Screening of Amino Acids and Surfactant as Hydrate Promoter for CO2 Capture from Flue Gas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Setup and Material
2.2. Methods
2.3. Experimental Data Processing
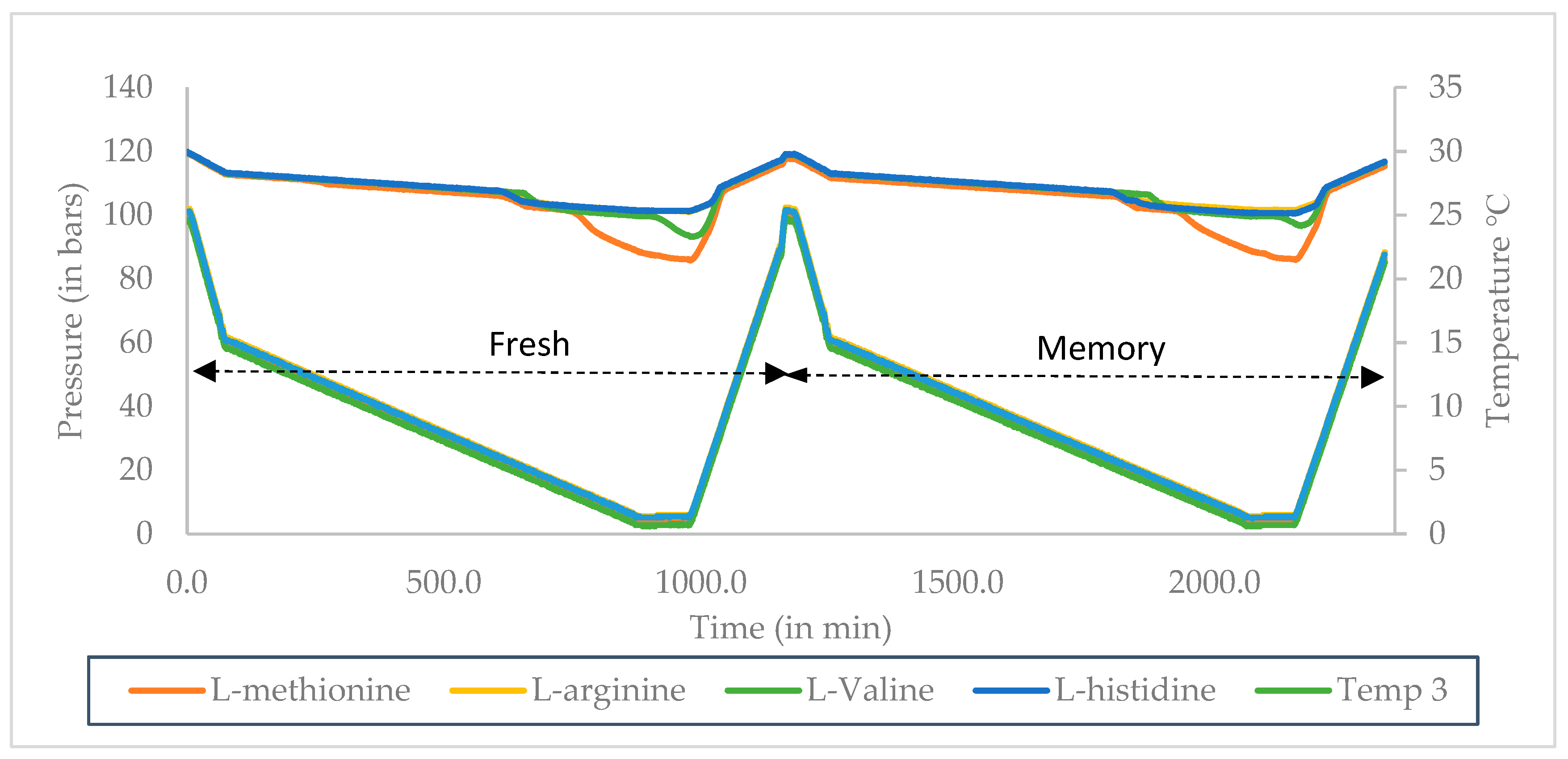
2.3.1. Constant Ramping Experiments
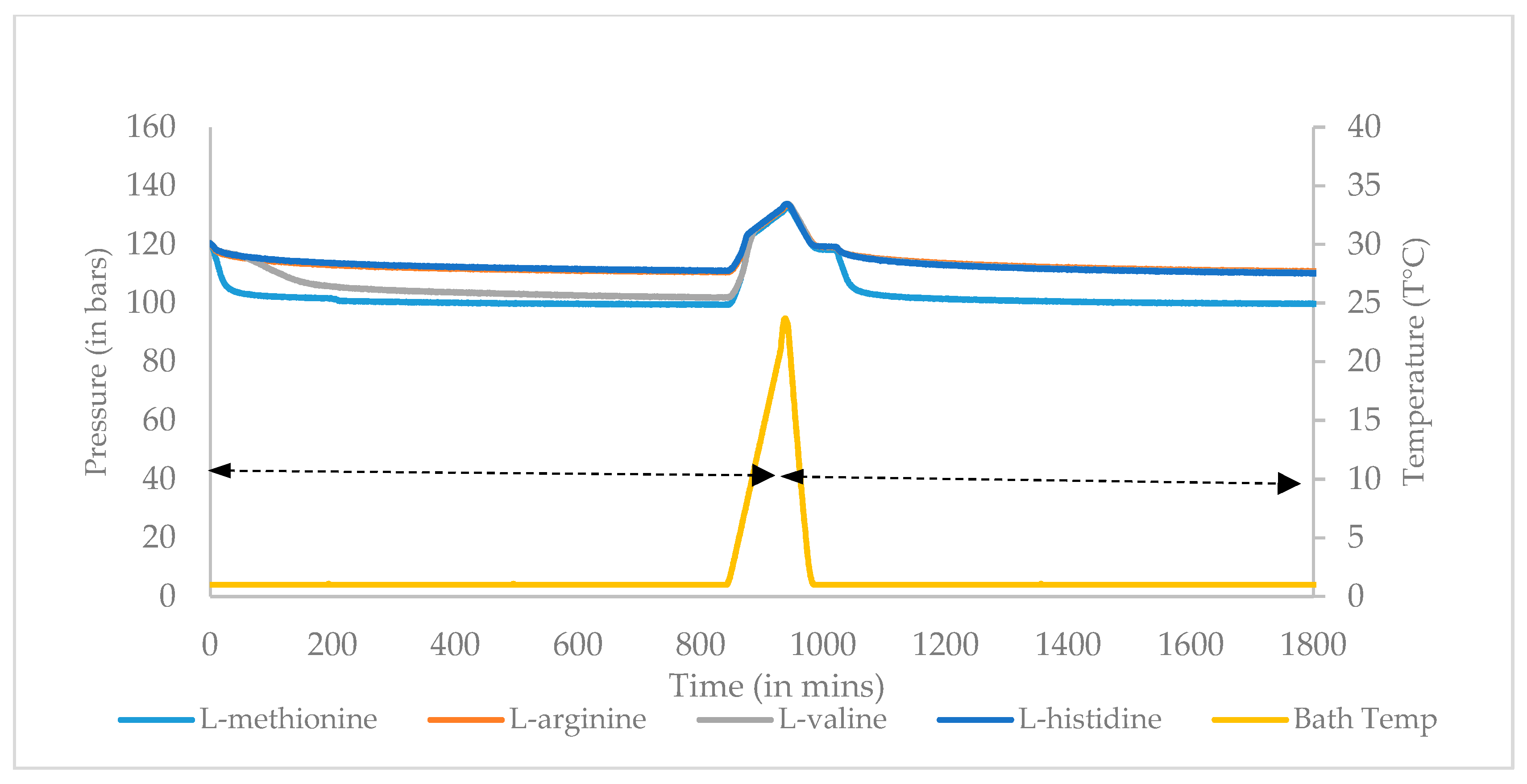
2.3.2. Isothermal Experiment
3. Results
3.1. Onset Temperature (T0) and Sub Cooling (ΔTsub) for Surfactant and Amino Acid
3.2. Induction Time Measurement for Surfactant and Amino Acids
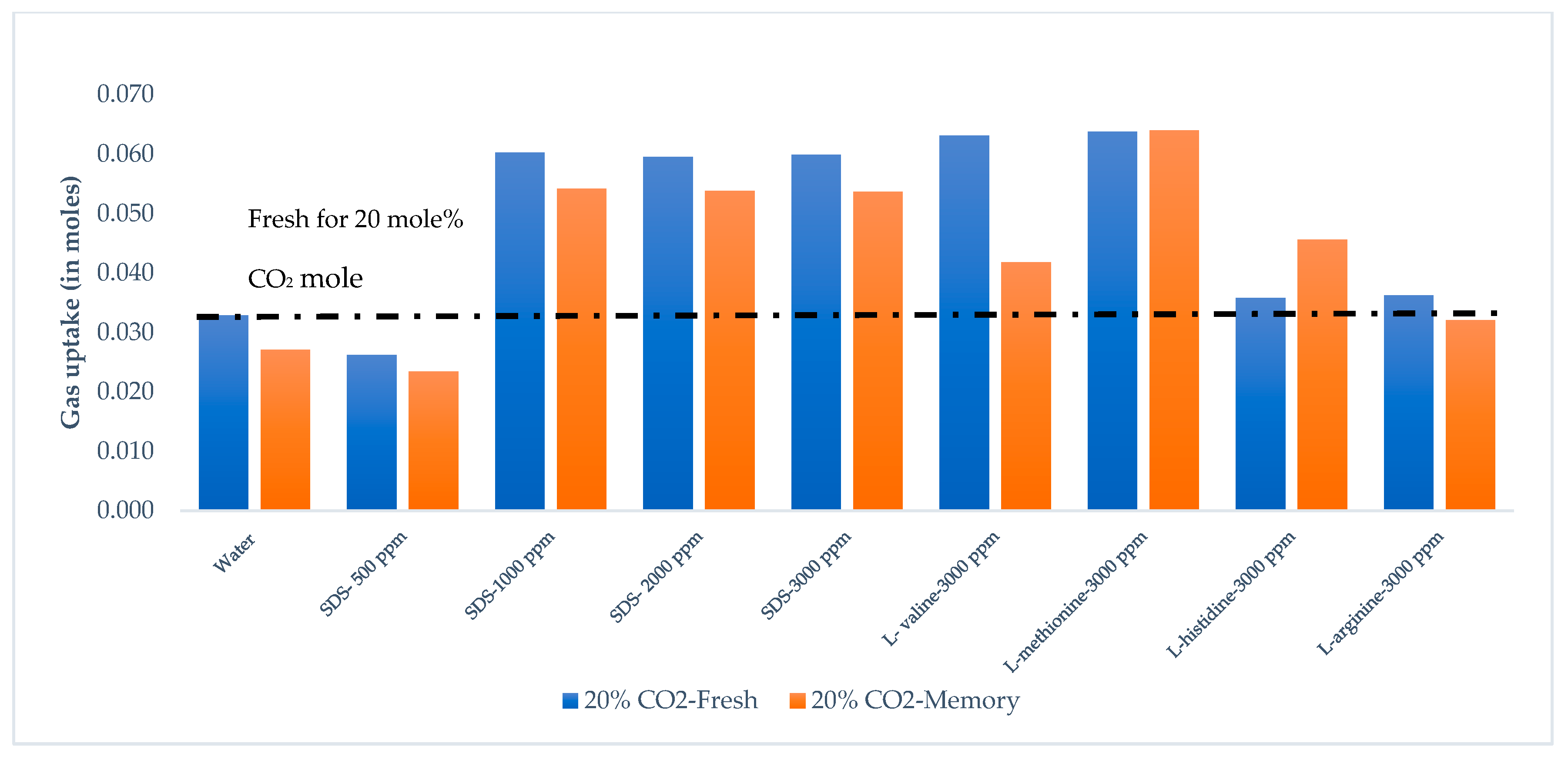
3.3. Gas Uptake and Growth Profile
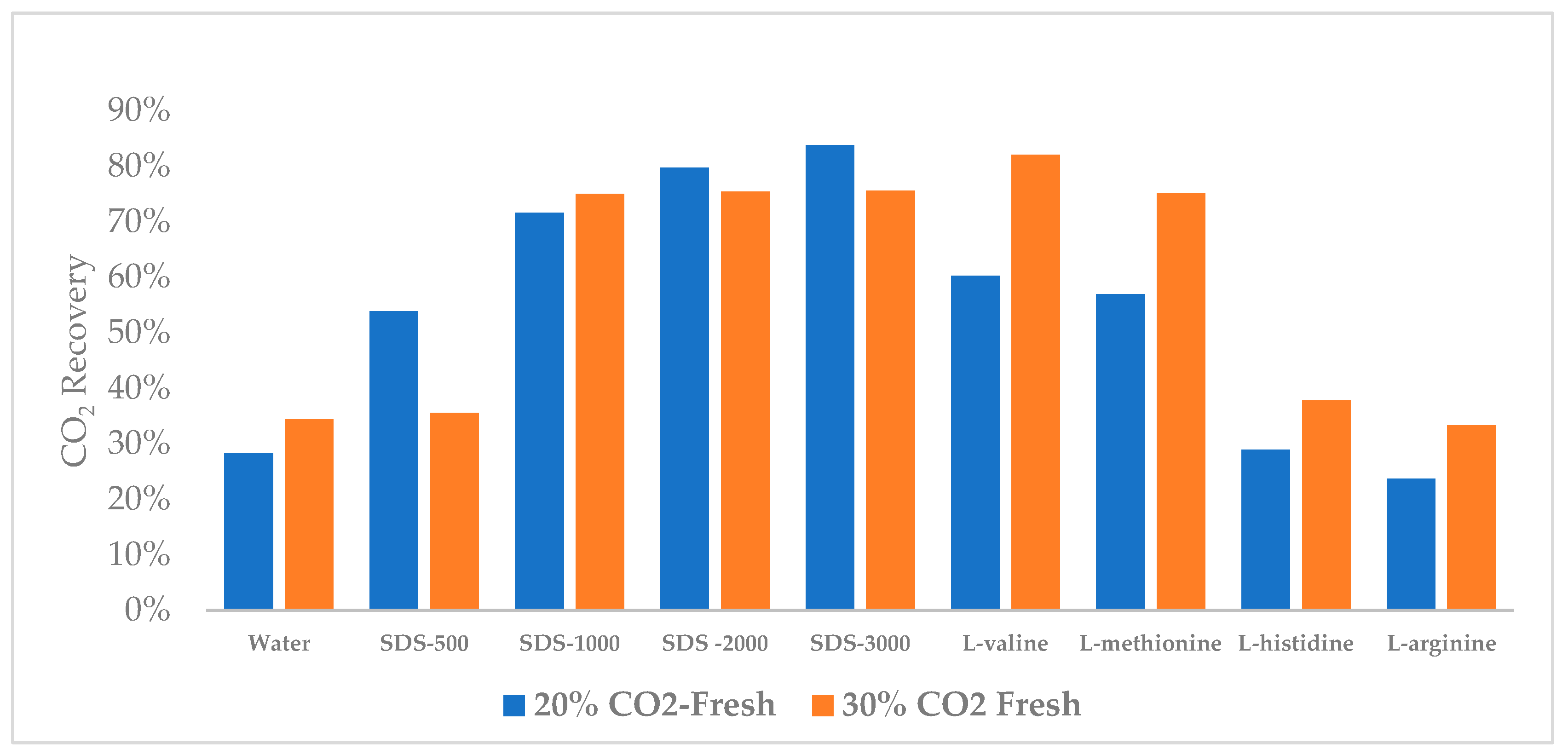
3.4. CO2 Recovery
3.5. Review of the Role of Amino Acids during CO2 Hydrate Formation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Boot-Handford, M.E.; Abanades, J.C.; Anthony, E.J.; Blunt, M.J.; Brandani, S.; Mac Dowell, N.; Fernández, J.R.; Ferrari, M.C.; Gross, R.; Hallett, J.P.; et al. Carbon capture and storage update. Energy Environ. Sci. 2014, 7, 130–189. [Google Scholar] [CrossRef]
- Parrenin, F.; Masson-Delmotte, V.; Köhler, P.; Raynaud, D.; Paillard, D.; Schwander, J.; Barbante, C.; Landais, A.; Wegner, A.; Jouzel, J. Synchronous change of atmospheric CO2and antarctic temperature during the last deglacial warming. Science 2013, 339, 1060–1063. [Google Scholar] [CrossRef]
- Dashti, H.; Yew, L.Z.; Lou, X. Recent advances in gas hydrate-based CO2 capture.pdf. J. Nat. Gas Sci. Eng. 2015, 23, 195–207. [Google Scholar] [CrossRef] [Green Version]
- Babu, P.; Linga, P.; Kumar, R.; Englezos, P. A review of the hydrate based gas separation (HBGS) process for carbon dioxide pre-combustion capture. Energy 2015, 85, 261–279. [Google Scholar] [CrossRef]
- Aaron, D.; Tsouris, C. Separation of CO2 from flue gas: A review. Sep. Sci. Technol. 2005, 40, 321–348. [Google Scholar] [CrossRef]
- Linga, P.; Kumar, R.; Englezos, P. The clathrate hydrate process for post and pre-combustion capture of carbon dioxide. J. Hazard. Mater. 2007, 149, 625–629. [Google Scholar] [CrossRef]
- Kumar, R.; Linga, P.; Englezos, P. Pre and post combustion capture of carbon dioxide via hydrate formation. In Proceedings of the 2006 IEEE EIC Climate Change Conference, Ottawa, ON, Canada, 10–12 May 2006; pp. 1–7. [Google Scholar]
- Kang, S.P.; Lee, H. Recovery of CO2 from flue gas using gas hydrate: Thermodynamic verification through phase equilibrium measurements. Environ. Sci. Technol. 2000, 34, 4397–4400. [Google Scholar] [CrossRef]
- Ho, L.C.; Babu, P.; Kumar, R.; Linga, P. HBGS (hydrate based gas separation) process for carbon dioxide capture employing an unstirred reactor with cyclopentane. Energy 2013, 63, 252–259. [Google Scholar] [CrossRef]
- Tang, J.; Zeng, D.; Wang, C.; Chen, Y.; He, L.; Cai, N. Study on the influence of SDS and THF on hydrate-based gas separation performance. Chem. Eng. Res. Des. 2013, 91, 1777–1782. [Google Scholar] [CrossRef]
- Lee, W.; Kim, Y.S.; Kang, S.P. Semiclathrate-based CO2capture from fuel gas in the presence of tetra-n-butyl ammonium bromide and silica gel pore structure. Chem. Eng. J. 2018, 331, 1–7. [Google Scholar] [CrossRef]
- He, Y.; Sun, M.-T.; Chen, C.; Zhang, G.-D.; Chao, K.; Lin, Y.; Wang, F. Surfactant-based promotion to gas hydrate formation for energy storage. J. Mater. Chem. A 2019, 7, 21634–21661. [Google Scholar] [CrossRef]
- Linga, P.; Kumar, R.; Englezos, P. Gas hydrate formation from hydrogen/carbon dioxide and nitrogen/carbon dioxide gas mixtures. Chem. Eng. Sci. 2007, 62, 4268–4276. [Google Scholar] [CrossRef]
- Daraboina, N.; Ripmeester, J.; Englezos, P. The impact of SO2 on post combustion carbon dioxide capture in bed of silica sand through hydrate formation. Int. J. Greenh. Gas Control 2013, 15, 97–103. [Google Scholar] [CrossRef]
- Kumar, A.; Sakpal, T.; Linga, P.; Kumar, R. Impact of fly ash impurity on the hydrate-based gas separation process for carbon dioxide capture from a flue gas mixture. Ind. Eng. Chem. Res. 2014, 53, 9849–9859. [Google Scholar] [CrossRef]
- Pandey, J.S.; Solms, N. von Hydrate Stability and Methane Recovery from Gas Hydrate through CH4–CO2 Replacement in Different Mass Transfer Scenarios. Energies 2019, 12, 2309. [Google Scholar] [CrossRef] [Green Version]
- Scott, M.J.; Jones, M.N. The biodegradation of surfactants in the environment. Biochim. Biophys. Acta Biomembr. 2000, 1508, 235–251. [Google Scholar] [CrossRef] [Green Version]
- Sa, J.H.; Lee, B.R.; Park, D.H.; Han, K.; Chun, H.D.; Lee, K.H. Amino acids as natural inhibitors for hydrate formation in CO2 sequestration. Environ. Sci. Technol. 2011, 45, 5885–5891. [Google Scholar] [CrossRef]
- Sa, J.H.; Kwak, G.H.; Lee, B.R.; Park, D.H.; Han, K.; Lee, K.H. Hydrophobic amino acids as a new class of kinetic inhibitors for gas hydrate formation. Sci. Rep. 2013, 3, 1–7. [Google Scholar] [CrossRef]
- Sa, J.H.; Kwak, G.H.; Lee, B.R.; Ahn, D.; Lee, K.H. Abnormal incorporation of amino acids into the gas hydrate crystal lattice. Phys. Chem. Chem. Phys. 2014, 16, 26730–26734. [Google Scholar] [CrossRef] [Green Version]
- Sa, J.H.; Kwak, G.H.; Han, K.; Ahn, D.; Lee, K.H. Gas hydrate inhibition by perturbation of liquid water structure. Sci. Rep. 2015, 5, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Alireza Bagherzadeh, S.; Alavi, S.; Ripmeester, J.A.; Englezos, P. Why ice-binding type i antifreeze protein acts as a gas hydrate crystal inhibitor. Phys. Chem. Chem. Phys. 2015, 17, 9984–9990. [Google Scholar] [CrossRef]
- Prasad, P.S.R.; Kiran, B.S. Are the amino acids thermodynamic inhibitors or kinetic promoters for carbon dioxide hydrates? J. Nat. Gas Sci. Eng. 2018, 52, 461–466. [Google Scholar] [CrossRef]
- Lal, B.; Mukhtar, H.; Bavoh, C.B.; Osei, H.; Sabil, K.M. A Review on the Role of Amino Acids in Gas Hydrate Inhibition, CO2 Capture and Sequestration, and Natural Gas Storage. J. Nat. Gas Sci. Eng. 2019, 64, 52–71. [Google Scholar]
- Pandey, J.S.; Daas, Y.J.; Solms, N. Von Insights into Kinetics of Methane Hydrate Formation in the Presence of Surfactants. Processes 2019, 7, 598. [Google Scholar] [CrossRef] [Green Version]
- Lone, A.; Kelland, M.A. Exploring kinetic hydrate inhibitor test methods and conditions using a multicell steel rocker rig. Energy Fuels 2013, 27, 2536–2547. [Google Scholar] [CrossRef]
- Kyte, J.; Doolittle, R.F. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 1982, 157, 105–132. [Google Scholar] [CrossRef] [Green Version]
- Roosta, H.; Dashti, A.; Mazloumi, S.H.; Varaminian, F. Inhibition properties of new amino acids for prevention of hydrate formation in carbon dioxide-water system: Experimental and modeling investigations. J. Mol. Liq. 2016, 215, 656–663. [Google Scholar] [CrossRef]
- Betts, M.J.; Russell, R.B. Amino Acid Properties and Consequences of Substitutions. In Bioinformatics for Geneticists; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2003; pp. 289–316. ISBN 9780470867303. [Google Scholar]
- Mukerjee, P.; Mysels, K.J. Critical micelle concentrations of aqueous surfactant systems. J. Pharm. Sci. 1972, 61, 319. [Google Scholar]
- Roosta, H.; Varaminian, F.; Khosharay, S. Experimental study of CO2 hydrate formation kinetics with and without kinetic and thermodynamic promoters. Sci. Iran. 2014, 21, 753–762. [Google Scholar]
- Molokitina, N.S.; Nesterov, A.N.; Podenko, L.S.; Reshetnikov, A.M. Carbon dioxide hydrate formation with SDS: Further insights into mechanism of gas hydrate growth in the presence of surfactant. Fuel 2019, 235, 1400–1411. [Google Scholar] [CrossRef]
- Kang, S.; Lee, J. Kinetic behaviors of CO 2 hydrates in porous media and effect of kinetic promoter on the formation kinetics. Chem. Eng. Sci. 2010, 65, 1840–1845. [Google Scholar] [CrossRef]
- Jiang, L.L.; Li, A.R.; Xu, J.F.; Liu, Y.J. Effects of SDS and SDBS on CO2Hydrate Formation, Induction Time, Storage Capacity and Stability at 274.15 K and 5.0 MPa. ChemistrySelect 2016, 1, 6111–6114. [Google Scholar] [CrossRef]
- Kelland, M.A. History of the development of low dosage hydrate inhibitors. Energy Fuels 2006, 20, 825–847. [Google Scholar] [CrossRef]
- Sloan, E.D.; Koh, C.A.; Koh, C. Clathrate Hydrates of Natural Gases, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Sloan, E.D.; Subramanian, S.; Matthews, P.N.; Lederhos, J.P.; Khokhar, A.A. Quantifying hydrate formation and kinetic inhibition. Ind. Eng. Chem. Res. 1998, 37, 3124–3132. [Google Scholar] [CrossRef]
- Seo, Y.T.; Moudrakovski, I.L.; Ripmeester, J.A.; Lee, J.W.; Lee, H. Efficient recovery of CO2 from flue gas by clathrate hydrate formation in porous silica gels. Environ. Sci. Technol. 2005, 39, 2315–2319. [Google Scholar] [CrossRef] [Green Version]
- Ide, M.; Maeda, Y.; Kitano, H. Effect of hydrophobicity of amino acids on the structure of water. J. Phys. Chem. B 1997, 101, 7022–7026. [Google Scholar] [CrossRef]
- Uchida, T.; Hirano, T.; Ebinuma, T.; Narita, H.; Gohara, K.; Mae, S.; Matsumoto, R. Raman spectroscopic determination of hydration number of methane hydrates. AIChE J. 1999, 45, 2641–2645. [Google Scholar] [CrossRef]
- Partoon, B.; Malik, S.N.A.; Azemi, M.H.; Phase, K.M.S. Experimental investigations on the potential of SDS as low-dosage promoter for carbon dioxide hydrate formation. ASIA-PACIFIC J. Chem. Eng. 2013, 8, 916–921. [Google Scholar] [CrossRef]
- Kumar, A.; Bhattacharjee, G.; Kulkarni, B.D.; Kumar, R. Role of Surfactants in Promoting Gas Hydrate Formation. Ind. Eng. Chem. Res. 2015, 54, 12217–12232. [Google Scholar] [CrossRef]
- Ohno, H.; Susilo, R.; Gordienko, R.; Ripmeester, J.; Walker, V.K. Interaction of antifreeze proteins with hydrocarbon hydrates. Chem. A Eur. J. 2010, 16, 10409–10417. [Google Scholar] [CrossRef]
- Sharp, K.A.; Madan, B. Hydrophobic effect, water structure, and heat capacity changes. J. Phys. Chem. B 1997, 101, 4343–4348. [Google Scholar] [CrossRef]
- Cai, Y.; Chen, Y.; Li, Q.; Li, L.; Huang, H.; Wang, S.; Wang, W. CO2 Hydrate Formation Promoted by a Natural Amino Acid l-Methionine for Possible Application to CO2 Capture and Storage. Energy Technol. 2017, 5, 1195–1199. [Google Scholar] [CrossRef]
- Bavoh, C.B.; Partoon, B.; Lal, B.; Gonfa, G.; Foo Khor, S.; Sharif, A.M. Inhibition effect of amino acids on carbon dioxide hydrate. Chem. Eng. Sci. 2017, 171, 331–339. [Google Scholar] [CrossRef]
- Prasad, P.S.R.; Sai Kiran, B. Clathrate Hydrates of Greenhouse Gases in the Presence of Natural Amino Acids: Storage, Transportation and Separation Applications. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
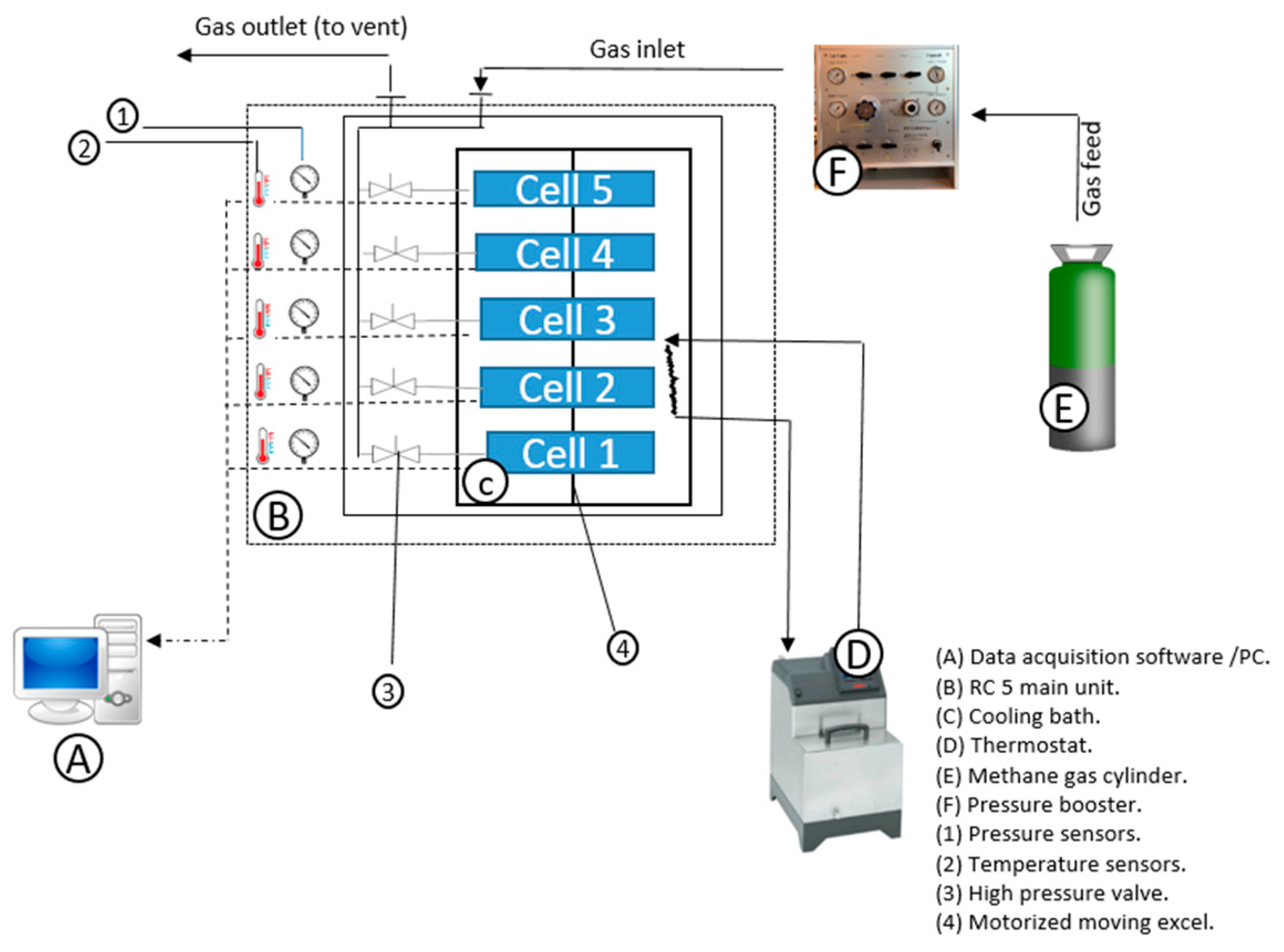

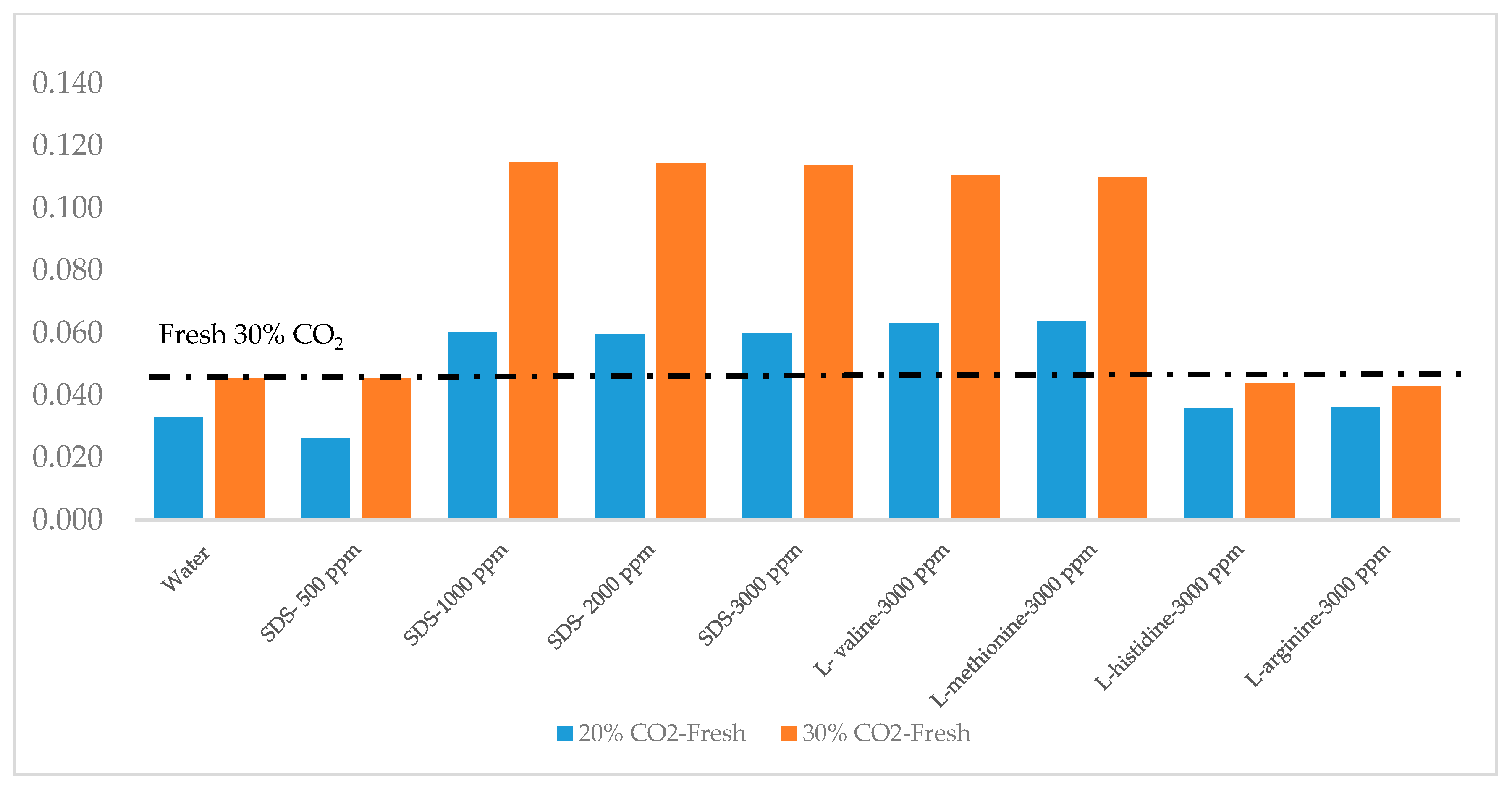

| # | Name | Side Chain Polarity | Side Chain | Molecular Formula | MWeight (g/mol) | Hydropathy Index Kyte et al. [27] |
|---|---|---|---|---|---|---|
| 1 | L-valine | Non-polar | -CH(CH3)2 | C5H11NO2 | 117.15 | 4.2 |
| 2 | L methionine | Non-polar | -CH3-S-(CH2)2 | C5H11NO2S | 149.21 | 1.9 |
| 3 | L-histidine | Basic polar. aromatic side chain | -CH2C3H3N2 | C6H9N3O2 | 155.16 | −3.2 |
| 4 | L-arginine | Basic polar. aliphatic side chain | HN=C(NH2)-NH(-CH2)3 | C6H14N4O2 | 174.20 | −4.5 |
| Name | Water Solubility (g/L) | Acidity (pH) | Pka Alpha Carboxy | Pka Alpha Amino | Pka Side Chain | pH | Isoelectric Point |
|---|---|---|---|---|---|---|---|
| L-valine | 58.5 | Neutral | 2.32 | 9.62 | 6.62 | 5.96 | |
| L-methionine | 56.6 | Neutral | 2.28 | 9.21 | 6.76 | 5.74 | |
| L-histidine | 45.6 | Basic (weak) | 1.82 | 9.17 | 6.04 | 7.64 | 7.59 |
| L-arginine | 182 | Basic (strong) | 2.17 | 9.04 | 12.48 | 10.31 | 11.15 |
| Promoters | 10% CO2/90% N2 | 20% CO2/80% N2 | 30% CO2/70% N2 |
|---|---|---|---|
| Trials | Trials | Trials | |
| Water | - | 4 | 4 |
| SDS | 1 | 2 | 1 |
| Amino Acid | 1 | 2 | 1 |
| 20% CO2/80% N2 | 30% CO2/70% N2 | ||||
|---|---|---|---|---|---|
| Concentration (ppm) | Fresh Runs | Fresh + Memory Run | Fresh Runs | Fresh + Memory Run | |
| Water | 0 | 4 | 4 | - | |
| SDS | 500 | 2 | 1 | 1 | - |
| 1000 | |||||
| 2000 | |||||
| 3000 | |||||
| Amino Acid | L-valine | 2 | 1 | 1 | - |
| L-methionine | |||||
| L-histidine | |||||
| L-arginine | |||||
| Onset Nucleation Temperature and Sucooling (ΔTo) in °C | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 20% CO2/80% N2 | 30% CO2/70% N2 | ||||||||
| Fr | Me | Fr | Me | Fr | Me | Fr | Me | ||
| Teq | 6.34 | 8.32 | |||||||
| Concentration (ppm) | ΔTsub,fr | ΔTsub,me | ΔTsub,fr | ΔTsub,me | |||||
| water | 0 | 4.10 | 4.10 | 2.24 | 2.24 | 5.80 | 5.70 | 2.52 | 2.62 |
| SDS | 500 | 3.85 | 3.65 | 2.49 | 2.69 | 4.90 | 4.80 | 3.42 | 3.52 |
| 1000 | 4.05 | 3.80 | 2.29 | 2.54 | 5.90 | 5.50 | 2.42 | 2.82 | |
| 2000 | 5.80 | 5.20 | 0.54 | 1.14 | 6.00 | 5.70 | 2.32 | 2.62 | |
| 3000 | 4.25 | 3.80 | 2.09 | 2.54 | 5.00 | 4.90 | 3.32 | 3.42 | |
| Amino Acid | L-valine | 5.40 | 5.30 | 0.94 | 1.04 | 5.80 | 5.70 | 2.52 | 2.62 |
| L-methionine | 4.60 | 4.65 | 1.74 | 1.69 | 5.70 | 5.40 | 2.62 | 2.92 | |
| L-histidine | 3.90 | 4.05 | 2.44 | 2.29 | 5.90 | 5.80 | 2.42 | 2.52 | |
| L-arginine | 4.25 | 4.25 | 2.09 | 2.09 | 5.80 | 5.70 | 2.52 | 2.62 | |
| Induction Time (to) (in mins) | |||||
|---|---|---|---|---|---|
| 20% CO2/80% N2 | 30% CO2/70% N2 | ||||
| Fresh | Memory | Fresh | Memory | ||
| Concentration (ppm) | |||||
| Water | 0 | No hydrae | No hydrate | No hydrate | - |
| SDS | 500 | No hydrae | No hydrate | No hydrate | - |
| 1000 | 72.5 | 70 | 27 | - | |
| 2000 | 14 | 4.5 | 13.5 | - | |
| 3000 | 4 | 1 | 2 | - | |
| Amino Acid | L-valine | 44 | 30 | 35 | - |
| L-methionine | 4.5 | 2 | 2 | - | |
| L-histidine | No hydrae | 35 | No hydrate | - | |
| L-arginine | No hydrae | 35.5 | No hydrate | - | |
| 20 % CO2/80 % N2 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Fresh Run | Memory Run | |||||||
| nFluegas | Pf (bar) | Pi-Pf (bar) | Δnfluegas (mole/mole) | Pf (bar) | Pi-Pf (bar) | Δnfluegas (mole/mole) | ||
| Pinj | 120 | |||||||
| Peq (20% CO2) | 56.85 | |||||||
| Pi-Peq | 63.16 | |||||||
| Concentration (ppm) | ||||||||
| 110.31 | 9.69 | 0.03 | 110.47 | 9.53 | 0.03 | |||
| Water | 0 | 0.18 | 110.18 | 9.82 | 0.03 | 109.95 | 10.05 | 0.02 |
| SDS | 500 | 0.18 | 98.29 | 21.71 | 0.06 | 98.32 | 21.68 | 0.05 |
| 1000 | 0.18 | 98.78 | 21.22 | 0.06 | 98.59 | 21.41 | 0.05 | |
| 2000 | 0.18 | 98.71 | 21.29 | 0.06 | 98.81 | 21.19 | 0.05 | |
| 3000 | 0.18 | 104.15 | 15.85 | 0.07 | 110.16 | 9.84 | 0.04 | |
| Amino acid | L-valine | 0.18 | 102.94 | 17.06 | 0.07 | 99.75 | 20.25 | 0.06 |
| L-methioine | 0.18 | 108.39 | 11.61 | 0.04 | 110.12 | 9.88 | 0.05 | |
| L-histidine | 0.18 | 109.06 | 10.94 | 0.03 | 110.63 | 9.37 | 0.03 | |
| L-arginine | 0.18 | 110.31 | 9.69 | 0.03 | 110.47 | 9.53 | 0.03 | |
| 20% CO2/80% N2 (Fresh) | 30% CO2/70% N2 (Fresh) | |||||||
|---|---|---|---|---|---|---|---|---|
| nFluegas | Pf (bar) | Pi-Pf (bar) | Δnfluegas (mole/mole) | Pf (bar) | Pi-Pf (bar) | Δnfluegas (mole/mole) | ||
| Pinj (bar) | 120 | |||||||
| Peq (20% CO2) | 56.84 | |||||||
| Peq (30% CO2) | 41.45 | |||||||
| Pi-Peq (20% CO2) | 63.16 | |||||||
| Pi-Peq (30% CO2) | 78.55 | |||||||
| Concentration (ppm) | ||||||||
| Water | 0 | 0.18 | 110.31 | 9.69 | 0.03 | 110.50 | 9.50 | 0.05 |
| SDS | 500 | 0.19 | 110.18 | 9.82 | 0.03 | 110.78 | 9.22 | 0.05 |
| 1000 | 0.19 | 98.29 | 21.71 | 0.06 | 90.40 | 29.60 | 0.11 | |
| 2000 | 0.19 | 98.78 | 21.22 | 0.06 | 90.68 | 29.32 | 0.11 | |
| 3000 | 0.19 | 98.71 | 21.29 | 0.06 | 90.31 | 29.69 | 0.11 | |
| Amino Acid | L-valine | 0.18 | 104.15 | 15.85 | 0.07 | 92.52 | 27.48 | 0.11 |
| L-methionine | 0.18 | 102.94 | 17.06 | 0.07 | 91.74 | 28.26 | 0.11 | |
| L-histidine | 0.18 | 108.39 | 11.61 | 0.04 | 111.96 | 8.04 | 0.04 | |
| L-arginine | 0.18 | 109.06 | 10.94 | 0.03 | 110.68 | 9.32 | 0.04 | |
| CO2 20%/80% N2 (Fresh) | CO2 30%/70% N2 (Fresh) | ||||||
|---|---|---|---|---|---|---|---|
| Concentration (ppm) | Moles of CO2 Injected | Mole of CO2 Remain in Gas | CO2 Recovery | Moles of CO2 Injected | A Mole of CO2 Remain in Gas | CO2 Recovery | |
| Water | 0 | 0.04 | 0.03 | 28% | 0.06 | 0.04 | 34% |
| SDS | 500 | 0.04 | 0.02 | 54% | 0.06 | 0.04 | 36% |
| 1000 | 0.03 | 0.01 | 72% | 0.06 | 0.01 | 75% | |
| 2000 | 0.03 | 0.01 | 80% | 0.06 | 0.01 | 75% | |
| 3000 | 0.03 | 0.01 | 84% | 0.06 | 0.01 | 76% | |
| Amino Acid | L-valine | 0.04 | 0.01 | 60% | 0.06 | 0.01 | 82% |
| L-methionine | 0.04 | 0.02 | 57% | 0.06 | 0.01 | 75% | |
| L-histidine | 0.04 | 0.03 | 29% | 0.06 | 0.04 | 38% | |
| L-arginine | 0.04 | 0.03 | 24% | 0.06 | 0.04 | 33% | |
| # | Amino Acid | Gas Tested | Test Type | Concentration | Pressure & Temperature | Reference | Remarks |
|---|---|---|---|---|---|---|---|
| 1.1 | L-valine | CO2 | THI | 0.1–0.5 mole % | 273.05–281.45 K, 14.1–35.2 bar | [18] | Phase equilibrium conditions reported, The shift in CO2 hydrate phase equilibrium to higher pressure and lower temperature is recorded L-valine solubility equal to 0.884 at 298.15 K and 0.763 at 273.15 K. Amino acid solubility in water affects the inhibition capability. THI function by lowering the water activity by associating with water molecules via hydrogen bonding. Increase in inhibiting effect increase with the increase in the size of the alkyl chain |
| 1.2 | L-valine | CO2 | KHI | 0.1 mole % | 36 bar, 284.05 K Isothermal 273.45 K | [19] | Inhibition is two types, absorption-based and perturbation inhibition Most of the amino acids are zwitterions during CO2 and interact with water due to strong electrostatic interaction generated by an electric charge on these molecules. The water structure around the electric charge becomes less ice-like. Less significant inhibition impacts due to the longer hydrophobic chain, thus increase hydrate promotion. |
| 1.3 | L-valine | CO2 | KHP | 0. 5 wt% | 33 bar, 298 K | [23] | The ability to inhibit the hydrate crystals decrease at higher mole fraction and at higher hydropathy index, reduction of THI capability observed. At higher concentrations, it is found that amino acids tend to crystallize. L-valine show 20% higher gas uptake than pure CO2 system under stirred conditions. |
| 2.1 | L-methionine | CO2 | KHP + XRD | 0.5 wt% | 53 bar, 275 K | [23,47] | Gas uptake 20% more than pure CO2 system. 90% of gas consumption in less than 45 min L-valine gas consumption takes double the time of the methionine system. L-methionine show highest gas uptake Near identical gas uptake in the stirred and unstirred system for L-methionine |
| 2.2 | L-methionine | CO2 | KHP | 0.02–1 wt% | 33 bar, 273.2 K | [45] | The presence of salt decreases the hydrate formation capability. Impurities in CO2 could have an impact on the CO2 hydrate formation. Significantly promote hydrate formation uptake without stirring Induction time equal to 15 min for 90% if gas consumption The amphiphilic molecule acts as a dispersant to prevent hydrate particle agglomeration, and the overall formation mechanism consists of hydrate film development at gas-liquid interface, nucleation at gas-liquid interface, growth of hydrate crystal as porous structure, and capillary action to promote growth. |
| 3.1 | L-histidine | CO2 | KHI+ Polarised Raman Spectroscopy | 0.1 mol % | 35 bar, 283.15 K | [21] | Experimental studies were carried out to study the perturbation of water in the presence of water. L-histidine display high hydrate inhibition effect caused by the perturbation of water structure. Perturbation is directly correlated with hydrophobicity. |
| 3.2 | L-histidine | CO2 | KHI + Spectroscopy | 0.5–2 wt% | 30 bar, 275.15 K | [28] | L-histidine show higher inhibition effect than glycine. The inhibition effect of the charged side chain is more than an uncharged side chain with a polar or non-polar group. Inhibition could be due to oxygen atom on carbonyl group forming a hydrogen bond with water or charged side chain forming van der Waal interaction/electrostatic with crystal surface and therefore interrupting nucleation/disrupt growth. The chemical affinity model is used to quantify the kinetic parameter. L-histidine based CO2 hydrate formation rate decrease with increasing concentration. Concentration range dependent on the amino acids. CO2 hydrate formation rate decrease with increasing hydrophobicity of un charge side chain in amino acids. |
| 4.1 | L-arginine | CO2 | THI | 10 wt% | 25.3–40 bar, 278.32–281.57 K | [46] | COSMO RS is used; the heat of dissociation is calculated to be 60.28 KJ/mole Inhibit CO2 hydrate formation, and inhibition effect is independent of the guest molecule and due to intermolecular forces. A higher alkyl chain in L-arginine leads to lower inhibition effect compare to glycine. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandey, J.S.; Daas, Y.J.; Solms, N.v. Screening of Amino Acids and Surfactant as Hydrate Promoter for CO2 Capture from Flue Gas. Processes 2020, 8, 124. https://doi.org/10.3390/pr8010124
Pandey JS, Daas YJ, Solms Nv. Screening of Amino Acids and Surfactant as Hydrate Promoter for CO2 Capture from Flue Gas. Processes. 2020; 8(1):124. https://doi.org/10.3390/pr8010124
Chicago/Turabian StylePandey, Jyoti Shanker, Yousef Jouljamal Daas, and Nicolas von Solms. 2020. "Screening of Amino Acids and Surfactant as Hydrate Promoter for CO2 Capture from Flue Gas" Processes 8, no. 1: 124. https://doi.org/10.3390/pr8010124






