stationary phase bearing polar headgroups for use in reversed-phase high-performance liquid chromatography. J. Chromatogr. A 2005, 1095, 40–49. [Google Scholar] [CrossRef]
- Umar, A.R.; Hussain, K.; Aslam, Z.; Haq, M.A.U.; Muhammad, H.; Sirajuddin; Shah, M.R. Ultra-trace level voltammetric sensor for MB in human plasma based on a carboxylic derivative of Calix[4]resorcinarene capped silver nanoparticles. J. Ind. Eng. Chem. 2022, 107, 81–92. [Google Scholar] [CrossRef]
- Faizal, C.K.M.; Kikuchi, Y.; Kobayashi, T. Molecular imprinting targeted for α-tocopherol by calix[4]resorcarenes derivative in membrane scaffold prepared by phase inversion. J. Membr. Sci. 2009, 334, 110–116. [Google Scholar] [CrossRef]
- Shumatbaeva, A.M.; Morozova, J.E.; Syakaev, V.V.; Shalaeva, Y.V.; Sapunova, A.S.; Voloshina, A.D.; Gubaidullina, A.T.; Bazanova, O.B.; Babaev, V.M.; Nizameev, I.R.; et al. The pH-responsive calix[4]resorcinarene-mPEG conjugates bearing acylhydrazone bonds: Synthesis and study of the potential as supramolecular drug delivery systems. Colloids Surf. A Physicochem. Eng. Asp. 2020, 589, 124453. [Google Scholar] [CrossRef]
- Sergeeva, T.Y.; Mukhitova, R.K.; Nizameev, I.R.; Kadirov, M.K.; Sapunova, A.S.; Voloshina, A.D.; Mukhametzyanov, T.A.; Ziganshina, A.Y.; Antipin, I.S. A Glucose-Responsive Polymer Nanocarrier Based on Sulfonated Resorcinarene for Controlled Insulin Delivery. Chempluschem 2019, 84, 1560–1566. [Google Scholar] [CrossRef] [PubMed]
- Ermakova, A.M.; E Morozova, J.; Shalaeva, Y.V.; Syakaev, V.V.; Gubaidullin, A.T.; Voloshina, A.D.; Zobov, V.V.; Nizameev, I.R.; Bazanova, O.B.; Antipin, I.S.; et al. Nanoconjugates of a calixresorcinarene derivative with methoxy poly(ethylene glycol) fragments for drug encapsulation. Beilstein J. Nanotechnol. 2018, 9, 2057–2070. [Google Scholar] [CrossRef]
- Shumatbaeva, A.M.; Morozova, J.E.; Shalaeva, Y.V.; Gubaidullin, A.T.; Saifina, A.F.; Syakaev, V.V.; Bazanova, O.B.; Sapunova, A.S.; Voloshina, A.D.; Nizameev, I.R.; et al. The novel calix[4]resorcinarene-PEG conjugate: Synthesis, self-association and encapsulation properties. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 570, 182–190. [Google Scholar] [CrossRef]
- Gao, C.; Wang, Y.; Zhu, W.-P.; Shen, Z.-Q. Resorcinarene-centered amphiphilic star-block copolymers: Synthesis, micellization and controlled drug release. Chin. J. Polym. Sci. 2014, 32, 1431–1441. [Google Scholar] [CrossRef]
- Shumatbaeva, A.M.; Morozova, J.E.; Shalaeva, Y.V.; Saifina, A.F.; Gubaidullin, A.T.; Syakaev, V.V.; Sapunova, A.S.; Voloshina, A.D.; Nizameev, I.R.; Kadirov, M.K.; et al. Synthesis of Ag-AgCl nanoparticles capped by calix[4]resorcinarene-mPEG conjugate and their antimicrobial activity. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 602, 125124. [Google Scholar] [CrossRef]
- Syakaev, V.V.; Morozova, J.E.; Bogdanov, A.V.; Shalaeva, Y.V.; Ermakova, A.M.; Voloshina, A.D.; Zobov, V.V.; Nizameev, I.R.; Kadirov, M.K.; Mironov, V.F.; et al. Solubilization of azo-dye-modified isatin derivative by amphiphilic carboxyresorcinarenes: The effect of macrocycle structure on the supramolecular association. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 553, 368–377. [Google Scholar] [CrossRef]
- Liu, J.-L.; Ye, X.-D.; Chen, L.-S.; Jia, A.-Q.; Zhang, Q.-F. The biological activity of tetrakis(benzoxazine) calix[4]resorcinarenes and as a host for small molecules. J. Incl. Phenom. Macrocycl. Chem. 2025, 105, 1–14. [Google Scholar] [CrossRef]
- Cristófalo, A.E.; Nieto, P.M.; Thépaut, M.; Fieschi, F.; Di Chenna, P.H.; Uhrig, M.L. Synthesis, self-assembly and Langerin recognition studies of a resorcinarene-based glycocluster exposing a hyaluronic acid thiodisaccharide mimetic. Org. Biomol. Chem. 2021, 19, 6455–6467. [Google Scholar] [CrossRef]
- Valladeau, J.; Ravel, O.; Dezutter-Dambuyant, C.; Moore, K.; Kleijmeer, M.; Liu, Y.; Duvert-Frances, V.; Vincent, C.; Schmitt, D.; Davoust, J.; et al. Langerin, a Novel C-Type Lectin Specific to Langerhans Cells, Is an Endocytic Receptor that Induces the Formation of Birbeck Granules. Immunity 2000, 12, 71–81. [Google Scholar] [CrossRef]
- Maarifi, G.; Czubala, M.A.; Lagisquet, J.; Ivory, M.O.; Fuchs, K.; Papin, L.; Birchall, J.C.; Nisole, S.; Piguet, V.; Blanchet, F.P. Langerin (CD207) represents a novel interferon-stimulated gene in Langerhans cells. Cell. Mol. Immunol. 2020, 17, 547–549. [Google Scholar] [CrossRef] [PubMed]
- Niño-Ramírez, V.A.; Maldonado, M.; Cuero-Amu, K.J.; García-Castañeda, J.E.; Rivera-Monroy, Z.J. Challenges in the Characterization and Purification of (Peptide)n-Calix[4]Resorcinarene Conjugates Synthesized via Thiol-Maleimide Reaction Using Liquid Chromatography. Processes 2025, 13, 222. [Google Scholar] [CrossRef]
- Pineda-Castañeda, H.M.; Maldonado-Villamil, M.; Parra-Giraldo, C.M.; Leal-Castro, A.L.; Fierro-Medina, R.; Rivera-Monroy, Z.J.; García-Castañeda, J.E. Peptide-Resorcinarene Conjugates Obtained via Click Chemistry: Synthesis and Antimicrobial Activity. Antibiotics 2023, 12, 773. [Google Scholar] [CrossRef] [PubMed]
- Padnya, P.; Shiabiev, I.; Pysin, D.; Gerasimova, T.; Ranishenka, B.; Stanavaya, A.; Abashkin, V.; Shcharbin, D.; Shi, X.; Shen, M.; et al. Non-Viral Systems Based on PAMAM-Calix-Dendrimers for Regulatory siRNA Delivery into Cancer Cells. Int. J. Mol. Sci. 2024, 25, 12614. [Google Scholar] [CrossRef]
- Mendoza-Cardozo, S.; Pedro-Hernández, L.D.; Organista-Mateos, U.; Allende-Alarcón, L.I.; Martínez-Klimova, E.; Ramírez-Ápan, T.; Martínez-García, M. In vitro activity of resorcinarene–chlorambucil conjugates for therapy in human chronic myelogenous leukemia cells. Drug Dev. Ind. Pharm. 2019, 45, 683–688. [Google Scholar] [CrossRef]

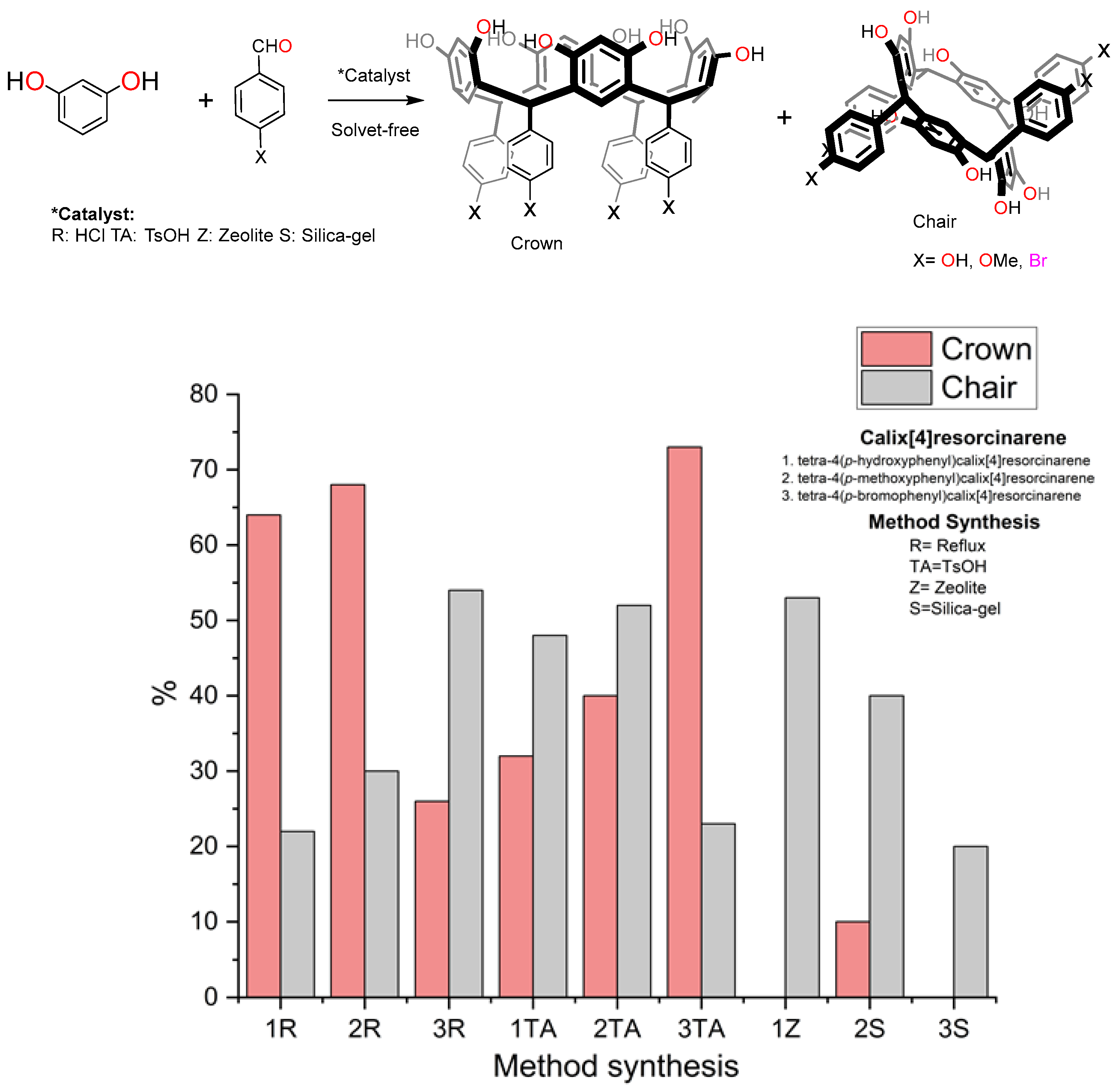
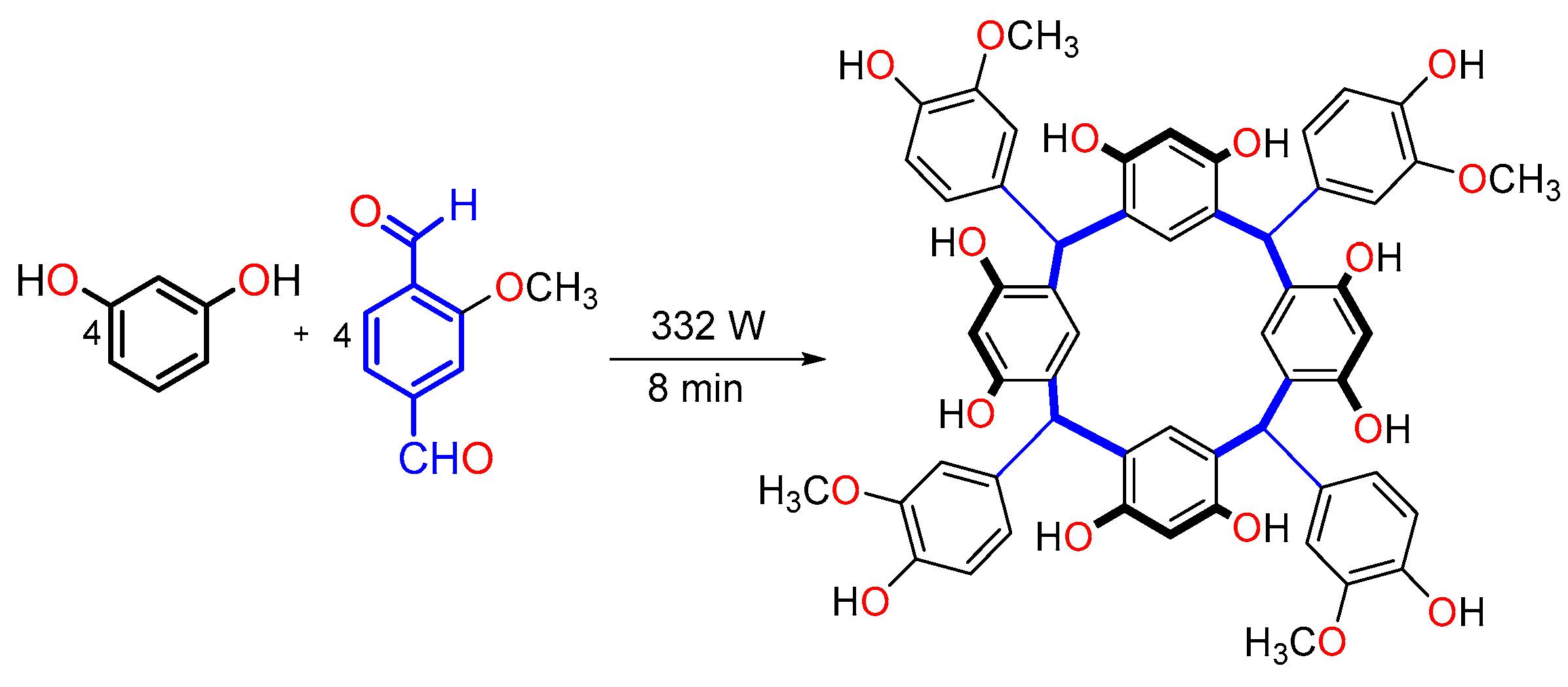

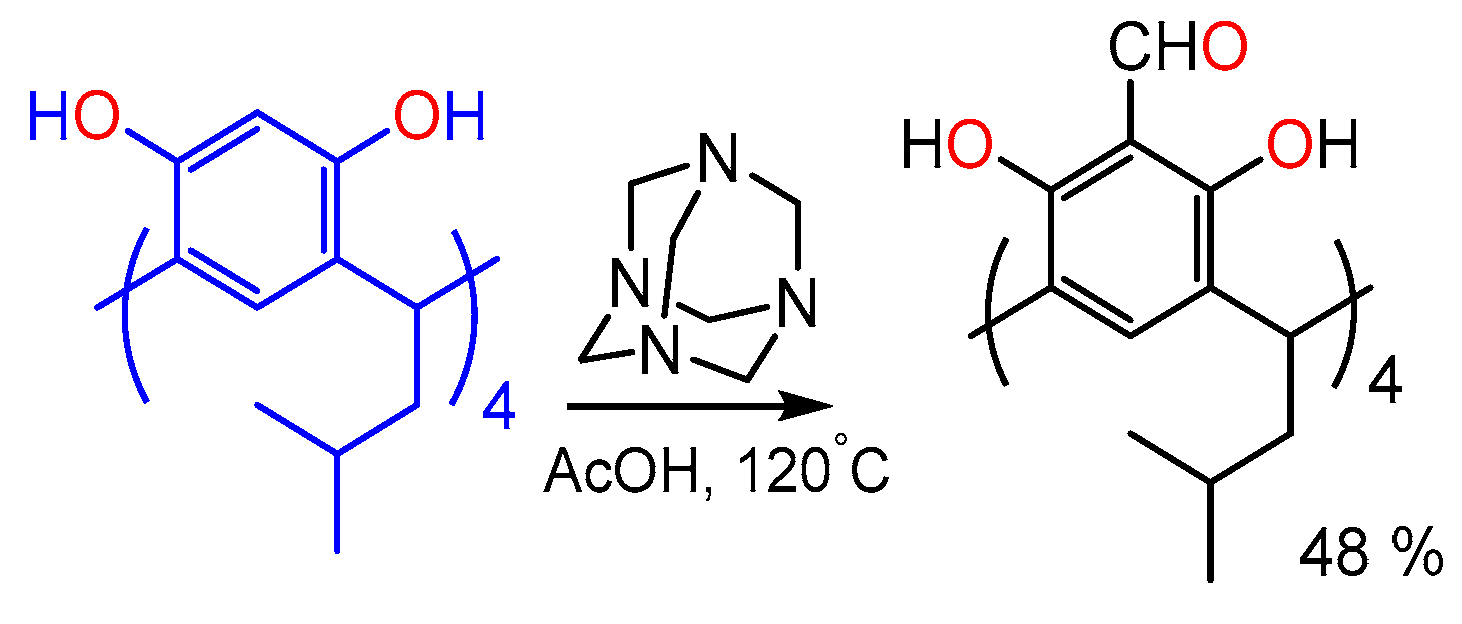
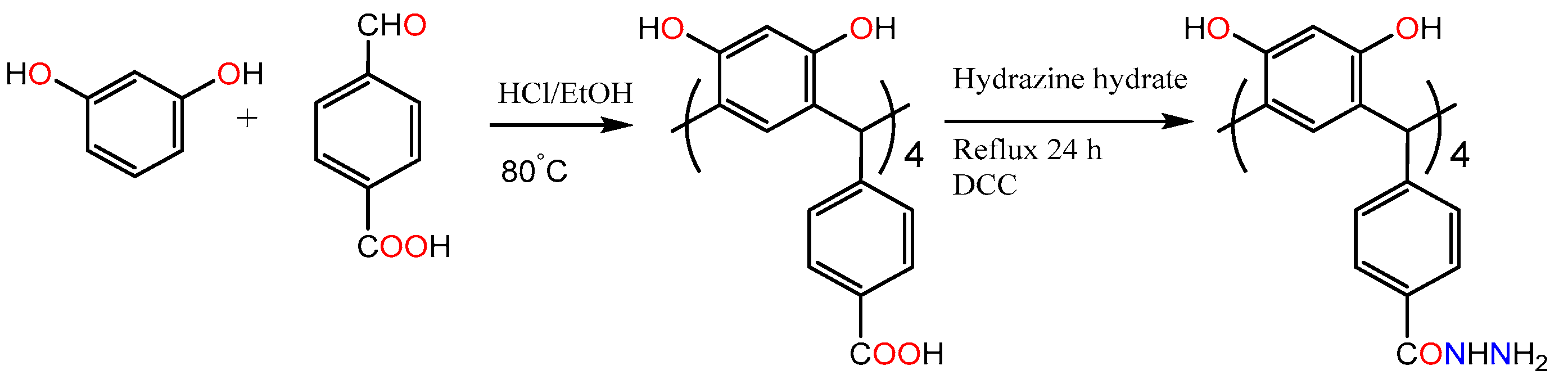



| Guest | KS (M−1) | ΔGo (kcal/mol) |
|---|---|---|
| Choline Chloride | 12,000 ± 2400 | 5.5 ± 0.1 |
| Acetylcholine Chloride | 4000 ± 800 | 4.9 ± 0.1 |
| L-carnitine | 15,000 ± 3000 | 5.6 ± 0.1 |
| Acetylcarnitine·HCl | 30 ± 6 | 2.0 ± 0.1 |
| Application | Examples | Advantages | Disadvantages |
|---|---|---|---|
| Controlled delivery drug | Resorcinarene centered on (SPCL-b-PEG) used in drug release [80]. | This conjugate delivery indomethacin stands out for its stability in aqueous solutions. | The synthesis of the conjugate is developed using a very unaffordable catalyst. |
| There is a strong interaction of the conjugate with the drug, so its delivery took 40 h. | This work can be complemented using hydrophobic drugs. | ||
| This study demonstrates their low hemolytic activity as cytotoxics against human hepatocyte cells. | |||
| Resorcinarene centered on methoxy-PEG conjugates [76]. | Doxorubicin was used as the base drug, whose release increases at acidic pH, which is relevant in tumor environments. | The conjugate structure contains acylhydrazone bonds, susceptible to unexpected hydrolysis. | |
| The associations of the drug with the conjugate showed an increase in toxicity against tumor cells in contrast to the free drug. | This work can be complemented with in vivo investigations. | ||
| Nanocarrier for glucose-regulated insulin for controlled insulin delivery [77]. | Conjugates exhibit low hemotoxicity and cytotoxicity in changing liver cells. | This study could evaluate the use of resorcinarene with aromatic substituents. | |
| The capacity of the nanocarrier for insulin encapsulation was 76% using a sulfonated resorcinarene. | This study can be complemented by assessing the interference of biomolecules. | ||
| This material is stable in blood plasma and water. | |||
| Pharmaceutical transport | Tetraundecylcalixresorcin-arene–mPEG encapsulates organic compounds and some drugs [78]. | The macrocycle obtained can form nano-associates in aqueous solution for drug encapsulation and has low hemotoxicity. | Macrocycle does not address long-term toxicity tests. |
| The critical association concentration of this conjugate is 0.01 mg/mL, which is the minimum value of the conjugate in micelle formation. | This study lacks biodegradability studies of the macrocycle. | ||
| This macrocycle, being amphiphilic, makes it possible to encapsulate both hydrophilic and hydrophobic drugs. | |||
| Dendrimer resorcinarene for gene-delivery systems [89]. | PAMAM-calix-dendrimers (PCD) can bind siRNA more efficiently and interact with cancer cells better than conventional PAMAM dendrimers. | There is an inefficient delivery of siRNA for the first generation (G1-alt). | |
| PCD shows high internalization capacity in HeLa cells. | This study could further investigate the effect of the interaction of the conjugate with some biological enzymes. | ||
| The larger the PCD size, the less toxic effect on blood cells has been found. | |||
| Therapeutic applications | Peptide resorcinarene with antibacterial activity potential [87,88]. | Broad therapeutic versatility and potential for controlled drug- and gene-delivery applications. Significant activity against specific bacterial strains is reported, supporting the therapeutic potential of the compound. Basis for the development of new antimicrobial agents. | The antibacterial activity of the conjugates studied against E. coli was low. The study is promising for further in vivo experiments. Limited toxicity assessment. |
| Chlorambucil resorcinarene utilized for therapy in human myelogenous leukemia cells [90]. | Basis for the development of new antimicrobial agents. | Limited toxicity assessment. | |
| Resorcinarene facilitates the internalization of chlorambucil in tumor cells. | Comparison with various chemotherapeutic agents is limited to free chlorambucil and cisplatin. | ||
| This work shows an effective way of retaining or improving the alkylating activity of chlorambucil. | Limited to in vitro results. | ||
| Therapeutic applications | Glycocluster-resorcinarene and thiodisaccharide: Langerin recognition studies [84]. | Creation of a glycocluster octavalent based on resorcinarene with hyaluronic acid mimetic. | Absence of advanced biological studies. |
| Specific interaction with Langerin demonstrated by STD-NMR, showing a multivalent effect. | The activity was verified only against Langerin; affinity against other relevant receptors was not studied. | ||
| Study the conjugates between resorcinarene dendrimers and ibuprofen [10]. | Biological activity tests showed that the synthesized compounds have high potential activity against cancer. | The biocompatibility of dendrimers is not comprehensively evaluated. | |
| They showed relevant cytotoxic effects against human glioblastoma and mammary adenocarcinoma cell lines. | Dendrimeric conjugates can be costly and difficult to scale up. | ||
| Calix[4]resorcinarene–mPEG and its antimicrobial activity [81]. | The macrocycle obtained has low hemotoxicity. High drug encapsulation capacity. Controlled drug release by self-assembly in nanostructures. | Dependence on the type of drug because not all compounds encapsulate with equal efficiency. Results are limited to in vitro tests. | |
| Analytic applications | Methacrylate resorcinarenes: Sorbent in noradrenalide extraction [69]. | Methacrylate-based polymers modified with resorcinarenes show significant potential for the micro-extraction of biomolecules. | Lack of studies with other analytes to validate the versatility of the material. |
| The chiral cavities introduced by the resorcinarenes allow enantioselective interactions. | Possible interference of other similar biomolecules in real biological matrices. | ||
| Resorcinarene-based sorbent butylmethacrylate and ethylenedimethacrylate in the enrichment of 3-hydroxy-4-trimethylaminobutyrate [71]. | The material demonstrates promising capability enrichment of L-carnitine from aqueous solutions. | Techniques based on the proposed copolymer can be improved, including the fabrication of sorbents for the preconcentration of other neurotransmitters. | |
| Conjugate can be used in several extraction cycles without significant loss of efficiency. | Specific conditions for improved performance. | ||
| New material, calix[4]resorcinarenes, as modifier of glycidyl methacrylate (GMA), promissory in extraction of amino acid [72]. | Enhanced surface polarity and the introduction of functionality present groups capable of interacting with peptides, which are about 60% at pH = 7.0. | The modifications could degrade under severe conditions (extreme pH or high temperatures). | |
| This is a promising stationary phase material for the separation of biomolecules by HPLC. | Specific reaction conditions (N2 atmosphere, DMF, basic agents) could restrict industrial scalability. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matiz, C.; Castellanos, K.; Maldonado, M. Resorcinarene-Based Polymer Conjugated for Pharmaceutical Applications. Processes 2025, 13, 1325. https://doi.org/10.3390/pr13051325
Matiz C, Castellanos K, Maldonado M. Resorcinarene-Based Polymer Conjugated for Pharmaceutical Applications. Processes. 2025; 13(5):1325. https://doi.org/10.3390/pr13051325
Chicago/Turabian StyleMatiz, Carlos, Karen Castellanos, and Mauricio Maldonado. 2025. "Resorcinarene-Based Polymer Conjugated for Pharmaceutical Applications" Processes 13, no. 5: 1325. https://doi.org/10.3390/pr13051325
APA StyleMatiz, C., Castellanos, K., & Maldonado, M. (2025). Resorcinarene-Based Polymer Conjugated for Pharmaceutical Applications. Processes, 13(5), 1325. https://doi.org/10.3390/pr13051325





